Abstract
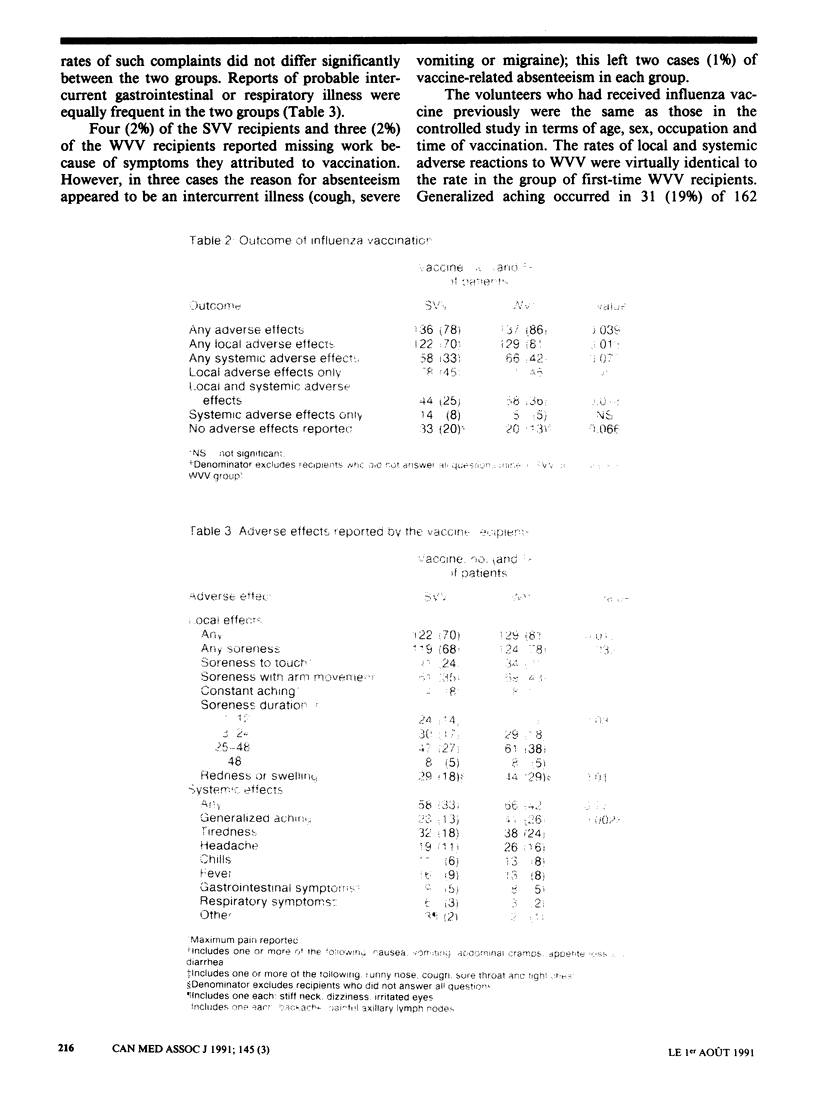
OBJECTIVE: To compare the adverse effects, particularly generalized aching, of a trivalent, inactivated whole-virion vaccine (WVV) and split-virion vaccine (SVV) for influenza in hospital personnel. DESIGN: Recipient-blinded study; first-time vaccinees were randomly assigned to receive either of the vaccines from one manufacturer in the 1989-90 influenza season. Subjects were asked to complete a symptom questionnaire during the 48 hours after immunization. SETTING: Annual influenza program for staff of a tertiary care children's hospital. PARTICIPANTS: Volunteers were sought among approximately 2200 members of the hospital staff. Of the 358 vaccinated for the first time, 333 (93%) returned the questionnaire. RESULTS: During the 48 hours after vaccination 13% of the SVV recipients reported generalized aching, as compared with 26% of the WVV recipients (p less than 0.01). Also, the SVV group reported fewer visible local reactions and more transient arm soreness, but the actual differences between the two groups were small. The occurrence of mild symptoms was equally common in the two groups (local reactions in at least 70% of cases, systemic reactions in at least 33%). In each group 1% of the subjects reported missing work because of the vaccination. CONCLUSIONS: The use of SVV reduces the rate of the most objectionable of the common adverse effects of influenza vaccination. Therefore, as with children, it might be more acceptable to health care workers than the current use of WVV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cate T. R., Couch R. B., Parker D., Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines - 1978. Rev Infect Dis. 1983 Jul-Aug;5(4):737–747. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- Girasek D. C. Increasing hospital staff compliance with influenza immunization recommendations. Am J Public Health. 1990 Oct;80(10):1272–1273. doi: 10.2105/ajph.80.10.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstein J. D., Smith L. J., Carter D. W., Engler R. J., Evans R., 3rd, Summers R. J. Comprehensive immunization delivery in conjunction with influenza vaccination. Arch Intern Med. 1986 Jun;146(6):1189–1192. [PubMed] [Google Scholar]
- Gross P. A., Ennis F. A., Gaerlan P. F., Denson L. J., Denning C. R., Schiffman D. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis. 1977 Nov;136(5):623–632. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- Hoffman P. C., Dixon R. E. Control of influenza in the hospital. Ann Intern Med. 1977 Dec;87(6):725–728. doi: 10.7326/0003-4819-87-6-725. [DOI] [PubMed] [Google Scholar]
- Jennings R., Clark A., Oxford J. S., Hockley D. J., Potter C. W. Reactogenicity and immunogenicity of whole and ether-Tween-split influenza A virus vaccines in volunteers. J Infect Dis. 1978 Nov;138(5):577–586. doi: 10.1093/infdis/138.5.577. [DOI] [PubMed] [Google Scholar]
- La Montagne J. R., Noble G. R., Quinnan G. V., Curlin G. T., Blackwelder W. C., Smith J. I., Ennis F. A., Bozeman F. M. Summary of clinical trials of inactivated influenza vaccine - 1978. Rev Infect Dis. 1983 Jul-Aug;5(4):723–736. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- Margolis K. L., Nichol K. L., Poland G. A., Pluhar R. E. Frequency of adverse reactions to influenza vaccine in the elderly. A randomized, placebo-controlled trial. JAMA. 1990 Sep 5;264(9):1139–1141. [PubMed] [Google Scholar]
- Mostow S. R., Eickhoff T. C., Chelgren G. A., Retailliau H. F., Castle M. Studies of inactivated influenza virus vaccines in hospital employees: reactogenicity and absenteeism. J Infect Dis. 1977 Dec;136 (Suppl):S533–S538. doi: 10.1093/infdis/136.supplement_3.s533. [DOI] [PubMed] [Google Scholar]
- Pachucki C. T., Pappas S. A., Fuller G. F., Krause S. L., Lentino J. R., Schaaff D. M. Influenza A among hospital personnel and patients. Implications for recognition, prevention, and control. Arch Intern Med. 1989 Jan;149(1):77–80. [PubMed] [Google Scholar]
- Scheifele D. W., Bjornson G., Johnston J. Evaluation of adverse events after influenza vaccination in hospital personnel. CMAJ. 1990 Jan 15;142(2):127–130. [PMC free article] [PubMed] [Google Scholar]
- Weingarten S., Riedinger M., Bolton L. B., Miles P., Ault M. Barriers to influenza vaccine acceptance. A survey of physicians and nurses. Am J Infect Control. 1989 Aug;17(4):202–207. doi: 10.1016/0196-6553(89)90129-6. [DOI] [PubMed] [Google Scholar]
- Weingarten S., Staniloff H., Ault M., Miles P., Bamberger M., Meyer R. D. Do hospital employees benefit from the influenza vaccine? A placebo-controlled clinical trial. J Gen Intern Med. 1988 Jan-Feb;3(1):32–37. doi: 10.1007/BF02595754. [DOI] [PubMed] [Google Scholar]
- Wise T. G., Dolin R., Mazur M. H., Top F. H., Jr, Edelman R., Ennis F. A. Serologic responses and systemic reactions in adults after vaccination with bivalent A/Victoria/75-A/New Jersey/76 and monovalent B/Hong Kong/72 influenza vaccines. J Infect Dis. 1977 Dec;136 (Suppl):S507–S517. doi: 10.1093/infdis/136.supplement_3.s507. [DOI] [PubMed] [Google Scholar]


