Abstract
Vernalization and photoperiod (PP) responses are developmental mechanisms that allow plants to synchronize their growth and reproductive cycles with the seasonal weather changes. Vernalization requirement has been shown to influence the length of time that low-temperature (LT)-induced genes are up-regulated when cereal species are exposed to acclimating temperatures. The objective of the present study was to determine whether expression of LT-induced Wcs and Wcor gene families is also developmentally regulated by PP response. The LT-tolerant, highly short-day (SD)-sensitive barley (Hordeum vulgare L. cv Dicktoo) was subjected to 8-h SD and 20-h long-day PPs at cold-acclimating temperatures over a period of 70 d. A delay in transition from the vegetative to the reproductive stage under SD resulted in an increased level and longer retention of LT tolerance. Similar WCS and WCOR protein homologs were expressed, but levels of expression were much higher in plants acclimated under SD, indicating that the poor LT tolerance of long-day plants was the result of an inability to maintain LT-induced genes in an up-regulated state. These observations indicate that the PP and vernalization genes influence the expression of LT-induced genes in cereals through separate pathways that eventually converge to activate genes controlling plant development. In both instances, the delay in the transition from the vegetative to the reproductive stage produces increased LT tolerance that is sustained for a longer period of time, indicating that the developmental genes determine the duration of expression of LT-induced structural genes.
Low-temperature (LT) tolerance in cereals is dependent upon a highly integrated system of structural, regulatory, and developmental genes. In regions with cold winters, vernalization requirement is an important adaptive feature that delays heading by postponing the transition from the vegetative to the reproductive phase. Time sequence studies have shown that the transition from the vegetative to the reproductive growth stage associated with the point of vernalization saturation is also a critical switch that initiates the down-regulation of LT tolerance genes. As a consequence, full expression of LT tolerance only occurs in the vegetative stage, whereas plants in the reproductive phase have a limited ability to cold acclimate (Fowler et al., 1996b; Mahfoozi et al., 2001). Similarly, photoperiod (PP) sensitivity allows plants to maintain LT tolerance for a longer period of time under short-day (SD) compared with long-day (LD) environments (Mahfoozi et al., 2000, 2001). In both instances, the delay in the transition from the vegetative to the reproductive stage produces increased LT tolerance that is sustained for a longer period of time.
In winter cereals, the expression of several LT-induced genes is positively correlated with the potential of genotypes to develop LT tolerance. Among these are the wcs120, the wcor410, and the wcs19 gene families. The wcs120 family encodes a group of highly abundant proteins ranging in size from 12 to 200 kD (Houde et al., 1995; Sarhan et al., 1997). This protein family is coordinately regulated by LT and accumulates in both the nucleus and the cytosol. The wcor410 family encodes peripheral acidic dehydrin proteins found near the plasma membrane. The products of these two dehydrin families accumulate in cells of the vascular transition zone, a critical region for plant survival and where freeze-induced dehydration is likely to be more severe (Danyluk et al., 1998). Based on their biochemical properties, abundance, and localization, it has been speculated that they are involved in the protection of the plasma membranes by replacing water and stabilizing membranes against freezing or dehydration stress. WCS19 is a nuclear-encoded chloroplast stroma protein whose abundance is regulated by LT and requires light for maximal accumulation. Although the function of this protein is not known, preliminary analyses suggest a possible role in the modulation of chloroplastic redox poise during photosynthetic acclimation to LT and the attainment of maximal LT50 (Chauvin et al., 1993; Gray et al., 1997; C. N′Dong and F. Sarhan, unpublished data).
According to the developmental theory of LT gene regulation (Fowler et al., 1999), level and duration of gene expression determine the degree of LT tolerance. The developmental genes act as the switches controlling the duration of expression of LT-induced structural genes, whereas the level of LT tolerance is determined by the length of time and degree that the structural genes are up-regulated. A close association between the point of vernalization saturation and the start of a decline in LT-induced wcs120 gene family mRNA signal and protein accumulation in plants maintained at 4°C has demonstrated the regulatory influence that vernalization genes have over LT-induced gene expression in winter cereals (Fowler et al., 1996a). However, the regulation of LT-induced genes by other plant development regulators has not been verified. As a consequence, the objective of this study was to determine whether LT-induced gene expression is developmentally regulated by PP response. To achieve this, the expression patterns of the WCS120, WCOR410, and WCS19 protein families were determined during LT acclimation of the very SD-sensitive barley (Hordeum vulgare L. cv Dicktoo) grown under LD and SD.
RESULTS AND DISCUSSION
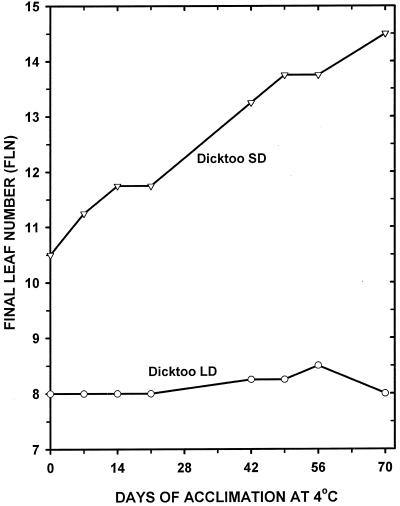
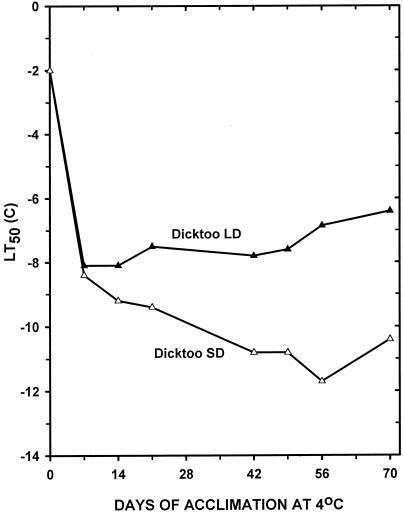
Analysis of variance for LT50 and final leaf number (FLN) showed that differences due to acclimation periods and PP and the acclimation period × PP interaction were all highly significant (P < 0.001). These results indicate that there were important differences in both the magnitude and pattern of FLN (Fig. 1) and LT50 (Fig. 2) response to temperature and PP.
Figure 1.
FLN of barley cv Dicktoo acclimated at 4°C under both LDs (20 h) and SDs (8 h) for 0 to 70 d and then moved to 20°C LD (se = 0.34).
Figure 2.
LT tolerance (se = 0.40) of barley cv Dicktoo acclimated at 4°C for 0 to 70 d under both LDs (20 h) and SDs (8 h).
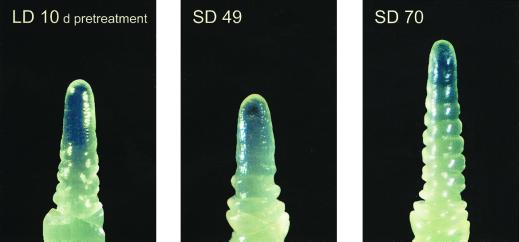
Double ridge formation, when leaf and spikelet initials are both apparent on the shoot apex, is a clear indication that transition to the reproductive phase has begun (McMaster, 1997). Double ridges were observed by d 10 of the 14-d LD pretreatment at 20°C (before the plants were exposed to 4°C) and at approximately 70 d at 4°C under SD, indicating that barley cv Dicktoo is very responsive to PP (Fig. 3). Under LD (20 h) conditions, barley cv Dicktoo reached its minimum leaf number without being exposed to acclimating temperatures (0 d LD, Fig. 1), indicating that it does not have a vernalization requirement. However, it produced 26 leaves when grown under continuous SD compared with 8.0 leaves under continuous LD conditions (data not shown) at constant 20°C (never exposed to 4°C), confirming that it was very sensitive to PP. At 4°C for 70 d, barley cv Dicktoo produced 6.5 more leaves under SD than LD conditions indicating that, although there were differences in magnitude as measured by FLN, sensitivity to PP was expressed under both warm and cold temperatures (Mahfoozi et al., 2000). These observations are in agreement with earlier reports that the activity of PP responsive genes is greater under warm than cool temperatures (Rahman and Wilson, 1978; Yan and Wallace, 1996).
Figure 3.
Apical development of barley cv Dicktoo grown under SD (8 h) and LD (20 h) PPs. Comparative phenological advancement to double ridge formation (LD, 10-d pretreatment; SD 70 d) is illustrated. Double ridges were observed by d 10 of the 14-d LD pretreatment at 20°C (before the plants were exposed to 4°C) and at approximately 70 d at 4°C.
Barley cv Dicktoo had a limited ability to LT acclimate when grown at 4°C under LD (Fig. 2), which is typical of the LT response of spring habit cultivars (Fowler et al., 1996b). In contrast, it acclimated to a lower temperature and retained its LT tolerance for a longer period of time under SD compared with LD. An increased expression of LT tolerance in SD plants (Fig. 2) was associated with the delayed transition from the vegetative to the reproductive stage (Fig. 1 and 3). This indicates that a longer acclimation response during the vegetative phase was the primary factor responsible for the superior LT tolerance of the SD compared with the LD plants in this SD-sensitive cultivar (Mahfoozi et al., 2000).
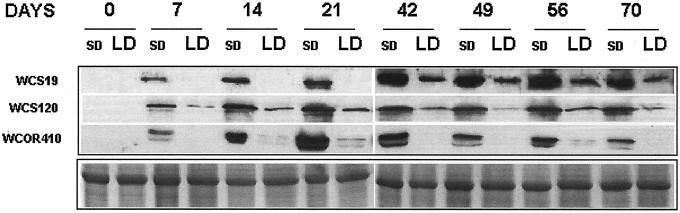
The availability of wheat (Triticum aestivum L. em Thell) LT-induced genes provided the opportunity to investigate the relationship between PP response and LT tolerance gene expression in barley. Using specific antibodies for WCS120, WCOR410, and WCS19, we identified the homologs of the three proteins in barley. The molecular masses of the three homologs were 60, 50, and 15 kD for WCS120, WCOR410, and WCS19, respectively, and their accumulation kinetics were similar to that found in wheat (Houde et al., 1992; Danyluk et al., 1998; C. N′Dong and F. Sarhan, unpublished data). The WCS120 and WCS19 protein accumulation patterns (Fig. 4) closely followed the LT acclimation curves (Fig. 2), whereas the WCOR410 protein is expressed at its highest level early in the LT acclimation regime, and its expression level declines with time (Fig. 5). The immunoblot in Figure 4 shows that the accumulation of all three protein homologs was much higher in plants LT acclimated under SD compared with LD conditions. This differential expression was related to the level of LT tolerance as determined by LT50 (Figs. 2 and 5), indicating that the mechanism regulating these LT-induced genes is associated with a gene(s) integrated into the developmental pathway.
Figure 4.
Immunoblot analyses of proteins identified by anti-WCS19, -WCS120, and -WCOR410 antibodies in barley cv Dicktoo after 0, 7, 14, 21, 42, 56, and 70 d of cold acclimation at 4°C under SD and LD PP. The molecular masses of the three homologs were 60, 50, and 15 kD for WCS120, WCOR410, and WCS19, respectively. Coomassie Brilliant Blue-stained gel shows protein load.
Figure 5.
Relative levels of WCS120, WCS19, and WCOR410 protein accumulation (se = 4.9) for barley cv Dicktoo grown at 4°C for 0 to 70 d. Signal strengths were normalized by setting maximum densitometer scans at 21 d under SD PP for WCS120 and WCOR410 and 56 d for WCS19 at 100%.
The PP sensitivity of barley cv Dicktoo caused it to remain in the vegetative state under SD conditions. The kinetic analysis shown in Figure 4 clearly indicates much higher protein accumulation over the entire 70-d acclimation period under SD conditions when the plants remained in the vegetative phase and continued to produce leaves (Fig. 1). Conversely, the LD-treated plants were in the reproductive phase when they entered the LT acclimation regime (Fig. 1), resulting in a down-regulation of the expression of all three genes. Therefore, in a single genotype, manipulation of development through the photoperiodic regulation of flowering was the major factor in determining the level of expression of LT-induced genes.
In wheat, the LT-induced Wcs120 and Wcor410 gene families have been mapped to the group 6 chromosomes of all three genomes (Limin et al., 1997; Danyluk et al., 1998), whereas the LT-induced, light-regulated, Wcs19 gene has been mapped to the group 2 chromosomes (Crosatti et al., 1999; Vagujfalvi et al., 2000). Wheat chromosome 5A, which carries the vrn-A1 vernalization gene, has been shown to regulate the expression of all members of the LT-induced gene families considered in this study (Limin et al., 1997; Danyluk et al., 1998; Sarhan and Danyluk, 1998). Similarly, we have demonstrated that the ppd alleles, which condition sensitivity to daylength and have been mapped to the group 2 chromosomes (Laurie, 1997; Law and Worland, 1997), also regulate the expression of LT tolerance genes in cereals by extending the vegetative phase under SD growth conditions (Mahfoozi et al., 2000, 2001). The regulatory control revealed in LT tolerance studies with PP-sensitive genotypes was verified at the molecular level by the increased accumulation and duration of expression of the LT-induced gene families in plants grown under SD compared with LD in the present study (Figs. 2 and 5). Of special note is the fact that the major vernalization (vrn) and daylength response (ppd) genes map to the fifth and second group chromosomes, respectively, indicating that the LT-induced genes are regulated by the interaction of genetic factors located on different chromosomes.
Developmental regulation of LT-induced genes from different gene families, on different chromosomes, targeted to completely different sites within the cell supports the conclusion that a global regulator is integrated into the developmental pathway downstream from the convergence of the PP and vernalization (Fowler et al., 1996b) pathways. As a consequence, use of the yeast (Saccharomyces cerevisiae) one-hybrid system to identify the specific DNA-binding proteins that interact with the promoter region of any of these three genes should enable us to identify the factors involved in their regulation and determine the LT signaling events associated with phenological development. This approach has been successfully used in the identification of the CBF/DREB transcription regulatory proteins involved in cold- and drought-regulated genes in Arabidopsis (Stockinger et al., 1997; Liu et al., 1998).
Clearly, LT tolerance gene expression is influenced not only by environment, but also by the pleiotropic effects of developmental genes. Vernalization requirement is an adaptive mechanism that maintains the plant in the vegetative growth phase until the severe winter stress period has passed. While in the vegetative phase, the plant is able to acclimate and maintain a high level of LT tolerance. In the absence of a vernalization requirement, daylength-sensitive genotypes use PP responses to extend the vegetative period and maintain LT-induced genes in an up-regulated state (Fig. 5). In this system, the developmental genes (vernalization and PP) act as the switches regulating the duration of expression of LT-induced structural genes, whereas the level of LT tolerance is determined by the length of time and degree that the structural genes are up-regulated.
The regulatory control of PP genes over the expression of the three LT-induced gene families considered in this study was similar to that reported for the vernalization genes in wheat (Fowler et al., 1996a) and supports the hypothesis that any factor that delays the transition from vegetative to reproductive stages increases the level of expression of LT tolerance genes in cereals exposed to acclimating temperatures (Fowler et al., 1996b). Threshold induction temperatures, time-temperature relationships for acclimation and de-acclimation, effectiveness of regulators, morphological adjustments to changes in light and temperature, and factors that influence the plants transition from the vegetative to the reproductive phase all appear to have an important influence on LT-induced gene regulation in this system. Studies in Arabidopsis (Reeves and Coupland, 2000) suggest that separate genetic pathways converge to control transition from the vegetative to the reproductive phases. The observations reported in the present study indicate that the PP and vernalization genes also influence the expression of LT-induced genes in cereals through separate pathways that eventually converge to activate genes controlling transition from the vegetative to the reproductive phase. Developmental regulation of LT tolerance gene expression by this means provides for a highly integrated system that permits multiple use of adaptive mechanisms and allows full expression of environmentally induced genes only when they are required in the plants life cycle.
MATERIALS AND METHODS
Plant Material and Experimental Design
Barley (Hordeum vulgare L. cv Dicktoo), which is an SD-sensitive cultivar without a vernalization requirement (Mahfoozi et al., 2000), was subjected to two PP treatments (8- and 20-h daylengths) and eight 4°C acclimation periods (0, 7, 14, 21, 42, 49, 56, and 70 d). FLN and LT50 (temperature at which 50% of the plants are killed by LT stress) were determined for each treatment. The experimental design was a 2 (PP) × 8 (acclimation period) factorial in a three-replicate (the experiment was repeated in its entirety three separate times) randomized complete block design.
The plants for LT50 determinations were grown hydroponically in continuously aerated one-half-strength modified Hoagland solution as outlined by Brule-Babel and Fowler (1988). Plants for FLN measurements were grown in 15-cm pots filled with Redi-earth (two plants/pot). The pots were wrapped in aluminum foil to help prevent radiant heat absorption from the lights, and the plants were uniformly fertilized with Osmocote sustained release fertilizer and a nutrient complete (Tune-up) water-soluble solution as required. Plants for both LT50 and FLN determinations were germinated and grown at 20°C in 8-h (SD) or 20-h (LD) d at a light intensity of 320 μmol m−2 s−1 for 14 d before being exposed to the eight 4°C acclimation periods under LD or SD conditions (0 d acclimation = start of 4°C acclimation period). Light intensity during LT acclimation at 4°C was 220 μmol m−2 s−1.
LT50 and Stage of Phenological Development
The procedure outlined by Limin and Fowler (1988) was used to determine the LT50 of each genotype at the end of each LT acclimation period in SD and LD treatments. Plant crowns were covered in moist sand in an aluminum weighing can and placed in a programmable freezer that was held at −3°C for 12 h. After 12 h, they were cooled at a rate of 2°C/h down to −17°C, then cooled at 8°C/h. Five crowns were removed at 2°C intervals for each of five test temperatures selected for each genotype in each treatment. The plant crowns were transplanted after slowly thawing and the LT50 was determined on the basis of regrowth after 3 weeks.
Stage of phenological development was determined by two methods. In the first method, PP sensitivity was determined by comparing the FLN (Wang et al., 1995) of potted plants grown continuously under SD and LD at 20°C (0 d at 4°C). Stages of phenological development were also determined for plants acclimated for 7, 14, 21, 42, 49, 56, and 70 d at 4°C under LD or SD. At the end of each acclimation period, plants grown in pots in the same cabinets as those used for the LT50 determinations outlined above were transferred to 20°C cabinets with 20-h (LD) PPs and a light intensity of 320 μmol m−2 s−1. Leaves on the main stem were numbered with a permanent marker, and the plants were grown until the flag leaf emerged and the FLN on the main shoot could be determined. Floral transition was considered to have been reached when the plant neither increased nor decreased its FLN. In the second method, stage of shoot apex development was determined on LD and SD crown samples of plants grown under the conditions for LT acclimation described above. At each freezing test time, at least two hydroponically grown plants were randomly sampled for dissection from the same populations used in the LT50 determinations and protein extraction. These plants were used to determine the phenological growth stages and identify the transition to the reproductive phase as indicated by double-ridge formation in the shoot apex (Kirby and Appleyard, 1987).
Protein Extraction, Separation, and Immunoblot Analysis
LT-induced acclimation in cereal leaf and crown tissues follow similar LT50 response patterns that are predetermined by the plant's genetic ability to respond to acclimating conditions (Limin and Fowler, 1985). Total proteins were extracted from leaf tissues of the plants that were grown for LT50 determinations using the phenol extraction procedure (Hurkman and Tanaka, 1986). Proteins extracted were resuspended in 2× SDS electrophoresis sample buffer (Laemmli, 1970). Equal amounts of proteins (8 μg) were separated on 10% (WCS120 and WCOR410) and 12% (WCS19) (w/v) SDS-PAGE and transferred electrophoretically for 1 h to nitrocellulose (NitroBind, Osmonics, Westborough, MA). After blocking with reconstituted skimmed milk (4% [w/v]) in phosphate-buffered saline containing 0.2% (w/v) Tween 20, the membrane was incubated with a 1:20,000 (v/v) dilution of the purified antibody (WCS19, C. N′Dong and F. Sarhan, unpublished data; WCS120, Houde et al., 1992; WCOR410, Danyluk et al., 1998). After washing with phosphate-buffered saline-Tween, the proteins recognized by the primary antibody were revealed with peroxidase coupled anti-rabbit IgG (Jackson Immunoresearch Inc., West Grove, PA) as a secondary antibody (1:20,000 [v/v] dilution). The complex was revealed using ECL chemiluminescence detection kit (Amersham Pharmacia Biotech, Uppsala) and X-OMAT-K film (Eastman-Kodak, Rochester, NY).
For western-blot signal quantification, Coomassie-stained proteins were first analyzed by densitometry using a CCD camera and AlphaEase 3.3a software (Alpha Innotech Corp., San Leandro, CA). Immunoblot signals were subsequently scanned with a densitometer and analyzed with ImageQuant 4.2 (Molecular Dynamics, Sunnyvale, CA). Immunoblot densitometric reading were adjusted against protein densitometric values and normalized by setting the maximum protein accumulation to 100%. For each antibody tested, the data represent the typical tendency curves from at least three independent immunoblots derived from two different extractions.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert technical assistance of Garcia Schellhorn and Khalil Kane.
Footnotes
This research was supported by a Natural Sciences and Engineering Research Council of Canada strategic grant.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010483.
LITERATURE CITED
- Brule-Babel AL, Fowler DB. Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop Sci. 1988;28:879–884. [Google Scholar]
- Chauvin LD, Houde M, Sarhan F. A leaf-specific gene stimulated by light during wheat acclimation to low-temperature. Plant Mol Biol. 1993;23:255–265. doi: 10.1007/BF00029002. [DOI] [PubMed] [Google Scholar]
- Crosatti C, Polverino de Laureto P, Bassi R, Cattivelli L. The interaction between cold and light controls the expression of the cold-regulated barley gene cor 14b and the accumulation of the corresponding protein. Plant Physiol. 1999;119:671–680. doi: 10.1104/pp.119.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Perron A, Houde M, Limin AE, Fowler DB, Benhamou N, Sarhan F. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell. 1998;10:623–638. doi: 10.1105/tpc.10.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Chauvin LP, Limin AE, Sarhan F. The regulatory role of vernalization in the expression of low-temperature-induced genes in wheat and rye. Theor Appl Genet. 1996a;93:554–559. doi: 10.1007/BF00417947. [DOI] [PubMed] [Google Scholar]
- Fowler DB, Limin AE, Ritchie JT. Low-temperature tolerance in cereals: model and genetic interpretation. Crop Sci. 1999;39:626–633. [Google Scholar]
- Fowler DB, Limin AE, Wang S-Y, Ward RW. Relationship between low-temperature tolerance and vernalization response in wheat and rye. Can J Plant Sci. 1996b;76:37–42. [Google Scholar]
- Gray GR, Chauvin LP, Sarhan F, Huner NPA. Cold acclimation and freezing tolerance. Plant Physiol. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Daniel C, Lachapelle M, Allard F, Laliberté S, Sarhan F. Immunolocalization of freezing-tolerance associated proteins in cytoplasm and nucleoplasm of wheat crown tissues. Plant J. 1995;8:583–593. doi: 10.1046/j.1365-313x.1995.8040583.x. [DOI] [PubMed] [Google Scholar]
- Houde M, Danyluk J, Laliberté J-F, Rassart E, Dhindsa DS, Sarhan F. A molecular marker to select for freezing tolerance in Gramineae. Mol Gen Genet. 1992;234:43–48. doi: 10.1007/BF00272343. [DOI] [PubMed] [Google Scholar]
- Hurkman MA, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby EJM, Appleyard M. Cereal Development Guide. Ed 2. Warwickshire, UK: Arable Unit, National Agriculture Centre; 1987. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurie DA. Comparative genetics of flowering time. Plant Mol Biol. 1997;35:167–177. [PubMed] [Google Scholar]
- Law CN, Worland AJ. Genetic analysis of some flowering time and adaptive traits in wheat. New Phytol. 1997;137:19–28. [Google Scholar]
- Limin AE, Danyluk J, Chauvin LP, Fowler DB, Sarhan F. Chromosome mapping of low-temperature induced Wcs120 family genes and regulation of cold-tolerance expression in wheat. Mol Gen Genet. 1997;253:720–727. doi: 10.1007/s004380050376. [DOI] [PubMed] [Google Scholar]
- Limin AE, Fowler DB. Cold-hardiness response of sequential winter wheat tissue segments to differing temperature regimes. Crop Sci. 1985;25:838–843. [Google Scholar]
- Limin AE, Fowler DB. Cold hardiness expression in interspecific hybrids and amphiploids of the Triticeae. Genome. 1988;30:361–365. [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB. Developmental regulation of low-temperature tolerance in winter wheat. Ann Bot. 2001;87:751–757. [Google Scholar]
- Mahfoozi S, Limin AE, Hayes PM, Hucl P, Fowler DB. Influence of photoperiod response on the expression of cold hardiness in wheat and barley. Can J Plant Sci. 2000;80:721–724. [Google Scholar]
- McMaster GS. Phenology, development, and growth of wheat (Triticum aestivum L.) shoot apex: a review. Adv Agron. 1997;59:63–118. [Google Scholar]
- Rahman MS, Wilson JH. Determination of spikelet number in wheat: III. Effect of varying temperature on ear development. Aust J Agric Res. 1978;29:495–467. [Google Scholar]
- Reeves PH, Coupland G. Response of plant development to environment: control of flowering by daylength and temperature. Curr Opin Plant Biol. 2000;3:37–42. doi: 10.1016/s1369-5266(99)00041-2. [DOI] [PubMed] [Google Scholar]
- Sarhan F, Danyluk J. Engineering cold-tolerant crops: throwing the master switch. Trends Plant Sci. 1998;3:289–290. [Google Scholar]
- Sarhan F, Ouelle F, Vasquez-Tello A. The wheat Wcs120 family: a useful model to understand the molecular genetics of freezing tolerance in cereals. Physiol Plant. 1997;101:439–445. [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagujfalvi A, Crosatti C, Galiba G, Dubcovsky J, Cattivelli L. Two loci on wheat chromosome 5A regulate the differential cold-dependent expression of the cor14b gene in frost tolerant and frost sensitive genotypes. Mol Gen Genet. 2000;263:194–200. doi: 10.1007/s004380051160. [DOI] [PubMed] [Google Scholar]
- Wang S-Y, Ward RW, Ritchie JT, Fischer RA, Schulthess U. Vernalization in wheat 1: a model based on the interchangeably of plant age and vernalization duration. Field Crop Res. 1995;41:91–100. [Google Scholar]
- Yan W, Wallace DH. A model of photoperiod × temperature interaction effects on plant development. Crit Rev Plant Sci. 1996;15:63–96. [Google Scholar]