Abstract
Myrosinase (EC 3.2.3.1) is a glucosinolate-degrading enzyme mainly found in special idioblasts, myrosin cells, in Brassicaceae. This two-component system of secondary products and degradative enzymes is important in plant-insect interactions. Immunocytochemical analysis of Arabidopsis localized myrosinase exclusively to myrosin cells in the phloem parenchyma, whereas no myrosin cells were detected in the ground tissue. In Brassica napus, myrosinase could be detected in myrosin cells both in the phloem parenchyma and in the ground tissue. The myrosin cells were similar in Arabidopsis and B. napus and were found to be different from the companion cells and the glucosinolate-containing S-cells present in Arabidopsis. Confocal laser scanning immunomicroscopy analysis of myrosin cells in B. napus embryos showed that the myrosin grains constitute a continuous reticular system in the cell. These findings indicate that in the two species studied, initial cells creating the ground tissue have different potential for making idioblasts and suggest that the myrosinase-glucosinolate system has at least partly different functions. Several myrosinases in B. napus extracts are recovered in complex together with myrosinase-binding protein (MBP), and the localization of MBP was therefore studied in situ. The expression of MBP was highest in germinating seedlings of B. napus and was found in every cell except the myrosin cells of the ground tissue. Rapid disappearance of the MBP from the non-myrosin cells and emergence of MBP in the myrosin cells resulted in an apparent colocalization of MBP and myrosinase in 7-d-old seedlings.
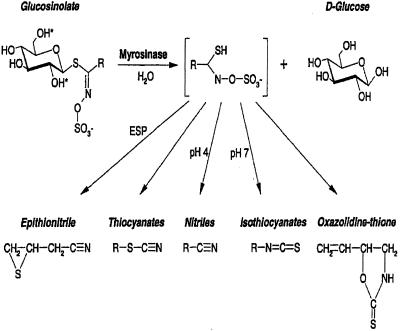
Glucosinolates constitute a group of secondary metabolites characteristic of the order Capparales but mainly found in the family Brassicaceae (Rodman et al., 1996; Rask et al., 2000). These compounds consist of a thioglucoside moiety linked to a variety of amino acid-derived side chains (Chew, 1988; Louda and Mole, 1991; Bones and Rossiter, 1996; Rosa et al., 1997). Whereas glucosinolates are sometimes regarded as being involved in intermediary metabolism as storage substances or precursors, the myrosinase-glucosinolate system is more often regarded as a defense system against generalist herbivores (Rask et al., 2000). The enzyme myrosinase (β-thioglucoside glucohydrolase, EC 3.2.3.1) catalyzes cleavage of glucosinolates to aglucons that decompose to form toxic products such as isothiocyanates, thiocyanates, nitriles, or epithionitriles (Fig. 1). In general, glucosinolates and myrosinase are thought to be brought together to interact (see below), either by a transport mechanism or by following tissue disruption, e.g. wounding caused by insect herbivory, breaking cellular boundaries.
Figure 1.
General structure of glucosinolates and their possible products after myrosinase cleavage. R denotes amino acid-derived side chains. Epithiospecifier protein (ESP) together with the pH and other factors are critical parameters determining which product is formed from the aglucone.
In Arabidopsis, two expressed myrosinase genes have been found (Xue et al., 1995). A more complex array of myrosinase genes has been reported for Brassica napus, which contains at least 20 genes divided into three subfamilies, MA, MB, and MC (Rask et al., 2000). In extracts from B. napus seeds and seedlings, myrosinases in the subfamilies MB and MC are found in complexes together with myrosinase-binding proteins (MBP; Lenman et al., 1990; Falk et al., 1995a; Taipalensuu et al., 1996; Geshi and Brandt, 1998). The levels of MBP transcripts are, like certain glucosinolates, induced in response to wounding and jasmonate treatment (Bodnaryk, 1992; Doughty et al., 1995; Taipalensuu et al., 1997a, 1997b).
Myrosinase has been found in all investigated organs of B. napus plants, mainly in idioblasts, also called myrosin cells (Thangstad et al., 1990, 1991; Höglund et al., 1991, 1992). Idioblasts are specialized cells that are scattered at low frequency and often as single cells among the other major cells in a tissue. Myrosin cells are anatomically characterized by a high protein content in the vacuole and thus are prone to react cytochemically with certain protein reagents. Ultrastructurally, the vacuolar content is fairly electron dense and the cytoplasm contains distended rough endoplasmic reticulum (rER) having a lumen with a similar electron density as the vacuoles (Jørgensen, 1981). In mature embryos of members of the Brassicaceae, myrosin cells can be distinguished from the surrounding cells by the absence of globoids in the protein bodies (Rest and Vaughan, 1972; Bones and Iversen, 1985; Höglund et al., 1992). Myrosinase has also been suggested to be present in other cell types, e.g. Bones et al. (1991) reported that myrosinase-containing cells in the vascular tissue most likely were companion cells.
The only glucosinolate that has been localized immunohistochemically is the highly abundant aliphatic glucosinolate sinigrin in Brassica juncea embryos (Kelly et al., 1998). Using light microscopy (LM) analysis, sinigrin was found to be present in vacuoles of aleurone-like cells but not in myrosin cell idioblasts. In Arabidopsis, glucosinolates have been found to be highly enriched in certain sulfur containing “S-cells” in the pedicel (flower stalk), located externally to the vascular system (Koroleva et al., 2000). The S-cells are giant cells that line the phloem between the vascular bundles and the endodermis (also denoted the starch sheath).
Today, Arabidopsis is the most useful model system in plant research. The recently available genome information further supports studies of various processes in this model system and will call for thorough investigations into special characteristics, e.g. the myrosinase-glucosinolate system. In this paper, we describe the cellular localization of myrosinase to be exclusively in myrosin cells in the phloem parenchyma in Arabidopsis and report ultrastructural characterization of those cells and the S-cells. We also show the cellular and subcellular localization of myrosinase in B. napus in myrosin cells in the phloem and the ground tissue. Finally, MBP was immunohistochemically detected in seedlings.
RESULTS
Myrosinase Expression in Arabidopsis
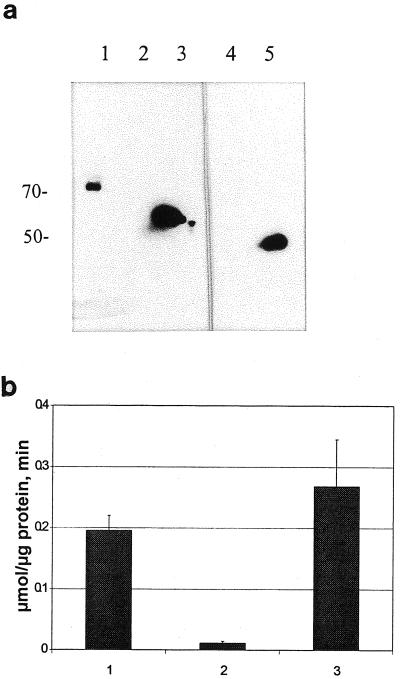
Using the previously characterized monoclonal antibody 3D7 specific to different plant myrosinases, we detected one band by western-blot analysis of protein extracts from leaves of Arabidopsis (Fig. 2a, lane 1). In the same tissue, we detected myrosinase activity (Fig. 2b, column 1). However, no western-blot signal was detected in extracts from Arabidopsis seeds (Fig. 2a, lane 2), and only very low myrosinase activity was found (Fig. 2b, column 2). As expected, myrosinase from B. napus seeds was readily detected by western-blot analysis (Fig. 2a, lane 3) and enzyme activity measurements (Fig. 2b, column 3). Thus, the activity measurements correlated with the western-blot analysis. The anti-MBP antibody did not recognize any protein in Arabidopsis leaf extracts (Fig. 2a, lane 4), whereas the positive control (B. napus seed extract) showed MBP expression (Fig. 2a, lane 5).
Figure 2.
Western-blot analysis (a) and myrosinase activity measurements (b) of protein extracts from Arabidopsis and B. napus. The monoclonal antibody 3D7 was used to detect myrosinase in leaves (a, lane 1) and seeds (lane 2) from Arabidopsis and seeds from B. napus (lane 3). The monoclonal antibody 34:14 was used to detect MBP from Arabidopsis leaves (lane 4) and B. napus seeds (lane 5). b, Specific myrosinase activity in leaves (column 1) and seeds (column 2) from Arabidopsis, and seeds from B. napus (column 3).
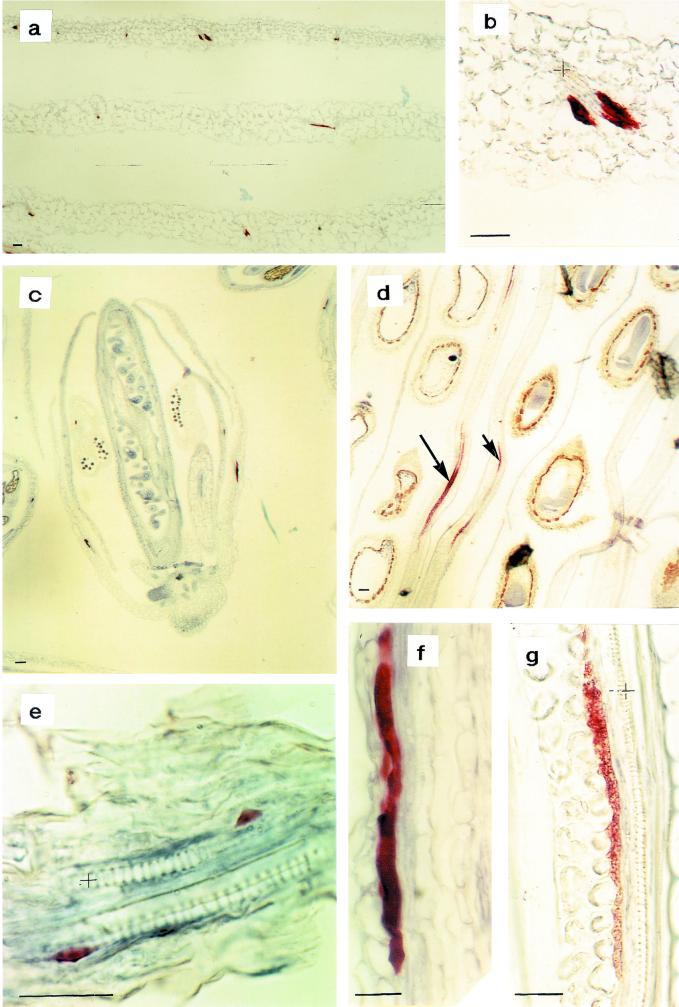
The spatial distribution of myrosinase in Arabidopsis was investigated using both the monoclonal antibody 3D7 and the polyclonal rabbit antiserum K505. Both antibodies showed the same staining characteristics confined to idioblastic cells in the phloem parenchyma of all tissues examined. In leaves from 25-d-old plants, myrosinase-containing cells often occurred symmetrically in the phloem parenchyma (Fig. 3, a and b). In flower buds, some cells in the developing petals and sepals contained myrosinase (Fig. 3c). In 10-d-old siliques, the myrosinase-containing phloem parenchyma cells were larger (Fig. 3d). In contrast, no myrosinase-containing cells were detected in the developing embryo or in near mature seeds. Five-day-old seedlings showed only small myrosinase-containing phloem parenchyma cells in the axis (Fig. 3e). In 9-d-old seedlings, it was possible to detect myrosinase also in large cells in the phloem parenchyma of the cotyledon. The staining was more granulated in myrosinase-containing cells from older tissues such as siliques (Fig. 3g) compared with younger tissues such as sepals (Fig. 3f), where the staining was more homogeneous. No staining could be observed in the negative control.
Figure 3.
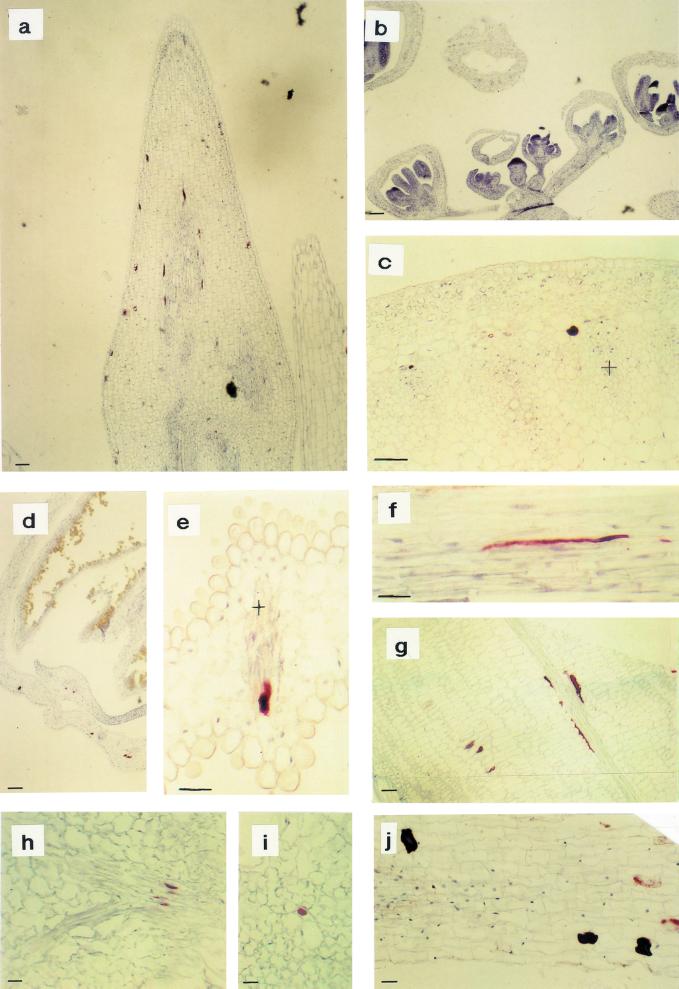
Immunohistochemical analysis of myrosinase in Arabidopsis using the monoclonal antibody 3D7. In fully expanded leaves from 25-d-old plants, myrosinase were found in often pair-wise-occurring idioblastic cells of the phloem parenchyma (a and b). b, Larger magnification of a. In flower buds, some cells contained myrosinase (c and f), even in the developing petal and sepal (c). In 10-d-old siliques, the myrosinase containing idioblastic phloem parenchyma cells were larger and the staining was more granulated (d and g). d, Arrows indicate long myrosinase-expressing cells. e, The yellowish staining outside the endosperm is retained unoxidized substrate and is therefore regarded as background. In 5-d-old seedlings, developing myrosinase-containing phloem cells were only found in the axis. Xylem is marked with + and the size bars correspond to 10 μm.
Myrosinase expression has been reported in young guard cells from B. napus (Höglund et al., 1991). However, radioactive in situ hybridization analysis with the myrosinase TGG1 probe (Xue et al., 1995) and immunohistochemical analysis with the anti-myrosinase antibodies 3D7 and K505 of tissue sections of Arabidopsis cotyledons and leaves of different ages did not reveal any signal from the transcript or the protein in the guard cells (results not shown).
Cytochemical and Ultrastructural Analysis in Arabidopsis
A correlation between staining with Millon's protein reagent (Jensen, 1962) of myrosin cells and immunostaining for myrosinase has been shown for B. napus seeds (Höglund et al., 1991). In an Arabidopsis leaf, the aniline blue black (ABB) protein reagent (Fisher, 1968) stained the material in the vacuole of the myrosin cell (Fig. 4a), and the same myrosin cell reacted with the antimyrosinase antibody 3D7 (Fig. 4b) in consecutive sections. This, together with the immunoelectron microscopy (IEM) study (Fig. 5a, see below), shows that the idioblastic myrosin cells present in the phloem parenchyma are the myrosinase-containing cells.
Figure 4.
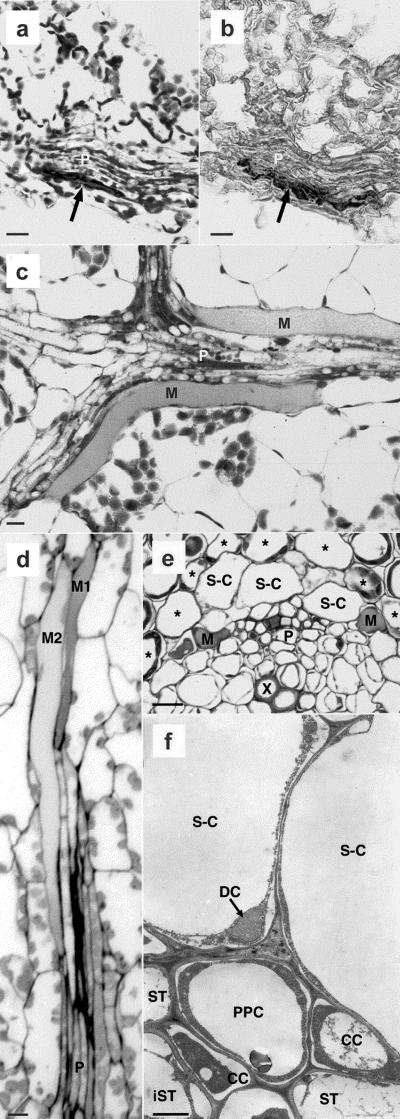
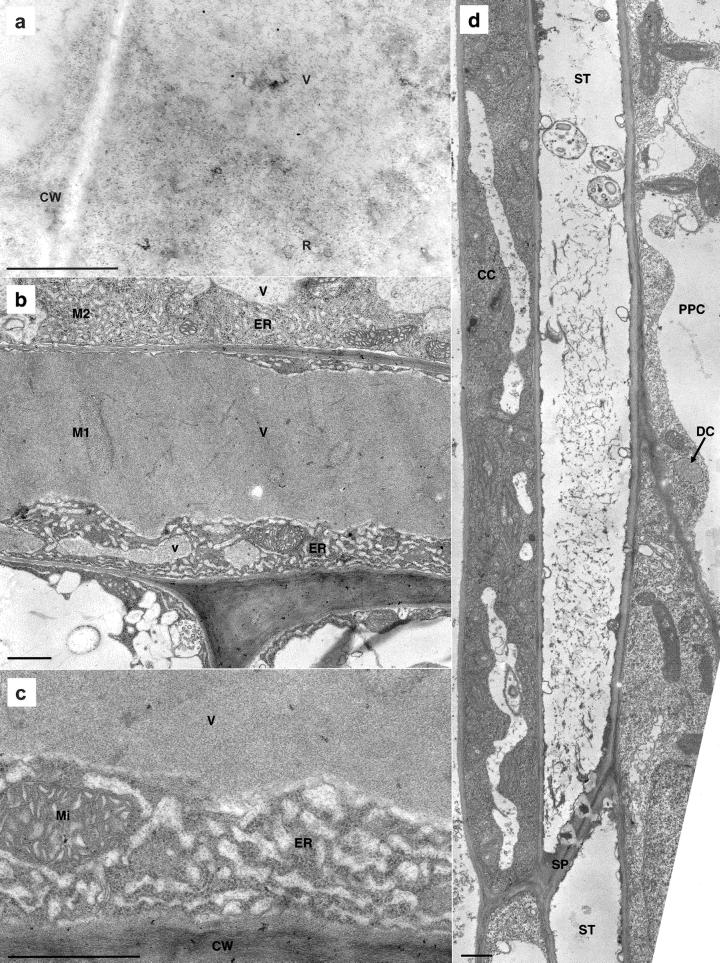
LM studies of Arabidopsis myrosin cells in young rosette leaf (a–d) and in pedicel (e). TEM study of S-cells in pedicel (f). a, ABB staining of paraffin-embedded material that included a myrosin cell (arrow) peripherally in the phloem (P). b, Immunohistochemical analysis of myrosinase on a section consecutive to the one shown in a. The antimyrosinase antibody 3D7 indicated myrosinase expression in the same myrosin cell (arrow) as in a. The leaf blade in c was embedded in glycol methacrylate, sectioned paradermally, and stained with ABB. It shows the phloem (P), including two long and relatively broad myrosin cells (M), and mesophyll cells. A paradermal section of a leaf blade (d) embedded in epoxy resin and stained with toluidine blue, including a vascular strand cut slightly obliquely longitudinally, showed phloem (P) and two adjacent myrosin cells (M1 and M2) located in the phloem parenchyma. e, Transverse section treated as d of a pedicel, with xylem (X), phloem (P), S-cells (S-C), and myrosin cells (M). The S-cells are located between the phloem and the cells (asterisks) of the starch sheath. The myrosin cell to the right is in direct contact with an S-cell. f, Two S-cells (S-C) and the outer part of the phloem with mature sieve-tube members (ST), immature members (iST), companion cells (CC), and phloem parenchyma cell (PPC) are shown in transverse section. The S-cells had large, empty vacuoles and a thin cytoplasmic layer in which a dilated cisternae (DC) of the endoplasmic reticulum (ER) was located. Size bars in a through e correspond to 10 μm and in f to 1 μm.
Figure 5.
TEM analysis of Arabidopsis phloem of rosette leaf (a–c) and pedicel (d). a, Immunogold labeling with the 3D7 antibody in a peripheral vacuole (V) surrounded by cytoplasm in a myrosin cell. Abundant ribosomes in the cytoplasm were sometimes organized as polysomes (R). To the left, two neighboring cells and the common cell wall (CW) are shown. b, The myrosin cells M1 and M2, also shown in Figure 4d, both exhibited the characteristic high protein content of vacuoles (V) in myrosin cells. The cytoplasm had abundant distended rER and mitochondria. Next to M1, two bundle sheath cells with cell sap in the vacuoles were located. c, Higher magnification of a myrosin cell with cell wall (CW), cytoplasm with mitochondria (Mi), distended rER (ER), and the vacuole (V) with electron-dense homogeneous, granular material. The distended ER had abundant ribosomes on the membrane, and the moderately electron-dense material inside the ER resembled the most peripheral part of the vacuolar material. d, The phloem with sieve tube members (ST), companion cell (CC), and phloem parenchyma cell (PPC). The sieve tube members were connected by a sieve plate (SP), and the upper member showed three sieve element plastids and P protein. The companion cells had a fairly electron-dense cytoplasm and long, narrow vacuoles. In the ordinary phloem parenchyma cell, the vacuole filled most of the cell lumen and in the cytoplasm was a small DC of the ER with granular content and ribosomes on the membrane. Size bars correspond to 1 μm.
The presence of structurally defined myrosin cells in the phloem parenchyma was investigated in plast-embedded young material of pedicels, leaves, and developing embryos from Arabidopsis. The myrosin cells always occurred peripherally in the phloem tissue as idioblasts among phloem parenchyma cells. No myrosin cells were found in the ground tissue, and we could not detect any stomatal myrosin cells. LM analyses of the phloem in young leaves showed that the myrosin cells generally were both broader and longer than the ordinary phloem parenchyma cells (Fig. 4, c and d) and often occurred in pairs (Fig. 4d). Figure 4c was stained with ABB and Figure 4d with toluidine blue after osmium tetroxide treatment. By both methods, vacuolar material stained strongly in the myrosin cells compared with the translucent vacuoles of the neighboring cells. Within the individual myrosin cell the vacuolar material seemed homogeneous but often a different degree of staining intensity was evident between myrosin cells (Fig. 4, c and d). In the pedicel myrosin cells could be seen in direct contact with the S-cells inside the starch sheath (Fig. 4e). We also detected very small myrosin cells in procambial strands of nearly mature embryos (data not shown) that could explain the very low myrosinase activity found in Arabidopsis seeds (Fig. 2b, column 2).
An IEM study of young leaves using colloidal gold labeling indicated that myrosinase was present in the vacuoles of the myrosin cell (Fig. 5a). The gold-labeled vacuole is located in the cell like the small vacuole (v) in Figure 5b. Transmission electron microscopy (TEM) analyses of the myrosin cells in the young leaf (Fig. 5, b and c) showed a central vacuole filled with finely granular material and a cytoplasm composed mainly of distended rER. The electron density of the vacuolar matrix often varied between larger and smaller vacuoles within the same cell and also between myrosin cells (Fig. 5b), reflecting the difference in staining intensity observed in LM (Fig. 4d, M1 and M2 are the same cells in LM and TEM). In the cytoplasm, mitochondria (Fig. 5c) and dictyosomes (not shown) were present, in addition to the dominating rER. The rER consisted of closely packed areas of distended sacs with a content resembling the material in the central and other vacuoles. There was a striking difference in vacuolar electron density between the myrosin cells and the neighboring bundle sheath cells (Fig. 5b). The ultrastructure of the other cells in the phloem, namely sieve tube members, companion cells, and ordinary phloem parenchyma cells is shown in Figure 5d. The sieve tube members contained p-protein and starch-containing sieve element plastids and were connected by simple sieve plates. The plasmodesmata between the sieve tube members and the companion cells were characteristically branched (data not shown) toward the companion cells, which had a rather electron-dense cytoplasm due to free ribosomes and narrow vacuoles. The phloem parenchyma cells had less electron-dense cytoplasm and larger vacuoles than the companion cells (Fig. 5d).
In the pedicel, S-cells were located outside the phloem (Fig. 4, e and f). S-cells had a large diameter in transverse section and were very long, with pointed ends (data not shown). A thin lining of cytoplasm surrounded a huge, electron-lucent central vacuole. Organelles in the cytoplasm were sparse, but organelle-like dilated cisterna (DC) of the ER (Bonnett and Newcomb, 1965; Behnke and Eschlbeck, 1978) containing homogeneous, granular protein surrounded by a ribosome-studded membrane were sometimes found. DCs were also found in ordinary phloem parenchyma cells (Fig. 5d).
Immunohistochemical Analysis of Myrosinase in B. napus
Immunohistochemical analyses of myrosinase expression in various B. napus organs were carried out by use of the monoclonal antibody 3D7, emphasizing organs and tissues that have not been carefully investigated earlier. Myrosinase was found in ground-tissue myrosin cells, as expected, but also in myrosin cells of the phloem parenchyma. In very young flower buds, no staining could be detected, but in the pedicel phloem-specific expression was present (Fig. 6b). In the seedling shoot (Fig. 6a), old flower buds (Fig. 6d), siliques (Fig. 6g), and leaves (Fig. 6, h and i), myrosinase could be detected in idioblastic cells in the ground tissue and also in the phloem parenchyma. In mature petals (Fig. 6e) and stems (Fig. 6, c and f) only phloem-specific expression was found. In roots, idioblasts containing myrosinase were present in the cortex (Fig. 6j). Myrosinase was detected in approximately 0.1% of the cells in the mesophyll of the seedling cotyledons and at a frequency of approximately 0.05% of the cells in the leaves. Further, expression of myrosinase in some of the guard cells in cotyledons of germinating embryos and in young leaves was found, using the antimyrosinase monoclonal antibody 3D7 and nonradioactive in situ hybridization with a myrosinase B-specific probe (data not shown). All observations are in agreement with an earlier study (Höglund et al., 1991), except that we could not detect any consistent staining in the xylem in petals and stem.
Figure 6.
Immunohistochemical analysis of myrosinase in B. napus by use of the monoclonal antibody 3D7. In undeveloped flower buds, no staining could be detected, but in the pedicel, phloem-specific expression could be found (b). In the shoot apex (a), old flower buds (d), siliques (g), and leaves (h and i), myrosinase could be detected in myrosin cells in the ground tissue and the phloem. In mature petals (e) and stems (c and f), only phloem-specific expression was found. c, Transverse section of a stem; f, Longitudinal section with the outside uppermost. In root, ground tissue idioblasts containing myrosinase were visible (j). Xylem is marked with + and the size bars correspond to 10 μm.
The Myrosin Grains Constitute a Continuous Reticular System in B. napus
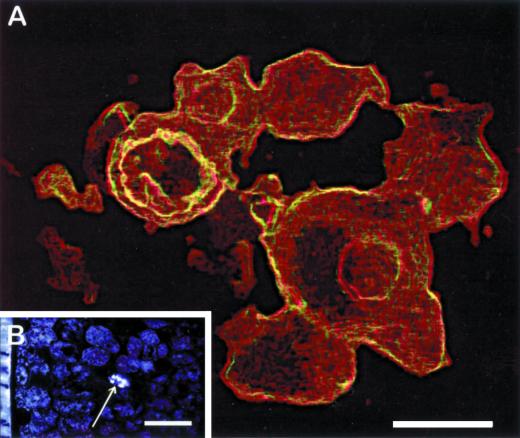
TEM analyses have shown that myrosin cells in embryos contain several vacuoles (Rest and Vaughan, 1972; Bones and Iversen, 1985). These vacuoles contain myrosinase as revealed by IEM (Thangstad et al., 1991; Höglund et al., 1992) and are denoted as myrosin grains. Here, we applied confocal laser scanning immunomicroscopy analysis to study the spatial organization of the myrosin grains in myrosin cells of the mature embryo. This analysis revealed that the myrosin grains within one cell seemed to constitute a continuous reticular system, a “myrosin body” (Fig. 7). The sizes of the different parts of the reticulum varied considerably, and the connections were sometimes very narrow. The myrosin body was present throughout the cell and comprised a significant portion of the cellular volume. The myrosinase staining was not homogeneous throughout the structure, and central portions with weak or no staining could sometimes be observed.
Figure 7.
Confocal microscopy analysis of a myrosin cell in B. napus. Myrosinase was detected by use of the 3D7 monoclonal antibody. A, Myrosin cell at high magnification showing the distribution of myrosinase in a reticular system rather than separate myrosin grains. This part is presented as a stereo anaglyph achieved as surface-extracted maximum intensity projection of a series of confocal images. B, Single confocal image presenting an overview of part of a cotyledon from a mature embryo. The arrow indicates the single myrosin cell shown in a. Size bars correspond to 5 μm (A) and 50 μm (B).
MBP and Myrosinase Expression in B. napus Seedlings
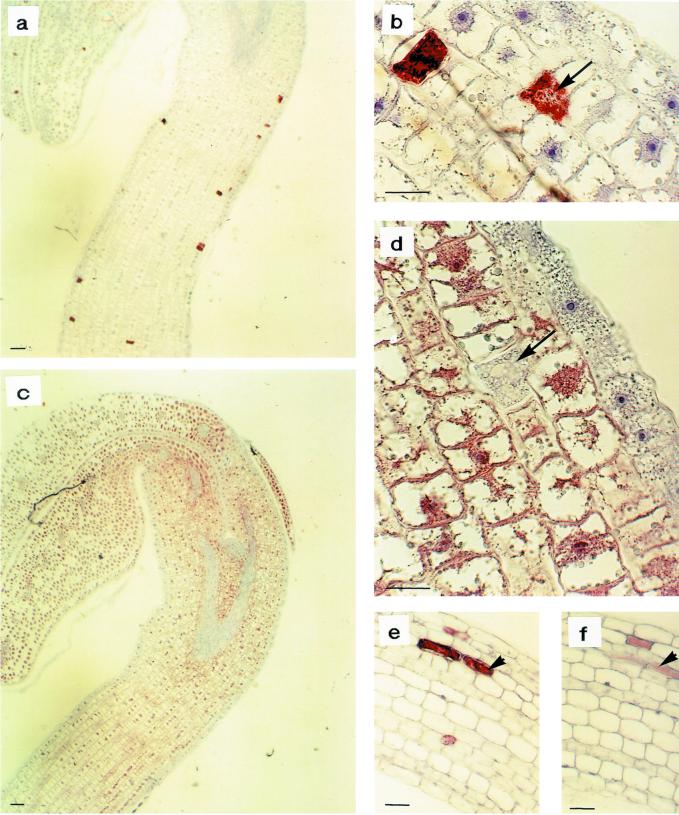
Immunohistochemical analyses of MBP expression in B. napus organs were carried out using the monoclonal antibody S4C6. In consecutive sections, both myrosinase (Fig. 8, a, b, and e) and MBP (Fig. 8, c, d, and f) were detected. In 2-d-old seedlings, myrosinase was found in idioblastic myrosin cells (Fig. 8, a and b) and MBP was found in all nonidioblastic ground tissue cells, but not in the vascular tissue, epidermis, or idioblastic myrosin cells (arrows indicate identical myrosin cell; Fig. 8, c and d). Thus, in 2-d-old seedlings, myrosinase and MBP were not colocalized. However, in 7-d-old seedlings, myrosinase (Fig. 8e) and MBP (Fig. 8f) were colocalized in idioblastic myrosin cells in the hypocotyl. Thus, in fully developed seedlings, MBP was confined to idioblastic structures, which suggests that myrosinase-MBP complexes might exist in vivo. However, it is likely that the myrosinase-MBP complexes do not exist in vivo in seeds.
Figure 8.
Immunohistochemical analysis of myrosinase and MBP expression in B. napus seedlings using the monoclonal antibodies 3D7 and S4C6, respectively. Myrosinase was detected in a, b, and e and MBP in consecutive sections in c, d, and f. In 2-d-old seedlings, myrosinase was found in myrosin cells (a and b) and MBP was found in all cells of the ground tissue except for the myrosin cells (c and d). In 7-d-old seedlings, myrosinase and MBP were colocalized (e and f). Size bars correspond to 10 μm. Arrows indicate the same myrosin cell in consecutive sections.
DISCUSSION
We have shown that myrosinase is present in similar idioblasts called myrosin cells in both Arabidopsis and B. napus. However, there is a difference in the localization of myrosinase between the two species. In Arabidopsis, myrosinase is confined to cells of the phloem parenchyma, whereas in B. napus myrosinase is expressed in both the ground tissue and the phloem tissue. The expression pattern of myrosinase in Arabidopsis matched in situ hybridization analysis of the published myrosinase genes in Arabidopsis, TGG1 and TGG2 (Xue et al., 1995). This expression pattern is also in agreement with an early investigation of myrosin cell localization, finding these cells in the vascular bundles of Arabidopsis (von Hayek, 1911). Our present data are also in agreement with recent microarray data (Ruan et al., 1998), in which myrosinase was found to be one of the highest expressed genes in the flower bud and the flower. The antibody used recognizes all characterized myrosinase subfamilies in B. napus (Lenman et al., 1990, 1993; Falk et al., 1995a), and in Arabidopsis, the TGG1 and TGG2 isoforms react with the 3D7 antibody, whereas the earlier suggested third myrosinase gene, tgg3 (Xue et al., 1992), was recently characterized and found to be a pseudogene (J. Zhang, B. Pontoppidan, J. Xue, L. Rask, and J. Meijer, unpublished data).
The glucosinolate-containing S-cells (Koroleva et al., 2000) with translucent vacuoles are structurally different from the phloem myrosin cells with protein-containing vacuoles. This probably means that myrosinase and glucosinolates are not colocalized in the same cells, at least not in pedicels of Arabidopsis. Kelly et al. (1998) also showed the presence of glucosinolates in non-myrosin cells in imbibed seeds of B. juncea. This is in contrast to the “mustard oil bomb” theory (Lüthy and Matile, 1984), which predicted myrosinase to be inactivated and localized in the same cell as glucosinolates. Actually, a recent study of uptake of radioactive glucosinolate precursors indicated accumulation of at least desulfoglucosinolates to occur in the myrosin cells present in the ground tissue of B. napus developing embryos (Thangstad et al., 2001). Because there is a difference in ontogenetic stage of the investigated tissues in the two Brassica spp. used by Thangstad et al. (2001) and Kelly et al. (1998), it is possible that during embryo development desulfoglucosinolates are transported into the myrosin cells and later the intact glucosinolates are transported into other cells or become degraded by myrosinase. Myrosinase probably does not degrade desulfoglucosinolates, and thus they can be colocalized without any reaction occurring. This could explain the available data and is in line with the transport of alkaloid-precursors to idioblasts suggested by St-Pierre et al. (1999). During germination, the glucosinolates may be transported with assistance of MBP to the myrosin cells for use as metabolites (see below). However, further experiments are needed to clarify this, e.g. in situ expression analysis of glucosinolate biosynthetic genes and cloning of the transporters.
The presence in Arabidopsis of glucosinolates in the S-cells and myrosinase in myrosin cells, with the cells adjacent and sometimes in contact with each other, is an important structural aspect considering the proposed functions of the system in nutrition and defense. In both cases, products may need to be rapidly distributed throughout the plant. Transport of secondary compounds have been shown in several species, e.g. for alkaloids (Hashimoto and Yamada, 1994; St-Pierre et al., 1999) and glucosinolates (Lykkesfeldt and Møller, 1993; Merritt, 1996; Brudenell et al., 1999). Glucosinolates have been proposed to be transported by the phloem and not by the xylem because myrosinase has been claimed to be present in the latter tissue (Brudenell et al., 1999). However, although we found no evidence for myrosinase in the xylem, myrosin cells in the phloem contained myrosinase. Ultrastructural studies did not indicate any differences between phloem and ground-tissue myrosin cells (Werker and Vaughan, 1976; Jørgensen et al., 1977; Jørgensen, 1981; this paper). It has been suggested that the myrosinase-containing cells in B. napus found in the vascular tissue are companion cells (Bones et al., 1991). This would imply a direct plasmodesmatal connection between the sieve tubes and myrosinase-containing cells. However, we found that the phloem myrosin cells are different from companion cells, which have small vacuoles and free ribosomes in the cytoplasm, whereas myrosin cells have large protein-rich vacuoles and mainly ER-bound ribosomes. On the other hand, plasmodesmata occur in myrosin cells (Werker and Vaughan, 1974; Burmeister et al., 1977; L.B. Jørgensen, unpublished data) making symplastic glucosinolate transport to the myrosin cell possible. Although the cells may have cytoplasmic connection, there is a need for tonoplast transporters because both glucosinolates and myrosinase are reported to be located in vacuoles (Thangstad et al., 1991; Höglund et al., 1992; Kelly et al., 1998).
It is generally believed that idioblasts have evolved to have different functions in different plant taxa (Mauseth, 1988). For example, tobacco (Nicotiana tabacum) idioblasts contain pathogenesis-related proteins (Dixon et al., 1991), and calcium-accumulating idioblasts are present in water lettuce (Pistia stratiotes; Franceschi et al., 1993). In Arabidopsis leaves, the idioblasts often occur next to each other (Figs. 3a and 4d), indicating that after idioblast determination, additional cell division events occur. This is also the case during differentiation of stomatal myrosin cells (Jørgensen, 1995). Different initial cells in the meristem give rise to the vascular system and the ground tissue. The ground tissue initial cells in Arabidopsis may not have the capacity to form idioblasts, and therefore myrosinase-containing cells are lacking in this tissue. The analysis of the subcellular organization of myrosinase in myrosin cells of B. napus embryos showed that the myrosin grains actually formed a continuous reticulum throughout the cell. Therefore, we suggest denoting the myrosinase-containing structure as a myrosin body rather than myrosin grains (Rest and Vaughan, 1972; Werker and Vaughan, 1974). This organization of the mature myrosin body may simply be a consequence of a protein-accumulating vacuole that slowly expands irregularly in the cell as proteins are accumulating. The relative concentration of myrosinase as judged from fluorescence intensity varied throughout the structure suggesting that additional molecules are present. Also, there seems to be an age-dependent effect on the reticular system in the mature plant because older myrosin cells from the siliques appeared to be more granulated than younger ones.
In general, members of the Brassicaceae are fast invaders that have many specialist enemies, i.e. insects that only attack plants from this family. Thus, it is logical to consider the special secondary metabolites, the glucosinolates, in this context. However, Arabidopsis produces many small seeds and often completes the life cycle often early in the season, thereby avoiding massive insect attack and accordingly can be regarded as a herbivory escaper. There are also differences in the myrosinase gene family between Arabidopsis and larger summer-living Brassicaceae members in the tribus Brassiceae such as B. napus and Sinapis alba (Xue et al., 1992). Arabidopsis seems to have only two myrosinase genes that are expressed exclusively in the phloem parenchyma, whereas the larger Brassicaceae members have approximately 20 genes expressed in both the ground tissue and the phloem parenchyma (Rask et al., 2000; Eriksson et al., 2001; this paper). The difference is especially pronounced in the seed wherein hardly any myrosinase is expressed in Arabidopsis, whereas in B. napus, members of all three myrosinase gene families are transcribed. Actually, the seed is the only organ in which all three myrosinase gene families are expressed in B. napus. In the Brassiceae (also including Raphanus sp., Brassica oleracea, and wild Rhynchosinapis cheiranthos), the levels of the myrosinase-glucosinolate system correlate better with the cost of herbivory than in Arabidopsis, if the seed is considered to be a costly organ to lose because it is essential for survival. In Arabidopsis seeds, the glucosinolates may be regarded as storage compounds used during later stages of germination, as suggested in B. napus by James and Rossiter (1991), because there is hardly any myrosinase present to produce toxic compounds although glucosinolates are abundant (Hogge et al., 1988; Haughn et al., 1991).
We have shown that the idioblastic myrosin cells change composition during seedling development because MBP expression changes considerably. Glucosinolates and MBP are probably colocalized in the B. napus seed (Kelly et al., 1998; this paper), but during germination MBP disappears outside myrosin cells, concominant with a dramatic decrease of mainly aliphatic glucosinolates (e.g. Clossais-Besnard and Larher, 1991). This is also in agreement with the observation by James and Rossiter (1991) that the myrosinase activity increased during this period. This might suggest a general storage protein-related function for MBP but also that MBP could be involved in the degradation of glucosinolates by being a part of the transport mechanism to the myrosin cells in which myrosinases degrade the glucosinolates into nutritional components. The presence of MBP in myrosin cells later during development may support the latter idea. However, MBPs constitute a gene family with several members in which the different genes are structurally distinct although certain motifs are shared (Taipalensuu et al., 1997b) The existence of MBP in Arabidopsis is not clear at the moment. Neither has any cross-reaction been detected with the B. napus anti-MBP antibody 34:14 in western-blot analysis (Fig. 2, Falk et al., 1995b), nor has any specific Southern-blot hybridization signal using two MBP probes (Taipalensuu et al., 1997b) been detected in Arabidopsis. A thorough analysis of the Arabidopsis genome for MBP homologs has indicated the presence of several genes containing various domains present in B. napus MBP but no full-length homolog (B. Pontoppidan and Johan Meijer, unpublished data). These results make it unlikely that a functional MBP homolog exists in Arabidopsis, and maybe MBPs are correlated to the presence of ground tissue myrosin cells, because B. napus, Brassica nigra, S. alba, and R. cheiranthos all contained MBPs and ground-tissue myrosin cells (Falk et al., 1995b; E. Andréasson and M. Lenman, unpublished data). This investigation calls for further studies to identify species-specific structures, regulation, and functions of the glucosinolate-myrosinase system, which may limit the usefulness of Arabidopsis as a model system for Brassicaceae.
MATERIALS AND METHODS
Plant Material
Brassica napus L. cv Karat (Svalöf Weibull AB, Svalöv, Sweden) and Arabidopsis ecotype Columbia (Nottingham Arabidopsis Stock Centre, Nottingham, UK) were used. The plants were grown in standard soil under greenhouse conditions or in a climate chamber with 70% relative humidity using a 16-h daylight/21°C and 8-h darkness/17°C regime. Investigations of the pedicel were carried out in ecotype Wassiljewskija because the reported characterization of S-cells was carried out using this ecotype (Koroleva et al., 2000).
Western-Blot Analysis
Extracts of seeds and leaves were prepared in SDS buffer (1.3 mm Tris, pH 8.8, 0.003% [w/v] bromphenol blue, 9% [w/v] Suc, 1.3% [v/v] SDS, and 8 mm dithiothreitol). Separation by SDS-PAGE (Minigel, Bio-Rad Laboratories, Hemel Hempstead, UK) and semidry transfer (Bio-Rad Laboratories) of the proteins to Hybond C nitrocellulose membrane (Amersham-Pharmacia Biotech, Uppsala) were carried out according to the manufacturer's instructions. A 3D7 mouse hybridoma supernatant at the dilution 1:300 was used to detect myrosinase and a 34:14 antibody hybridoma supernatant at the dilution 1:1,000 was used to detect MBP (Lenman et al., 1990; Falk et al., 1995b). The further detection was carried out as described (Chen and Halkier, 1999), except that the developing reagent was purchased from Pierce (Rockford, IL).
Myrosinase Activity Assay
Leaves and seeds were extracted in 0.5 mL of 50 mm Tris-HCl, pH 7.5, 0.2 m NaCl, 1 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride for 1 h, and the supernatant was passed through a Sephadex G-25 column (Amersham-Pharmacia Biotech) equilibrated with the extraction buffer. Myrosinase activity was measured by monitoring Glc release after sinigrin hydrolysis using a kit (Randox Laboratories Ltd, Ardmore, UK) in 50 mm citrate buffer, pH 4.5, and 0.3 mm ascorbate. Protein concentration was determined by the Bradford method according to the manufacturer using bovine serum albumin as the standard (Bio-Rad Laboratories).
Immunohistochemical Analysis
The plant material was fixed for 16 to 48 h at 4°C in 4% (w/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde. Paraffin embedding, binding of the anti-myrosinase antibody, and staining using the peroxidase-antiperoxidase procedure were carried out as previously described (Höglund et al., 1991), except that 4% (w/v) bovine serum albumin was used as blocking agent. The ascites propagated antibodies 3D7 at the dilution 1:500 to 1:2,000 and S4C6 at the dilution 1:100 were used to detect myrosinase and MBP, respectively (Lenman et al., 1990; Geshi et al., 1998). The antimyrosinase polyclonal antisera K505 was diluted 1:12,000 (Lenman et al., 1990; Höglund et al., 1991). Negative control stainings with an ascites anticruciferin antibody with the same total protein amount as the specific antibodies were also analyzed. Sections, consecutive to the 3D7 antibody treated, were stained with ABB (see below) and dehydrated in ethanol before mounting. These 3D7 antibody treated sections were processed with DAKO AEC+High sensitivity substrate-chromogen system (DAKO, Carpinteria, CA) for visualization of the peroxidase-antiperoxidase reaction before mounting in glycerin gelatin.
Cytochemical and Ultrastructural Analysis
Standard methods were used for ultrastructural analysis by TEM. Small pieces of tissue were fixed overnight at 5°C in 2.5% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 m phosphate buffer, pH 7.0, rinsed in buffer, and postfixed in 1% (w/v) OsO4 in the same buffer for 2 h at room temperature. After rinsing, the material was dehydrated in a graded acetone series before infiltration and embedding in Spurr's epoxy resin (Spurr, 1969). Ultrathin sections were cut on a Supernova ultramicrotome (Reichert-Jung, Heidelberg), collected on grids, and contrasted with uranyl acetate and lead citrate before being examined in a JEM 100CX microscope (JEOL, Tokyo). For LM analysis with a Reichert-Jung Polyvar microscope, 1- to 2-μm-thick sections of the material embedded in Spurr were stained in 0.05% (w/v) toluidine blue (Toluidinblau O, Merck, Darmstadt, Germany) in 1% (w/v) borax (sodium tetra borate), pH 8.4. Also, 3-μm-thick sections of tissue embedded in glycol methacrylate were analyzed after fixation as above in the aldehyde-mixture and dehydration with methoxyethanol, ethanol, propanol, and butanol before infiltration with the plastic (Feder and O'Brien, 1968). These sections were stained with the protein reagent ABB (Naphtol Blue Black, Sigma, St. Louis) for cytochemical identification of myrosin cells in green tissue as described by Jørgensen et al. (1977) and Jørgensen (1995).
IEM
The fixation and incubation with the 3D7 antibody was done according to Höglund et al. (1992), except that the dilution of the 3D7 antibody was 1:300. The blocking was achieved in 4% (w/v) bovine serum albumin and collodial gold-labeled protein A (20 nm) from Sigma was used to detect antibody bounding. The grids were contrasted with uranyl acetate and lead citrate and examined in a JEOL JEM 100CX microscope.
Immunofluorescence Staining and Confocal Laser Scanning Microscopy Analysis
Whole seeds of B. napus (a dihaploid line 20516-K of cv Karat) were fixed in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 12 h at 4°C. Samples were dehydrated through a graded series of ethanol followed by incubation in xylene before infiltration and embedding in Histowax (Histolab, Göteborg, Sweden). Sections (5 μm thick) were placed on chromogelatine-coated coverslips. Before staining, sections were xylene treated, followed by rehydration in decreasing concentrations of ethanol and distilled water followed by a wash in PBS. After treatment with blocking buffer (normal goat serum 1:10 in PBS with 4% [w/v] bovine serum albumin) for 30 min, sections were incubated for 30 min with the monoclonal antibody 3D7 directed against myrosinase (1:400) and followed by an incubation with a tetramethyl rhodamine isothiocyanate-labeled rabbit anti-mouse immunoglobulin antiserum (1:200; Dakopatts AB, Älvsjö, Sweden) for 30 min. Sections were mounted in Moviol. Images were recorded using a system equipped with a Nikon inverted microscope and argon/krypton laser, purchased from Molecular Dynamics (Sunnyvale, CA). Fluorochrome-labeled samples were excited by a 543-nm line laser.
ACKNOWLEDGMENTS
We are grateful to Ulla Pihlgren and Lis Munk Frederiksen for technical assistance and Leif Bolding for graphic assistance. Bo Pontoppidan is thanked for Figure 1.
Footnotes
This study was supported by grants from the Nordic Joint Committee for Agricultural Research, from the Swedish Council for Forestry and Agricultural Research, from the Swedish Foundation for Strategic Research, from Nilsson-Ehles, and from Lamms Stiftelse.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010334.
LITERATURE CITED
- Behnke HD, Eschlbeck G. Dilated cisternae in Capparales: an attempt towards the characterization of a specific endoplasmic reticulum. Protoplasma. 1978;97:351–363. [Google Scholar]
- Bodnaryk RP. Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry. 1992;31:2671–2677. [Google Scholar]
- Bones A, Iversen TH. Myrosin cells and myrosinase. Isr J Bot. 1985;34:351–376. [Google Scholar]
- Bones A, Rossiter JT. The myrosinase-glucosinolate system, its organization and biochemistry. Physiol Plant. 1996;97:194–208. [Google Scholar]
- Bones A, Thangstad OP, Haugen OA, Espevik T. Fate of myrosin cells: characterization of monoclonal antibodies against myrosinase. J Exp Bot. 1991;42:1541–1549. [Google Scholar]
- Bonnett HT, Newcomb EH. Polyribosomes and cisternal accumulations in root cells of radish. J Cell Biol. 1965;27:423–432. doi: 10.1083/jcb.27.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudenell AJP, Griffiths H, Rossiter JT, Baker DA. The phloem mobility of glucosinolates. J Exp Bot. 1999;50:745–756. [Google Scholar]
- Burmeister WP, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B. The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure. 1997;5:663–675. doi: 10.1016/s0969-2126(97)00221-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Halkier BA. Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Prot Exp Purif. 1999;17:414–420. doi: 10.1006/prep.1999.1158. [DOI] [PubMed] [Google Scholar]
- Chew FS. Biological effects of glucosinolates. In: Cutler HG, editor. Biologically Active Natural Products for Potential Use in Agriculture. Washington, DC: American Chemical Society; 1988. pp. 155–181. [Google Scholar]
- Clossais-Besnard N, Larher F. Physiological role of glucosinolates in Brassica napus: concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J Sci Food Agric. 1991;56:25–38. [Google Scholar]
- Dixon DC, Cutt JR, Klessig DF. Differential targeting of the tobacco PR-1 pathogenesis-related proteins to the extracellular space and vacuoles of crystal idioblasts. EMBO J. 1991;10:1317–1324. doi: 10.1002/j.1460-2075.1991.tb07650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty K, Kiddle G, Pye B, Wallsgrove R, Pickett J. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry. 1995;38:347–350. [Google Scholar]
- Eriksson S, Ek B, Xue J, Rask L, Meijer J. Identification and characterization of soluble and insoluble myrosinases in different organs of Sinapis alba. Physiol Plant. 2001;111:353–364. doi: 10.1034/j.1399-3054.2001.1110313.x. [DOI] [PubMed] [Google Scholar]
- Falk A, Ek B, Rask L. Characterization of a new myrosinase in Brassica napus. Plant Mol Biol. 1995a;27:863–874. doi: 10.1007/BF00037015. [DOI] [PubMed] [Google Scholar]
- Falk A, Taipalensuu J, Ek B, Lenman M, Rask L. Characterization of rapeseed myrosinase-binding protein. Planta. 1995b;195:387–395. doi: 10.1007/BF00202596. [DOI] [PubMed] [Google Scholar]
- Feder N, O'Brien TP. Plant microtechnique: some principles and new methods. Am J Bot. 1968;55:123–142. [Google Scholar]
- Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemistry. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Li X, Zhang D, Okita TW. Casequestrinlike calcium-binding proteins is expressed in calcium-accumulating cells of Pistia stratiotes. Proc Natl Acad Sci USA. 1993;90:6986–6990. doi: 10.1073/pnas.90.15.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshi N, Andréasson E, Meijer J, Rask L, Brandt A. Myrosinase and myrosinase-binding proteins are co-localized in grains of myrosin cells in cotyledon of Brassica napus L. seedlings. Plant Physiol Biochem. 1998;36:583–590. [Google Scholar]
- Geshi N, Brandt A. Two jasmonate-inducible myrosinase-binding proteins from Brassica napus L. seedlings with homology to jacalin. Planta. 1998;204:295–304. doi: 10.1007/s004250050259. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Yamada Y. Alkaloid biogenesis: molecular aspects. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:257–285. [Google Scholar]
- Haughn GW, Davin L, Giblin M, Underhill EW. Biochemical genetics of plant secondary metabolites in Arabidopsis thaliana: the glucosinolates. Plant Physiol. 1991;97:217–226. doi: 10.1104/pp.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge LR, Reed DW, Underhill EW, Haughn GW. HPLC separation of glucosinolates from leaves and seeds of Arabidopsis thaliana and their identification using thermospray liquid chromatography/mass spectrometry. J Chromatogr Sci. 1988;26:551–556. [Google Scholar]
- Höglund AS, Lenman M, Falk A, Rask L. Distribution of myrosinase in rapeseed tissues. Plant Physiol. 1991;95:213–221. doi: 10.1104/pp.95.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund AS, Lenman M, Rask L. Myrosinase is localized to the interior of myrosin grains and is not associated to the surrounding tonoplast membrane. Plant Sci. 1992;85:165–170. [Google Scholar]
- James DC, Rossiter JT. Development and characteristics of myrosinase in Brassica napus during early seedling growth. Physiol Plant. 1991;82:163–170. [Google Scholar]
- Jensen WA. Botanical Histochemistry: Principles and Practice. W.H. San Francisco: Freeman; 1962. pp. 228–229. [Google Scholar]
- Jørgensen LB. Myrosin cells and dilated cisternae of the endoplasmic reticulum in the order Capparales. Nord J Bot. 1981;1:433–445. [Google Scholar]
- Jørgensen LB. Stomatal myrosin cells in Caricaceae: taxonomic implications for a glucosinolate-containing family. Nord J Bot. 1995;15:523–540. [Google Scholar]
- Jørgensen LB, Behnke HD, Mabry TJ. Protein-accumulating cells and dilated cisternae of the endoplasmic reticulum in three glucosinolate-containing genera: Armoracia, Capparis, Drypetes. Planta. 1977;137:215–224. doi: 10.1007/BF00388153. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Bones A, Rossiter JT. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta. 1998;206:370–377. doi: 10.1007/s004250050412. [DOI] [PubMed] [Google Scholar]
- Koroleva OA, Davies A, Deeken R, Thorpe MR, Tomos AD, Hedrich R. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 2000;124:599–608. doi: 10.1104/pp.124.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman M, Falk A, Rödin J, Höglund AS, Ek B, Rask L. Differential expression of myrosinase gene families. Plant Physiol. 1993;103:703–711. doi: 10.1104/pp.103.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman M, Rödin J, Josefsson LG, Rask L. Immunological characterization of rapeseed myrosinase. Eur J Biochem. 1990;194:747–753. doi: 10.1111/j.1432-1033.1990.tb19465.x. [DOI] [PubMed] [Google Scholar]
- Louda S, Mole S. Glucosinolates, chemistry and ecology. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. San Diego: Academic Press; 1991. pp. 123–164. [Google Scholar]
- Lüthy B, Matile P. The mustard oil bomb: rectified analysis of the subcellular organisation of the myrosinase system. Biochem Physiol Pflanz. 1984;179:5–12. [Google Scholar]
- Lykkesfeldt J, Møller BL. Synthesis of benzylglucosinolate in Tropaeolum majus L.: isothiocyanates as potent enzyme inhibitors. Plant Physiol. 1993;102:609–613. doi: 10.1104/pp.102.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauseth JD. Plant Anatomy. Menlo Park, CA: Benjamin/Cummings; 1988. [Google Scholar]
- Merritt SZ. Within plant variation in concentrations of amino acids, sugar, and sinigrin in phloem sap of black mustard, Brassica nigra (L) Koch (Cruciferae) J Chem Ecol. 1996;22:1133–1145. doi: 10.1007/BF02027950. [DOI] [PubMed] [Google Scholar]
- Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivory defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Rest JA, Vaughan GJ. The development of protein and oil bodies in the seed of Sinapis alba L. Planta. 1972;105:245–262. doi: 10.1007/BF00385396. [DOI] [PubMed] [Google Scholar]
- Rodman JE, Karol KG, Price RA, Sytsma KJ. Molecules, morphology, and Dahlgren's expanded order Capparales. Syst Bot. 1996;21:289–307. [Google Scholar]
- Rosa EAS, Heaney RK, Fenwick GR, Portas CAM. Glucosinolates in crop plants. Hortic Rev. 1997;19:99–215. [Google Scholar]
- Ruan Y, Gilmore J, Conner T. Towards Arabidopsis genome analysis: monitoring expression profiles of 1400 genes using cDNA microarrays. Plant J. 1998;15:821–833. doi: 10.1046/j.1365-313x.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastr Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, Vazquez-Flota A, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of pathway intermediate. Plant Cell. 1999;11:887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipalensuu J, Eriksson S, Rask L. The myrosinase-binding protein from Brassica napus seeds possesses lectin activity and has a highly similar vegetatively expressed wound-inducible counterpart. Eur J Biochem. 1997a;250:680–688. doi: 10.1111/j.1432-1033.1997.00680.x. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Falk A, Ek B, Rask L. Myrosinase-binding proteins are derived from a large wound-inducible and repetitive transcript. Eur J Biochem. 1997b;243:605–611. doi: 10.1111/j.1432-1033.1997.t01-1-00605.x. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Falk A, Rask L. A wound- and methyl jasmonate-inducible transcript coding for a myrosinase-associated protein with similarities to an early nodulin. Plant Physiol. 1996;110:483–491. doi: 10.1104/pp.110.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangstad OP, Bones AM, Holtan S, Moen L, Rossiter J. Microautoradiographic localization of a glucosinolate precursor to specific cells in Brassica napus L. embryos indicates a separate transport pathway into myrosin cells. Planta. 2001;213:207–213. doi: 10.1007/s004250000491. [DOI] [PubMed] [Google Scholar]
- Thangstad OP, Evjen K, Bones A. Immunogold-EM localization of myrosinase in Brassicaceae. Protoplasma. 1991;161:85–93. [Google Scholar]
- Thangstad OP, Iversen T-H, Slupphaug G, Bones A. Immunocytochemical localization of myrosinase in Brassica napus L. Planta. 1990;180:245–248. doi: 10.1007/BF00194003. [DOI] [PubMed] [Google Scholar]
- von Hayek A. Entwurf eines Cruciferen-Systems auf phylogenetischer Grundlage. Beihefte Bot Centralblatt. 1911;27:127–335. [Google Scholar]
- Werker E, Vaughan JG. Anatomical and ultrastructural changes in aleurone and myrosin cells of Sinapis alba during germination. Planta. 1974;116:243–255. doi: 10.1007/BF00390230. [DOI] [PubMed] [Google Scholar]
- Werker E, Vaughan JG. Ontogeny and distribution of myrosin cells in the shoot of Sinapis alba L.: a light- and electron-microscope study. Isr J Bot. 1976;25:140–151. [Google Scholar]
- Xue J, Jørgensen M, Pihlgren U, Rask L. The myrosinase gene family in Arabidopsis thaliana: gene organization, expression and evolution. Plant Mol Biol. 1995;27:911–922. doi: 10.1007/BF00037019. [DOI] [PubMed] [Google Scholar]
- Xue J, Lenman M, Falk A, Rask L. The glucosinolate-degrading enzyme myrosinase in Brassicaceae is encoded by a gene family. Plant Mol Biol. 1992;18:387–398. doi: 10.1007/BF00034965. [DOI] [PubMed] [Google Scholar]