Abstract
In contrast to angiosperms, pines and other gymnosperms form well-developed suspensors in somatic embryogenic cultures. This creates a useful system to study suspensor biology. In a study of gene expression during the early stages of conifer embryogenesis, we identified a transcript, PtNIP1;1, that is abundant in immature loblolly pine (Pinus taeda) zygotic and somatic embryos, but is undetectable in later-stage embryos, megagametophytes, and roots, stems, and needles from 1 year-old seedlings. Analysis of PtNIP1;1 transcript in embryo proper and suspensor tissues by reverse transcription-polymerase chain reaction suggests preferential expression in the suspensor. Based on comparisons of derived amino acid sequences, PtNIP1;1 belongs to the nodulin-like members of the major intrinsic protein superfamily branch of the aquaporin (major intrinsic protein) superfamily. Through heterologous expression in Xenopus laevis oocytes and the yeast (Saccharomyces cerevisiae) fps1− mutant, PtNIP1;1 has been shown to be an active aquaglyceroporin.
Embryogenesis is a critical period in the earliest stages of the sporophytic generation of plants. Following fusion of haploid male and female gametes to form the diploid zygote, embryo development and concomitant cellular differentiation commence. Subsequent early events in embryogenesis establish an embryo proper region and a separate suspensor region.
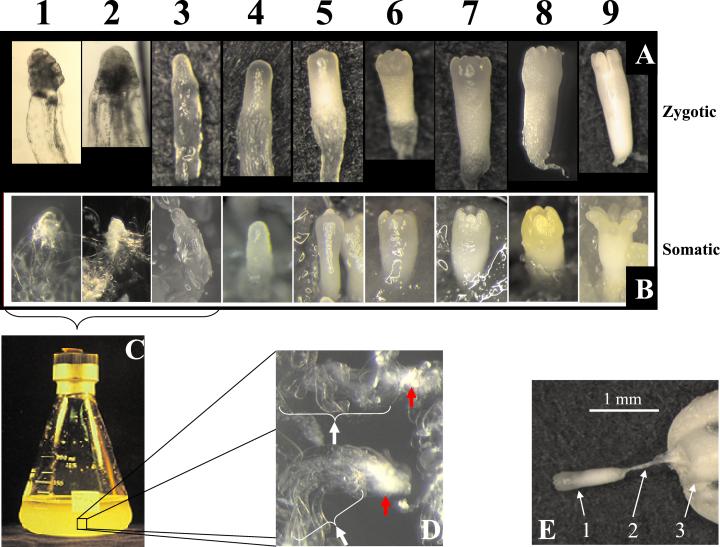
Although angiosperm and gymnosperm embryo ontogeny share many features, gymnosperms have notable unique features. For example, in contrast to the double fertilization event and triploid endosperm characteristic of angiosperms, gymnosperm embryogenesis proceeds via a single fertilization of the female oocyte. Embryos, therefore, develop in a haploid female tissue, the megagametophyte. In angiosperms, the first zygotic division determines the basal cell, which gives rise to the suspensor, and a terminal cell, which gives rise to the embryo proper. Gymnosperms, however, undergo a free-nuclear phase where several nuclear divisions occur (three divisions in Pinus spp. to yield eight nuclei) before cell wall formation. Another round of division produces a four-tiered, 16-celled proembryo. With respect to the mycropylar end of the seed, the four cells in the distal tier give rise to the embryo proper, and the next tier form the suspensor. Also common in gymnosperm embryogeny is a phenomenon called cleavage polyembryony, whereby each embryo proper can cleave into four individual embryos, each with its own intact suspensor (Spurr, 1949). Ultimately, one of the embryos dominates and the others degenerate. Finally, although not unique to gymnosperms, their embryos and suspensors tend to be large (Fig. 1E), which is conducive to facile dissection, visualization, and molecular analysis.
Figure 1.
Loblolly pine zygotic (A) and somatic (B) embryos from the nine developmental stages. The earliest two to three stages of somatic embryos typically are grown in liquid suspension culture (LSC) maintenance medium (C); stages 3 through 9 are on gelled, semi-solid maturation medium. D, Tissue in liquid cultures is characterized by dense embryo proper-like cell clusters (red arrows) surrounded by abundant, vacuolated suspensor-like cells (white arrows). E, Stage 7 zygotic embryo being dissected from megagametophyte (arrows: 1, embryo; 2, suspensor; and 3, megagametophyte).
The role of the suspensor in embryogenesis has been studied almost exclusively in angiosperms (for review, see Schwartz et al., 1997). Suspensor elongation and development is rapid, usually preceding embryo development. The suspensor stimulates growth of the embryo by synthesizing growth factors such as gibberellins (Cionini, 1987) and by acting as a conduit for nutrients from the surrounding cells or medium to the growing embryo (Yeung, 1980). Later, the suspensor undergoes programmed cell death and is absent or shrunken in the mature seed. Support of embryo growth appears to be achieved in a variety of ways, as suspensors exhibit a wide variety of shapes (filamentous, columnar, spherical, or irregular), sizes (minute, unicellular to large, and multicellular), ploidy, and metabolic activity. Smaller suspensors appear to promote growth via nutrient transport (e.g. suspensors of Capsella spp. have structural modifications to facilitate nutrient transport (Schulz and Jensen, 1969). Larger suspensors may serve as a storage tissue, and they appear to be more involved in macromolecular biosynthesis; thereby providing nutritional support for the embryo proper (Yeung and Meinke, 1993; Panitz et al., 1995; Cairney et al., 2000). From a genetic perspective, recently isolated developmental mutants are providing insights into suspensor function (for review, see Schwartz et al., 1997; Yadegari and Goldberg, 1997).
Suspensors usually fail to develop when somatic embryos of angiosperms are produced in culture (Yeung and Meinke, 1993). However, when pines and other conifers undergo somatic embryogenesis in culture, embryos develop with an attached suspensor that can readily be isolated from somatic embryos (Fig. 1, C and D). This creates a unique system to study suspensor molecular and cellular biology. This system has, so far, seen limited exploitation by molecular biologists (Cairney et al., 2000).
From an applied perspective, somatic embryogenesis is of particular interest to forest products industries as a method for mass-producing elite genotypes of commercially important coniferous species (Timmis, 1998; Grossnickle and Sutton, 1999). For loblolly pine (Pinus taeda), the predominating timber species of the southeastern United States (Schultz, 1999), the technology remains inefficient. The biochemical and metabolic reasons that underlie aberrant somatic embryo development are largely unknown. To better understand molecular events that are critical to proper embryogenesis, we have been exploring stage-specific gene expression during zygotic and somatic embryogenesis of loblolly pine, treating zygotic embryogenesis as the model against which somatic embryogenesis is judged (Cairney et al., 1999, 2000). For accurate comparison of somatic and zygotic embryos, we use a nine-stage system that is based on embryo morphology (Pullman and Webb, 1994; Fig. 1, A and B).
We have used differential display reverse transcription (RT)-PCR (Liang and Pardee, 1992) to search for genes expressed in very young somatic and zygotic embryos, before the formation of cotyledons. Here we report cloning, expression analysis, and functional characterization of one early expressed message, PtNIP1;1, that is very similar to members of the nodulin-like (NIP) members of the major intrinsic protein (MIP) superfamily. The mRNA appears exclusive to early embryo development. Evidence from functional analyses suggests that PtNIP1;1 forms an aquaporin channel upon expression in Xenopus laevis oocytes and, similar to AtNLM1 (Weig and Jakob, 2000a), functions as a glycerol permease upon expression in fps1− yeast (Saccharomyces cerevisiae). Taking advantage of large, easily dissectable loblolly pine embryos and suspensors, we performed RT-PCRs on embryo proper and suspensor tissues. Results suggest PtNIP1;1 expression is at least preferential for the suspensor. This result is consistent with previous reports of up-regulated MIP expression during cell elongation (Ludevid et al., 1992; Schünmann and Ougham, 1996; Smart et al., 1998; Weig and Eisenbarth, 2000) and may suggest a role for PtNIP1;1 in suspensor elongation. Alternatively, as a channel protein in suspensor cells, PtNIP1;1 may play a role in the transport of nutrients to the developing embryo proper.
RESULTS
PtNIP1;1 mRNA Is Detected Early in Embryogenesis and Is Most Homologous to the NIP Branch of the MIP Superfamily
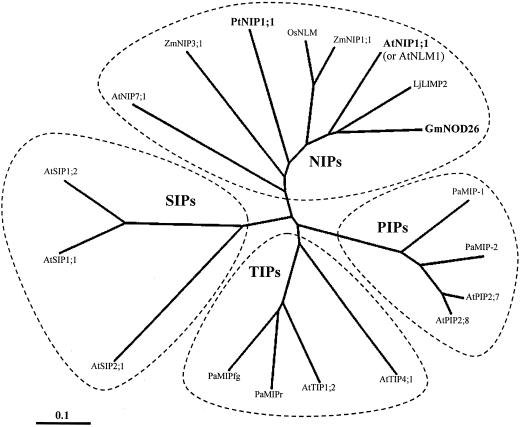
To identify genes involved in early events of loblolly pine embryogenesis, we performed a modified differential display procedure (Xu et al., 1997) on somatic embryos from all stages of development and cloned several cDNAs that appeared to be exclusive to early stage embryos. To confirm early stage somatic embryo expression, northern blots of LSC and late-stage somatic embryo RNA were probed with radiolabeled differential display expressed sequence tags (ESTs). One particular EST displayed very striking early embryogenesis specificity, so using a biotin-streptavidin bead strategy (Ciavatta and Cairney, 2000), its full-length cDNA was obtained. Blastx searches with the cDNA sequence against the National Center for Biotechnology Information GenBank database revealed significant primary amino acid sequence homology to NIPs (Weig and Jakob, 2000b). To reflect recently proposed MIP nomenclature (Johanson et al., 2001), this full-length cDNA was subsequently named PtNIP1;1. The predicted PtNIP1;1 amino acid sequence is very similar to other functionally characterized NIPs, sharing 41% identity + 21% similarity to Arabidopsis AtNLM1, 43% identity + 21% similarity to soybean (Glycine max) GmNOD26, 43% identity + 21% similarity to Lotus japonicus LjLIMP2, and near identity to AtNLM1, GmNOD26, and LjLIMP2 at five key positions that are significant for aquaporins or glycerol permeases (Dean et al., 1997; Rivers et al., 1997; Weig et al., 1997; Froger et al., 1998; Guenther and Roberts, 2000; Weig and Jakob, 2000a; Fig. 2). A cladogram of a multiple sequence alignment of PtNLM1;1 with selected MIPs demonstrates proper assignment of PtNIP1;1 to the NIP branch of the MIP superfamily (Fig. 3).
Figure 2.
Alignment of PtNIP1;1, AtNLM1, and GmNOD26 to emphasize similarity of PtNIP1;1 to other aquaglyceroporins. According to a survey of more than 150 MIPs that identified five residues that are significant for either aquaporins or glycerol permeases (Froger et al., 1998), the NIPs are aquaporin-like at P 2–4 and glycerol permease-like at P 1 and P 5. The alignment was assembled with CLUSTALW (Thompson et al., 1994), and the shading was performed with BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html).
Figure 3.
Phylogenetic analysis of PtNIP1;1 with selected MIPs from the four classes: NIPs, plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPS), and small and basic intrinsic proteins (SIPs). The scale indicates nucleotide substitutions per amino acid position. Organism abbreviations are as follows: At, Arabidopsis; Gm, G. max; Lj, L. japonicus; Os, Oryza sativa; Pa, P. abies; Pt, P. taeda; Zm, Z. mays. Accession numbers are: AtNIP7;1, AAF30303; ZmNIP3;1, AF326486; PtNIP1;1, AY055751; OsNLM, BAA04257; ZmNIP1;1, AF326483; AtNIP1;1 (referred to as AtNLM1 elsewhere in text), CAA16760; LjLIMP2, AAF82791; GmNOD26, AAA02946; PaMIP-1, CAB06080; PaMIP-2, CAB07783; AtPIP2;7, CAA17774; AtPIP2;8, AAC64216; AtTIP5;1, CAB51216; AtTIP1;2, BAB01832; PaMIPr, CAA06335; PaMIPfg, CAB39758; AtSIP2;1, CAB72165; AtSIP1;1, AAF26804; and AtSIP1;2, BAB09487.
To characterize expression further, we performed northern blots and slots blots with different embryo and vegetative tissues. Closer analysis of PtNIP1;1 expression throughout somatic embryogenesis revealed a drastic drop in expression that coincides with embryo maturation (Fig. 4A). Because our somatic embryo maturation protocol involves a switch from liquid maintenance medium to gelled, semi-solid maturation medium, we were interested to know whether the drop in PtNIP1;1 expression was triggered by this change from a submerged, aqueous environment to a more arid growth plate environment. To check whether this environmental switch had an effect on PtNIP1;1 expression, cells from LSC were plated on maintenance medium that had been amended with a gelling agent. After 4 weeks on gelled, semi-solid maintenance medium, however, northern analysis of RNA from the plate-grown tissue revealed no change in expression of PtNIP1;1 (not shown; i.e. PtNIP1;1 was still abundantly expressed in early stage somatic embryos growing on plates). Because the environmental switch did not appear to influence expression of PtNIP1;1, we were interested to know whether the PtNIP1;1 expression profile during somatic embryogenesis (high early, sharp decline, and undetectable late) would be conserved during zygotic embryogenesis. Upon analysis of zygotic embryos, PtNIP1;1 mRNA was again detected in early, but not late-stage embryos (Fig. 4B). Finally, to broaden the scope of our expression analyses, more northerns were done with nonembryo tissues. These northerns indicated that PtNIP1;1 expression was not detected in megagametophytes throughout embryogenesis (Fig. 4C), nor was it detected in roots, stems, and needles of 1-year-old loblolly pine seedlings (Fig. 4D). Therefore, all expression analyses detected PtNIP1;1 mRNA only in young, precotyledonary somatic and zygotic embryos. To our knowledge, no aquaporin-like gene has been shown to be expressed this early in embryogenesis.
Figure 4.
Expression analyses of PtNIP1;1 in P. taeda tissues. All blots were hybridized with a 32P-labeled 3′-untranslated region (UTR) fragment. A, Somatic embryo northern analysis. Each lane contained 5 μg of somatic embryo total RNA. B, Zygotic embryo slot blot. Each slot was loaded with 2 μg of zygotic embryo total RNA. C, Megagametophyte northern analysis. Each lane contained 10 μg of either somatic LSC or megagametophyte total RNA. D, Vegetative tissue northern analysis. Each lane contained 10 μg of either somatic embryo total RNA (LSC, late stage) or vegetative total RNA from 1-year-old seedlings (R, S, and N). RNA designations: L, late stage somatic embryo; R, root; S, stem; N, needle; numbers refer to embryo stages except for megagametophyte northern where numbers refer to stages of embryos removed prior to megagametophyte RNA isolation.
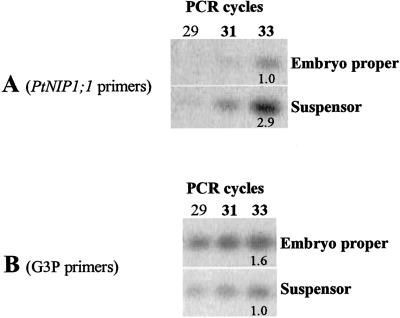
Aquaporin expression has been shown to be up-regulated in regions of cellular elongation (Ludevid et al., 1992; Schünmann and Ougham, 1996; Smart et al., 1998; Weig and Eisenbarth, 2000) and then down-regulated when cells became fully elongated (Weig and Eisenbarth, 2000). During early stages of conifer zygotic embryogenesis, suspensors undergo extensive elongation as the embryo proper advances into the corrosion cavity in the megagametophyte. Likewise, somatic embryo cultures are replete with elongating suspensor-like structures (Fig. 1D). However, as the embryo proper reaches middle to later developmental stages, suspensor cells of zygotic (Jones and Dangl, 1996) and somatic embryos (Filonova et al., 2000) undergo programmed cell death. When RNA was isolated from embryo tissues in preparation for northern and slot blots (Fig. 4, A–D), no dissection was made to separate embryo proper from embryo suspensor. Instead, the embryos proper with attached embryo suspensors were used for RNA isolations. To examine the site of PtNIP1;1 expression, we dissected zygotic embryo suspensors from embryo propers of stage 3 embryos and performed RT-PCR with PtNIP1;1-specific primers on 1 ng of poly(A+) RNA isolated from each tissue. The results indicate greater PtNIP1;1 mRNA abundance in suspensor cells than in embryo proper cells (Fig. 5). This result needs to be verified via mRNA in situ hybridization on early stage somatic and zygotic embryos.
Figure 5.
RT-PCR analysis of embryo proper and embryo suspensor total RNA. Aliquots for electrophoresis, ethidium bromide staining, and subsequent quantification were removed after 29, 31, and 33 cycles (see “Materials and Methods” for PCR conditions). Numbers beneath PCR products (1.0, 2.9, 1.6, and 1.0) are normalized signal intensities. Comparison of normalized signal intensities at equal numbers of PCR cycles during the linear range of the PCR reaction provides an estimate of PtNIP1;1 mRNA relative abundance in the original embryo proper and suspensor RNA samples. A, After background correction, measurement of ethidium bromide staining intensity indicates suspensor PtNIP1;1 PCR product was nearly 3-fold greater than embryo proper products after 33 cycles. B, As further support that approximately equal amounts of embryo proper and suspensor RNA were used in their respective RT reactions, embryo proper and suspensor RT products were PCR amplified with glyceraldehyde-3-phosphodehydrogenase (G3P) primers. Results indicate slightly higher expression of G3P in embryo proper compared with suspensor. Together, these results suggest a greater expression of PtNIP1;1 in suspensor than in embryo proper.
PtNIP1;1 Is an Aquaglyceroporin
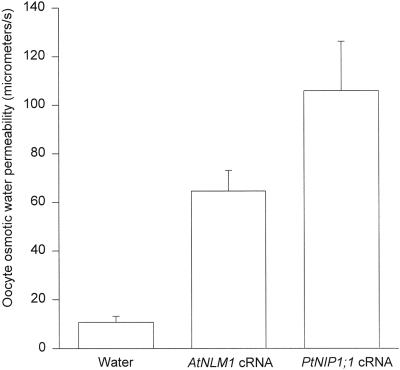
In light of primary amino acid sequence similarity, we were interested to know whether PtNIP1;1 would function similarly to AtNLM1 and GmNOD26. Because AtNLM1 and GmNOD26 were previously characterized as aquaporins (Rivers et al., 1997; Weig et al., 1997) and glyceroporins (Dean et al., 1997; Weig and Jakob, 2000a), we assessed the aquaporin and glyceroporin function of PtNIP1;1. To test aquaporin function, PtNIP1;1 cRNA, AtNLM1 cRNA (positive control), and water (negative control) were injected into X. laevis oocytes, and the average oocyte membrane osmotic water permeability, Pos, was determined for the three treatments. Results showed that Pos of PtNIP1;1-expressing oocytes was about 10 times greater than the Pos of the negative control (water-injected oocytes) and slightly greater than the Pos of the positive control (AtNLM1-expressing oocytes), suggesting PtNIP1;1 does function as an aquaporin (Fig. 6).
Figure 6.
Average osmotic water permeability, Pos, of oocytes injected with either cRNA (PtNIP1;1 and AtNLM1 [positive control]) or nuclease-free water (negative control). After measuring the rate of change of oocyte volume in response to a 5-fold drop in external osmolarity, oocyte Pos was calculated according to the formula in “Materials and Methods.” Each treatment (injection of PtNIP1;1, AtNLM1, or nuclease-free water) was repeated three times with five to seven oocyte measurements per repeat. For each treatment, height of bars in the figure represents a mean of all oocyte measurements and error bars represent one sd from the mean.
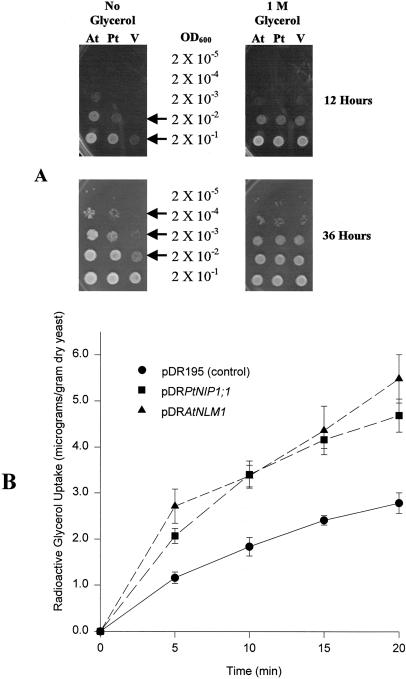
To test glyceroporin function, we used a strategy that had been previously used by Weig and Jakob (2000a) to demonstrate glycerol permease ability for AtNLM1. For these experiments, PtNIP1;1 was expressed in a yeast strain that lacks the glycerol facilitator protein, Fps1. Because of this mutation, fps1− yeast cannot rapidly modulate their internal glycerol concentration, and are consequently sensitive to hypo-osmotic shock (Tamás et al., 1999). Yeast transformants were tested for complementation of osmosensitivity and for increased ability to take up [3H]glycerol from surrounding medium. Results from the complementation experiments showed that PtNIP1;1 had a rescuing effect comparable to that of a proven aquaglyceroporin, AtNLM1 (Fig. 7A). Similarly, glycerol uptake experiments indicated that fps1− cells expressing PtNIP1;1 accumulated glycerol faster than vector transformed controls, and about as well as cells expressing AtNLM1 (Fig. 7B). Together, complementation and glycerol uptake experiments suggest that PtNIP1;1 can function as a glyceroporin.
Figure 7.
Demonstration of glyceroporin function for PtNIP1;1. Wild-type yeast have a plasma membrane glycerol facilitator protein, Fps1, to modulate glycerol efflux and thereby maintain osmotic balance with the environment (Tamás et al., 1999). Lacking this protein, fps− yeast display sensitivity to hypo-osmotic shock (i.e. slowed growth, presumably due to rapid influx of water, after a sudden decrease in external osmolarity). A, Complementation of yeast fps1− osmosensitivity by PtNIP1;1 and AtNLM1. fps1− yeast transformed with PtNIP1;1, AtNLM1 or vector-only (see “Materials and Methods” for plasmid construction) were grown to a common OD 600 in synthetic minimal medium amended with 1 m glycerol. For each culture, 10-fold serial dilutions were made and 5 μL of each dilution were spotted on synthetic minimal medium plates with 1 m glycerol (control) and without added glycerol (hypo-osmotic shock). Plates were incubated at 30°C for 12 and 36 h prior to photography. Upon hypo-osmotic shock, yeast that expressed PtNIP1;1 or AtNLM1 grew significantly better than vector-transformed yeast (arrows). All transformants grew equally well on plates with 1 m glycerol. B, Radioactive glycerol uptake by fps1− yeast that express PtNIP1;1 and AtNLM1. Yeast transformants were grown in synthetic minimal medium (no added glycerol) to OD 600 of 1.0. Fifty milliliters of each culture was washed twice and concentrated to 5 mL in 50 mm sodium phosphate buffer, pH 5.5. At 30°C and constant stirring, glycerol concentration was adjusted to 0.1 mm, 1% of which was 3H-labeled glycerol. At 5, 10,15, and 20 min, 100-μL samples, three samples at each time point, were removed, filtered, and washed. Radioactivity of each sample was determined and converted to mass of glycerol. Data in B are from one experiment of three that gave consistent results.
DISCUSSION
The distinctive aspects of conifer embryogenesis render the system worthy of careful study for the light shed on gymnosperm embryogenesis and plant embryogenesis in general. Somatic embryogenesis in conifers is an established, tractable system that facilitates study; certain cell lines produce healthy embryos capable of germination, other cell lines show consistent patterns of aberrant development. The system also offers a unique opportunity to explore embryo suspensor development in vitro—a fact that, until recently (Cairney et al., 2000), has been largely unexploited. Here, we report the identification and cDNA cloning of an mRNA expressed in the earliest stages of loblolly pine embryogenesis. The mRNA encodes a novel aquaglyceroporin whose expression pattern differs from previously identified members of the MIP superfamily. Evidence from RT-PCR experiments suggests that this mRNA may be located preferentially in the suspensor.
An Aquaglyceroporin That Is Expressed Exclusively during Early Embryogenesis
Major intrinsic proteins (MIPs) are integral membrane proteins that facilitate transmembrane movement of small polar molecules. These proteins belong to a superfamily that is ubiquitous throughout bacteria, fungi, plants, and animals. In plants, the family is large with 35 members identified in Arabidopsis (Johanson et al., 2001) and 34 in maize (Zea mays; Chaumont et al., 2001). The superfamily is divided into PIPS, TIPs, NIPs, and SIPs. Crystallographers recently have been able to resolve the membrane conformation of AQP1 and GlpF (Fu et al., 2000; Murata et al., 2000). These reports have shown which amino acid residues are important in forming the transmembrane channel pore and provide a basis for envisioning how water or solute molecules traverse the membrane.
Expression of MIPs has been shown to be an integral part of the embryogenesis program. Reports of embryogenesis-related MIPs, however, are limited to the late embryogenesis-expressed α-TIP, a seed- and embryo-specific aquaporin that has been described in several plants such as Phaseolus vulgaris, Arabidopsis, and spruce (Picea abies; Johnson et al., 1989; Höfte et al., 1992; Oliviusson and Hakman, 1995). This protein is situated in protein storage vacuolar membranes and may therefore play an important role in stock-piling nutrients necessary for proper embryo maturation and germination. There is also a report of MIP expression in reproductive tissues of maize where two NIPs, three SIPs, and four TIPs were shown to be exclusively or nearly exclusively identified in cDNA libraries from different developmental stages of reproductive tissues (Chaumont et al., 2001). However, it is not clear to what extent embryo tissue contributed to the reproductive tissues from which the cDNA libraries were derived.
In contrast to α-TIPs that are expressed in late-stage angiosperm and gymnosperm embryos and megagametophytes (Johnson et al., 1989; Höfte et al., 1992; Oliviusson and Hakman, 1995), PtNIP1;1 transcript is detected only in the earliest, precotyledonary-stage loblolly pine somatic and zygotic embryos. It is interesting that when an antiserum against the seed-specific α-TIP of P. vulgaris was used to probe western blots of protein preparations from spruce whole ovules, a low molecular mass band (approximately 26 kD) was detected from about the time of fertilization to early embryo stages (Oliviusson and Hakman, 1995). As embryos matured, detection of the higher molecular mass α-TIP (approximately 27 kD) became apparent, whereas the earlier-expressed band vanished. The early expression profile of the lower molecular mass protein noted by Oliviusson coincides with the mRNA expression profile of PtNIP1;1, which raises the possibility that the α-TIP antiserum from P. vulgaris cross reacts with a PtNIP1;1-like protein in spruce (PtNIP1;1 shares 29% identities plus 16% similarities to the original P. vulgaris α-TIP from which the antiserum was raised and 29% identities plus 17% similarities to the spruce MIPfg). If the α-TIP antiserum is cross-reacting to a PtNIP1;1-like protein in spruce, this would imply that it is smaller than the PtNIP1;1 predicted molecular mass of 28–29 kD. Alternatively, the antiserum may simply be recognizing another early embryogenesis TIP isoform from embryos, megagametophytes, or both.
Examples of tissue-specific expression, although not abundant, are not uncommon for plant MIPs (Johnson et al., 1989; Weig and Jakob, 2000b). More specifically, within the NIP subgroup (accessions listed at http://mbclserver.rutgers.edu/CPGN/AquaporinWeb/Aquaporin.Table.html), four analyzed maize cDNAs show different expression patterns (ZmNIP2-1 and ZmNIP2-2 are exclusive to aereal vegetative tissues and ZmNIP1-1 and ZmNIP3-1 are nearly exclusive to reproductive tissues; Chaumont et al., 2001), and similarly, Arabidopsis AtNLM1(AtNIP1;1) and AtNIP4;1 seem to be exclusive to roots (Weig and Jakob, 2000b). The specific expression pattern of PtNIP1;1 is consistent, therefore, with what has previously been reported for some MIPs.
In light of NIP diversity within plants (five NIPs identified in maize [Chaumont et al., 2001], nine in Arabidopsis [Johanson et al., 2001]), other NIPs would be expected in loblolly pine. A search of the Pine Gene Discovery Program EST database (http://www.cbc.umn.edu/ResearchProjects/Pine/DOE.pine/index.html) returned a 350-bp EST derived from loblolly pine normal xylem. Alignment and cladogram of the theoretical translation of the xylem EST with other MIPs revealed 85% nucleotide identity to PtNIP1;1 spanning 292 bases, 77% amino acid identity spanning 114 amino acids, and clearly established the xylem EST as a NIP (not shown). Perhaps not coincidentally, this is another example of MIP expression in a region characterized by cell elongation. The 85% nucleotide identity of the xylem EST to PtNIP1;1 over 292 bases would suggest a reasonably high degree of 3′-UTR similarity. It is curious, however, that we fail to detect a band in northern blots of 1-year-old stem tissue probed with the PtNIP1;1 3′-UTR (Fig. 4D). This might suggest a very low abundance of the xylem NIP in our 1-year-old seedling stem RNA samples and/or significant divergence in the 3′-UTR regions of the loblolly pine embryogenesis (PtNIP1;1) and xylem NIPs.
Opportunities for Deciphering the Biological Function of PtNIP1;1
Multiple members in a gene family and tissue-specific expression such as that seen for PtNIP1;1 argues for specialized function. Knowledge of such biological function for PtNIP1;1 should prove useful to understanding embryogenesis in greater detail. More information is needed, however, to establish a biological role. Narrowing the expression profile to a few weeks early in embryogenesis begs finer localization on a cellular level (embryo suspensor versus embryo proper) and subcellular level (vacuolar, plasma, or other membrane) so the exact location of PtNIP1;1 channels can be determined.
Work by Panitz et al. (1995) in broad bean (Vicia faba), showed transient accumulation of storage proteins and their mRNAs in suspensor and endosperm preceded synthesis in the embryo proper. We have observed differential accumulation of an array of transcripts in suspensor, megagametophyte, and embryo proper of loblolly pine (Cairney et al., 2000; J. MacKay and C. Perfetti, unpublished data). During embryo dissections in preparation for our RT-PCRs, contamination of embryo proper tissue with suspensor tissue and vice versa was unavoidable. Despite problems of accurately separating embryo proper from embryo suspensor, present RT-PCR results (Fig. 5) indicate greater PtNIP1;1 expression in the suspensor. More qualitative expression analyses with earlier stages of development also show preferential PtNIP1;1 expression in the suspensor (not shown). The profile and localization of PtNIP1;1 expression is now being studied in greater detail to determine whether localization of expression varies over development, although present expression analyses (Fig. 4, A and B) would seem to preclude the type of sus-pensor-expression-early followed by embryo-proper-expression-late pattern described by Panitz et al. (1995).
Several possible roles can be envisioned for PtNIP1;1 channels in the suspensor. A role in suspensor elongation could be envisioned as aquaporins have been shown to be up-regulated in regions of cellular elongation (Ludevid et al., 1992; Schünmann and Ougham, 1996; Smart et al., 1998; Weig and Eisenbarth, 2000). That PtNIP1;1 fluxes glycerol in addition to water raises the possibility that it is a multifunctional solute channel as other plant (Rivers et al., 1997; Gerbeau et al., 1999) and mammalian (Ishibashi et al., 1997; Tsukaguchi et al., 1998) aquaglyceroporins appear to be permeable to small, uncharged solutes when expressed in oocytes. As a solute channel, PtNIP1;1 may play a role in transporting nutrients to the developing embryo, or solute flux through PtNIP1;1 might be critical to maintaining turgor as suspensors elongate. In addition, permeation of NH3 in peribacteroid membrane vesicles was shown to be partially mediated by proteinaceous channels (Niemietz and Tyerman, 2000), raising the question of GmNOD26 involvement in NH3 flux. Assuming GmNOD26 and other NIPs like PtNIP1;1 flux NH3, an immediate role could be envisioned for PtNIP1;1, because embryo maturation is marked by storage protein accumulation and suspensors likely synthesize storage proteins (Panitz et al., 1995; Cairney et al., 2000), both of which may require additional capacity for shuttling nitrogen.
Whatever the true biological function of PtNIP1;1 it is clear that more work is needed to establish its role in embryogenesis. Fortunately, established conifer embryo research programs are well positioned to decipher biological functions of embryogenesis genes by exploiting virtues of somatic embryogenesis. That conifer somatic embryogenic material is amenable to genetic transformation (for review, see Ahuja, 2000) makes it a potentially workable system to study embryo- and suspensor-specific genes through transformation-dependent strategies (e.g. RNA interference, promoter-reporter fusions, etc.), and because large amounts of tissue can be rapidly generated, ample transgenic tissue is readily available for physiological and genome-wide expression studies (Cairney et al., 1999, 2000). In addition, the rather large size of conifer somatic embryos has proven useful for sectioning and mRNA in situ hybridization (Cantón et al., 1999; Sabala et al., 2000; Avila et al., 2001). Last, development of somatic embryos with attached suspensors creates a useful system to study gene function during suspensor development (e.g. PtNIP1;1) and suspensor biology in general.
MATERIALS AND METHODS
Plant Tissue
Loblolly pine (Pinus taeda) somatic embryo cultures were initiated as described by Becwar and Pullman (1995) with modifications. Somatic embryos were subsequently grown in liquid maintenance medium 16 and on gelled maturation medium 240 (Pullman and Webb, 1994). Weekly, aliquots of LSC were filtered with Miracloth (Calbiochem, San Diego) to remove excess liquid medium, placed in 50-mL tubes, immediately frozen in liquid nitrogen, and stored at −70°C. For northern analysis to compare PtNIP1;1 expression in early stage somatic embryos maintained in LSC versus gelled medium, 1 mL of LSC was plated on gelled maintenance medium (identical composition to LSC plus 0.25% [w/v] Phytagel [Sigma, St. Louis]). Weekly, the mass of early stage embryos was transferred to fresh gelled maintenance medium. After 4 weeks, tissue was collected and frozen in liquid nitrogen in preparation for total RNA isolation. For later stage embryos growing on gelled maturation medium, embryos were judged for stage of development under a dissecting microscope according to Pullman and Webb (1994), selected from plates, plunged into liquid nitrogen, and stored at −70°C. Zygotic embryo tissue was collected from cones of mother tree UC5-1036 (generously supplied by Union Camp Corporation, Bellville, GA). Cones were packed on ice and shipped overnight, and seeds were extracted upon receipt. Embryos were dissected from seeds, judged for stage of development according to Pullman and Webb (1994), frozen in liquid nitrogen, and stored at −70°C until RNA extraction. No attempt was made to separate embryo proper from embryo suspensor for any differential display or northern analysis.
Differential Display, PtNIP1;1 Cloning, and Sequence Analysis
Poly(A+) RNA was extracted from early stage somatic embryo tissue maintained in LSC and more mature somatic embryos maintained on gelled maturation medium using oligo(dT)-coated beads (Dynal, Lake Success, New York). Differential display was performed essentially as described previously (Xu et al., 1997). The full-length PtNIP1;1 cDNA was captured from SMART cDNA synthesized from somatic embryo LSC RNA as described previously (Ciavatta and Cairney, 2000), cloned into pGEM T Easy (Promega, Madison, WI), and sequenced by the dideoxy chain termination method. Sequence alignments were performed with CLUSTALW (Thompson et al., 1994). The shaded alignment was constructed with BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html). The tree was assembled with TreeView(Win32) 1.6.5 (Page, 1996).
Northern Analyses
Total RNA for expression analysis was isolated by two methods. A modified hexadecyltrimethylammonium bromide procedure (Chang et al., 1993) was used for early stage somatic embryos from LSC and vegetative tissues. For all zygotic and somatic embryo tissue other than LSC, the RNeasy Plant Mini Kit (Qiagen, Valencia, CA) was used with 1% (w/v) polyvinylpyrrolidone, 30K (Acros Organics, Fisher Scientific, Pittsburgh) added to RNA extraction buffer. For northern blots, total RNA was separate on a formaldehyde-containing agarose gel and transferred to Hybond N+ membrane (Amersham Biosciences, Piscataway, NJ) according to the method in Ausubel et al. (1995). For slot blots, 2 μg of total RNA per slot was attached to Hybond N+ membrane (Amersham Biosciences) with a PR 648 Slot Blot Filtration Manifold (Hoefer Scientific Instruments, San Francisco) according to the method in Ausubel et al. (1995). The RNAs were UV cross-linked to nylon membranes, prehybridized at 65°C for >3 h in several changes of hybridization buffer (0.5 m sodium phosphate buffer [pH 7.2], 5% [w/v] SDS, 10 mm EDTA [pH 8.0], and 1% [w/v] BSA; Church and Gilbert, 1984). Probes were prepared from 50 to 100 ng of DNA from the PtNIP1;1 3′-UTR and 5 μL of α-[32P]dATP (10 mCi mL−1; Amersham Biosciences) with Ready-To-Go DNA Labeling Beads (Amersham Biosciences) according to manufacturer's instructions. Before hybridization, probes were purified with Nick Columns (Amersham Biosciences), heat denatured, and placed on ice. All hybridizations were done overnight in the above hybridization buffer at 65°C. Blots were washed as follows: two times, 5 min each wash in 2× SSC and 0.1% (w/v) SDS at room temperature; two times, 5 min each wash in 0.2× SSC and 0.1% (w/v) SDS at room temperature; and two times, 15 min each wash in 0.2× SSC and 0.1% (w/v) SDS at 65°C. Blots were exposed overnight to a phosphorimaging plate, images were read with a BAS1800 (Fuji Photo Film Co., Ltd., Kanagawa, Japan), and images were manipulated with ImageGauge (version 2.54, Fuji Photo Film Co.).
Embryo Proper and Suspensor RT-PCR
Thirty stage 3 zygotic embryos were removed from megagametophytes, dissected into embryo proper and suspensor regions, and frozen in liquid nitrogen. Poly(A+) RNA was extracted from the two tissues with oligo(dT)-coated beads (Dynal, Lake Success, New York). RNA concentration was measured with Ribogreen RNA quantitation reagent (Molecular Probes, Eugene, OR). In 20-μL reactions, 1 ng of each RNA was primed with oligo(dT12–18), reverse transcribed with Superscript II, and treated with RNase OUT (Invitrogen Life Technologies, Carlsbad, CA) according to manufacturer's instructions. For PCRs, 4 μL of reverse transcription products were PCR amplified in 100-μL reactions with either forward and reverse PtNIP1;1 gene-specific primers (0.2 μm each) or forward and reverse G3P primers (0.2 μm each), dNTPs (0.2 mm each dNTP), 10× buffer (10 μL), and Advantage cDNA polymerase mix (2.0 μL; CLONTECH, Palo Alto, CA). Reaction conditions were: 94°C, 2 min; 35× (94°C, 15 s; 67.3°C, 30 s; 72°C, 1 min); 72°C, 5 min for PtNIP1;1 primers and 94°C, 2 min; 35× (94°C, 15 s; 60.0°C, 30 s; 72°C, 30 s); 72°C, 5 min for G3P primers. Aliquots (8 μL) were removed after every other cycle starting at cycle 17 and separated in 1.5% (w/v; PtNIP1;1 products) or 2.0% (w/v; G3P products) agarose, stained with ethidium bromide (0.5 μg mL−1), and photographed under UV illumination. Digitized signals were quantitated with ImageGauge (version 2.54, Fuji Photo Film Co.). All RT-PCRs were repeated three times to verify consistent results.
Complementation Test and Glycerol Uptake Assays
Complementation and uptake experiments were performed in yeast (Saccharomyces cerevisiae) strain YSH6.114.-2A kindly donated by Dr. Alfons Weig. The PtNIP1;1 ORF was excised from pGEM T Easy with NotI and subcloned into the NotI site of the yeast expression vector pDR195 (Rentsch et al., 1995). Proper sense orientation with respect to the PMA1 promoter was determined by restriction digestion and corroborated with dideoxy sequencing. Empty pDR195 (negative control), PtNIP1;1-containing vector, and AtNLM1-containing vector (positive control) were introduced into yeast cells via the SC EasyComp Transformation Kit (Invitrogen) and transformants were selected with synthetic minimal medium lacking Leu and uracil (SC −Ura, −Leu; required amino acids, 2% dextrose, and 0.67% yeast nitrogen base without amino acids). Complementation tests and radioactive glycerol uptake experiments were conducted essentially as described by Weig et al. (2000) with the amendment that glycerol uptake is expressed on a per gram of dry yeast basis.
Expression of PtNIP1;1 in Xenopus laevis Oocytes
To prepare the PtNIP1;1 ORF for subcloning, the plasmid was linearized with AvaI and blunted with Klenow fragment, and the insert was released with SpeI. For directional cloning, the resulting PtNIP1;1 ORF fragment was ligated into pAW2 (contains the 5′- and 3′-untranslated sequences of the Xenopus β-globin gene) that had been digested with SpeI and EcoRV. Resulting plasmid DNA was sequenced to verify construction and linearized with NaeI. Capped RNA was made with the T3 RNA polymerase from the mMessage mMachine kit according to manufacturer's instructions (Ambion, Austin, TX).
Oocyte Osmotic Water Permeability Assay
X. laevis oocytes (stages V and VI) were prepared as described previously (Zhang and Verkman, 1991) and incubated overnight at 16°C in ND96 buffer (96 mm NaCl, 5.0 mm HEPES, 1.0 mm MgCl2, 2.0 mm KCl, 1.8 mm CaCl2, 5.0 mm sodium pyruvate, and 0.1 mg mL−1 gentamicin) prior to injection. Oocytes were injected with 50 nL of 0.5 ng nL−1 in vitro synthesized transcripts PtNIP1;1 or AtNLM1 (positive control) or nuclease-free water (negative control) and kept at 16°C. ND96 buffer was changed and dead oocytes were removed daily until swelling assays were conducted. Three days after injection, oocyte osmotic water permeability (Pos) was determined. At room temperature, individual oocytes experienced a 5-fold drop in external osmolarity (200 mosmol to 40 mosmol) while oocyte images were captured every 5 s for 1.5 min with Scion Image software. Assuming oocytes were perfect spheres, Pos was calculated for each oocyte by: Pos = Vo(d(V/Vo)/dt) S−1Vw−1(Osmin − Osmout)−1 where Vo is the initial oocyte volume (determined for each oocyte), d(V/Vo)/dt is the relative rate of volume change determined by the initial slope of V/Vo versus time, S is the initial oocyte surface area (determined for each oocyte), Vw is the molar volume of water (18 cm3 mol−1), Osmin is the osmolarity inside the oocyte (2.0 × 10−4 mol cm−3) and Osmout is the medium osmolarity (4.0 × 10−5 mol cm−3; Zhang and Verkman, 1991). Injections and subsequent swelling assays were conducted on three separate replicates of oocyte preparations and five to seven oocytes were measured in each replicate.
Note Added in Proof
Since the acceptance of this manuscript, Weterings et al. (2001), working with scarlet runner bean (Phaseolus coccineus), have described the isolation of two genes of unknown function, G564 and C541, whose mRNAs accumulate specifically in the suspensor of globular-stage embryos. Gene G564 is transcriptionally regulated, and its promoter directs expression of GUS to the suspensor transgenic tobacco embryos containing a G564/GUS chimeric gene. Our recent work has demonstrated that an 880-bp DNA fragment from loblolly pine, which includes 450 bp 5' of the putative transcriptional start site of NIP1;1, directs GUS expression specifically to the embryonal tube cells and suspensor of somatic embryos of transgenic Norway Spruce containing a NIP1;1/GUS chimeric gene (V.T. Ciavatta, U. Egertsdotter, D. Clapham, S. von Arnold, and J. Cairney, unpublished data).
ACKNOWLEDGMENTS
We would like to thank Dr. Sarah Covert for the G3P primers, Dr. John MacKay for assistance with embryo dissections, Dr. Alfons Weig for control plasmids and helpful comments, and Dr. Julian Schroeder for use of oocyte injection materials.
Footnotes
This work was supported by the Member Companies of Institute of Paper Science and Technology (studentship to V.T.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010793.
LITERATURE CITED
- Ahuja MR. Genetic engineering in forest trees: state of the art and future perspectives. In: Jain SM, Minocha SC, editors. Molecular Biology of Woody Plants. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 31–49. [Google Scholar]
- Ausubel FM, Brent R, Kinston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. Ed 3. New York: John Wiley & Sons; 1995. [Google Scholar]
- Avila C, Suárez MF, Gómez-Maldonado J, Cánovas FM. Spatial and temporal expression of two cytosolic glutamine synthetase genes in scots pine: functional implications on nitrogen metabolism during early stages of conifer development. Plant J. 2001;25:93–102. doi: 10.1046/j.1365-313x.2001.00938.x. [DOI] [PubMed] [Google Scholar]
- Becwar MR, Pullman GS. Somatic embryogenesis in loblolly pine (Pinus taedaL.) In: Jain SM, Gupta PK, Newton RJ, editors. Somatic Embryogenesis in Woody Plants. Vol. 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 287–301. [Google Scholar]
- Cairney J, Xu N, Pullman GS, Ciavatta VT, Johns B. Natural and somatic embryo development in loblolly pine. Appl Biochem Biotechnol. 1999;77–79:5–17. [Google Scholar]
- Cairney J, Xu N, MacKay J, Pullman J. Transcript profiling: a tool to assess the development conifer embryos. In Vitro Cell Dev Biol Plant. 2000;36:155–162. [Google Scholar]
- Cantón FR, Suárez MF, Josè-Estanyol M, Cánovas FM. Expression analysis of a cytosolic glutamine synthetase gene in cotyledons of scots pine seedlings: developmental, light regulation and spatial distribution of specific transcripts. Plant Mol Biol. 1999;40:623–634. doi: 10.1023/a:1006219205062. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatta V, Cairney J. Isolation of full-length cDNA clones using SMARTTMcDNA and a biotin-streptavidin bead system. Biotechniques. 2000;29:444–450. doi: 10.2144/00293bm08. [DOI] [PubMed] [Google Scholar]
- Cionini PG. The suspensor and its role in embryo development in Phaseolus(Papilionaceae): a review. Atti Soc Toxc Sci Nat Mem. 1987;94:151–161. [Google Scholar]
- Dean RM, Rivers RL, Zeidel M, Roberts DM. Purification and functional reconstitution of soybean nodulin 26: an aquaporin with water and glycerol transport properties. Biochemistry. 1997;38:347–353. doi: 10.1021/bi982110c. [DOI] [PubMed] [Google Scholar]
- Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S. Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci. 2000;113:4399–4411. doi: 10.1242/jcs.113.24.4399. [DOI] [PubMed] [Google Scholar]
- Froger A, Tallur D, Thomas D, Delamarche C. Prediction of functional residues in water channels and related proteins. Protein Sci. 1998;7:1458–1468. doi: 10.1002/pro.5560070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Gerbeau P, Güçlü J, Ripoche P, Maurel C. Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J. 1999;18:577–587. doi: 10.1046/j.1365-313x.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Grossnickle SC, Sutton BCS. Applications of biotechnology for forest regeneration. New Forests. 1999;17:213–226. [Google Scholar]
- Guenther JB, Roberts DM. Water-selective and multifunctional aquaporins form Lotus japonicusnodules. Planta. 2000;210:741–748. doi: 10.1007/s004250050675. [DOI] [PubMed] [Google Scholar]
- Höfte H, Hubbard L, Reizer J, Ludevid D, Herman EM, Chrispeels MJ. Vegetative and seed-specific forms of tonoplast intrinsic protein in the vacuolar membrane of Arabidopsis thaliana. Plant Physiol. 1992;99:561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Herman EM, Chrispeels MJ. An abundant, highly conserved tonoplast intrinsic protein in seeds. Plant Physiol. 1989;91:1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Dangl JM. Logjam at the Styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Ludevid D, Höfte H, Himelblau E, Chrispeels MJ. The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thalianais correlated with cell enlargement. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000;465:110–114. doi: 10.1016/s0014-5793(99)01729-9. [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Hakman I. A tonoplast intrinsic protein (TIP) is present in seeds, roots and somatic embryos of Norway spruce (Picea abies) Physiol Plant. 1995;95:288–295. [Google Scholar]
- Page RDM. TREEVIEW: an application to display phylogenetic trees on personal computers. Comp Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Panitz R, Borijuk L, Manteuffel R, Wobus U. Transient expression of storage-protein genes during early embryogenesis of Vicia faba: synthesis and metabolization of vicilin and legumin in the embryo, suspensor and endosperm. Planta. 1995;196:765–774. [Google Scholar]
- Pullman GS, Webb DT. Proceedings of the TAPPI R&D Division Biological Sciences Symposium, October 3–6, Minneapolis, MN. Atlanta, GA: Technical Association of the Pulp and Paper Industry Press; 1994. An embryo staging system for comparison of zygotic and somatic embryo development; pp. 31–34. [Google Scholar]
- Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB. NTRI encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 1995;370:264–268. doi: 10.1016/0014-5793(95)00853-2. [DOI] [PubMed] [Google Scholar]
- Rivers RL, Dean RM, Chandy G, Hall JE, Roberts DM, Zeidel ML. Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J Biol Chem. 1997;272:16256–16261. doi: 10.1074/jbc.272.26.16256. [DOI] [PubMed] [Google Scholar]
- Sabala I, Elfstrand M, Farbos I, Clapham D, von Arnold S. Tissue-specific expression of Pa18, a putative lipid transfer protein gene, during embryo development in norway spruce (Picea abies) Plant Mol Biol. 2000;42:461–478. doi: 10.1023/a:1006303702086. [DOI] [PubMed] [Google Scholar]
- Schultz RP. Loblolly: the pine for the twenty-first century. New Forests. 1999;17:71–88. [Google Scholar]
- Schulz P, Jensen WA. Capsellaembryogenesis: the suspensor and the basal cell. Protoplasma. 1969;67:139–163. [Google Scholar]
- Schünmann PHD, Ougham HJ. Identification of three cDNA clones expressed in the leaf extension zone mutant of barley: tonoplast intrinsic protein, a putative structural protein and protochlorophyllide oxidoreductase. Plant Mol Biol. 1996;31:529–537. doi: 10.1007/BF00042226. [DOI] [PubMed] [Google Scholar]
- Schwartz BW, Vernon DM, Meinke DW. Development of the suspensor: differentiation, communication, and programmed cell death during plant embryogenesis. In: Larkins BA, Vasil IK, editors. Cellular and Molecular Biology of Plant Seed Development. The Netherlands: Kluwer Academic Publishers; 1997. pp. 53–72. [Google Scholar]
- Smart LB, Vojdani F, Maeshima M, Wilkins TA. Genes involved in osmoregulation during turgor-driven cell expansion of developing cotton fibers are differentially regulated. Plant Physiol. 1998;116:1539–1549. doi: 10.1104/pp.116.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. Histogenesis and organization of the embryo in Pinus strobusL. Am J Bot. 1949;36:629–641. [Google Scholar]
- Tamás MJ, Luyten K, Sutherland FCW, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol. 1999;31:1087–1104. doi: 10.1046/j.1365-2958.1999.01248.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis R. Bioprocessing for tree production in the forest industry: conifer somatic embryogenesis. Biotechnol Prog. 1998;14:156–166. [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, Hediger MA. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- Weig AR, Deswarte C, Chrispeels MJ. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997;114:1347–1357. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig AR, Eisenbarth D. Role of aquaporins during elongation growth of castor bean seedlings. In: Hohmann S, Nielsen S, editors. Molecular Biology and Physiology of Water and Solute Transport. Dordrecht, New York: Kluwer Academic/Plenum Publishers; 2000. pp. 357–364. [Google Scholar]
- Weig AR, Jakob C. Functional identification of the glycerol permease activity of Arabidopsis thaliana NLM1 and NLM2 proteins by heterologous expression in Saccharomyces cerevisiae. FEBS Lett. 2000a;481:293–298. doi: 10.1016/s0014-5793(00)02027-5. [DOI] [PubMed] [Google Scholar]
- Weig AR, Jakob C. Functional characterization of Arabidopsis thaliana aquaglyceroporins. In: Hohmann S, Nielsen S, editors. Molecular biology and physiology of water and solute transport. Dordrecht, The Netherlands: Kluwer Academic/Plenum Publishers; 2000b. pp. 365–372. [Google Scholar]
- Xu N, Johns B, Pullman GS, Cairney J. Rapid and reliable differential display from minute amounts of tissue: mass cloning and characterization of differentially expressed genes from loblolly pine embryos. Plant Mol Biol Rep. 1997;15:377–391. [Google Scholar]
- Yadegari R, Goldberg RB. Embryogenesis in dicotyledonous plants. In: Larkins BA, Vasil IK, editors. Cellular and Molecular Biology of Plant Seed Development. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 3–52. [Google Scholar]
- Yeung EC. Embryogeny of Phaseolus: the role of the suspensor. Z Pflanzenphysiol. 1980;96:17–28. [Google Scholar]
- Yeung EC, Meinke DW. Embryogenesis in angiosperms: development of the suspensor. Plant Cell. 1993;5:1371–1381. doi: 10.1105/tpc.5.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Verkman AS. Water and urea permeability properties of Xenopusoocyte expression of mRNA from toad urinary bladder. Am J Physiol. 1991;260:C26–C34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]