Abstract
The rice plant (Oryza sativa L. cv Oochikara) is known to be a Si accumulator, but the mechanism responsible for the high uptake of Si by the roots is not well understood. We investigated the role of root hairs and lateral roots in the Si uptake using two mutants of rice, one defective in the formation of root hairs (RH2) and another in that of lateral roots (RM109). Uptake experiments with nutrient solution during both a short term (up to 12 h) and relatively long term (26 d) showed that there was no significant difference in Si uptake between RH2 and the wild type (WT), whereas the Si uptake of RM109 was much less than that of WT. The number of silica bodies formed on the third leaf in RH2 was similar to that in WT, but the number of silica bodies in RM109 was only 40% of that in WT, when grown in soil amended with Si under flooded conditions. There was also no difference in the shoot Si concentration between WT and RH2 when grown in soil under upland conditions. Using a multi-compartment transport box, the Si uptake at the root tip (0–1 cm, without lateral roots and root hairs) was found to be similar in WT, RH2, and RM109. However, the Si uptake in the mature zone (1–4 cm from root tip) was significantly lower in RM109 than in WT, whereas no difference was found in Si uptake between WT and RH2. All these results clearly indicate that lateral roots contribute to the Si uptake in rice plant, whereas root hairs do not. Analysis of F2 populations between RM109 and WT showed that Si uptake was correlated with the presence of lateral roots and that the gene controlling formation of lateral roots and Si uptake is a dominant gene.
Si is a beneficial element for higher plants. Si can stimulate canopy photosynthesis by improving leaf erectness, decrease susceptibility to disease and insect damage, prevent lodging, and alleviate water and various mineral stresses (for review, see Epstein, 1999; Ma et al., 2001). The beneficial effects of Si characteristically differ with the plant species. Usually the effects are more obvious in plants that accumulate Si in their shoots. The more Si accumulates in the shoots, the larger is the effect that is gained. This is because most effects of Si are expressed through the formation of silica gel, which is deposited on the surface of leaves, stems, and other organs of plants (Ma et al., 2001). The beneficial effects of Si also characteristically vary with the growth conditions. The effects are usually expressed more clearly when plants are under various abiotic and biotic stresses (Savant et al., 1997; Epstein, 1999; Ma et al., 2001). In addition, Si is the only element that does not damage plants when accumulated in excess due to its properties of un-dissociation at physiological pH and polymerization (Ma et al., 2001).
All plants growing in soil contain Si (Takahashi et al., 1990; Epstein, 1999), but the Si concentration of plant shoots varies greatly between plant species; ranging from about 0.1% to 10% (w/w) Si on a dry weight basis (Takahashi et al., 1990; Epstein, 1994). This variation is largely due to different capacities for Si uptake by plant roots. Three uptake modes have been suggested: active, passive, and rejective uptake (Takahashi et al., 1990). The mode of uptake employed is dependent on the particular plant species (Takahashi et al., 1990; Ma et al., 2001). Rice (Oryza sativa) is a typical plant that shows active uptake of Si. Rice roots take up Si in the form of silicic acid, an uncharged molecule (Takahashi and Hino, 1978). The uptake rate of Si by rice roots is much faster than that of water, resulting in a quick decrease in Si concentration of external solution (Okuda and Takahashi, 1962a). The Si uptake is not affected by the transpiration, but is inhibited by a respiratory inhibitor such as NaCN (Okuda and Takahashi, 1962a) and metabolic inhibitors such as 2,4-dinitrophenol, iodo-acetate, and 2,4-D (Okuda and Takahashi, 1962b). Unlike other plant species, rice roots take up Si much faster than other nutrients (Takahashi, 1995). These findings suggest that a specific transport system for silicic acid such as a Si transporter, which is energy dependent, exists in the rice roots. A gene family encoding a Si transporter from the marine diatom (Cylindrotheca fusiformis), which requires Si as an essential element, has been cloned (Hildebrand et al., 1997). However, neither genes encoding the Si transporter nor those encoding the transporter protein have been isolated in rice. In the present study, we investigated the role of root hairs and lateral roots in the Si uptake using two rice mutants of rice cv Oochikara, one defective in the formation of root hairs (RH2) and another in that of lateral roots (RM109), and the wild type (WT). Our results clearly show that lateral roots contribute to the Si uptake in the rice, whereas root hairs do not.
RESULTS
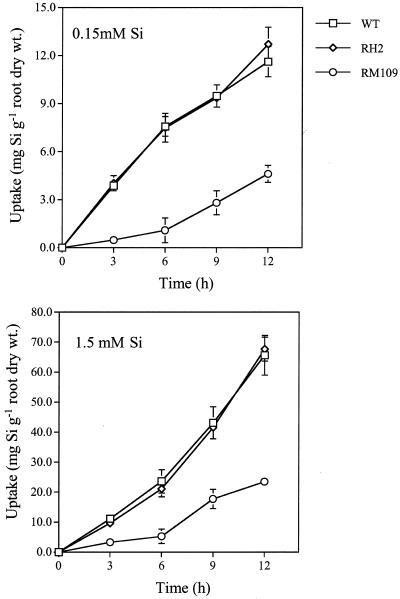
The uptake rate of Si during a short term was examined in RH2, RM109, and WT (Fig. 1). The Si uptake increased with time in RH2 and WT at both low (0.15 mm) and high (1.5 mm) Si levels (Fig. 2). There was no difference in Si uptake between RH2 and WT. However, the uptake of Si by RM109 was much less than that by either WT or RH2. At 12 h, the Si uptake by RM109 was about 40% of that by WT at either Si level. The Si concentration in the treatment solution decreased with time in WT and RH2 (data not shown), showing rapid uptake in these plants (Takahashi, 1995). However, the Si concentration in the treatment solution of RM109 hardly changed by uptake. No significant difference was observed in the transpiration rate between the three lines (data not shown).
Figure 1.
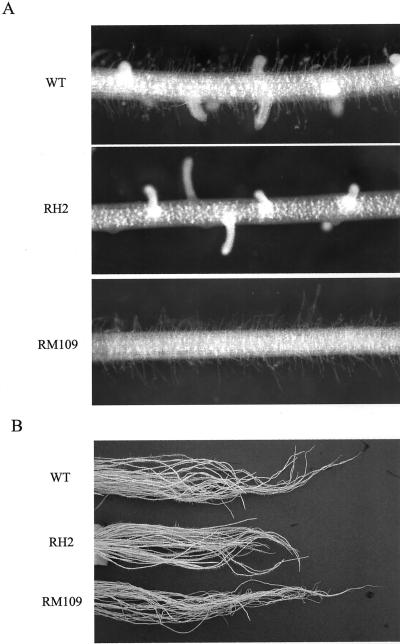
Root of WT rice cv Oochikara and two root mutants (RH2 and RM109). RH2 and RM109 are defective in the formation of root hairs and lateral roots, respectively. A, Individual root. B, Root system.
Figure 2.
Uptake rate of Si by WT rice cv Oochikara and two root mutants without root hairs (RH2) and lateral roots (RM109). Two-week-old seedlings were placed in a nutrient solution containing 0.15 and 1.5 mm Si as silicic acid, respectively. Error bars represent ±sd (n = 3).
In a relatively long-term uptake experiment with solution culture, the Si uptake by RH2 was similar to that by WT when Si was supplied at either a low or high concentration (Table I). However, the Si uptake by RM109 from low and high Si solutions was only 70% and 45% of that by WT, respectively. The dry weight of the shoot was similar between WT and RH2, whereas that of RM109 was smaller (Table I).
Table I.
Si concentration, dry weight, and Si uptake of a wild type (cv Oochikara) of rice and two root mutants without root hairs (RH2) and lateral roots (RM109)
| Treatment | Line
|
||
|---|---|---|---|
| WT | RH2 | RM109 | |
| mg Si g−1 dry wt | |||
| Si concentration | |||
| 0.15 mm Si | |||
| Shoot | 11.0 ± 0.2 | 11.8 ± 1.1 | 9.5 ± 1.1 |
| Root | 0.4 ± 0.2 | 0.3 ± 0.0 | 0.4 ± 0.1 |
| 1.5 mm Si | |||
| Shoot | 37.9 ± 0.7 | 35.1 ± 2.4 | 22.6 ± 1.0 |
| Root | 1.2 ± 0.4 | 1.2 ± 0.1 | 0.9 ± 0.0 |
| g | |||
| Dry weight | |||
| 0.15 mm Si | |||
| Shoot | 2.52 ± 0.06 | 2.14 ± 0.15 | 1.22 ± 0.30 |
| Root | 0.54 ± 0.04 | 0.39 ± 0.04 | 0.32 ± 0.08 |
| 1.5 mm Si | |||
| Shoot | 2.68 ± 0.17 | 2.16 ± 0.10 | 1.34 ± 0.10 |
| Root | 0.50 ± 0.05 | 0.36 ± 0.01 | 0.33 ± 0.03 |
| mg Si g−1 root dry wt | |||
| Si uptake | |||
| 0.15 mm Si | 51.7 ± 3.1 | 65.7 ± 8.2 | 36.7 ± 3.8 |
| 1.5 mm Si | 203.3 ± 7.8 | 211.6 ± 9.5 | 92.2 ± 9.1 |
The three lines were grown in a nutrient solution containing 0.15 or 1.5 mm Si as silicic acid for 26 d. Values are means ± sd of three replicates.
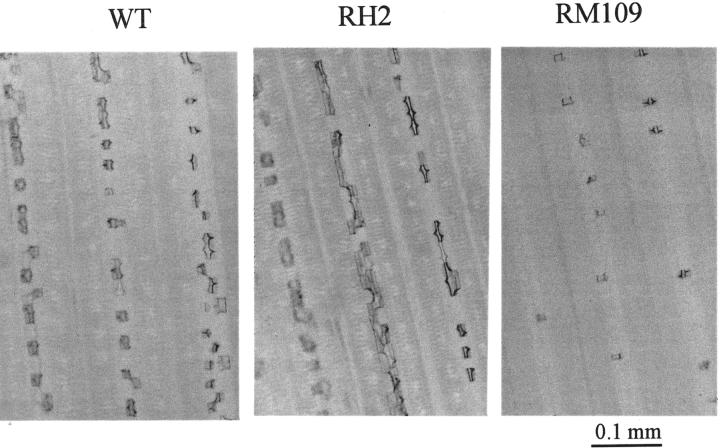
When the plants were grown in soil under flooded condition, there was no significant difference in the Si concentration of the shoot between WT and RH2, regardless of Si application (Table II). However, the shoot Si concentration in RM109 was lower than that in WT. This trend was more obvious when the plants were grown in a soil amended with Si. WT and RH2 had a similar number of silica bodies on the third leaf when grown in a soil either with Si or without Si amendment (Table III; Fig. 3). However, RM109 had only 40% of that of WT when grown in a soil amended with Si.
Table II.
Si concentration of the shoot of a WT (cv Oochikara) of rice and two root mutants, one without root hairs (RH2) and another without lateral roots (RM109)
| Treatment | Shoot Si Concentration
|
||
|---|---|---|---|
| WT | RH2 | RM109 | |
| mg Si g−1 | |||
| −Si | 20.6 ± 1.2 | 21.5 ± 0.4 | 17.8 ± 0.7 |
| +Si | 32.3 ± 1.6 | 34.2 ± 0.9 | 19.6 ± 1.2 |
Three lines were grown in a soil amended with or without 2 g Na2SiO3 kg−1 soil. After a 1-month growth period, the shoot was harvested, and the Si concentration of the shoot was measured. Values are means ± sd of three replicates.
Table III.
Number of silica bodies in a WT (cv Oochikara) of rice, a mutant without root hairs (RH2) and a mutant without lateral roots (RM109)
| Treatment | Number of Silica Bodies
|
||
|---|---|---|---|
| WT | RH2 | RM109 | |
| −Si | 19.3 ± 0.9 | 19.0 ± 9.1 | 12.9 ± 6.1 |
| +Si | 71.0 ± 6.3 | 82.6 ± 8.4 | 26.9 ± 11.2 |
Three lines were grown on a soil amended with or without Si (2 g Na2SiO3 kg−1 soil) for 1 month. After staining, silica bodies around 2 cm from the tip of the third leaf were counted under a microscope. Values are means ± sd of nine views from three replicates.
Figure 3.
Silica bodies on the third leaf of WT rice cv Oochikara and two root mutants, one without root hairs (RH2) and one without lateral roots (RM109). Three lines were grown in a soil amended with Si (2 g Na2SiO3 kg−1 soil) for 1 month. Silica bodies were observed around 2 cm from the leaf tip under a microscope.
To further investigate the role of root hairs in the Si uptake, the Si uptake by WT and RH2 from soil under upland condition was also examined. After 1 month of growth, the dry weight of the shoots was 2.77 ± 0.21 g for WT and 3.07 ± 0.28 g for RH2. No difference in the shoot Si concentration was observed between WT and RH2; the Si concentration of the shoots of WT and RH2 was 14.08 ± 1.05 and 15.76 ± 0.35 mg Si g−1 dry wt, respectively.
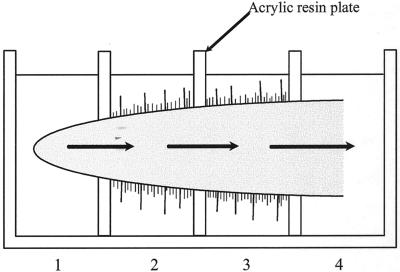
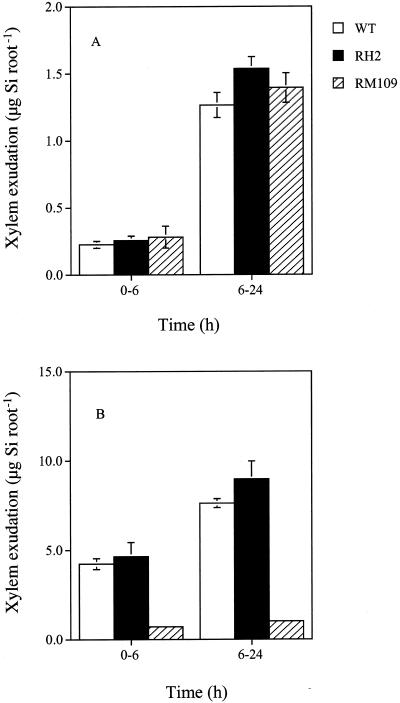
Si uptake by individual roots was investigated using a multi-compartment transport box (Fig. 4). This technique makes it possible to examine the uptake by different root zones. The uptake was estimated by determining the Si concentration in compartment 4, where Si was exuded from the xylem (Fig. 4). When Si was applied only to root apexes (0–1 cm), the amount of Si exuded was similar in RH2, RM109, and WT at different time points (Fig. 5A). When Si was applied to the mature zone (1–4 cm), there was no difference in the xylem exudation of Si between WT and RH2, but the Si exuded from RM109 was much less compared with WT at both 6 and 24 h (Fig. 5B).
Figure 4.
Schematic diagram of multi-compartment transport box. Each compartment is isolated by acrylic resin plates sealed with vaseline and supplied with 4 mL of treatment solution containing Si or not. Root was placed with apex in compartment 1 and the cut end in compartment 4. Si exuded from the xylem in compartment 4 was measured.
Figure 5.
Uptake of Si by different root zones. Ten excised roots (4 cm long) from WT rice cv Oochikara and two root mutants, one without root hairs (RH2) and one without lateral roots (RM109), were placed in the multi-compartment transport box. Four milliliters of treatment solution containing 1.5 mm Si as silicic acid was applied to compartment 1(A, root apex) or compartments 2 and 3 (B, mature zone). The remaining compartments were filled with the solution without Si. At 6 and 24 h, the Si exuded from the xylem in compartment 4 was measured. Error bars represent ±sd (n = 3).
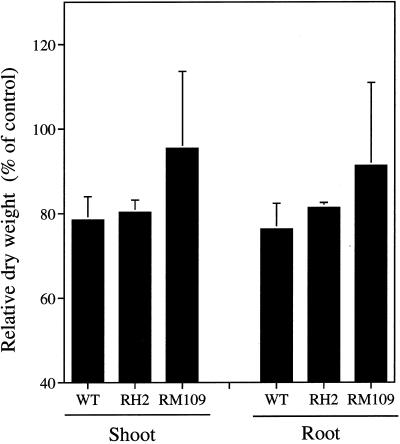
Ge is an analog element of Si. Evidence has shown that rice roots cannot distinguish Ge from Si in terms of uptake (Takahashi et al., 1976). However, in contrast to Si, Ge is toxic to the plant when it enters the cells, resulting in brown spots on leaves and stems. This implies that plants taking less Si are tolerant of Ge toxicity. On d 3 following exposure to Ge, brown spots were observed on the stem of WT and RH2, but not in RM109. On d 5 after the treatment, the brown spots were spread the leaves of WT and RH2. However, the brown spot on the leaves of RM109 was only observed on d 10 after exposure to Ge. The dry weights of both the shoots and roots were decreased by 20% due to exposure to Ge in WT and RH2 (Fig. 6). However, in RM109, the growth was hardly inhibited by Ge. This result is in agreement with the difference in Si uptake between the mutants and WT (Fig. 2; Table I).
Figure 6.
Effect of exposure to Ge on the growth of WT rice cv Oochikara and two root mutants, one without root hairs (RH2) and one without lateral roots (RM109). One-month-old seedlings were exposed to a nutrient solution containing 0 or 20 μm Ge for 10 d. Data are presented as percent dry weight with Ge treatment/dry weight without Ge treatment × 100. Error bars represent ±sd (n = 3).
Genetic analysis was performed using F2 populations between WT and RM109. Among 103 seedlings tested, 30 seedlings have lateral roots, whereas 73 seedlings did not have lateral roots (Table IV). The Si uptake by individual seedlings was investigated. The formation of lateral roots was correlated with higher Si uptake. The Si uptake during 24 h was 61.0 ± 14.2 mg Si g−1 root dry wt for the seedlings without lateral roots, and 152.6 ± 35.0 mg of Si for those with lateral roots. The Si uptake by WT and RM109 was 136.0 ± 22.8 and 61.1 ± 20.0 mg Si g−1 root dry wt, respectively. As the roots without lateral roots (low Si uptake) segregated to the roots with lateral roots (high Si uptake) at a 3:1 ratio (Table IV), it is suggested that the gene controlling the formation of lateral roots and subsequent Si uptake is a dominant gene.
Table IV.
Segregation ratio of progeny resulting from genetic crosses between a WT (cv Oochikara) of rice and a root mutant without lateral roots (RM109)
| Phenotype of Progeny
|
Ratio Tested | χ2 | P | ||
|---|---|---|---|---|---|
| Without lateral roots | With lateral roots | ||||
| WT×RM109 F2 | 73 | 30 | 3:1 | 0.73 | 0.3–0.5 |
| Si uptake (mg Si g−1 root dry wt 24 h−1) | 61.1 ± 14.2 | 152.6 ± 35.0 | |||
Si uptake by each seedling in a nutrient solution containing 1.5 mm Si was determined during 24 h.
DISCUSSION
Rice is a well-known Si accumulator (Takahashi et al., 1990). The Si concentration in rice leaf blades can reach more than 10% (w/w) Si, which is much higher than the concentration of other essential macronutrients such as N, P, and K (Takahashi, 1995; Savant et al., 1997). High accumulation of Si by rice has been ascribed to active uptake by the roots (Okuda and Takahashi, 1962a; Takahashi et al., 1990; Ma et al., 2001). The root system consists of primary roots, lateral roots, and root hairs. In the present study, we investigated the role of root hairs and lateral roots in the Si uptake by using two mutants, one defective in the formation of root hairs and another in that of lateral roots.
Root hairs form from root epidermal cells. It is presumed that root hairs contribute to the adhesion of the growing root to the rhizosphere and assist in the uptake of nutrients and water from the soil by increasing the absorptive surface area (Peterson and Farquhar, 1996). The role of root hairs in phosphorus uptake has been studied intensively, although it remains controversial. In soils low in available P, the contribution to P uptake by root hairs has been calculated to be up to 90% of total uptake (Fohse et al., 1991). Direct evidence of the participation of root hairs in phosphorus uptake from soil was provided by Gahoonia and Nielsen (1998), who found that root hairs contributed up to 63% of the total 32P uptake in shoots. In Arabidopsis, the response of increased root hair growth under low phosphorus availability is important in increasing phosphorus acquisition (Bates and Lynch, 2000). On the other hand, no relationship was found between root hairs and P influx of wheat lines differing in root hair length (Bole, 1973). A corn mutant defective in root hair growth showed normal growth and development under field conditions (Wen and Schnable, 1994), which raises questions about the importance of root hairs in phosphorus acquisition under field conditions. However, no information is available for the role of root hairs in the Si uptake. In the present study, the role of root hairs in the Si uptake by rice was examined by comparing WT with RH2, which is defective in the formation of root hairs, but has normal lateral roots (Fig. 1). Uptake experiments with both a short term (up to 12 h) and relatively long term (26 d) in a nutrient solution revealed a similar uptake of Si in WT and RH2 (Fig. 2; Table I). The uptake of Si by WT was also similar to that by RH2, when plants were grown in a soil under either flooded (Tables II and III) or upland conditions. Experiment using a multi-compartment transport box clearly showed that lack of root hairs did not affect the uptake of Si by the individual root (Fig. 5, A and B). All these results consistently suggest that root hairs are dispensable for the Si uptake.
The contribution of root hairs to nutrient uptake may vary with nutritional status. When Arabidopsis was grown under different levels of P, root hairs played a role in phosphorus uptake under moderate-to-low phosphorus availability but not at a high phosphorus level (Bates and Lynch, 2000). In the present study, the Si uptake at a low Si concentration was also examined in both solution and soil culture (flooded and upland conditions). The concentration of Si (0.15 mm) used for the uptake experiments is similar to that present in irrigation water, and the soil used has never been amended with Si fertilizers. However, no difference in Si uptake was observed between WT and RH2 grown either in a nutrient solution with low Si or in a soil without Si amendment under both flooded and upland conditions (Fig. 2; Tables I and II).
The contribution of root hairs to nutrient uptake may also differ with element and culture method. In soil, root hairs may be very important for the uptake of nutrients that move only a small distance by diffusion, such as phosphorus, potassium, and the micronutrient metals (Marschner, 1995). It is, however, difficult to observe the role of root hairs in such nutrient uptake in solution culture because the nutrients diffuse freely to the root surface in solution culture and the added advantage of having longer root hairs is lost (Clarkson, 1991; Gahoonia and Nielsen, 1997). However, it should be noted that in contrast to other nutrients except B, Si is present as an uncharged molecule in the soil solution and its concentration is usually much higher than that of other nutrients (Marschner, 1995). Furthermore, paddy rice is usually cultivated under flooded conditions. Therefore, the diffusion of Si to the root surface is not rate limiting. This might explain why root hairs of rice did not play a role in the Si uptake in both solution and soil cultures.
The uptake of Si by rice roots is suggested to be mediated by a specific transport system (Ma et al., 2001). Voltage-dependent potassium channels, which may function as a major component of a low-affinity potassium uptake system at K+ concentrations above 0.5 mm, are present in the plasma membrane of root hairs of wheat (Gassmann and Schroeder, 1994). A K+ uptake channel from tomato root hairs has also been cloned (Hartje et al., 2000). However, our results suggest that unlike the primary and lateral roots, a specific transport system for silicic acid is unlikely to be present in the root hairs. The low Si uptake by RM109, which has normal root hairs, also supports this speculation (Fig. 2; Tables I and II). Therefore, it seems that the rice root hairs do not play any role in the Si uptake because they lack a specific transport system for silicic acid.
Lateral roots originate in the root pericycle and are a major component of root systems. The role of lateral roots in the Si uptake by rice was investigated by comparing WT and RM109, which is defective in the formation of lateral roots, but has normal root hairs (Fig. 1). Lack of lateral roots resulted in a significant decrease in the uptake of Si in both short-term and relatively long-term experiments (Fig. 2; Tables I and II). Auxin has been suggested to be involved in the formation of lateral roots (Reed et al., 1998), which may result in a smaller plant size in RM109 (Table I). However, the differences observed in Si uptake between WT and RM109 do not result from the reduced growth of RM109. This is supported by the multi-compartment transport box experiment, which clearly showed that lack of lateral roots lead to a lower Si uptake by individual root (Fig. 5B). Analysis of F2 populations between RM109 and WT showed that the seedlings with lateral roots have high Si uptake similar to WT, whereas the seedlings without lateral roots have low Si uptake similar to RM109 (Table IV). These results suggest that lateral roots play an important role in the Si uptake. The structure of lateral roots resembles the primary roots (Blakely and Evans, 1979; Reed et al., 1998), suggesting that a specific transport system for the Si uptake is present in the lateral roots.
In conclusion, root hairs do not play any demonstrable role in the Si uptake, but lateral roots contribute to the Si uptake in rice plant.
MATERIALS AND METHODS
Plant Materials and Growth Condition
Two rice (Oryza sativa L. cv Oochikara) root mutants, RH2 and RM109, which are defective in the formation of root hairs and lateral roots, respectively, were used in this study (Fig. 1). The mutants were screened from M2 seeds (60,000 each) of rice that were treated with 10−3 M of sodium azide for 6 h at 25°C (Hao and Ichii, 1999; Ichii et al., 2000).
Seeds of WT rice cv Oochikara, RH2, and RM109 were surface sterilized in 0.5% (v/v) NaOCl for 15 min, rinsed, and soaked in water overnight at 25°C in the dark. The seeds were then transferred to a net floated on 0.5 mm CaCl2 solution in a plastic container. On d 5, the seedlings were transferred to a 3-L plastic pot containing one-half-strength of Kimura B nutrient solution. The nutrient solution contained the macronutrients 0.18 mm (NH4)2SO4, 0.27 mm MgSO4·7H2O, 0.09 mm KNO3, 0.18 mm Ca(NO3)2·4H2O, and 0.09 mm KH2PO4, and the micronutrients 20 μm NaEDTA-Fe·3H2O, 6.7 μm MnCl2·4H2O, 9.4 μm H3BO3, 0.015 μm (NH4)6Mo7O24·4H2O, 0.15 μm ZnSO4·7H2O, and 0.16 μm CuSO4·5H2O. The pH of this solution is 5.5 without adjustment and the solution was renewed every 2 d. Si was supplied as silicic acid, which was prepared by passing potassium silicate through cation-exchanges resin (Amberlite IR-120B, H+ form). Addition of silicic acid to the nutrient solution did not cause any pH change. Unless stated otherwise, the plants were grown in a controlled-environment growth chamber (CFH-400, TOMI, Osaka) under a 14-h/25°C day and a 10-h/20°C night regime and a light intensity of 40 W m−2. Each experiment was repeated at least twice.
Si Uptake Experiments
The uptake rate of Si was examined by a short-term (up to 12 h) uptake experiment. Two seedlings each (2 weeks old) were placed in a 50-mL plastic bottle containing one-half concentration of Kimura B solution with 0.15 mm or 1.5 mm Si. The bottle was wrapped with aluminum foil. At points indicated in Figure 2, a 1-mL aliquot of uptake solution was taken for determination of Si concentration. Transpiration (water loss) was also recorded at each sampling time. After the uptake experiment, the roots and shoots were harvested separately, and their fresh and dry weights were recorded.
Si accumulation in the shoot was examined by a relatively long-term culture using both water and soil culture. Six-day-old seedlings of the three lines were transplanted to a 1-L plastic pot (two seedlings per pot) containing one-half concentration of Kimura B solution with 0.15 or 1.5 mm Si. The solution was renewed every other day. The plants were grown in a green house in April, 2000. After 26 d, the plants were harvested. Soil used for growing rice was taken from the experimental farm of Kagawa University. The soil pH was 6.02. Seven-day-old seedlings were planted in a 1-L plastic pot (two seedlings per pot) filled with soil. Prior to transplanting, the soil was amended with (NH4)2SO4 (0.5 g kg−1 soil), KCl (0.2 g kg−1 soil), and KH2PO4 (0.2 g kg−1 soil). For Si application, sodium silicate was added to the soil at 2 g kg−1 soil. The plants were cultured in a greenhouse in a flooded condition from April to May, 2000. Deionized water was supplied daily. One month later, the shoot was harvested. The third leaf blade was sampled for the observation of silica bodies as described below.
Soil culture under upland condition was also conducted to further investigate the role of root hairs in the Si uptake. Both WT and RH2 were grown in soil without Si amendment under upland condition from July to August, 2001 in a greenhouse. Soil moisture was kept at field capacity daily with tap water. One month later, the shoot was harvested, and Si concentration was determined as described below.
Multi-Compartment Transport Box Experiment
The uptake of Si by individual roots and by different root zones of root was examined using a multi-compartment transport box (Fig. 4; Kawasaki et al., 1984). The box consisted of four compartments, and each compartment (1.4 cm height × 4.7 cm length × 1.0 cm width) was isolated by acrylic resin plates (0.4 cm wide) sealed with white vaseline. Four milliliters of treatment solution was added in each compartment. For the experiment on Si uptake by root apexes (0–1 cm), where neither root hair nor lateral roots are formed, 10 excised roots (4 cm long) from 1-week-old seedlings were placed in the box. Root apexes in compartment 1 were exposed to one-half concentration of Kimura B solution with 1.5 mm Si, whereas the remaining roots were exposed to the same nutrient solution without Si. At 6 and 24 h, the solution in each compartment was replaced with fresh solution, and the Si concentration in compartment 4 (cut end) as well as the other compartments, was determined. The Si uptake by lateral roots and root hairs was compared in a similar experiment except that the mature part of the roots in compartments 2 and 3, where root hairs and lateral roots formed, were exposed to Si solution. Compartments 1 and 4 were filled with the nutrient solution without Si. Roots used for this experiment were excised from 18-d-old seedlings. After the experiment, no leakage was found using a dye (Eriochrome cyanine R).
Ge Resistance
Seedlings cultured in the nutrient solution for 1 month were exposed to one-half concentration of Kimura B solution with or without 20 μm Ge as GeO (Wako, Tokyo). The treatment solution was renewed once every 3 d. After 10 d, the roots and shoots were harvested separately, and the fresh and dry weights were recorded.
Si Analysis
Plant samples were dried at 70°C in an oven for at least 2 d and then ground to powders. The Si concentration in the solution was determined by the colorimetric molybdenum blue method after the samples were melted with Na2CO3 and then dissolved in water (Okuda and Takahashi, 1961).
Observation of Silica Bodies
The leaf blade was immersed in 70% (v/v) ethanol for 1 d to remove chlorophyll. The leaf blade was boiled in phenol with several drops of safranine for 5 min. Silica bodies around 2 cm from the tip were observed and counted under a microscope (Olympus BX50-PH, Tokyo). Photomicrographs of the silica bodies were taken on Fuji 400 color film (Fuji Photo Film, Tokyo).
Genetic Analysis
F2 populations between WT and RM109 were used for lateral root observation and Si uptake determination. Si uptake of a total of 103 seedlings was tested in a nutrient solution with 1.5 mm Si as described above. The uptake period was 24 h. After the uptake experiment, the formation of lateral roots was observed. Chi-square analysis was performed.
ACKNOWLEDGMENTS
We thank Dr. Mineo Shibasaka for use of the multi-compartment transport box. We are also grateful to Prof. Eiichi Takahashi for his critical reading of this manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010271.
LITERATURE CITED
- Bates TR, Lynch JP. Plant growth and phosphorus accumulation of wild type and two root mutants of Arabidopsis thaliana (Brassicaceae) Am J Bot. 2000;87:958–963. [PubMed] [Google Scholar]
- Blakely LM, Evans TA. Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci Lett. 1979;14:79–83. [Google Scholar]
- Bole JB. Influence of root hairs in supplying soil phosphorus to wheat. Can J Soil Sci. 1973;53:169–175. [Google Scholar]
- Clarkson DT. Root structure and site of ion uptake. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant Roots: The Hidden Half. New York: Marcel Dekker; 1991. pp. 483–510. [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proc Natl Acad Sci USA. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:641–644. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Fohse D, Claassen N, Jungk A. Phosphorus efficiency of plants: II. Significance of root radius, root hairs and cation-anion balance for phosphorus influx in seven plant species. Plant Soil. 1991;132:261–272. [Google Scholar]
- Gahoonia TS, Nielsen NE. Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica. 1997;98:177–182. [Google Scholar]
- Gahoonia TS, Nielsen NE. Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil. 1998;198:147–152. [Google Scholar]
- Gassmann W, Schroeder JI. Inward-rectifying K+ channels in root hairs of wheat: a mechanism for aluminum-sensitive low-affinity K+ uptake and membrane potential control. Plant Physiol. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Ichii M. A mutant RM109 of rice (Oryza sativa L.) exhibiting altered lateral root initiation and gravitropism. Jpn J Crop Sci. 1999;68:245–252. [Google Scholar]
- Hartje S, Zimmermann S, Klonus D, Mueller-Roeber B. Functional characterization of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta. 2000;210:723–731. doi: 10.1007/s004250050673. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Volcani BE, Gassmann W, Schroeder JI. A gene family of silicon transporters. Nature. 1997;385:688–689. doi: 10.1038/385688b0. [DOI] [PubMed] [Google Scholar]
- Ichii M, Kawamura Y, Yang L, Taketa S. Characterization of root hair defective mutant in rice. Breeding Res. 2000;2:137. [Google Scholar]
- Kawasaki T, Moritsugu M, Shimizu G. The absorption and translocation of ions in excised barley roots: a multi-compartment transport box experiment. Soil Sci Plant Nutr. 1984;30:417–425. [Google Scholar]
- Ma JF, Miyake Y, Takahashi E. Silicon as a beneficial element for crop plants. In: Datonoff L, Korndorfer G, Snyder G, editors. Silicon in Agriculture. New York: Elsevier Science Publishing; 2001. pp. 17–39. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. Ed 2. San Diego: Academic Press; 1995. [Google Scholar]
- Okuda A, Takahashi E. Studies on the physiological role of silicon in crop plants: 1. Discussion on the silicon deficient culture method. J Sci Soil Manure Jpn. 1961;32:475–480. [Google Scholar]
- Okuda A, Takahashi E. Studies on the physiological role of silicon in crop plants: 8. Some specific behaviors of rice plants in silicic acid uptake. J Sci Soil Manure Jpn. 1962a;33:217–221. [Google Scholar]
- Okuda A, Takahashi E. Studies on the physiological role of silicon in crop plants: 9. Effect of metabolic inhibitors on silicic acid uptake by rice plants. J Sci Soil Manure Jpn. 1962b;33:453–455. [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev. 1996;62:2–33. [Google Scholar]
- Savant NK, Snyder GH, Datnoff LE. Silicon management and sustainable rice production. Adv Agron. 1997;58:151–199. [Google Scholar]
- Takahashi E. Uptake mode and physiological functions of silica. Sci Rice Plant. 1995;2:58–71. [Google Scholar]
- Takahashi E, Hino K. Effect of different Si forms on silicic acid uptake. J Sci Soil Manure Jpn. 1978;33:453–455. [Google Scholar]
- Takahashi E, Ma JF, Miyake Y. The possibility of silicon as an essential element for higher plants. Comments Agric Food Chem. 1990;2:99–122. [Google Scholar]
- Takahashi E, Matsumoto H, Syo S, Miyake Y. Variation of Ge uptake in different species. Jpn J Soil Sci Plant Nutri. 1976;47:217–221. [Google Scholar]
- Wen TJ, Schnable PS. Analysis of mutant of three genes that influences root hair development in Zea mays (Graminae) suggests that root hairs are dispensable. Am J Bot. 1994;81:833–842. [Google Scholar]