Abstract
An Arabidopsis mitochondrial proteome project was started for a comprehensive investigation of mitochondrial functions in plants. Mitochondria were prepared from Arabidopsis stems and leaves or from Arabidopsis suspension cell cultures, and the purity of the generated fractions was tested by the resolution of organellar protein complexes applying two-dimensional blue-native/N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine (Tricine) sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The Arabidopsis mitochondrial proteome was analyzed by two-dimensional isoelectric focusing/ Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 650 different proteins in a pI range of pH 3 to 10 were separated on single gels. Solubilization conditions, pH gradients for isoelectric focusing, and gel staining procedures were varied, and the number of separable proteins increased to about 800. Fifty-two protein spots were identified by immunoblotting, direct protein sequencing, and mass spectrometry. The characterized proteins cooperate in various processes, such as respiration, citric acid cycle, amino acid and nucleotide metabolism, protection against O2, mitochondrial assembly, molecular transport, and protein biosynthesis. More than 20% of the identified proteins were not described previously for plant mitochondria, indicating novel mitochondrial functions. The map of the Arabidopsis mitochondrial proteome should be useful for the analysis of knockout mutants concerning nuclear-encoded mitochondrial genes. Considerations of the total complexity of the Arabidopsis mitochondrial proteome are discussed. The data from this investigation will be made available at http://www.gartenbau.uni-hannover.de/genetik/AMPP.
Mitochondria play a pivotal role in energy metabolism of eukaryotic cells. Mitochondria are the location of numerous catabolic reactions, many of which are coupled to the reduction of NAD+, they are the location of the respiratory chain that reoxidizes NAD+, transfers electrons to molecular O2, and generates a proton gradient across the inner mitochondrial membrane, and they are the site of ADP phosphorylation by the ATP synthase complex. Furthermore, mitochondria are involved in several anabolic reactions: Mitochondria can synthesize amino acids, nucleotides, lipids, and prosthetic groups, such as heme, biotin, and lipoic acid. Mitochondria have their own genetic system and protein biosynthesis machinery. Finally, mitochondria seem to have central regulatory functions for the eukaryotic cell, e.g. in apoptosis (Gottlieb, 2000). To perform all of the addressed functions, mitochondria need a large number of different proteins, most of which are nuclear encoded and post-translationally transported into the organelle (Lithgow, 2000).
Mitochondria from plants have additional functions (Mackenzie and McIntosh 1999; Rasmusson et al., 1999). Plant mitochondria indirectly participate in photosynthesis, because an important step of the photorespiratory pathway—the decarboxylation of Gly—takes place in mitochondria (Raghavendra et al., 1998). Plant mitochondria have special ways for malate oxidation, which are based on the presence of an NAD-dependent malic enzyme (Winning et al., 1994). Plant mitochondria are capable of synthesizing Met, folate, and thymidylate (Neuburger et al., 1996; Rébeillé et al., 1997; Ravanel et al., 1998). The respiratory chain of plant mitochondria is much more branched than in other organisms: There is a cyanide-insensitive respiration, which is based on the alternative oxidase, and there are also alternative NADH dehydrogenases, which can use internal and external NADH or NADPH as substrates (Vanlerberghe and McIntosh, 1997; Rasmusson et al., 1999). The cytochrome c reductase of the respiratory chain of plants is a bifunctional enzyme because it comprises a protease activity that is responsible for the removal of presequences of nuclear-encoded mitochondrial proteins (Braun et al., 1992). The preprotein translocase of the outer mitochondrial membrane (also called the TOM complex) contains fewer preprotein receptors with broader substrate specificity (Jänsch et al., 1998; Braun and Schmitz, 1999; Werhahn et al., 2001). Finally, the genetic system of plant mitochondria is very special (for review, see Brennicke et al., 1999; Mackenzie and McIntosh, 1999). The plant mitochondrial genome is comparatively large, transcripts in plant mitochondria undergo editing before they are translated, and some transcripts are generated by trans-splicing. The genome of plant mitochondria undergoes rearrangements, which can have important implications, e.g. in causing cytoplasmic male sterility (Janska et al., 1998). To identify further functions of plant mitochondria and to better understand their complex role in plant cells, a comprehensive characterization of the plant mitochondrial proteome is necessary.
Recently, proteome analyses became a powerful tool for the investigation of complex cellular processes (for review, see Lottspeich, 1999; Görg et al., 2000) and were also successfully used for genetic and physiological studies in plants (for review, see Thiellement et al., 1999). Most proteomic studies are based on the resolution of protein mixtures by two-dimensional (2D) gel electrophoresis and subsequent identification of the resolved proteins by protein sequencing or mass spectrometry. However, the resolution capacity of 2D gel electrophoresis is still insufficient to monitor entire protein sets of eukaryotic cells. Hence, proteome research often is based on a subset of proteins of eukaryotic cells called “subproteome” (Cordewell et al., 2000; Jung et al., 2000). In plant biology, very successful subproteomic analyses were carried out for the cell wall, the plasma membrane, and the thylakoids (Robertson et al., 1997; Santoni et al., 1998, 2000; Peltier et al., 2000; Prime et al., 2000; van Wijk, 2000). During these investigations, hundreds of proteins were separated, several of which were identified for the first time.
Here, we report the characterization of a new subproteome of Arabidopsis, the mitochondrial proteome. Shortly before the completion of the Arabidopsis genome-sequencing project, we started a systematic approach to separate and identify the protein components of plant mitochondria. Optimization of mitochondrial preparations from green Arabidopsis tissues and Arabidopsis suspension cell cultures are reported. More than 50 mitochondrial proteins could be identified by mass spectrometry, direct protein sequencing, and immunoblotting, several of which were previously not described for plant mitochondria. 2D resolutions of mitochondrial proteins under varying conditions are used to define the total complexity of the mitochondrial proteome from Arabidopsis.
RESULTS
Purification of Mitochondria from Arabidopsis Stems and Leaves and from Arabidopsis Suspension Cell Cultures
The separation of mitochondria and chloroplasts from green plant cells is a difficult task that in the past often was overcome by the preparation of mitochondria from nongreen tissues such as tubers or etiolated seedlings. However, mitochondria from green tissue have to be prepared to understand the functions of mitochondria during photosynthesis. Some protocols were published that allow the separation of mitochondria and chloroplasts (Jackson et al., 1979; Day et al., 1985; Hamasur et al., 1990), but these protocols work for only specific plants. In an attempt to establish an efficient procedure for the preparation of Arabidopsis mitochondria from green tissue, several available protocols were tested but turned out to be of limited suitability. Arabidopsis mitochondria and chloroplasts are not well resolved on the three-step Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) gradients often used for plant mitochondrial preparations. In fact, it proved to be essential to separate the two classes of organelles by multiple differential centrifugations before gradient centrifugation as described in “Materials and Methods.”
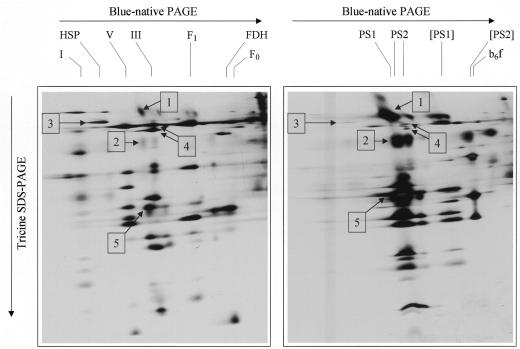
The purity of mitochondrial preparations usually is monitored by milligrams of chlorophyll per milligram of mitochondrial protein or by the activity of enzymes specific for mitochondria or chloroplast metabolism (Jackson et al., 1979; Day et al., 1985; Hamasur et al., 1990). However, monitoring organellar contamination by enzymes that are characteristic of either mitochondria or chloroplasts is a very predictive method that draws conclusions from looking at a minor segment of organellar proteins. Furthermore, contamination of mitochondrial fractions with different subcompartments of chloroplasts can vary considerably. We therefore established a novel procedure for monitoring the purity of mitochondria that is based on resolution of the characteristic mitochondrial or chloroplastic protein complexes by 2D blue-native/ N-[tris(hydroxymethyl)methyl]glycine (Tricine) SDS-PAGE (Fig. 1). The fractions of purified Arabidopsis mitochondria (Fig. 1, left) contained four dominant protein complexes: the NADH dehydrogenase, the HSP60 complex, the F0F1-ATP synthase (partially dissociated into the F1 and F0 parts), and the cytochrome c reductase, which were identified on the basis of their subunit compositions as characterized previously (Jänsch et al., 1996). The fractions of Arabidopsis chloroplasts (Fig. 1, right) contained the two photosystems and the b6f complex as identified by Kügler et al. (1997; the photosystem II forms a dimer on our gels, which was described for other native gel systems before [Boekema et al., 1995]). Mitochondrial HSP60 and the two subunits of the mitochondrial processing peptidase (MPP), which form part of the cytochrome c reductase complex (arrows 3 and 4 on Fig. 1), are very abundant in the mitochondrial fraction and are hardly detectable in the chloroplast fraction. A complementary situation is observed for the large subunits of the two photosystems (arrows 1 and 2 on Fig. 1). Based on the 2D gels, the purity of the mitochondrial fraction is estimated to be significantly >90%. However, slight contaminations of the mitochondrial fraction by chloroplast proteins were visible under all purification conditions tested, as exemplified by a light-harvesting complex I protein (arrow 5 on Fig. 1).
Figure 1.
Documentation of the purity of mitochondria prepared from green tissue (leaves and stems) of Arabidopsis. Mitochondria were isolated as described in “Materials and Methods” by differential centrifugation and density gradient centrifugation on Percoll step gradients (18%, 29%, 45% Percoll). A fraction containing mitochondria was isolated from the 29%/45% interphase and a fraction containing thylakoids was isolated from the 18%/29% interphase. The protein complexes of both fractions were separated by 2D blue-native/Tricine SDS-PAGE and visualized by silver staining. Left, Mitochondria; right, thylakoids. The designations on the top indicate the identity of the separated protein complexes: I, NADH dehydrogenase; V, F0F1 ATP synthase complex; III, cytochrome c reductase; F1, F0, F1, and F0 parts of the ATP synthase complex; FDH, formate dehydrogenase; HSP, heat stress protein 60; PS1, photosystem 1; PS2, photosystem 2; [PS1], subcomplex of photosystem 1; [PS2], subcomplex of photosystem 2, b6f, cytochrome b6f complex. The numbers on the gels mark the following proteins: 1, the PsaA/B proteins of photosystem 1 (approximately 80 kD); 2, the CP47/43 proteins of photosystem 2 (approximately 45 kD); 3, mitochondrial HSP60 (approximately 60 kD); 4, the MPP subunits of the mitochondrial cytochrome c reductase (approximately 55 kD); 5, light-harvesting chlorophyll protein of photosystem 1 (approximately 22 kD). The identifications are taken from Jänsch et al. (1996 [mitochondrial proteins]) and Kügler et al. (1997 [chloroplast proteins]).
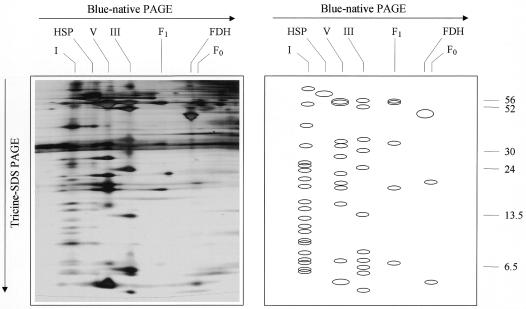
Because a proteomic characterization of cellular compartments should be based on extremely pure fractions, we decided to start our investigations with mitochondria prepared from suspension cell cultures grown in the dark. Arabidopsis suspension cultures were established previously and shown to be a valuable tool for the investigation of basic cell functions (May and Leaver, 1993; Zook, 1998). The mitochondria prepared from suspension cell cultures proved to be very pure as demonstrated by 2D blue-native/Tricine SDS-PAGE (Fig. 2). The main mitochondrial protein complexes are visible, whereas protein complexes from other cellular compartments are absent. The NADH dehydrogenase from Arabidopsis can be resolved into >20 subunits, and the ATP synthase and cytochrome c reductase complexes can be resolved into >10 subunits. (For unknown reasons, the cytochrome c oxidase complex is not visible on the 2D gels, most likely because of insolubility of this protein complex under the conditions applied.)
Figure 2.
Resolution of the mitochondrial protein complexes from Arabidopsis cell suspension cultures by 2D blue-native/Tricine SDS-PAGE. The gel was silver stained. A scheme of the gel is presented on the right. Designations on top refer to the identity of the resolved protein complexes (see Fig. 1), and the numbers on the right refer to the molecular masses of standard proteins (in kD).
Characterization of the Mitochondrial Proteome from Arabidopsis
The mitochondrial proteome from Arabidopsis prepared from suspension cell cultures was analyzed by 2D isoelectric focusing (IEF)/Tricine SDS-PAGE using immobilized nonlinear pH gradients of pH 3 to 10. Silver staining of the gels reproducibly revealed 650 different spots (Fig. 3). The majority of mitochondrial proteins has molecular masses between 30 and 60 kD and isoelectric points (IEPs) between pH 4.5 and 8.0. There are about 40 dominant spots, representing abundant proteins that are well solubilized under the conditions applied. The identities of >50 spots, which were selected randomly and which represent abundant as well as rare proteins, were successfully determined by immunoblotting, direct protein sequencing, and mass spectrometry (identified spots are marked by arrows in Fig. 3 and listed in Table I). For unknown reasons, spot identifications of another 25 proteins gave no interpretable data. Direct sequence determination by Edman degradation nearly always revealed sequence information indicating unmodified N termini, which can be explained by the fact that the N termini of most mitochondrial proteins are freshly generated by the mitochondrial processing peptidase shortly after import of proteins into the organelle. The determination of peptide masses of trypsinated spots by matrix-assisted laser-desorption ionization-mass spectrometry also led to successful identification of proteins, because >98% of the genomic sequence of Arabidopsis is now available in the public sequence databases, allowing the comprehensive definition of a theoretical Arabidopsis proteome including the trypsin fragments of all its components. If an identification of protein spots by matrix-assisted laser-desorption ionization-mass spectrometry was uncertain, electrospray ionization-mass spectrometry was used to generate partial sequence information by electrospray ionization tandem mass spectrometry. Identified proteins were grouped into 10 functional categories. Thirty percent of the proteins have a role in respiration and 25% in primary metabolism such as pyruvate decarboxylation, citric acid cycle, and amino acid and nucleotide metabolism. Five identified proteins represent chaperones; others are involved in molecular transport or protection against O2. Only two identified proteins form part of the mitochondrial protein biosynthesis machinery, probably indicating that proteins of this functional category are of low abundance. More than 20% of the identified proteins were not described for plant mitochondria previously: Protein 37 exhibits significant sequence similarity to an NADH-cytochrome b5 reductase from yeast, which is localized in the mitochondrial intermembrane space and transfers electrons from external NADH to the respiratory chain (Hahne et al., 1994). Protein 100 is similar to a chaperone from N. crassa, which was reported to be bound to a subcomplex of the NADH dehydrogenase during assembly of this protein complex (Kuffner et al., 1998). Protein 6 seems to represent an inosine 5-monophosphate dehydrogenase of the guanine synthesis pathway, protein 16 very much resembles bacterial peroxiredoxin and possibly has thioredoxin peroxidase activity, protein 1 resembles subunit d of the ATP synthase from rat, and protein 96 is similar to a recently discovered 17.2-kD subunit of complex I from beef (Skehel et al., 1998). Proteins 4, 18, 74, 84, 97, and 98 could not be unambiguously identified on the basis of sequence comparisons but clearly represent mitochondrial proteins based on subcellular localization predictions of computer programs. Proteins 18 and 74 resemble proteins involved in the regulation of cell development, protein 84 resembles an integral membrane protein described for M. musculus, and proteins 97 and 98 are similar to a putative gene product described for R. prowazekii. Only two of the identified spots might represent non-mitochondrial proteins: protein 75 is a 3-ketoacyl-CoA thiolase involved in the peroxisomal β-oxidation of fatty acids, and protein 82 represents a cytosolic aconitase. Hence, the analyzed mitochondrial fractions from Arabidopsis can be assumed to be very pure.
Figure 3.
2D map of mitochondrial proteins from Arabidopsis. Mitochondrial proteins (50 μg) were solubilized by lysis solution A (8 m urea, 4% Triton X-100, and 50 mm DTT) and IEF was carried out using a nonlinear IPG stripe of pH 3 to 10. Proteins were detected by silver staining. The numbers above the gel indicate pI values, and the numbers on the right indicate molecular masses of standard proteins. The arrows mark spots identified by direct protein sequencing, mass spectrometry, or immunoblotting (spot numbers correspond to the numbers in Table I).
Table I.
Identified proteins of the mitochondrial proteome from Arabidopsis
| No.a | Identity | Accession No.b | Calculated Molecular Mass | Calculated IEPc | P for Mitochondrial Localizationd | Identificatione | Sequences Determinedf | Remarks |
|---|---|---|---|---|---|---|---|---|
| kD | ||||||||
| Respiratory chain and ATP synthase complex | ||||||||
| 3 | Complex I, 18-kD subunit | AV553957 | 17.9 | 4.61 | 0.892 | ED | N: AKVKQTTGIVGLDVV | – |
| 83 | Complex I, 75-kD subunit | AB025630 | – | – | 0.989 | MS | I: NPAIIVGAGLFNR | – |
| 96 | Putative complex I, 17-kD subunit | AC012328 | – | – | 0.239 | MS | I: CLPDGNLLQTK | Similar to 17.2-kD subunit of complex I from beef (AJ011400); no presequence |
| 85 | Complex II, subunit α | O82663 | – | – | 0.941 | MS | I: AFGGQSLDFGK | Succinate dehydrogenase flavoprotein |
| 49 | Complex III, β-MPP subunit | AAF14827 | – | – | 0.991 | IgG | – | – |
| 50 | Complex III, α-MPP subunit | AC006216 | – | – | 0.955 | IgG | – | Occurrence of two mito |
| AC001645 | 0.944 | IgG | chondrial isoforms | |||||
| 1 | Putative ATP synthase, subunit d | AL132972 | 19.5 | 5.09 | 0.530 | ED, MS | N: XGAXKKIADVXFKAS | No presequence, Met |
| I: EAYDSIEIPK | absent; similar to ATPd | |||||||
| I: AFDEVNTQLQTK | from rat (P31399) | |||||||
| 2 | ATP synthase, subunit δ′ | D88376 | 18.8 | 5.12 | 0.985 | ED | N: STELPSTLDSTFVEA | – |
| 13 | ATP synthase, subunit δ | D88375 | 22.2 | 8.01 | 0.811 | ED | N: ATASAQTTANVKVPI | Apparent IEP at pH7 |
| 21 | ATP synthase, 24-kD subunit | AC007019 | 24.0 | 5.30 | 0.900 | ED | N: AKEAARPTFKGDEML | – |
| 22 | ATP synthase, 24-kD subunit | AC007019 | 24.0 | 5.30 | 0.900 | ED | N: AKEAARPTFKG | – |
| 30 | ATP synthase, subunit β | At5g08670 | 54.2 | 5.37 | 0.996 | ED | N: ATSXPASXAAPSSAP | Three very similar isoforms (At5g08670, |
| At5g08690 | 54.2 | 5.37 | 0.997 | At5g08680, and At5g08690) | ||||
| 32 | ATP synthase, subunit β | At5g08670 | 54.2 | 5.37 | 0.996 | ED | N: ATSSPAXXAA | Three very similar isoforms (At5g08670, |
| At5g08690 | 54.2 | 5.37 | 0.997 | At5g08680, and At5g08690) | ||||
| 55 | ATP synthase, subunit α | CAA69802 | 55.0 | 6.23 | – | ED | N: MELSPRAAELTNL… | Mitochondrial encoded |
| 93 | ATP synthase, inhibitor protein | AC005824 | – | – | 0.977 | MS | I: QGPGEQAAGSASEAKV… | – |
| 37 | Putative cytochrome-b5 reductase | AF296836 | 27.3 | 5.58 | 0.995 | ED | N: DQAKEETGPKTALN | Similar to NADH-cytochrome-b5 reductase from yeast Saccharomyces cerevisiae (S37800); bipartite presequence: localized in mitochondrial intermembrane space; apparent and calculated IEP differ by ∼pH 1.0 |
| Pyruvate decarboxylation and citric acid cycle | ||||||||
| 26 | Pyruvate dehydrogenase, subunit E1β | U09137 | 35.2 | 5.10 | 0.910 | ED | N: VRDALNSAIDEEMSADP | – |
| 80 | Pyruvate dehydrogenase, subunit E3 | AF228640 | – | – | 0.986 | MS | I: RTPFTSXXDLEK | Lipoamide dehydrogenase; occurrence of two mitochondrial isoforms |
| AC023673 | 0.984 | |||||||
| 29 | Succinyl-coenzyme A (CoA)-ligase, β-subunit | AJ001808 | 42.4 | 5.41 | 0.934 | ED | N: LNIHEYQGAELMGKY | – |
| 35 | Succinyl-CoA-ligase, α-subunit | AJ001807 | 31.7 | 6.73 | 0.971 | ED | N: AXDPHPPAAVFVDK | – |
| 33 | NAD-dependent malate dehydrogenase | AJ131205 | 33.3 | 6.00 | 0.998 | ED | N: SXXVVPERKVAI | Apparent and calculated IEP differ by ∼pH 0.25 |
| 34 | NAD-dependent malate dehydrogenase | AJ131205 | 33.3 | 6.00 | 0.998 | ED | N: SXGSVPERKVAILGA | – |
| Amino acid metabolism, N- and S-metabolism | ||||||||
| 48 | 3-mercaptopyruvate sulfurtransferase | CAB64716 | – | – | 0.987 | IgG | – | – |
| 77 | Glutamate dehydrogenase 2 | U37771 | – | – | 0.623 | MS | I: GAFTLGVNR | – |
| I: IVAVSXXTGALK | ||||||||
| 78 | Cys synthase | AB024282 | – | – | 09946 | MS | I: TPLVFLNK | Beta-cyano-Ala synthase |
| Nucleotide metabolism | ||||||||
| 9 | Nucleoside diphosphate kinase | AF044265 | 17.1 | 7.07 | 0.997 | ED | N: AEMERTFIAIKPDGVQRG | Localized in mt intermembrane space; bipartite presequence; two mitochondrial isoforms |
| AV536236 | 17.1 | 8.67 | ||||||
| 10 | Putative inosine 5-monophosphate dehydrogenase | CAB96841 | 18.6 | 7.18 | 0.855 | ED | N: ESTQPARMEESGFES | Similar to IMPDH from Methanopyrus kandleri (P50100) |
| 6 | Putative inosine 5-monophosphate dehydrogenase | CAB96841 | – | – | – | ED | N: ESTQPARMEESGF | Fragment of protein no. 10 |
| 7 | Nucleoside diphosphate kinase | AF044265 | – | – | – | ED | N: IKVIVPSKDFAQKH… | Fragment of protein no. 9 |
| Transport across membranes | ||||||||
| 40 | Mitochondrial porin | ATH131391 | 29.1 | 7.98 | – | ED, IgG | N: VKGPGLYTEIGKKAR | Two mitochondrial isoforms; presumably corresponds to isoform 1 (IEP: 7.98, Mr: 29.1) |
| 41 | Mitochondrial porin | ATH131391 | 29.1 | 7.98 | – | ED, IgG | N: VKGPGLY | Two mitochondrial isoforms; presumably corresponds to isoform 1 (IEP: 7.98, Mr: 29.1) |
| 42 | Mitochondrial porin | AC010676 | 29.3 | 8.77 | – | ED, IgG | N: XKGPGLYT | Two mitochondrial isoforms; presumably corresponds to isoform 2 (IEP: 8.77, Mr: 29.3) |
| 43 | Mitochondrial porin | AC010676 | 29.3 | 8.77 | – | ED, IgG | N: VKGPGLY… | Two mitochondrial isoforms; presumably corresponds to isoform 2 (IEP: 8.77, Mr: 29.3) |
| 46 | TOM40 subunit of the preprotein translocase | Q9LHE5 | 34.1 | 6.36 | – | MS | N: ADLLPPLTAAQVDAK | N-terminal Met absent |
| I: DVTASVGYDYMLR | ||||||||
| 47 | TOM20 subunit of the preprotein translocase | AJ296024 | – | – | – | IgG | – | Mitochondrial preprotein receptor, occurrence of four mitochondrial isoforms |
| Chaperones | ||||||||
| 8 | CPN10 | P34893 | 10.8 | 6.74 | 0.962 | MS | – | No presequence, blocked N terminus |
| 36 | Prohibitin | U69155 | 30.4 | 6.99 | 0.501 | MS | – | No presequence; blocked N terminus; apparent and calculated IEP differ by ∼pH 0.5; one membrane-spanning helix |
| 51 | HSP70 | AF217458 | – | – | 0.988 | IgG | – | – |
| 52 | HSP60 | P29197 | – | – | 0.989 | IgG | – | – |
| 100 | Putative chaperone for complex I assembly | AC007843 | – | – | 0.910 | MS | I: VLGMSLSVNAEGGAVG… | Similar to complex I intermediate associated protein from Neurospora crassa (O42636) |
| DNA replication, transcription, translation, DNA-RNA-binding proteins | ||||||||
| 5 | Gly-rich RNA-binding protein | AJ002892 | 11.6 | 5.19 | 0.743 | ED | N: TKLFIGGLSXXTDD… | Similar to mitochondrial ribosomal protein S19 (X77989) |
| 79 | Elongation factor TU | T01400 | – | – | 0.874 | MS | I: VGEEVEILGLR | – |
| Protection against oxygen | ||||||||
| 14 | Superoxide dismutase | AF061518 | 22.5 | 6.06 | 0.960 | ED, MS | N: IQTFTLPDLPYDYGA | – |
| IgG | I: LVVDTTANQDPLVTK | |||||||
| 16 | Putative peroxyredoxin | AAF66133 | 18.3 | 6.29 | 0.985 | ED | N: SKLAEGT… | Thioredoxin peroxidase activity? |
| Unknown mitochondrial proteins | ||||||||
| 4 | Unknown mitochondrial protein | AC007519 | 18.4 | 4.62 | 0.3842 | ED, MS | N: SEDVSHMPEMXD | Mitochondrial protein as predicted by TargetP (score: 0.787; Emanuelsson et al. 2000): abundant protein |
| I: AAEAVEEFGGILTSIK | ||||||||
| I: VTVLGTSGLSGSYVEQR | ||||||||
| 18 | Unknown mitochondrial protein | AC055769 | 19.7 | 5.84 | 0.994 | ED | N: RMDRSGGSYS | Two mitochondrial isoforms, similar to dal1 protein [O24657] from Arabidopsis, role in cell development? |
| AC004667 | 19.7 | 5.83 | 0.998 | |||||
| 74 | Unknown mitochondrial protein | AP000370 | – | – | 0.990 | MS | I: WVLPDSYLDVR | Similar to the DAG protein (CAA65064) from Antirrhinum majus, which plays a role in chloroplast differentiation |
| 84 | Unknown mitochondrial protein | AB018115AL035602 | – | – | 0.963 | MS | I: IVEALNVAAK | Similarity to the integral membrane protein stomatin from Mus musculus (JC5221); two mitochondrial isoforms |
| 97 | Unknown mitochondrial protein | AB007649 | – | – | 0.998 | MS | I: SILEAGSVVPPGR | Similar to unknown protein from Rickettsia prowazekii (gene RP516) |
| 98 | Unknown mitochondrial protein | T46212 | – | – | 0.996 | MS | I: IPSGELWGGNPAR | Similar to unknown protein from R. prowazekii (gene RP516) |
| I: TLTNEETLEIPK | ||||||||
| I: VAVATT | ||||||||
| Proteins of other cellular compartments | ||||||||
| 75 | 3-Ketoacyl-CoA thiolase | AB008854 | – | – | 0.518 | MS | I: TSLYGDDVVIVAAH | Role in peroxisomal β-oxidation of fatty acids |
| 82 | Aconitase (aconitate hydratase) | Q42560 | – | – | 0.243 | MS | I: RILLESAIR | Cytoplasmic protein? |
| I: KTSLAPGSGVVTKY | ||||||||
The nos. refer to the spot numbers as given in Figure 3.
The accession nos. represent protein entries if available (until December 2000); otherwise, entries for corresponding nucleic acids.
Calculated molecular masses and isoelectric points are given for proteins with known mature N termini. Apparent molecular masses and isoelectric points for all proteins can be extracted from Figure 3.
Probability for mitochondrial localization was calculated with MitoProt (Claros et al. 1996) except for mitochondrial-encoded proteins and proteins located in the outer mitochondrial membrane.
Methods of protein identification: ED, Edman degradation; MS, mass spectrometry; and IgG.
Partial amino acid sequences as determined by Edman degradation or electrospray ionization tandem mass spectrometry. N, N-terminal amino acid sequence; I, internal amino acid sequence.
The identified mitochondrial proteins are localized in all four mitochondrial subcompartments: the outer mitochondrial membrane (≥5 proteins), the inner mitochondrial membrane (>15 proteins), the mitochondrial intermembrane space (2 proteins), and the mitochondrial matrix (>20 proteins). Except for the α-subunit of the mitochondrial ATP synthase complex (protein 55), all identified proteins are nuclear encoded and imported into the organelle. Some proteins were identified more than once, most likely because of the presence of isoforms. In one case, the occurrence of isoforms could be verified by peptide sequences (proteins 97/98). For 29 different proteins, the N-terminal amino acid sequence was determined, enabling us to calculate theoretical molecular masses and pI values (Table I). In at least five cases, apparent and calculated IEPs of proteins differ significantly, probably reflecting post-translational modifications (proteins 13, 33, 34, 36, and 37).
Most proteins have a presequence for mitochondrial targeting, which is removed by the mitochondrial processing peptidase. Exceptions are mitochondrial encoded proteins, nuclear-encoded proteins destined for the outer mitochondrial membrane, such as porin or TOM40 (proteins 40–43, 46), and a few nuclear-encoded proteins destined for other mitochondrial subcompartments, such as the 17-kD subunit of complex 1 (protein 96), the subunit d of the ATP synthase complex (protein 1), or the chaperone CPN10 (protein 8). Comparisons between N-terminal sequences of mature mitochondrial proteins and the amino acid sequences deduced from the corresponding genes enabled us to newly define presequences (Table II). The average presequence length is 41.4 amino acids; the average content of Ser lies at 15.9%, the content of basic amino acids at 16%, and the content of acidic amino acids at only 1.3%. These data are in line with previous calculations, which were carried out on the basis of a large set of individually defined presequences (Sjöling and Glaser, 1998). In most proteins, an Arg is located at position +2 or +3 with respect to the cleavage site (Table II). Proteins 9 and 37 have a bipartite presequence for targeting to the mitochondrial intermembrane space.
Table II.
Presequence properties of identified mitochondrial proteins from Arabidopsis
| No.a | Protein | Length of Presequence | Ser | Arg/Lys | Glu/Asp | Arg at Cleavage Site? | P of Mitochondria (MITOPROT)b |
|---|---|---|---|---|---|---|---|
| no. of amino acids | % | ||||||
| 2 | ATP synthase, subunit δ′ | 26 | 23 | 19 | 0 | +3 | 0.985 |
| 3 | Complex I, 18-kD subunit | 11 | 0 | 18 | 0 | +4 | 0.892 |
| 4 | Unknown mitochondrial protein | 89 | 9 | 13 | 3 | +2 | 0.384 |
| 5 | Gly-rich RNA-binding protein | 34 | 15 | 9 | 0 | +4 | 0.743 |
| 9 | Nucleoside diphosphate kinasec | 85 | 19 | 11 | 4 | – | 0.997 |
| 10 | Inosine 5-monophosphatedehydrogenase | 39 | 15 | 10 | 0 | +2 | 0.855 |
| 13 | ATP synthase, subunit δ | 36 | 19 | 17 | 3 | +3 | 0.881 |
| 14 | Superoxide dismutase | 27 | 12 | 26 | 4 | +2 | 0.960 |
| 16 | Peroxyredoxin | 28 | 21 | 18 | 0 | +3 | 0.985 |
| 18 | Unknown mitochondrial protein | 58 | 19 | 16 | 0 | +2 | 0.989 |
| 21 | ATP synthase, 24-kD subunit | 32 | 9 | 12 | 0 | +3 | 0.900 |
| 26 | Pyruvate dehydrogenase E1β | 37 | 8 | 19 | 5 | – | 0.910 |
| 29 | Succinyl-CoA-ligase, β-subunit | 26 | 15 | 23 | 0 | +2 | 0.934 |
| 32 | ATP synthase, subunit β | 51 | 20 | 18 | 2 | +5 | 0.997 |
| 33 | NAD-dependent malatedehydrogenase | 22 | 23 | 23 | 0 | +2 | 0.998 |
| 35 | Succinyl-CoA-ligase, α-subunit | 42 | 26 | 14 | 2 | +3 | 0.971 |
| 37 | Cytochrome b5 reductasec | 60 | 17 | 8 | 0 | – | 0.995 |
| Average | 41.4 | 15.9 | 16.1 | 1.35 | – | 0.904 | |
The nos. refer to the nos. given in Figure 3.
The probability for mitochondrial targeting was calculated using MITOPROT (Claros and Vincens, 1996).
These proteins are targeted to the mitochondrial intermembrane space and have a bipartite presequence.
IEF is known to be complicated for hydrophobic proteins. Nevertheless, about 50% of the identified mitochondrial proteins from Arabidopsis represent proteins localized in the outer or inner mitochondrial membrane. However, most of these proteins form part of large protein complexes and lack hydrophobic membrane-spanning helices. Other membrane proteins form β-barrels for membrane insertion and can easily be analyzed by IEF, e.g. mitochondrial porin or TOM40 (proteins 40–43, 46). Some of the identified membrane proteins have one membrane-spanning helix, e.g. TOM20 (protein 47). Indeed, hydrophobic proteins with more than one membrane-spanning helix were not identified in the course of the Arabidopsis mitochondrial proteome project. Therefore, different procedures for the solubilization and resolution of mitochondrial proteins from Arabidopsis were systematically tested.
Analysis of the Arabidopsis Mitochondrial Proteome under Varying Conditions: How Many Mitochondrial Proteins from Arabidopsis Can Be Separated by 2D IEF/SDS-PAGE?
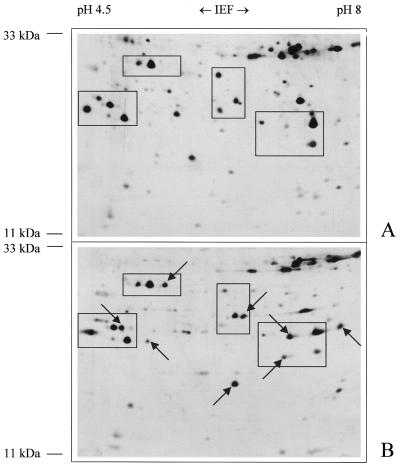
2D resolutions of Arabidopsis mitochondrial proteins under varying conditions were used to obtain information concerning potentials and limitations of 2D gel systems for the characterization of the Arabidopsis mitochondrial proteome. Solubilization of proteins with lysis solution B, which contains urea, thiourea, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS), sulfobetaine (SB) 3–10, and tributyl phosphine (TBP) and which was reported to be suitable for the solubilization of hydrophobic proteins (Molloy et al., 1998), allowed to visualize new proteins on the 2D maps (exemplified for the IEP range pH 4.5–8 and molecular mass range 11–33 kD in Fig. 4). Overall, solubilization with lysis solution B revealed comparatively few proteins spots, most of which were also visible after solubilization with lysis solution A (containing urea, Triton X-100, and dithiothreitol [DTT]). However, about 50 additional proteins became visible in the IEP range of pH 3 to 10 with respect to solubilization with lysis solution A (data not shown).
Figure 4.
2D pattern of mitochondrial proteins from Arabidopsis with IEP values between 4.5 and 8 and molecular masses between 11 and 33 kD after solubilization of proteins in lysis solution A (8 m urea, 2% Triton X-100, 20 mm DTT; A) or lysis solution B (5 m urea, 2 m thiourea, 2% CHAPS, 2% SB 3–10, 2 mm TBP; B). The gels were silver stained. The arrows mark proteins that are much better solubilized with lysis solution B.
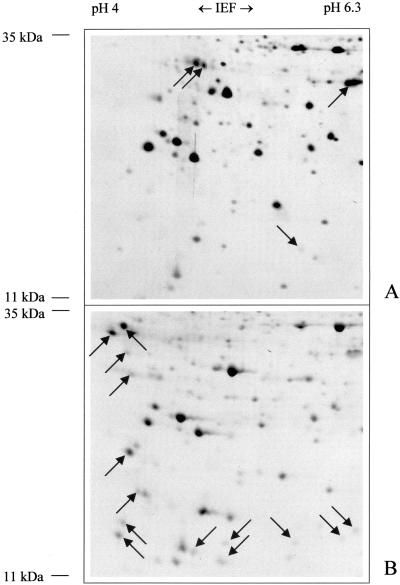
Visualization of proteins after gel electrophoresis depends on various staining procedures that are known to give different results with individual proteins. To analyze the effect of protein staining on the representation of the Arabidopsis mitochondrial proteome two 2D gels were stained in parallel with either Coomassie Blue or silver (Fig. 5). Most of the protein spots were visible on both gels. However, Coomassie Blue stained acidic and very small proteins better (silver-staining intensities of proteins are directly related to mole percentage of Lys; Dion and Pomenti, 1983). On the other hand, some specific proteins strongly reacted with silver but were hardly detectable on the Coomassie-stained gel. Overall, Coomassie staining revealed about 20 new spots not visible by silver staining in the IEP range pH 3 to 10. Length of silver staining also proved to be critical for protein visualization. Prolonged staining causes problems due to spot overlapping in gel areas with high spot density. In other gel areas, prolonged staining allowed us to visualize additional proteins of very low abundance (Fig. 6). The increase in spot number due to prolonged silver staining was estimated in representative gel areas and found to be 12%.
Figure 5.
2D pattern of mitochondrial proteins from Arabidopsis with IEP values between 4 and 6.3 and molecular masses between 11 and 35 kD after silver staining (A) or Coomassie Blue staining (B). Proteins were solubilized with lysis solution A (8 m urea, 4% Triton X-100, 50 mm DTT). Fifty micrograms of mitochondrial protein was loaded onto the silver-stained gel, and 1,000 μg of protein was loaded on the Coomassie-stained gel. Arrows mark protein spots not stained with Coomassie or silver.
Figure 6.
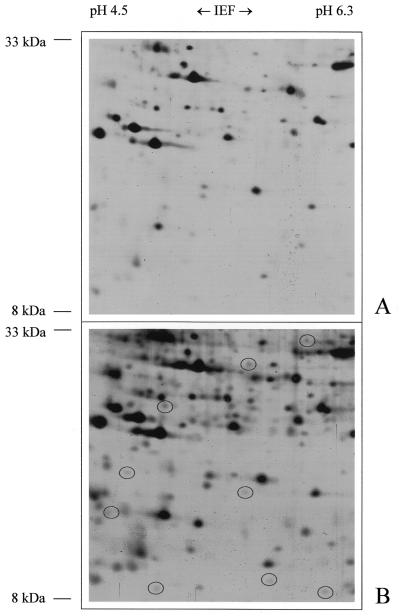
2D pattern of mitochondrial proteins from Arabidopsis with IEP values between 4.5 and 6.3 and molecular masses between 8 and 33 kD after silver staining for 10 min (A) or for 30 min (B). Both gels were loaded with 50 μg of protein. Circles indicate protein spots visible only after prolonged silver staining.
Finally, the influence of the immobilized pH gradients used for IEF was tested with respect to resolution capacity. Broad-range pH gradients are convenient for proteome analyses because they allow the parallel visualization of most proteins. However, narrow-range pH gradients can improve resolutions significantly, especially in gel regions with very high spot density. In the case of the mitochondrial proteome from Arabidopsis, the effect of varying pH gradients is documented in Figure 7. Because the spot density of separated mitochondrial proteins from Arabidopsis is moderate after use of broad-range pH gradients, narrow-range pH gradients only slightly improve resolution. After evaluation of representative gel areas, the resolution improvement of narrow-range pH gradients was found to be approximately 10%.
Figure 7.
2D pattern of mitochondrial proteins from Arabidopsis with IEP values between 5.1 and 5.9 and molecular masses between 15 and 100 kD after separation on IPG stripes with a pH gradient of 3 to 10 (A), of 4 to 7 (B), or of 5 to 6 (C). Boxes/arrows indicate regions/spots of improved resolution on narrow-range pH gradients.
Solubilization, resolution, and visualization of all mitochondrial proteins from Arabidopsis cannot be achieved on single gels. About 650 protein spots are visible after electrophoresis under standard conditions (solubilization with lysis solution A, nonlinear pH gradient pH 3–10, silver staining). Because of contamination or proteolytic fragmentations, the true number of distinct mitochondrial proteins might be 10% lower. On the other hand, variation of conditions for solubilization, resolution, and visualization increases the number of spots by about 30% and, therefore, the total number of separable mitochondrial proteins is estimated to be approximately 800 (Table III). However, several mitochondrial proteins might not be visible under all conditions applied.
Table III.
No. of detectable proteins forming part of the mitochondrial proteome from Arabidopsis
| No. of protein spots detectable on the 2D gel in Figure 3 between pH 3 and 10 | 650 |
| Positive corrections | |
| No. of protein spots only solubilized by lysis solution B (Fig. 4) | +7% |
| No. of protein spots only stained by Coomassie Blue (Fig. 5) | +3% |
| No. of protein spots only detectable after prolonged silver staining (Fig. 6) | +12% |
| No. of protein spots separated only on narrow-range pH gradients (Fig. 7) | +10% |
| Negative corrections | |
| No. of identified protein spots representing fragments (2/52) | −4% |
| No. of identified protein spots representing contaminations (2/52) | −4% |
| Sum of corrections | +24% |
| Total no. of different mitochondrial proteins detectable on 2D gels between pH 3 and 10 under the conditions applied | ∼800 |
DISCUSSION
Because Arabidopsis became the model organism for plant biology some years ago, progress in understanding the structure of its genomes was fast. In 1997, the sequence of the Arabidopsis mitochondrial genome was announced (Unseld et al., 1997), 2 years later, the complete sequence of the Arabidopsis chloroplast genome was published (Sato et al., 1999) and, by December 2000, the nearly complete sequence of the nuclear genome was presented (The Arabidopsis Genome Initiative, 2000). To analyze gene functions, new approaches have been started, including the establishment of large Arabidopsis “knock out” populations and the development of DNA chips to monitor gene expression. Meanwhile, proteome projects were initiated to systematically characterize gene products of defined subproteomes from Arabidopsis (Robertson et al., 1997; Santoni et al., 1998, 2000; Peltier et al., 2000). The present paper reports the initiation of an Arabidopsis mitochondrial proteome project.
Purity of Mitochondrial Fractions of Arabidopsis
A critical parameter for every subproteome project is the purity of the subcellular compartment to be analyzed. There were previous reports of the isolation of mitochondria from green Arabidopsis tissues (e.g. Turano et al., 1997; Berkemeyer et al., 1998; Yasuno and Wada, 1998; Fan et al., 1999). However, some of the generated mitochondrial fractions were not tested for chloroplast contamination, some were tested and found to contain chloroplast proteins, and some others were tested and found to be pure, but the results are based on monitoring single enzymes of single chloroplast subcompartments in the mitochondrial fractions. In the present study, the purity of the prepared mitochondrial fractions from green Arabidopsis tissue was demonstrated by 2D blue-native/Tricine SDS-PAGE, which is a very sensitive procedure that allows one to generate a “fingerprint” of the subunit compositions of characteristic mitochondrial and chloroplast protein complexes that are involved in photosynthesis or respiration. With this sensitive procedure, the purity of the mitochondrial fractions was found to be >90%. Preparation of mitochondrial fractions from green Arabidopsis tissue with higher purity might not be possible because some plastids exhibit sizes and densities like typical mitochondria.
Very pure mitochondrial fractions can be isolated from Arabidopsis suspension cell cultures as demonstrated by blue-native/Tricine SDS-PAGE. Indeed, the identification of protein spots on our 2D gels revealed only two proteins from other cellular compartments. Mitochondria isolated from suspension cell cultures represent a valuable starting material for studying all basic functions of mitochondria, such as respiration, citric acid cycle, amino acid and nucleotide metabolism, protein biosynthesis, molecular transport, and so on. Nongreen Arabidopsis cells also occur naturally in roots and flowers. On the other hand, suspension cell cultures represent an artificial system that might not reflect physiological conditions. Furthermore, studying mitochondria prepared from green tissue will be very important to investigate the role of mitochondria during photosynthesis.
Proteomics as a Tool for the Identification of Novel Mitochondrial Functions
The analysis of proteins in the course of this study allowed us to identify several proteins not described previously for plant mitochondria. A plant mitochondrial NADH-cytochrome b5 reductase may be an important enzyme for the oxidation of external NADH in concert with the rotenone-insensitive alternative NADH dehydrogenases that were characterized before (Rasmusson et al., 1999). The protein has a typical bipartite presequence as described previously for nuclear-encoded mitochondrial proteins destined to the mitochondrial intermembrane space. It is interesting that in yeast mitochondria the enzyme exists in two forms that are encoded by a single gene and generated by differential processing: a membrane-bound form on the outer mitochondrial membrane of the mitochondrion and a soluble form in the intermembrane space (Hahne et al., 1994; Hauke et al., 1997). Nucleotide metabolism in plant mitochondria is poorly understood, and the identification of a putative mitochondrial inosine 5-monophosphate dehydrogenase represents a starting point for the investigation of mitochondrial guanine biosynthesis. Peroxiredoxins play an important role in O2 protection of various cellular compartments. The newly identified plant mitochondrial peroxiredoxin might be a counterpart of yeast mitochondrial peroxiredoxin, which was discovered very recently and which was shown to exhibit thioredoxin peroxidase activity (Pedrajas et al., 2000). Another identified protein is presumably important for mitochondrial development because it exhibits sequence similarity to the chloroplast DAG protein of A. majus, which was shown to be important for chloroplast differentiation (Chatterjee et al., 1996). Some of the newly identified proteins seem to be involved in unknown mitochondrial functions because they represent abundant proteins on our 2D gels but exhibit no sequence similarity to any database entry. Hence, proteomics is a powerful tool for the discovery of novel mitochondrial proteins. Nevertheless, individual experiments have to be carried out to characterize all newly described proteins to verify their predicted functions and determine their biological roles. One promising strategy could be the comparison of mitochondrial proteomes of knock-out mutants for unknown mitochondrial proteins with the mitochondrial proteomes of corresponding wild-type plants. Proteomics in combination with genome information also allows one to newly define presequences for mitochondrial targeting and post-translational modifications.
How Many Proteins Form Part of the Mitochondrial Proteome from Arabidopsis?
Analyzing the Arabidopsis mitochondrial proteome by 2D gel electrophoresis and silver staining under standard conditions reveals about 650 different protein spots. If different protein lysis and staining procedures and different pH gradients are combined, about 800 different mitochondrial proteins can be separated. However, the total number of proteins forming part of the mitochondrial proteome from Arabidopsis might be twice as high for the following reasons: (a) Very hydrophobic proteins are known to be absent on gels after IEF. Although most mitochondrial membrane proteins form part of large protein complexes and, consequently, are not necessarily hydrophobic, there is a remaining class of hydrophobic membrane proteins that was not detected during this study including members of the mitochondrial metabolite carrier superfamily. (b) Very basic proteins (IEP > 10) were not analyzed in this study because of the pH gradients used for IEF. Studies of the theoretical proteomes of completely sequenced bacteria indicate that about 10% of bacterial proteins have pI values above pH 10 (Link et al., 1997a, 1997b; van Bogelen et al., 1997). This also might be true for mitochondria. (c) The abundance of some mitochondrial proteins is extremely low, preventing detection by silver staining. Examples might be transcription factors or other proteins with regulatory functions. (d) Some proteins might have identical molecular masses and pI values resulting in exactly overlapping spots. (e) Several mitochondrial proteins have functions related to photosynthesis or to other tissue-specific processes and might be absent in mitochondria prepared from Arabidopsis suspension cell cultures.
Based on these considerations, we estimate the total number of Arabidopsis genes encoding mitochondrial proteins to be in the range of 1,500 to 2,000. Recently, the total number of mitochondrial proteins of Arabidopsis was calculated by a new version of TargetP, a subcellular localization prediction software (Emanuelsson et al., 2000). According to this study, about 10% of all Arabidopsis genes would encode proteins destined to mitochondria. This result implies about 2,500 mitochondrial proteins because the total number of Arabidopsis genes is approximately 25,000 (The Arabidopsis Genome Initiative, 2000). The same study predicted about 14% Arabidopsis genes encoding chloroplast proteins (2,800–3,500 different proteins). Slightly smaller values were predicted by a different study of the Arabidopsis chloroplast proteome that was based on evolutionary considerations (1,900–2,500 different proteins, Abdallah et al., 2000). In yeast, the size of the mitochondrial proteome is estimated to be in the range of 400 to 500 different proteins (Karlberg et al., 2000; Lithgow et al., 2000), which represents 6.6% to 8.3% of the 6,000 postulated proteins of this organism. Hence, plants seem to have significantly more mitochondrial proteins than yeast, which should be due to the presence of isoforms of several mitochondrial enzymes and to the occurrence of additional mitochondrial functions in plants.
This paper reports the characterization of a plant mitochondrial proteome. Similar investigations were carried out for the human and rat mitochondrial proteome (Rabilloud et al., 1998; Lopez et al., 2000). In both projects, mitochondrial proteins were isolated and separated by 2D gel electrophoresis, and 46/92 proteins were identified. It is interesting that a large number of mitochondrial proteins were likewise identified in the two studies of the mammalian mitochondrial proteome on one side and the Arabidopsis mitochondrial proteome on the other. Often these proteins even have similar positions on the 2D gels. The mammalian mitochondrial proteome projects were initiated to better understand mitochondrial dysfunctions that have been implicated in numerous diseases. Similar promises might become true for the analyses of plant mitochondrial proteomes (Mihr et al., 2001). Alterations of mitochondrial functions were found to cause cytoplasmic male sterility, an attribute widely used for the generation of hybrid seeds in plant breeding. Characterizations of plant mitochondrial proteomes also will be a powerful tool for the investigation of knock-out mutants concerning nuclear-encoded mitochondrial proteins. Regular updates on additional identified proteins of the Arabidopsis mitochondrial proteome will be made available at http://www.gartenbau.uni-hannover.de/genetik/ AMPP.
MATERIALS AND METHODS
Isolation of Mitochondria/Chloroplasts
Arabidopsis was cultivated in a greenhouse under long-day conditions for 3 to 4 weeks. Starting material for organelle preparations were 100 g of green tissue (leaves and stems). The cells were disrupted using a grinder, and mitochondria were isolated by differential centrifugation and Percoll density gradient centrifugation as described by Werhahn et al. (2001) with the following modifications. Differential centrifugation consisted of five steps: four steps to purify the mitochondria from particles with higher sedimentation coefficients (10 min at 3,500g, two times 5 min at 3,500g, 5 min at 6,000g) and a final step to pellet a fraction enriched in mitochondria (10 min at 17,000g). The three-step Percoll gradients for density gradient centrifugation contained 18%, 29%, and 45% Percoll in 0.3 m Suc, 10 mm 3-(N-morpholino)propanesulfonic acid (MOPS)/KOH, pH 7.2. After gradient centrifugation (45 min at 70,000g), mitochondria were isolated from the 29%/45% interphase, and chloroplasts/thylakoids were isolated from the 18%/29% interphase. Purification of mitochondria from Arabidopsis cell lines was carried out as described previously (Werhahn et al., 2001). To efficiently prevent proteolysis, single organelle preparations were carried out in the presence of the protease inhibitor cocktail Complete (Roche Molecular Biochemicals, Mannheim, Germany).
2D Blue-Native/Tricine SDS-PAGE
The purity of organelle preparations was tested by 2D blue-native/Tricine SDS-PAGE (Schägger et al., 1994). About 100 μg of protein was resuspended in 75 μL of ACA buffer (0.5 mm EDTA, 750 mm aminocaproic acid, 50 mm Bis-Tris, pH 7.0) supplemented with 15 μL of 10% n-dodecyl maltoside. After centrifugation for 30 min at 20,000g, the supernatant was combined with 20 μL of a Coomassie Blue solution (5% Serva Blue G, 750 mm aminocaproic acid) and loaded onto a 4.5% to 16% acrylamide gradient gel. Electrophoresis was carried out as described by Jänsch et al. (1996).
2D IEF/Tricine SDS-PAGE
The mitochondrial proteome was analyzed by 2D IEF/Tricine SDS-PAGE. IEF was carried out with the IPGphor system Amersham Pharmacia Biotech AB (Uppsala, Sweden) using Immobiline DryStrip gels (18 cm) with nonlinear pH gradients (pH 3–10) according to the manufacturer's instructions (Berkelman and Stenstedt, 1998). Proteins (100 μg) were resuspended either in 10 μL of lysis solution A (8 m urea, 4% Triton X-100, 40 mm Tris base, 50 mm DTT, 0.1 mm phenylmethylsulfonyl fluoride) or in 10 μL of lysis solution B (5 m urea, 2 m thiourea, 2% CHAPS, 2% SB 3–10, 2 mm TBP, and 40 mm Tris base), incubated for 1 h at room temperature, and subsequently supplemented with 340 μL of the corresponding DryStrip rehydration solution (rehydration solution A: 8 m urea, 2% Triton X-100, 0.5% of a carrier ampholyte mixture [IPG buffer; Amersham Pharmacia Biotech, Piscataway, NJ], a trace of bromphenol blue, 20 mm DTT; rehydration solution B: 5 m urea, 2 m thiourea, 2% CHAPS, 2% SB 3–10, 2 mm TBP, and 0.5 m IPG buffer). In the case of protein identification by mass spectrometry or Edman degradation, 1,000 μg of mitochondrial protein was loaded onto the gel strips and Triton X-100 was substituted with CHAPS in the lysis and rehydration solutions. Focusing conditions were as described by Werhahn et al. (2001). For the second gel dimension, the gel strips were incubated with equilibration buffer 1 (50 mm Tris-HCl, pH 8.8, 6 m urea, 30% glycerol, 2% SDS, 66 mm DTT, a trace of bromphenol blue) and equilibration buffer 2 (50 mm Tris-HCl, pH 8.8, 6 m urea, 30% glycerol, 2% SDS, 135 mm iodoacetamide, a trace of bromphenol blue) for 15 min and subsequently placed horizontally onto a Tricine-SDS polyacrylamide gel as described by Berkelman and Stenstedt (1998). Tricine SDS-PAGE was carried out in the Protean II Xi cell from Bio-Rad (Munich; gel dimensions 20 × 18 × 0.1 cm) according to the protocol given by Schägger and von Jagow (1987). The 2D gels were silver stained (Heukeshoven and Dernick, 1986).
Protein Identification by Immunoblotting and Edman Degradation
The proteins separated on acrylamide gels were blotted onto nitrocellulose membranes for antibody stainings or onto polyvinylidene difluoride membranes for direct protein sequencing using the Trans-Blot Cell from Bio-Rad. Blotting onto nitrocellulose membranes was carried out in transfer buffer A (20 mm Tris base, 20% methanol, 150 mm Gly) for 6 h at 200 mA, and blotting onto polyvinylidene difluoride membranes was carried out in transfer buffer B (20 mm Tris-HCl, pH 8.8, 0.04% SDS, 1 mm DTT, 20% methanol) for 12 h at 500 mA. Immunostainings were performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. For sequencing, blotted proteins were stained with Ponceau S, cut out, and directly subjected to Edman degradation on a Procise-HAT protein sequencer (model ABI 494A, Applied Biosystems, Foster City, CA).
Protein Preparations for Mass Spectrometry
For mass spectrometry, gels were colloidal stained with Coomassie Blue (Neuhoff et al., 1985, 1990). Single proteins were cut out, transferred into an Eppendorf vessel, and incubated once in 250 μL of 25 mm NH4HCO3 and twice in 250 μL of 50% acetonitrile/25 mm NH4HCO3 for 30 min. Subsequently, the proteins were lyophilized and incubated with 20 μL of digestion solution (0.5 μg of trypsin [Promega, Madison, WI] in 20 μL of 50 mm NH4HCO3) for 4 h at 37°C. Samples were supplemented with 100 μL of 50 mm NH4HCO3 and incubated for 30 min. Afterward, supernatants were taken and stored. The gel pieces were incubated twice with 100 μL of 60% acetonitrile/0.5% formic acid, and all supernatants were pooled and lyophilized. Purification of the generated peptides was achieved using ZipTips (Millipore, Bedford, MA) according to the manufacturer's instructions.
Matrix-Assisted Laser Desorption Ionization/Time of Flight Mass Spectrometry
Determination of the molecular masses of Zip Tip-purified peptides was carried out by positive-ion matrix-assisted laser desorption ionization/time of flight mass spectrometry using a reflex instrument equipped with delayed-extraction and N2 laser (337 nm; Bruker, Newark, DE). For each sample 1 μL of matrix solution (19 mg of α-cyano-4-hydroxycinnamic acid in 1 mL of 60% methanol/0.1% formic acid) was placed on the Scout ion source and crystallized as a thin layer. One to 2 μL of sample was placed directly on the top of the thin matrix layer, and cocrystallization was carried out at room temperature. Spectra were recorded in reflection mode with an acceleration voltage of 20 kV and a reflection voltage of 21.5 kV. Monoisotopic masses from spectra were selected manually and used for protein identification with the help of GPMAW (Lighthouse Data, Lodbjerg, Denmark).
Electrospray Ionization Tandem Mass Spectrometry
For peptide sequencing, 3 μL of Zip Tip-purified sample was filled into Au/Pd-coated nanospray glass capillaries (Protana, Odense, Denmark). The tip of the capillary was placed orthogonally in front of the entrance hole of a quadrupole time-of-flight mass spectrometry instrument (Micromass, Manchester, UK) equipped with a nanospray ion source. A capillary voltage between 750 and 1,000 V and a cone voltage of 30 V was applied. Two-fold charged peptides were chosen for collision-induced dissociation experiments, and the corresponding parent ions were selectively transmitted from the quadrupole mass analyzer into the collision cell. Ar was used as the collision gas, and the kinetic energy was set between 20 and 40 eV. The resulting daughter ions were separated by an orthogonal time-of-flight mass analyzer. Peptide sequencing and protein identification was carried out with the programs PeptideSequencing and ProteinProbe of the BioLynx software package (version 3.4, Micromass).
Bioinformatics
2D gels were compared using the ImageMaster 2D Elite software (Amersham Pharmacia Biotech). All sequence comparisons were carried out at The Arabidopsis Information Resource (http://www.Arabidopsis.org/). Query sequences were compared with GenBank entries for Arabidopsis including expressed sequence tags and bacteria artificial chromosome ends with the help of TBLASTN and TFASTX3. Translations of nucleotide sequences were performed with TRANSLATE, and calculations of the molecular mass and the pI of proteins were performed with COMPUTE pI/Mw (both available at the ExPASy Molecular Biology Server, Geneva [http://www.expasy.ch/]). Alignments were calculated using SIM + LALNVIEW (ExPASy) or CLUSTALW (http://www2.ebi.ac.uk/clustalw/). Protein-coding regions of genomic DNA were predicted with GENEBUILDER (http://www.itba.mi.cnr.it/webgene/). For predictions of subcellular locations of proteins, the programs MITOPROT (http://www.mips. biochem.mpg.de/cgi-bin/proj/medgen/mitofilter/) and TargetP (http://www.cbs.dtu.dk/services/TargetP/) were used.
ACKNOWLEDGMENTS
We thank Professor Udo Schmitz for constant support and for critical reading of the manuscript. Thanks are also due to Gabi Kühne and Dagmar Lewejohann for the cultivation of Arabidopsis suspension cell cultures and expert technical assistance.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010474.
LITERATURE CITED
- Abdallah F, Salamini F, Leister D. A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Sci. 2000;5:141–142. doi: 10.1016/s1360-1385(00)01574-0. [DOI] [PubMed] [Google Scholar]
- Berkelman T, Stenstedt T. 2-D Electrophoresis using Immobilized pH Gradients: Principles and Methods. Piscataway, NJ: Amersham Pharmacia Biotech; 1998. [Google Scholar]
- Berkemeyer M, Scheibe R, Ocheretina O. A novel, non-redox-regulated NAD-dependent malate dehydrogenase from chloroplasts of Arabidopsis thalianaL. J Biol Chem. 1998;273:27927–27933. doi: 10.1074/jbc.273.43.27927. [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Hankamer B, Bald D, Kruip J, Nield J, Boonstra AF, Barber J, Rögner M. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc Natl Acad Sci USA. 1995;92:175–179. doi: 10.1073/pnas.92.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun HP, Emmermann M, Kruft V, Schmitz UK. The general mitochondrial processing peptidase from potato is an integral part of cytochrome creductase of the respiratory chain. EMBO J. 1992;11:3219–3227. doi: 10.1002/j.1460-2075.1992.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun HP, Schmitz UK. The protein import apparatus of plant mitochondria. Planta. 1999;209:267–274. doi: 10.1007/s004250050632. [DOI] [PubMed] [Google Scholar]
- Brennicke A, Marchfelder A, Binder S. RNA editing. FEMS Microbiol Rev. 1999;23:297–316. doi: 10.1111/j.1574-6976.1999.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C. DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO J. 1996;15:4194–4207. [PMC free article] [PubMed] [Google Scholar]
- Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem, 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- Cordewell SJ, Nouwens AS, Verrills NM, Basseal DJ, Walsh BJ. Subproteomics based upon protein cellular location and relative solubilities in conjunction with composite two-dimensional electrophoresis. Electrophoresis. 2000;21:1094–1103. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1094::AID-ELPS1094>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Day DA, Neuburger M, Douce R. Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol. 1985;12:219–228. [Google Scholar]
- Dion AS, Pomenti AA. Ammoniacal silver staining of proteins: mechanism of glutaraldehyde enhancement. Anal Biochem. 1983;129:490–496. doi: 10.1016/0003-2697(83)90582-1. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Cui D, Wang X. Subcellular distribution and tissue expression of phospholipase Dα, Dβ, and Dγ in Arabidopsis. Plant Physiol. 1999;119:1371–1378. doi: 10.1104/pp.119.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21:1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA. Mitochondria: execution central. FEBS Lett. 2000;482:6–12. doi: 10.1016/s0014-5793(00)02010-x. [DOI] [PubMed] [Google Scholar]
- Hahne K, Haucke V, Ramage L, Schatz G. Incomplete arrest in the outer membrane sorts NADH-cytochrome b5 reductase to two different submitochondrial compartments. Cell. 1994;79:829–839. doi: 10.1016/0092-8674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Hamasur B, Birgersson U, Eriksson AC, Glaser E. Large-scale purification procedure of spinach leaf mitochondria: isolation and immunological studies of the F1-ATPase. Physiol Plant. 1990;78:367–373. [Google Scholar]
- Hauke V, Ocana CS, Honlinger A, Tokatlidis K, Pfanner N, Schatz G. Analysis of the sorting signals directing NADH-cytochrome b5 reductase to two locations within yeast mitochondria. Mol Cell Biol. 1997;17:4024–4032. doi: 10.1128/mcb.17.7.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R. Silver staining of proteins. In: Radola BJ, editor. Elektrophoresis Forum 1986. Munich: Technische Universität München; 1986. pp. 22–27. [Google Scholar]
- Jackson C, Dench JE, Hall DO, Moore AL. Separation of mitochondria from contaminating subcellular structures utilizing silicea sol gradient centrifugation. Plant Physiol. 1979;64:150–153. doi: 10.1104/pp.64.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänsch L, Kruft V, Schmitz UK, Braun HP. New insights into the composition, molecular mass and stoichiometry of the protein complexes of plant mitochondria. Plant J. 1996;9:357–368. doi: 10.1046/j.1365-313x.1996.09030357.x. [DOI] [PubMed] [Google Scholar]
- Jänsch L, Kruft V, Schmitz UK, Braun HP. Unique composition of the preprotein translocase of the outer mitochondrial membrane from plants. J Biol Chem. 1998;273:17251–17257. doi: 10.1074/jbc.273.27.17251. [DOI] [PubMed] [Google Scholar]
- Janska H, Sarria R, Woloszynska M, Arrieta-Montiel M, Mackenzie SA. Stoichiometric shifts in the common bean mitochondrial genome leading to male sterility and spontaneous reversion to fertility. Plant Cell. 1998;10:1163–1180. doi: 10.1105/tpc.10.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E, Heller M, Sanchez JC, Hochstrasser DF. Proteomics meets cell biology: the establishment of subcellular proteomes. Electrophoresis. 2000;21:3369–3377. doi: 10.1002/1522-2683(20001001)21:16<3369::AID-ELPS3369>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Karlberg O, Canbäck B, Kurland CG, Andersson SGE. The dual origin of the yeast mitochondrial proteome. Yeast. 2000;17:170–187. doi: 10.1002/1097-0061(20000930)17:3<170::AID-YEA25>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner R, Rohr A, Schmiede A, Krull C, Schulte U. Involvement of two novel chaperones in assembly of mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Mol Biol. 1998;23:409–417. doi: 10.1006/jmbi.1998.2114. [DOI] [PubMed] [Google Scholar]
- Kügler M, Jänsch L, Kruft V, Schmitz UK, Braun HP. Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis. Photosynth Res. 1997;53:35–44. [Google Scholar]
- Link AJ, Hays LG, Carmack EB, Yates JR. Identifying the major proteome components of Hemophilus influenzaetype-strain NCTC8143. Electrophoresis. 1997a;18:1314–1334. doi: 10.1002/elps.1150180808. [DOI] [PubMed] [Google Scholar]
- Link AJ, Robison K, Church GM. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coliK2. Electrophoresis. 1997b;18:1259–1313. doi: 10.1002/elps.1150180807. [DOI] [PubMed] [Google Scholar]
- Lithgow T. Targeting of proteins to mitochondria. FEBS Lett. 2000;476:22–26. doi: 10.1016/s0014-5793(00)01663-x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Kristal BS, Chernokalskaya E, Lazarev A, Shestopalov AI, Bogdanova A, Robinson M. High-throughput profiling of the mitochondrial proteome using affinity fractionation and automation. Electrophoresis. 2000;21:3427–3440. doi: 10.1002/1522-2683(20001001)21:16<3427::AID-ELPS3427>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lottspeich F. Proteome analysis: a pathway to the functional analysis of proteins. Angew Chem Int Ed. 1999;38:2476–2492. doi: 10.1002/(sici)1521-3773(19990903)38:17<2476::aid-anie2476>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mackenzie S, McIntosh L. Higher plant mitochondria. Plant Cell. 1999;11:571–585. doi: 10.1105/tpc.11.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Leaver C. Oxidative stimulation of glutathione synthesis in Arabidopsis thalianasuspension cultures. Plant Physiol. 1993;103:621–627. doi: 10.1104/pp.103.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihr C, Baumgärtner M, Dieterich JH, Schmitz UK, Braun HP. Proteomic approach for investigation of cytoplasmic male sterility (CMS) in Brassica. J Plant Physiol. 2001;158:787–794. [Google Scholar]
- Molloy MP, Herbert BR, Walsh BJ, Tyler MI, Traini M, Sanchez JC, Hochstrasser DF, Williams KL, Gooley AA. Extraction of membrane proteins by differential solubilization for the separation using two-dimensional gel electrophoresis. Electrophoresis: 1998;19:837–844. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- Neuburger M, Rébeillé F, Jourdain A, Nakamura S, Douce R. Mitochondria are the major site for folate and thymidylate synthesis in plants. J Biol Chem. 1996;271:9466–9472. doi: 10.1074/jbc.271.16.9466. [DOI] [PubMed] [Google Scholar]
- Neuhoff V, Stamm R, Eibl H. Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis. 1985;6:427–448. [Google Scholar]
- Neuhoff V, Stamm R, Pardowitz I, Arold N, Ehrhardt W, Taube D. Essential problems in quantification of proteins following colloidal staining with Coomassie Brilliant Blue dyes in polyacrylamide gels, and their solution. Electrophoresis. 1990;11:101–117. doi: 10.1002/elps.1150110202. [DOI] [PubMed] [Google Scholar]
- Pedrajas JR, Miranda-Vizuete A, Javanmardy N, Gustafsson JA, Spyrou G. Mitochondria of Saccharomyces cerevisiaecontain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J Biol Chem. 2000;275:16296–16391. doi: 10.1074/jbc.275.21.16296. [DOI] [PubMed] [Google Scholar]
- Peltier JB, Friso G, Kalume DE, Roepstorff P, Nilsson F, Adamska I, van Wijk KJ. Proteomics of the chloroplast: systematic identification and targeting analysis of lumenal and peripheral thylakoid proteins. Plant Cell. 2000;12:319–341. doi: 10.1105/tpc.12.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime TA, Sherrier DJ, Mahon P, Packman LC, Dupree P. A proteomic analysis of organelles from Arabidopsis thaliana. Electrophoresis. 2000;21:3488–3499. doi: 10.1002/1522-2683(20001001)21:16<3488::AID-ELPS3488>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rabilloud T, Kieffer S, Procaccio V, Louwagie M, Courchesne PL, Patterson SD, Martinez P, Garin J, Lunardi J. Two-dimensional electrophoresis of human placental mitochondria and protein identification by mass spectrometry: toward a human mitochondrial proteome. Electrophoresis. 1998;19:1006–1014. doi: 10.1002/elps.1150190616. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Reumann S, Heldt HW. Participation of mitochondrial metabolism in photorespiration. Plant Physiol. 1998;116:1333–1337. doi: 10.1104/pp.116.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Svensson AS, Knoop V, Grohmann L, Brennicke A. Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: two enzymes are present in potato mitochondria. Plant J. 1999;20:79–87. doi: 10.1046/j.1365-313x.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Ravanel S, Gakiere B, Job D, Douce R. The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci USA. 1998;95:7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébeillé F, Macherel D, Mouillon JM, Garin J, Douce R. Folate biosynthesis in higher plants: purification and molecular cloning of a bifunctional 6-hydroxymethyl-7, 8-dihydropterin pyrophosphokinase/7,8-dihydropteroate synthase localized in mitochondria. EMBO J. 1997;16:947–957. doi: 10.1093/emboj/16.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Mitchell GP, Gilroy JS, Gerrish C, Bolwell GP, Slabas AR. Differential extraction and protein sequencing reveals major differences in patterns of primary cell wall proteins from higher plants. J Biol Chem. 1997;272:15841–15848. doi: 10.1074/jbc.272.25.15841. [DOI] [PubMed] [Google Scholar]
- Santoni V, Kieffer S, Desclaux D, Masson F, Rabilloud T. Membrane proteomics: use of additive main effects with multiplicative interaction model to classify plasma membrane proteins according to their solubility and electrophoretic properties. Electrophoresis. 2000;21:3329–3344. doi: 10.1002/1522-2683(20001001)21:16<3329::AID-ELPS3329>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Santoni V, Rouquié D, Doumas P, Mansion M, Boutry M, Degand H, Dupree P, Packman L, Sherrier J, Prime T. Use of a proteome strategy for tagging proteins present at the plasma membrane. Plant J. 1998;16:633–641. doi: 10.1046/j.1365-313x.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S. Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 1999;29:283–290. doi: 10.1093/dnares/6.5.283. [DOI] [PubMed] [Google Scholar]
- Schägger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sjöling S, Glaser E. Mitochondrial targeting peptides in plants. Trends Plant Sci. 1998;3:136–140. [Google Scholar]
- Skehel JM, Fearnley IM, Walker JE. NADH:ubiquinone oxidoreductase from bovine heart mitochondria: sequence of a novel 17.2-kDa subunit. FEBS Lett. 1998;438:301–305. doi: 10.1016/s0014-5793(98)01317-9. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Thiellement H, Bahrman N, Damerval C, Plomion C, Rossignol M, Santoni V, Vienne D, Zivy M. Proteomics for genetic and physiological studies in plants. Electrophoresis. 1999;20:2013–2026. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2013::AID-ELPS2013>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Turano FJ, Thakkar SS, Fang T, Weisemann JM. Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiol. 1997;113:1329–1341. doi: 10.1104/pp.113.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unseld M, Marienfeld JR, Brandt P, Brennicke A. The mitochondrial genome of Arabidopsis thalianacontains 57 genes in 366924 nucleotides. Nat Genet. 1997;15:57–61. doi: 10.1038/ng0197-57. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- van Bogelen RA, Abshire KZ, Moldover B, Olson ER, Neidhardt FC. Escherichia coliproteome analysis using the gene-protein database. Electrophoresis. 1997;18:1243–1251. doi: 10.1002/elps.1150180805. [DOI] [PubMed] [Google Scholar]
- van Wijk KJ. Proteomics of the chloroplast: experimentation and prediction. Trends Plant Sci. 2000;5:420–425. doi: 10.1016/s1360-1385(00)01737-4. [DOI] [PubMed] [Google Scholar]
- Werhahn W, Niemeyer A, Jänsch L, Kruft V, Schmitz UK, Braun HP. Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis thaliana: identification of multiple forms of TOM20. Plant Physiol. 2001;125:943–954. doi: 10.1104/pp.125.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning BM, Bourguignon J, Leaver CJ. Plant mitochondrial NAD+-dependent malic enzyme: cDNA cloning, deduced primary structure of the 59- and 62-kDa subunits, import, gene complexity and expression analysis. J Biol Chem. 1994;269:4780–4786. [PubMed] [Google Scholar]
- Yasuno R, Wada H. Biosynthesis of lipoic acid in Arabidopsis: cloning and characterization of the cDNA for lipoic acid synthase. Plant Physiol. 1998;118:935–943. doi: 10.1104/pp.118.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook M. Biosynthesis of camalexin from tryptophan pathway intermediates in cell-suspension cultures from Arabidopsis. Plant Physiol. 1998;118:1389–1393. doi: 10.1104/pp.118.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]