Abstract
Sodium uptake from the soil is a major cause of salinity toxicity in plants, yet little is known about the mechanisms that underlie Na+ influx. We have characterized voltage independent channels (VICs) in Arabidopsis roots that are thought to contribute to Na+ entry. VICs showed no selectivity among monovalent cations, and their gating was found to be voltage independent. However, VIC open probability showed sensitivity to cyclic nucleotides. The presence of micromolar concentrations of cAMP or cGMP at the cytoplasmic side of the plasma membrane evoked a rapid decrease in channel open probability. In accord with predictions from electrophysiological data, our results show that short-term unidirectional Na+ influx is also reduced in the presence of cyclic nucleotides. Moreover, addition of membrane permeable cyclic nucleotides during growth assays improved plant salinity tolerance, which corresponded with lower levels of Na+ accumulation in plants. In summary, these data imply that Arabidopsis plants may contain a cyclic nucleotide-based signaling pathway that directly affects Na+ transport via VICs.
Around 6% of the global land area suffers from salinization due to natural causes or irrigation, posing a major strain on agricultural production. It is estimated that irrigation-related salinization leads to the abandonment of 107 hectares of agricultural land annually (Flowers and Yeo, 1995). The detrimental effects of salt toxicity in plants comprise ionic and osmotic components, both causing diminished growth rates. Although most major crop species are salt sensitive, other plant species are salt tolerant, relying on minimized influx, extrusion, compartmentation, and translocation of Na+ to preclude excessive damage (Flowers and Yeo, 1995).
One of the key questions regarding salt toxicity in plants is related to the identity of the pathway for Na+ entry into the symplast. Very little is known about the nature of this pathway and how it is regulated. The search for Na+ entry pathways focused initially on voltage-dependent cation selective ion channels because these constitute the dominant component of the plasma membrane conductance, and on the basis that K+ and Na+ may compete for the same transport site. However, inward- and outward-rectifying voltage dependent cation channels were generally found to be highly selective for K+ with a negligible Na+ permeability (for review, see Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999), and a substantial Na+ flux via this pathway was ruled out.
More recently it has been suggested that voltage-independent cation channels (VICs) identified in species such as wheat (Triticum aestivum), barley (Hordeum vulgare), and maize (Zea mays) are involved in Na+ uptake (Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999).
In animals, many nonselective ion channels function in the transduction of sensory input and in Ca2+ signaling (e.g., Biel et al., 1996). Typically, such channels are not voltage dependent, but are gated via binding of cAMP or cGMP to a domain near the C terminus. Additional regulation of channel activity is provided by a calmodulin-binding site that alters the channel affinity for cyclic nucleotides (Finn et al., 1996).
Putative cyclic nucleotide gated channels (CNGCs) have recently been cloned in plants. Initially cloned from barley on the basis of its capacity to bind calmodulin (Schuurink et al., 1998), HvCBT1 was shown to contain a cyclic nucleotide-binding site and to reside in the plasma membrane. CBT homologs have now been identified in tobacco (Nicotiana tabacum; Arazi et al., 1999), and Arabidopsis (Kohler et al., 1999). In tobacco, overexpression of NtCBP4 led to hypersensitivity to Pb2+, and it was concluded that this protein may be involved in transport of heavy metals and possibly Ca2+ (Arazi et al., 1999). Leng et al. (1999) characterized one of the Arabidopsis homologues (CNGC2) after heterologous expression in yeast (Saccharomyces cerevisiae) and oocytes. They reported that activation of CNGC2 requires the presence of cyclic nucleotides and that the channel shows permeability for various monovalent cations. Nevertheless, little is known about the transport properties and physiological role(s) of such transporters in planta.
In this study, we characterized VICs that are present in Arabidopsis root cells. We report here on their role as a Na+ uptake pathway and on the regulation of these transporters by cyclic nucleotides. The properties of cyclic nucleotide-regulated VICs of Arabidopsis root cells are discussed in comparison with other ion channels.
RESULTS
Arabidopsis Root Cells Contain Nonselective Ion Channels
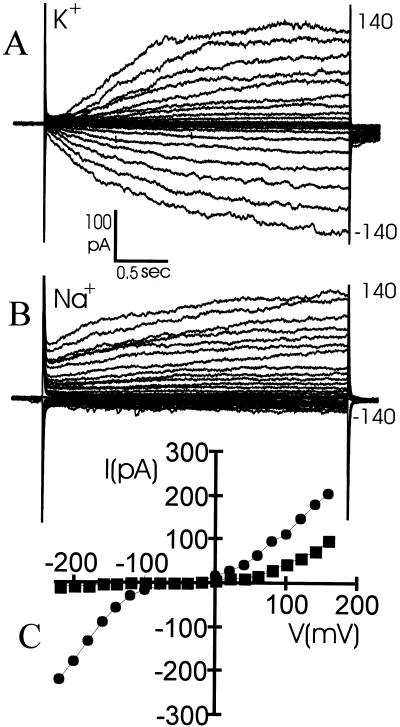
As has been established for many plant species, the dominant channel types in Arabidopsis root cells are voltage and time dependent and K+ selective (Maathuis and Sanders, 1995). The previously determined relatively high PNa+:PK+ of the main inward rectifier suggests that this channel could allow a substantial inward Na+ flux at low external K+:Na+ ratios. However, this ratio was determined with excised patches in bionic conditions by linear interpolation of the I/V relationship through the reversal potential. This approach may have led to an underestimation of the actual PK+:PNa+. Therefore, we measured whole-cell time-dependent inward currents in the respective presence of K+ or Na+. Figure 1 shows that whereas outward K+ currents are comparable, substitution of external K+ for Na+ drastically reduces inward currents, yielding a K+:Na+ current ratio of over 30 at a physiological membrane potential of −170 mV. This result confirms that voltage-dependent channels are unlikely to present a major pathway for Na+ uptake.
Figure 1.
A, Time-dependent currents during a whole-cell recording on an Arabidopsis root protoplast. The intracellular and external media contained 50 mm KCl and membrane potentials were clamped from −140 to 140 mV in 3-s, 10-mV steps. B, Same cell and clamping protocol with the external medium containing 50 mm NaCl instead of KCl. C, Current voltage relationship for A (●) and B (▪).
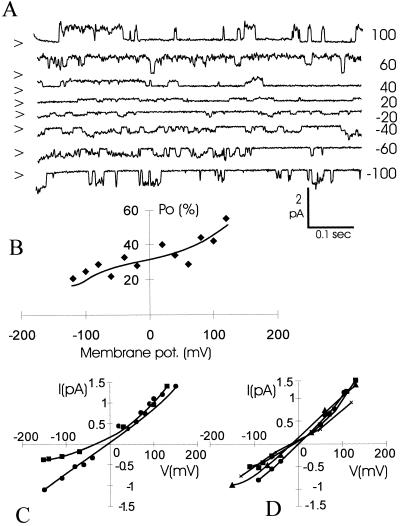
In addition to voltage-dependent ion channels, Arabidopsis root cells contain ion channels that show little or no discrimination between monovalent cations (Fig. 2). In contrast to the above mentioned inward- and outward-rectifying channels, both of which show an appreciable voltage dependence (Maathuis and Sanders, 1995), no or only weak voltage dependence was associated with the nonselective current (Fig. 2B), and we will henceforth refer to the underlying transporters as VICs.
Figure 2.
A, Single channel traces obtained from an outside-out excised membrane patch from an Arabidopsis root cell protoplast. The pipette and bath solution contained 50 mm KCl. Membrane potentials (mV) are expressed with reference to the extracellular compartment and are noted on the right. Closed levels are noted on the left by arrows. B, Open probability as a function of membrane potential, expressed as percentage of open time relative to total sampled time. Open probability is defined as the accumulative relative open time of all channels present in the patch sampled over 10-s periods for each membrane voltage. C, Current/voltage relationship showing cation selectivity. The pipette solution contained 50 mm KCl, and the bath solution contained 50 mm KCl (●) or zero KCl (▴). D, Current/voltage relationships with various monovalent cations. The pipette solution contained 50 mm KCl, and the bath solution contained, respectively, 50 mm KCl (●), NaCl (♦), CsCl (+), and RbCl (▴).
A reversal potential of around −30 mV was recorded in 50:0 mm KCl (cytoplasmic:external) solutions (Fig. 2C), whereas substitution of external K+ for equimolar Na+, Rb+, or Cs+ did not cause a discernible shift in the reversal potential (Fig. 2D). Although various (sub)conductance levels are frequently observed, the most prevalent unitary conductance level is around 8 pS in the presence of 50:50 mm KCl solutions.
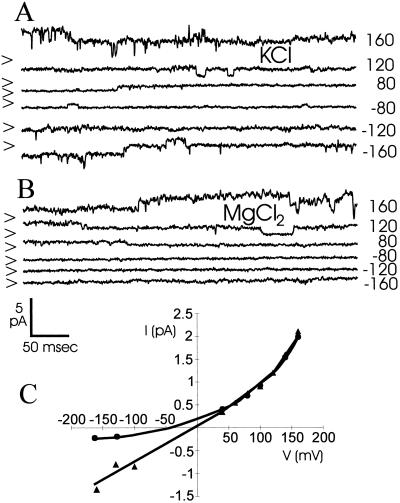
As Figure 3 shows, the channel is virtually impermeable to Mg2+. However, as reported previously for VICs characterized in cereals (Amtmann and Sanders, 1999), Arabidopsis VICs are insensitive to the K+ channel blockers Cs+ (Fig. 2D), tetraethylammonium (5 mm), and quinidine (1 mm). In addition, we found no effect of the anion channel inhibitors flufenemic acid (0.1 mm) and nitro-(phenylpropylamino) benzoic acid (0.05 mm).
Figure 3.
VIC currents in an excised, outside-out patch with 50 mm KCl (A) or 25 mm MgCl2 (B) in the external medium. C, Current/voltage for A (▴) and B (●).
VIC Gating Is under Control of Cyclic Nucleotides
Opening and closing of VICs is clearly not under control of the membrane voltage, and therefore, the mechanism by which plant cells control VIC-mediated membrane permeability remains a central question. One general mechanism for the modulation of nonselective conductances in animal cell plasma membranes consists of alterations in cytoplasmic cAMP or cGMP concentration. These cyclic nucleotides modify channel open probability via direct binding to the channel protein. The presence in plants (Schuurink et al., 1998; Kohler et al., 1999; Leng et al., 1999) of genes that encode homologs of animal CNGCs prompted us to study the response of Arabidopsis VICs to cyclic nucleotides.
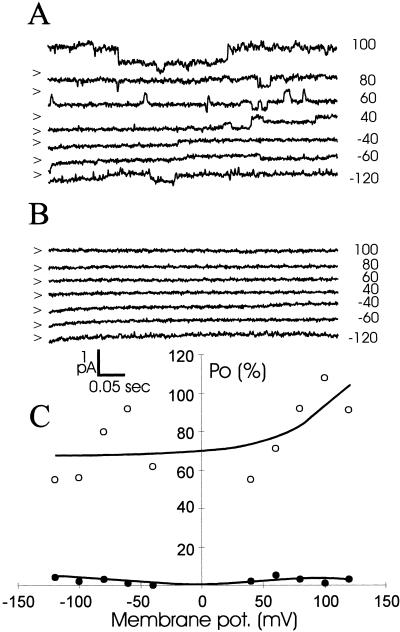
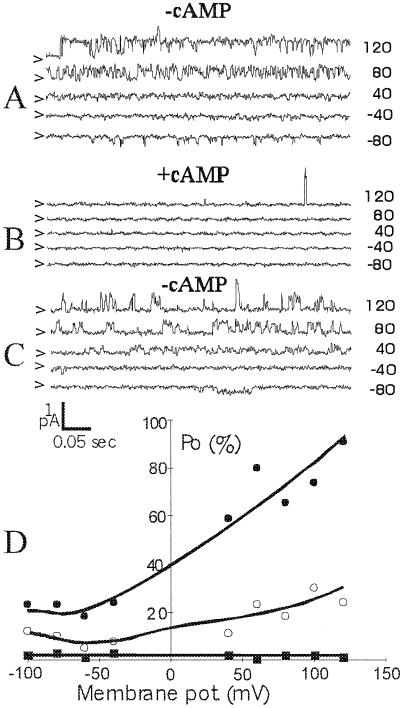
Figure 4A shows VIC-mediated currents observed in an inside-out excised patch. Addition of cGMP (final concentration of 100 μm) to the cytosolic side of the membrane caused a decrease in channel activity (Fig. 4B). This finding is in stark contrast to what is generally observed for animal nonselective ion channels, where a rise in cytoplasmic concentration of cyclic nucleotides usually leads to channel activation. In addition, VICs sensitive to cAMP were observed (Fig. 5): Exposure to cAMP (final concentration of 30 μm) led to an almost complete cessation of channel activity (Fig. 5B), an effect that was largely reversed after washout of cAMP (Fig. 5C). Using inside-out patches, channel deactivation is almost instantaneous after cyclic nucleotides are applied to the cytosolic side of the membrane. This suggests that cAMP and cGMP directly interact with the channel.
Figure 4.
Single channel traces from an inside-out excised patch showing VIC activity. The pipette solution contained 10 mm KCl, and the bath solution contained 50 mm NaCl. A, Channel activity in the absence of cyclic nucleotides. B, Channel activity recorded 5 s after the introduction of 100 μm cGMP to the cytosolic side of the membrane. Membrane potentials are noted on the right and closed levels are noted on the left by arrows. C, Open probability as a function of membrane potential, expressed as percentage of open time relative to total sampled time. Open probability is defined as in Figure 2.
Figure 5.
Single channel traces from an inside-out excised patch showing VIC activity. Pipette (50 mm KCl) and bath (50 mm NaCl) solutions were similar to those in Figure 2. A, Single channel activity in the absence of cyclic nucleotides. B, Channel activity recorded 10 s after addition of 30 μm cAMP to the cytosolic side of the membrane. C, Channel activity recorded 0.5 to 1 min after removal of cAMP. Membrane potentials are noted on the right and closed levels are noted on the left by arrows. D, Open probability as a function of membrane potential, expressed as percentage of open time relative to total sampled time. Open probability is defined as in Figure 2.
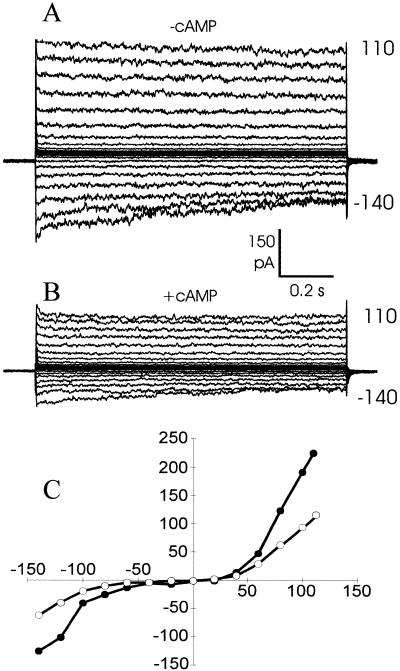
Reduced currents in the presence of cyclic nucleotides were observed in inside-out patches, and, using membrane-permeable cyclic nucleotides, in outside-out patches and the whole-cell configuration (Table I). In whole-cell recordings, exposure to (membrane-permeable) cyclic nucleotides reduced instantaneous currents up to 50% (Fig. 6). However, in all configurations, we observed VIC type currents that were completely insensitive to cyclic nucleotides (Table I), suggesting that cyclic nucleotide-sensitive VICs may only occur in a subpopulation of cells.
Table I.
Observed reduction of VIC-mediated currents in inside-out patch (IOP), outside-out patch (OOP), and whole cell (WC) patch-clamp experiments
| Patch-Clamp Configuration | No. of Experiments with cNMP | No. of Observations of Reduced Current (cAMP) | No. of Observations of Reduced Current (cGMP) |
|---|---|---|---|
| IOP | 39 | 8 | 6 |
| OOP | 11 | 2 | 1 |
| WC | 8 | 2 | 2 |
Observations of reduced currents were counted whenever VIC-mediated currents were less than 90% of the control (minus cNMP) level after addition of cAMP or cGMP. cAMP and cGMP were used in various concentrations ranging from 1 to 300 μm and were added in membrane-permeable form for OOP and WC configuration.
Figure 6.
Whole cell, instantaneous currents in the absence (A) and presence (B) of 0.5 mm dibutyryl cGMP. The recording shown in B was made approximately 1 min after the addition of cyclic nucleotide. C, Current/voltage graph for A (●) and B (○). Pipette and bath solution contained 50 mm NaCl.
Cyclic Nucleotides Improve Arabidopsis Salinity Tolerance
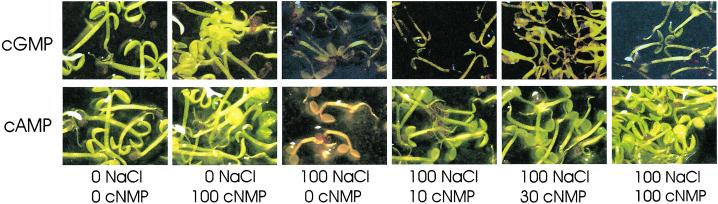
Our data show that the VIC-mediated Na+ currents are down-regulated by cGMP and cAMP. Therefore, we tested whether membrane-permeable analogs of these nucleotides would modify the level of salinity tolerance of Arabidopsis. Arabidopsis plants were germinated and grown suspended in growth medium for 6 to 8 d after which plants were exposed to NaCl in the presence or absence of cGMP/cAMP. Exposure to 100 mm NaCl in these conditions leads to bleaching of leaf tissue and subsequent plant death within 4 to 7 d. However, in the presence of cGMP or cAMP, plant survival is considerably extended (Fig. 7). The ameliorating effect of exogenously applied nucleotides is apparent at external concentrations as low as 10 μm, it is dose dependent, and no effect of nucleotides was apparent in control plants (zero NaCl) provided concentrations remained less than 500 μm (Table II). Improved plant growth in the presence of cyclic nucleotides was not observed when NaCl was substituted for an equiosmolar amount of sorbitol.
Figure 7.
The presence of membrane permeable cGMP or cAMP improves salt tolerance in Arabidopsis seedlings. Plants were grown suspended in Murashige and Skoog medium for approximately 7 d after which they were exposed for 5 d to treatment with NaCl and the cyclic nucleotides (cNMP) 8-bromo-cAMP or 8-bromo-cGMP. Box 1 to 6 for each row, respectively, shows control without NaCl and cyclic nucleotide, control without NaCl plus 100 μm cyclic nucleotide, 100 mm NaCl treatment without cyclic nucleotide, and 100 mm NaCl treatments with 10, 30, or 100 μm cyclic nucleotide. For each treatment, 40 to 60 seedlings were used.
Table II.
Fresh wt (mg/100 seedlings ± se) of Arabidopsis seedlings exposed for 5 d to various concentrations of NaCl and cyclic nucleotides (cNMP)
| Treatment
|
||||||
|---|---|---|---|---|---|---|
| −NaCl/−cNMP | −NaCl/100 cNMP | 100 NaCl/−cNMP | 100 NaCl/+10 cNMP | 100 NaCl/+30 cNMP | 100 NaCl/+100 cNMP | |
| cGMP | 56 ± 2.9 | 55 ± 2.2 | 15 ± 1.2 | 19 ± 1.1 | 22 ± 3.0 | 29 ± 3.1 |
| cAMP | 54 ± 2.3 | 53 ± 1.8 | 16 ± 2.0 | 28 ± 3.1 | 25 ± 2.3 | 35 ± 3.0 |
All plants were grown on 50% Murashige and Skoog medium for 8 d before treatment, after which fresh wt was 31 ± 1.8 mg. cNMP (cAMP and cGMP) concentrations are given in micromoles and were added to the growth medium as membrane-permeable analogs. NaCl concentrations are 0 or 100 mm.
To some extent, the effects of cAMP and cGMP can be mimicked by modulators of cyclic nucleotide metabolism: Forskolin (an adenyl cyclase agonist) reduced Na+ accumulation (Table III). Also in accordance with the above results is the exacerbating effect of micromolar amounts in the growth medium of LY83583 (a guanyl cyclase inhibitor) with regard to sodium toxicity symptoms (results not shown).
Table III.
Whole-tissue Na+ contents on a tissue water basis of Arabidopsis seedlings grown for 3 to 4 d on one-half-strength Murashige and Skoog media containing 100 mm NaCl
| Tissue Na+
| |||
|---|---|---|---|
| No Treatment | cAMP | cGMP | Forskolin |
| mm | |||
| 96 ± 8.3 | 77 ± 13 | 68 ± 8.0 | 78 ± 7.1 |
NaCl treatments were carried out in the presence or absence of membrane-permeable cyclic nucleotides or the adenyl cyclase agonist Forskolin. Data are ±se for three to six independent experiments. cAMP/cGMP were added as Na+ salts of the membrane-permeable analog 8-Br-cAMP/cGMP with a concentration of 100 μm, and Forskolin was added at a final concentration of 10 μm.
We then tested whether the observed effects of cAMP/cGMP on plant growth in the presence of salt, and the above described inhibitory action of cAMP/cGMP on Na+ currents would translate into different plant Na+ contents. Hence, the total tissue Na+ contents in seedlings were determined. From Table III it becomes clear that externally supplied cyclic nucleotides reduce the amount of Na+ that is accumulated in plants. The smaller amounts of tissue Na+ in cyclic nucleotide-treated plants may contribute to improving plant salinity tolerance.
Sodium Influx Is Inhibited by Cyclic Nucleotides
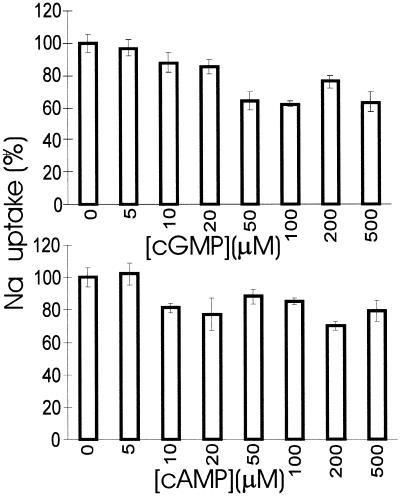
Patch clamp data show that Arabidopsis VIC activity is down-regulated by cAMP and cGMP. Growth experiments show that cyclic nucleotides improve salinity tolerance, possibly as a result of less Na+ accumulation in the plant tissues. A decrease in Na+ accumulation could be achieved by a reduction in Na+ uptake, an enhanced Na+ efflux, or a mixture of both. Therefore, we measured the unidirectional Na+ uptake by using radioactive 22Na+ and we tested the effect of exogenously applied membrane-permeable cyclic nucleotides on Na+ uptake in intact plants. The results of these short-term (30 min) uptake experiments show a clear inhibition of Na+ uptake in the presence of cGMP and, to a lesser extent, cAMP (Fig. 8). Inhibitory effects were seen at concentrations as low as 10 to 20 μm. These results are consistent with the notion that a proportion of Na+ uptake is mediated by a cyclic nucleotide-regulated pathway.
Figure 8.
The effect of membrane-permeable cyclic nucleotides on unidirectional Na+ influx in intact Arabidopsis seedlings. Na+ uptake proceeded for 30 min in uptake medium containing 50 mm NaCl in the absence or presence of various concentrations of 8-bromo-cAMP or 8-bromo-cGMP. The control uptake rate was 8.8 (± 2.9) μmol g−1 h−2 fresh weight.
DISCUSSION
Roles of Cyclic Nucleotides in Plants
The presence of cGMP and cAMP in plant cells has unequivocally been demonstrated for a number of species (Newton et al., 1999). Furthermore, evidence has been reported that plants contain adenylate cyclase and cyclic nucleotide phosphodiesterase, two key enzymes of cAMP metabolism (Bolwell, 1995). The role of cyclic nucleotides as second messengers in animal cells is well established: cAMP and cGMP have as main targets specific kinases whose activity is modulated by binding of regulatory subunits. Subsequent protein phosphorylation by such kinases is involved in the modification of enzyme activities. In addition, cyclic nucleotides can directly alter gene expression by binding to specific proteins. The latter occurs in the activation of transcription factors such as cAMP response element-binding proteins, which bind to many promoters. Direct interaction is also prominent in cyclic nucleotide gated ion channels where activity is directly controlled by binding/dissociation of cAMP or cGMP (Newton et al., 1999).
Although the roles of cyclic nucleotides in plants are less well understood, increasing evidence points to their pivotal role: cGMP has been shown to be involved in phytochrome signal transduction (Bowler et al., 1994) and also in hormone signaling in barley aleurone cell layers (Penson et al., 1996). cAMP has been suggested to play a role in cell cycle progression (Ehsan et al., 1998), the activation of transcription factors (Katagiri et al., 1989), cell signaling in response to pathogenic attack (Kurosaki and Nishi, 1993), and stomatal movement (Curvetto et al., 1994). As in animal cells, plant cells, too, may contain ion channels that form cAMP or cGMP targets.
The Role of Ion Channels in Sodium Uptake
The predominant type of ion channel that is present in the plasma membrane of plant cells is voltage dependent and K+ selective. The high selectivity for K+ excludes the occurrence of a considerable Na+ flux through this pathway and, therefore, we investigated the presence in Arabidopsis roots of nonselective VICs, as were previously described in other species (for review, see Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999). Little is known about the physiological function of this type of transporter. However, based on the similarity of Ca2+-dependent block of VIC current and Na+ influx in intact tissue, VICs have been proposed to form a major pathway for Na+ entry into plants (Amtmann and Sanders, 1999; Tyerman and Skerrtt, 1999; White, 1999; Davenport and Tester, 2000).
In Arabidopsis root protoplasts, VICs were observed in a small proportion of cells and found to have little or no selectivity between monovalent cations (Fig. 2), and classical cation and anion channel blockers failed to affect the channel open probability or the open channel conductance. Significantly, VIC activity does not depend, or does so only weakly, on membrane voltage and, hence, the presence of an alternative regulatory mechanism is required.
VIC Activity Is Modulated by Cyclic Nucleotides
Because membrane voltage only weakly affects VIC open probability, we tested whether cyclic nucleotides could be involved in the regulation of VIC-mediated currents. In a previous study Davenport and Tester (2000), using wheat membranes incorporated in lipid bilayers, characterized a nonselective Na+ conducting channel. No effect of cyclic nucleotides was observed on this conductance, possibly indicating that their object of study consisted of a different type of channel than the ones reported here. Alternatively, wheat roots may not contain cyclic nucleotide-regulated VICs. In contrast, we observed in a number of cases VIC-mediated currents in Arabidopsis root cells that were sensitive to cGMP (Fig. 4) and to cAMP (Fig. 5). In the presence of micromolar levels of either cyclic nucleotide, VIC open probability was drastically and rapidly decreased. We always observed current reduction after addition of cyclic nucleotides, which is in stark contrast to what is generally observed for animal nonselective ion channels involved in sensory signal transduction. However, the Ca2+ activated nonspecific cation channel of insect olfactory receptor neurons is completely blocked by 10 μm cytoplasmic cGMP (Zufall et al., 1997). In contrast, we never observed any effect of cyclic nucleotides on the voltage-dependent inward- or outward-rectifying K+ channels described previously (Maathuis and Sanders, 1995). VIC deactivation by cyclic nucleotides frequently occurred within seconds and was most prevalent using excised membrane patches. These results strongly suggest that cyclic nucleotides exert their effect via direct interaction with the channel protein, and they argue against indirect channel modulation through, for example, the action of kinases.
In about 50% of attempts to find effects of cyclic nucleotides on VIC currents, no change in current magnitude occurred. In other cases, currents were found to be sensitive to cGMP or cAMP (Figs. 4 and 5). The shorter mean open time (around 2 ms) of the cAMP-sensitive current compared with cGMP-sensitive currents (mean open time around 10 ms) and the slightly stronger voltage dependence of the cAMP-sensitive currents may suggest that Arabidopsis root cells contain more than one type of cyclic nucleotide-regulated VIC. Results obtained in the whole-cell recording mode showed reduction in current ranging from 0% to approximately 40% in various cells. In contrast, results with excised patches showed an almost complete cessation of channel activity. These results indicate the presence of multiple VIC types and/or the presence of strong phophodiesterase activity as was previously concluded for guard cells (Li et al., 1994). The presence of various VICs with different sensitivities for cyclic nucleotides may provide a large degree of flexibility in regulating ion fluxes in the plant root. Alternatively, our data may reflect the presence of different categories of VIC in different cell types. However, as yet it cannot be ruled out that the open probability of only one type of VIC is affected, albeit with different affinities, by cAMP and cGMP as has been described for animal cells (Li and Lester, 1999).
Cyclic Nucleotides Affect Sodium Uptake
In agreement with observations made during patch clamp experiments, we measured a significantly reduced Na+ influx in intact seedlings when cyclic nucleotides were added to the uptake medium. cAMP and cGMP were able to inhibit Na+ influx by up to 40% (Fig. 8). This suggests that the remaining fraction of Na+ influx is mediated by VICs that are not sensitive to cyclic nucleotides or through alternative, non-VIC type pathways.
Reduced Na+ influx was reflected in a smaller total amount of Na+ that was accumulated in plants grown in the presence of cyclic nucleotides as compared with plants grown without cyclic nucleotides (Table III). The lower Na+ contents are likely to contribute to plant survival under salinity stress (Fig. 7) and they may be a direct effect of cyclic nucleotide action on specific ion channels (Figs. 4 and 5). Yet, alternative mechanisms may be operating in salinity stress responses where cyclic nucleotides function in signaling cascades without ion channels being direct targets. For example, in the yeast S. cerevisiae, cAMP is involved in cell tolerance toward moderate osmotic/salinity stress (Marquez and Serrano, 1996). In this organism, salinity causes a decrease in cellular levels of cAMP, which in turn leads to a deactivation of PKA that normally suppresses expression of the Na+ efflux pump ENA.
Which Genes Encode Sodium-Permeable VICs?
Members of the AKT and KAT channel families do contain putative cyclic nucleotide binding sites, but their high degree of selectivity for K+ excludes them from mediating significant Na+ transport. Putative nonselective ion channels were initially cloned from barley (Schuurink et al., 1998), and subsequently from Arabidopsis (Kohler et al., 1999) and tobacco (Arazi et al., 1999). In tobacco, overexpression of NtCBP4 caused tolerance to Ni2+ and hypersensitivity to Pb2+, but showed no phenotype when exposed to Na+, Ba2+, Cd2+, and a range of other metal ions. Characterization of AtCNGC2 after heterologous expression (Leng et al., 1999) showed CNGC2 activation in the presence of cyclic nucleotides. Surprisingly, CNGC2 resembles KAT/AKT type channels in displaying considerable voltage-dependence and inward rectification. Although CNGC2 showed permeability for various monovalent cations, it did not conduct Na+. These observations appear to exclude the possibility that VICs described in this study are encoded by AtCNGC2.
In summary, three lines of investigation (patch clamp, flux, and growth experiments) all point in the same direction: Cyclic nucleotides down-regulate Na+ influx. From the flux and growth experiments it cannot be concluded whether the cyclic nucleotides act directly on channel proteins, are part of a cyclic nucleotide based signaling cascade, or both. Patch clamp experiments, though, strongly suggest a direct interaction between cyclic nucleotides and nonselective cation channels.
MATERIALS AND METHODS
Plant Growth
Seeds of Arabidopsis cv Columbia were germinated in soil and were hydroponically grown as described elsewhere (Maathuis and Sanders, 1995). Plants used for flux and growth studies were grown as follows. Surface-sterilized seeds of Arabidopsis cv Columbia were grown for 8 to 10 d in 50-mm petri dishes containing 8 mL of 50% (w/v) strength Murashige and Skoog medium. Seedlings were grown suspended in the growth medium or supported on a nylon grid (100-μm mesh size). Growth conditions were 16 h of light/8 h of darkness, a temperature of 24°C/20°C degrees during the light/dark periods, and 40 μmol m−2 s−1 light intensity. After 8 to 10 d of growth in standard Murashige and Skoog medium, plants for growth assays were exposed for 4 to 6 d to treatment with NaCl and/or membrane-permeable cyclic nucleotides.
Plant Sodium Contents
The total Na+ content in seedlings was determined as follows. Plants were washed twice for 8 min in ice-cold 20 mm CaCl2 to exchange cell wall-bound Na+. Plant fresh weight was determined and plants were dried at 100°C for 24 h. Total Na+ from the tissue was then extracted by incubation of the dried tissue for 3 h in a solution containing 20 mm CaCl2 and 2 mm Tris-HCl, pH 8. Subsequently, Na+ measurements were made in the 20-mm CaCl2 solution with a Na+-selective electrode (Russell, Fife, UK) against standards in the same solution. Data are expressed on a tissue water (fresh weight minus dry weight) basis.
22Na+ Flux Experiments
Seedlings grown for 8 to 10 d as described above were acclimatized for 30 min to the Na+ uptake solution, which comprised (in millimoles) 50 NaCl, 2 MES [2-(N-morpholino)ethanesulfonic acid]/Tris, pH 6.0, and 2 CaCl2. Uptake of 22Na+ was started by adding 0.2 μCi mL−1 of 22Na+ and it was allowed to proceed for 30 min at room temperature. After the uptake period, two 8-min washes were carried out in an ice-cold solution of the same composition but without radiotracer. Subsequently, plants were blotted dry and weighed into scintillation vials containing 2.5 mL of scintillation liquid. Radioactivity in the tissue was liberated through a 2-h exposure to the scintillation liquid, after which samples were analyzed on a scintillation counter. Results are the average (± se) of four to seven independent experiments for each treatment.
Patch-Clamp Experiments
Protoplasts were isolated from root tissue as described in Maathuis and Sanders (1995). Standard extracellular solutions contained (in millimoles) 10 KCl or NaCl, 1 CaCl2, 1 MgCl2, and 2 MES/Tris, pH 5.5. The standard solution facing the cytoplasmic side of the membrane contained (in millimoles) 50 KCl or NaCl, 1 CaCl2 buffered with EGTA (free Ca2+ of 200–700 nm), 2 MgCl2, and 2 MES/Tris, pH 7.5. Total osmolarity of all solutions was adjusted to 500 mOsM with sorbitol, and all solutions were filtered (0.2-μm pore size) before use. Pipette fabrication, giga-Ω seal formation, and data sampling were as described in Maathuis and Sanders (1995).
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010502.
LITERATURE CITED
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Adv Bot Res. 1999;29:75–112. [Google Scholar]
- Arazi T, Sunkar R, Kaplan B, Fromm H. A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 1999;20:171–182. doi: 10.1046/j.1365-313x.1999.00588.x. [DOI] [PubMed] [Google Scholar]
- Biel M, Zong XG, Hofmann F. Cyclic nucleotide-gated cation channels: molecular diversity, structure, and cellular functions. Trends Cell Motil. 1996;6:274–280. doi: 10.1016/S1050-1738(96)00105-3. [DOI] [PubMed] [Google Scholar]
- Bolwell GP. Cyclic AMP, the reluctant messenger in plants. Trends Biochem Sci. 1995;20:492–495. doi: 10.1016/s0968-0004(00)89114-8. [DOI] [PubMed] [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Curvetto N, Darjania L, Delmastro S. Effect of two cAMP analogues on stomatal opening in Vicia faba: possible relationship with cytosolic calcium concentration. Plant Physiol Biochem. 1994;32:365–372. [Google Scholar]
- Ehsan H, Reichheld JP, Roef L, Witters E, Lardon F, VanBockstaele D, VanMontagu M, Inzé D, VanOnckelen H. Effect of indomethacin on cell cycle-dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Lett. 1998;422:165–169. doi: 10.1016/s0014-5793(97)01610-4. [DOI] [PubMed] [Google Scholar]
- Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol. 1995;22:875–884. [Google Scholar]
- Gaymard F, Cerutti M, Horeau C, Lemaillet G, Urbach S, Ravallec M, Devauchelle G, Sentenac H, Thibaud JB. The baculovirus/insect cell system as an alternative to Xenopus oocytes. J Biochem. 1996;271:22863–22870. doi: 10.1074/jbc.271.37.22863. [DOI] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Lam E, Chua NH. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989;340:727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Kohler C, Merkle T, Neuhaus G. Characterization of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 1999;18:97–104. doi: 10.1046/j.1365-313x.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Kurosaki F, Nishi A. Stimulation of calcium influx and calcium cascade by cyclic AMP in cultured carrot cells. Arch Biochem Biophys. 1993;302:144–151. doi: 10.1006/abbi.1993.1192. [DOI] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Yao WZ, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lester HA. Single-channel kinetics of the rat olfactory cyclic nucleotide-gated channel expressed in Xenopus oocytes. Mol Pharmacol. 1999;55:883–893. [PubMed] [Google Scholar]
- Li W, Luan S, Schreiber SL, Assmann SM. Cyclic AMP stimulates K+ channel activity in mesophyll cells of Vicia faba L. Plant Physiol. 1994;106:957–961. doi: 10.1104/pp.106.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, May ST, Graham NS, Bowen HC, Jelitto TC, Trimmer P, Bennett MJ, Sanders D, White PJ. Cell marking in Arabidopsis thaliana and its application to patch-clamp studies. Plant J. 1998;15:843–851. doi: 10.1046/j.1365-313x.1998.00256.x. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Contrasting roles in ion transport of two K+-channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Mechanisms of potassium absorption by higher plant roots. Physiol Plant. 1996;96:158–168. [Google Scholar]
- Marquez JA, Serrano R. Multiple transduction pathways regulate the sodium-extrusion gene pmr2/ena1 during salt stress in yeast. FEBS Lett. 1996;382:89–92. doi: 10.1016/0014-5793(96)00157-3. [DOI] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1668. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RP, Roef L, Witters E, VanOnckelen H. Tansley review no. 106: cyclic nucleotides in higher plants: the enduring paradox. New Phytol. 1999;143:427–455. doi: 10.1046/j.1469-8137.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL. cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Skerrett IM. Root ion channels and salinity. Sci Hort. 1999;78:175–235. [Google Scholar]
- White PJ. The molecular mechanism of sodium influx to root cells. Trends Plant Sci. 1999;4:245–246. doi: 10.1016/s1360-1385(99)01435-1. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Ryan PR, Tyerman SD. Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol. 2001;125:1459–1472. doi: 10.1104/pp.125.3.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Shepherd GM, Barnstable CJ. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr Opin Neurobiol. 1997;7:404–412. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]