Abstract
Many plants invest carbon to form isoprene. The role of isoprene in plants is unclear, but many experiments showed that isoprene may have a role in protecting plants from thermal damage. A more general antioxidant action has been recently hypothesized on the basis of the protection offered by exogenous isoprene in nonemitting plants exposed to acute ozone doses. We inhibited the synthesis of endogenous isoprene by feeding fosmidomycin and observed that Phragmites australis leaves became more sensitive to ozone than those leaves forming isoprene. Photosynthesis, stomatal conductance, and fluorescence parameters were significantly affected by ozone only in leaves on which isoprene was not formed. The protective effect of isoprene was more evident when the leaves were exposed for a long time (8 h) to relatively low (100 nL L−1) ozone levels than when the exposure was short and acute (3 h at 300 nL L−1). Isoprene quenched the amount of H2O2 formed in leaves and reduced lipid peroxidation of cellular membranes caused by ozone. These results indicate that isoprene may exert its protective action at the membrane level, although a similar effect could be obtained if isoprene reacted with ozone before forming active oxygen species. Irrespective of the mechanism, our results suggest that endogenous isoprene has an important antioxidant role in plants.
Isoprene (C5H8) is emitted by many plants around the world (Kesselmeier and Staudt, 1999), and research is aimed at finding whether the cost of this carbon emission is matched by the accomplishment of a biological function. There have been many indications that isoprene and other isoprenoids enhance leaf thermotolerance (Sharkey and Singsaas, 1995; Singsaas et al., 1997; Loreto et al., 1998). Such a protective action has not been found in excised leaves (Logan and Monson, 1999) and, in some cases, in nonemitting leaves fumigated with exogenous isoprene (Singsaas et al., 1997). More recent work indicated that the thermotolerance is achieved mainly after short and repeated heat bursts (Singsaas and Sharkey, 1998). When present, thermotolerance has been attributed to a stabilization of membrane lipid bilayer, which is sensitive and often denatured by exposure to high temperatures (Gounaris et al., 1984). This effect may be exclusive to chloroplast membranes in which isoprene is formed (Sharkey, 1996; Sharkey and Yeh, 2001). However, no enhancement of stabilization by isoprene has been observed when using artificial membranes (Logan et al., 1999).
Isoprene is a very reactive compound, and the reaction products can be multiple depending on the other substrates (Fuentes et al., 2000). In a very oxidizing environment isoprene can scavenge oxidative species (Sauer et al., 1999). The reaction between isoprene and ozone in the leaves was considered to be unimportant on the basis of a mathematical model (Chameides, 1989). But Salter and Hewitt (1992) pointed out that the concentration of isoprene inside leaves should be by far higher than that indicated in Chameides' formulation. They concluded that, at physiological concentrations, isoprene may effectively react with ozone forming hydroxymethyl hydroperoxide and aggravating the ozone induced damage. Recently, however, Loreto et al. (2001) demonstrated that fumigation with exogenous isoprene dramatically reduces the damage caused by acute (300 nL L−1) and short (3 h) ozone treatments in leaves of plants that do not emit isoprene endogenously. They therefore suggested that isoprene may have a very strong antioxidant role in plants, perhaps related to the membrane strengthening action of this compound.
To test whether isoprene effectively exerts this action in nature, however, measurements must be repeated in isoprene-emitting species and in conditions that modulate or inhibit the endogenous emission of isoprene. Nowadays the best way to modulate isoprene emission is by using fosmidomycin, a powerful and specific inhibitor of the deoxy-xylulose-phosphate pathway of isoprenoid biosynthesis in chloroplasts (Lichtenthaler et al., 1997). Fosmidomycin fed through leaf petiole quenches isoprene emission by more than 90% in less than 1 h (Zeidler et al., 1998), but does not impair the photosynthetic process for several hours (Sharkey et al., 2001).
Phragmites australis, the common reed, is a strong and widespread isoprene-emitting plant (Kesselmeier and Staudt, 1999). We studied the effect of isoprene inhibition by fosmidomycin on the resistance of P. australis leaves to different concentrations of ozone and to short or long exposure to ozone. We show physiological and biochemical data indicating that isoprene production and emission is a powerful defense against ozone damage and suggesting that isoprene efficiently protects membranes against denaturation in a oxidative environment.
RESULTS
Isoprene emission was almost totally inhibited by fosmidomycin. The emission was reduced to less than 10% within an hour (Fig. 1) and remained very low during the time course of the reported experiments (data not shown). Fosmidomycin did not inhibit photosynthesis (Figs. 1 and 2), stomatal conductance (Fig. 2), or the photosynthetic electron transport rate (Figs. 2 and 3) during short and long treatment. However, there was a small but significant increase in the amount of non-photochemical quenching in fosmidomycin-fed leaves with respect to controls (Fig. 4). The content of H2O2 also increased in leaves fed with fosmidomycin with respect to controls (Fig. 5). The same effect, though to a lower extent, was observed in the level of malonyldialdehyde (MDA; an indicator of lipid peroxidation) in leaves (Fig. 6).
Figure 1.
Time course of the inhibition of isoprene emission (●) after feeding on 20 μmol of fosmidomycin. The inhibitor feeding through the petiole was started at time = 0. The photosynthetic rate of the same leaves during the experiment is also shown (○). Symbols and error bars represent means ± se (n = 6).
Figure 2.
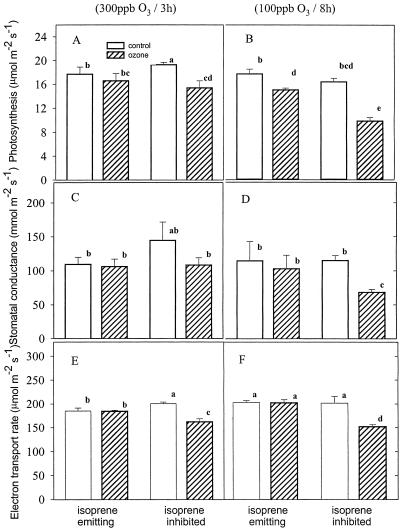
Effect of short-term (3 h, 300 nL L−1; left) and long-term (8 h, 100 nL L−1; right) ozone treatments on photosynthesis (A and B), stomatal conductance (C and D), and electron transport rate (E and F) of P. australis leaves. Each panel shows two pairs of bars representing photosynthesis of control (white) and ozone-treated (striped) leaves emitting isoprene (first pair) and nonemitting isoprene because of fosmidomycin feeding (second pair). Mean ± se (n = 3) is shown. Means were statistically separated by a Tukey's test, and means significantly different at the 5% level are identified by different letters.
Figure 3.
Photochemical efficiency, estimated by the fluorescence parameter ΔF/Fm′, during the long-term ozone treatment (8 h, 100 nL L−1 O3) in leaves emitting isoprene (black) or leaves in which isoprene emission was inhibited by fosmidomycin (white). Mean ± se (n = 6) is shown.
Figure 4.
Effect of short-term (3 h, 300 nL L−1; A) and long-term (8 h, 100 nL L−1; B) ozone treatments on the non-photochemical quenching of P. australis leaves. Each panel shows two pairs of bars representing photosynthesis of control (white) and ozone-treated (striped) leaves emitting isoprene (first pair) and nonemitting isoprene because of fosmidomycin feeding (second pair). Mean ± se (n = 3) is shown. Means were statistically separated by a Tukey's test, and means significantly different at the 5% level are identified by different letters.
Figure 5.
Effect of short-term (3 h, 300 nL L−1; A) and long-term (8 h, 100 nL L−1; B) ozone treatments on the content of H2O2 in P. australis leaves. Each panel shows two pairs of bars representing photosynthesis of control (white) and ozone-treated (striped) leaves emitting isoprene (first pair) and nonemitting isoprene because of fosmidomycin feeding (second pair). Mean ± se (n = 3) is shown. Means were statistically separated by a Tukey's test, and means significantly different at the 5% level are identified by different letters.
Figure 6.
Effect of short-term (3 h, 300 nL L−1; A) and long-term (8 h, 100 nL L−1; B) ozone treatments on the content of MDA in P. australis leaves. Each panel shows two pairs of bars representing photosynthesis of control (white) and ozone-treated (striped) leaves emitting isoprene (first pair) and nonemitting isoprene because of fosmidomycin feeding (second pair). Mean ± se (n = 3) is shown. Means were statistically separated by a Tukey's test, and means significantly different at the 5% level are identified by different letters.
Exposure to ozone decreased photosynthesis of P. australis leaves. This effect was not significant after short and acute treatment, but it was significant after a long-term treatment (Fig. 2, A and B). When isoprene synthesis was inhibited by fosmidomycin, ozone significantly affected photosynthesis both after short and long exposures. The negative effect of ozone was particularly evident after a long-term treatment.
Stomatal conductance was not significantly influenced by short-term ozone exposure when isoprene synthesis was allowed (Fig. 2, C and D). In fosmidomycin-fed leaves, the stomatal conductance was slightly reduced by the ozone treatment. However, the stomatal conductance of these leaves was comparable with those of leaves emitting isoprene. Therefore we argue that stomatal conductance was not significantly influenced by short-term ozone exposure, even when isoprene synthesis is inhibited. However, after a long-term ozone treatment, stomatal conductance was negatively affected by ozone, and the effect was statistically significant in fosmidomycin-fed leaves.
The electron transport rate calculated from fluorescence measurements confirmed the gas-exchange indications that ozone negatively affected the leaf properties only when fosmidomycin was concurrently fed and particularly when the leaves were exposed to a long-term treatment (Fig. 2, D and E). However, the fluorescence yield in dark-adapted leaves was not affected by ozone indicating no permanent damage to the photochemical apparatus (data not shown). The use of chlorophyll fluorescence also allowed us to noninvasively follow changes in photochemical efficiency during the ozone treatments. In both short- (not shown) and long-term ozone treatments (Fig. 3), the photochemical efficiency, as estimated by ΔF/Fm′, did not change in isoprene emitting leaves. However, in fosmido-mycin-fed leaves, the photochemical efficiency dropped 1 h after starting the ozone treatments, and progressively decreased until the end of the treatments. The non-photochemical quenching of fluorescence was slightly increased by ozone, but the effect of ozone became more evident in fosmidomycin-fed leaves (Fig. 4). As in the other fluorescence and gas-exchange parameters, the effect was more dramatic in the long-term than in the short-term treatment.
The amount of H2O2 that accumulated in the leaves after the short-term ozone treatment was slightly higher than in controls and not significantly different from that in fosmidomycin-fed leaves (Fig. 5). However, the long-term treatment with ozone caused a significantly higher accumulation of H2O2, and the effect was greatly enhanced in leaves concurrently fed with fosmidomycin.
The levels of MDA were also affected, but to a different extent depending on the treatments (Fig. 6). The MDA content after the short-term ozone treatment was higher than in control, and the content after the long-term treatment was further enhanced. In fosmidomycin-fed leaves, the MDA content was always higher than in isoprene-emitting leaves exposed to the same treatment. The highest content of MDA among all treatments was observed in fosmidomycin-fed leaves after a long-term exposure to ozone.
DISCUSSION
The finding that fosmidomycin specifically inhibits isoprene synthesis (Fig. 1; Lichtenthaler et al., 1997; Zeidler et al., 1998; Sharkey et al., 2001) gave us the opportunity to test whether isoprene functions as a strong antioxidant in leaves, as suggested by fumigations with exogenous isoprene on nonemitting leaves (Loreto et al., 2001). We found that the negative effects of ozone on the photosynthetic characteristics of the isoprene-emitting plant P. australis were strongly enhanced when isoprene synthesis was inhibited by fosmidomycin. This clearly indicates that isoprene is involved in the defense mechanism against ozone damage. The short-term and acute treatment used in our experiment dramatically affected the physiology and anatomy of tobacco and birch leaves that do not emit isoprene, whereas it was unable to reduce photosynthesis in the isoprene-emitting poplar leaves (Loreto et al., 2001). Therefore, the absence of ozone damage to photosynthesis after the short-term treatment in P. australis is interpreted as a confirmation that isoprene-emitting leaves are naturally protected against ozone.
The long-term treatment with 100 nL L−1 of ozone had a significantly more negative effect on photosynthesis of fosmidomycin-fed P. australis than the short-term and acute treatment. However, photosynthesis was only slightly reduced, and stomatal conductance and the electron transport rate were unaltered at the end of the treatment in isoprene-emitting leaves. By measuring the fluorescence yield of fosmidomycin-fed leaves during the treatment, we found that ozone did not damage photosynthesis during the 1st h, whereas thereafter the damage was progressive until the end of the treatment (Fig. 3). Thus the protective effect of isoprene seems to be especially important when the oxidative stress is prolonged.
Ozone may have several negative effects on leaves, and fosmidomycin-fed P. australis leaves showed many of them. Ozone damage is thoroughly described in the literature (e.g. Pell et al., 1997) but there is no consensus on the mechanisms and on the order in which the damage occurs. Ozone may directly induce stomatal closure, and photosynthetic inhibition could be caused by the lowered CO2 concentration in the mesophyll (Heath, 1994). We show that ozone causes stomatal closure only in fosmidomycin-fed leaves. Stomata stay open when ozone or fosmidomycin are supplied separately. Thus a direct inhibition of stomatal opening by ozone is unlikely.
There is evidence that ozone directly lowers the carboxylation efficiency by reducing either the amount or the activity of Rubisco and, consequently, photosynthesis (Farage et al., 1991; Pell et al., 1994). However, there is no apparent reason why such an effect should not occur in isoprene emitting leaves but only when isoprene synthesis is inhibited.
Ozone reacts rapidly with cellular structures generating active oxygen species (O′2, OH′, and H2O2) that are toxic and cause the peroxidation and denaturation of membrane lipids (Pell et al., 1997). Isoprene, in turn, has been postulated to stabilize the membrane lipid bilayer (Sharkey, 1996; Sharkey and Yeh, 2001). Thus, isoprene may counteract the ozone damaging effect on membranes. Our results show that the amount H2O2 increased to very high levels in fosmidomycin-fed leaves after the long-term ozone exposure, indicating that more oxidative products are formed when isoprene is absent. It should be noted that H2O2 also significantly increased in fosmidomycin-fed leaves that were not exposed to ozone, with respect to controls. This suggests that isoprene quenches oxidative products even when these are physiologically formed, for instance from the direct photoreduction of oxygen.
Membrane denaturation consequent to the attack of H2O2 and other active oxygen species produced by ozone results in the accumulation of end products of lipid peroxidation such as MDA (Heath and Parker, 1968). We found that an increase of MDA content was associated with H2O2 increase in all treatments. Higher levels of MDA were found in fosmidomycin-fed leaves, with respect to the leaves emitting isoprene, either in control conditions or after the ozone treatments. This demonstrates that lipid peroxidation is enhanced when isoprene is absent and indicates that isoprene may effectively protect membranes against denaturation. The high level of H2O2 and MDA in fosmidomycin-fed leaves unexposed to ozone or exposed to the short-term treatment and in isoprene-emitting leaves exposed to the long-term treatment were not associated with significant changes of photosynthetic properties. This indicates that most of the observed changes do not have direct negative consequences on leaf physiology. Perhaps other mechanisms are activated to combat the accumulation of active oxygen species. We found an increase in the non-photochemical quenching of fluorescence associated to H2O2 and MDA increase. The non-photochemical quenching reflects the de-epoxidation status of xanthophylls (Demmig-Adams and Adams, 1996). It is possible that this other class of isoprenoids successfully acts as a defensive agent in most of the cases but is unable to limit the ozone damage when isoprene is absent and the oxidative pressure is strong and prolonged.
Loreto et al. (2001) suggested that isoprene may quench ozone by directly reacting with it in the intercellular spaces. This would have the same effect as if isoprene functions as a membrane stabilizer reducing the quantity of ozone products and their oxidative pressure over the membranes. Thus, our experiments cannot prove whether isoprene is acting as a compound embedded within the membranes or as a gas reacting with ozone in every compartment of the leaf.
Isoprene reaction with ozone produces hydroperoxides in the atmosphere and this has been suggested to be a possible cause of damage for leaves (Hewitt et al., 1990; Sauer et al., 1999). Isoprene reaction can also indirectly form H2O2 in plants (Salter and Hewitt, 1992). This is apparently contradictory with the protective effect against ozone of exogenous isoprene in nonemitting leaves (Loreto et al., 2001). Our results show that H2O2 is always lower in the presence of isoprene than when isoprene is absent. Thus, in contrast to previous indications, isoprene reaction with ozone within the leaves may not form these dangerous products. A low yield of H2O2 was recently suggested from the reaction between isoprene and ozone in the atmosphere (Fuentes et al., 2000). It is possible that toxic hydroperoxides would be generated if the reaction between isoprene and ozone occur in presence of polluted air, whereas we used a mixture of clean gases. This has not yet been tested. It is also possible that H2O2 is formed but that isoprene embedded in membranes limits the damage and eventually reduces the final concentration of reactive oxygen species.
In conclusion, we demonstrated that endogenous isoprene has an important antioxidant role in plants. When the oxidation potential becomes high, such as under acute or prolonged ozone exposures, isoprene quenches ozone-dependent reactive oxygen species reducing the damage at the membrane level and probably the consequent damage at biochemical and physiological levels.
MATERIALS AND METHODS
Plant Material
Ten plants of Phragmites australis were grown from rhizome cuttings in 10-L pots filled with clay nuts. The pots were immersed in water fertilized with a very diluted (one-tenth strength) Hoagland solution. The water was continuously stirred and oxygenated by bubbling air through it, and was changed every other day. Plants were grown in a cabinet (Fitotron, Sanyo-Gallenkamp, Uxbridge, UK) on which the air temperature was maintained at 30°C during the 14-h photoperiod and at 20°C during the dark period. The photon flux density at the level of the leaves was 700 μmol m−2 s−1 and the relative humidity was 80%.
Gas-Exchange and Fluorescence Measurements
P. australis leaves were cut under water and maintained in a plastic vial with 30 mL of distilled water during the measurements. A round portion (4.91 cm2) of the leaf was clamped in a gas-exchange cuvette coated with Teflon to avoid ozone uptake by the aluminum body and with glass windows on both sizes. The characteristics of the cuvette are detailed by Loreto et al. (2001). The leaf disc was exposed to a 500 mL min−1 flow of synthetic air containing 80% N2, 20% O2, 330 μL L−1 CO2, and no ozone, isoprenoids, or other trace-gases and contaminants (Loreto et al., 2001). Water from a thermostated water bath was circulated through the aluminum body of the cuvette to control the leaf temperature. The illumination was provided by concentric arrays of light-emitting red diodes located 1 cm from the cuvette upper window and providing a homogenous light throughout the entire exposed leaf surface with intensities varying between 0 and 1500 μmol m−2 s−1. This light source was reported to have the same effect as white light on CO2, H2O, and isoprene gas exchanges between leaf and air (Tennessen et al., 1994). During all measurements the leaf temperature was maintained at 30°C and the light intensity at 700 μmol m−2 s−1. Leaf temperature was measured with a thermocouple firmly appressed to abaxial leaf surface and light intensity was measured with a LI-COR quantum meter (Lincoln, NE). CO2 and H2O exchange were measured when steady before and after ozone fumigation by using a LI-COR 6262 infrared gas analyzer. The analyzer was disconnected during the ozone treatment to avoid damages to its internal parts. Chlorophyll fluorescence was measured with a PAM 2000 (Walz, Effeltrich, Germany) modulated fluorometer. The fluorescence probe was inserted in the middle of the light-emitting diode arrays with the tip reaching the upper window. The ratio between variable and maximal fluorescence (Fv/Fm) was measured in dark-adapted (15 min) leaves at the beginning of each measurement and after ozone fumigation. In the illuminated leaves, the steady-state fluorescence in response to the 700 μmol m−2 s−1 light intensity (Fs) and the maximal fluorescence in response to a saturating (10000 μmol m−2 s−1) pulse of white light (Fm′) were measured and the ratio between (Fm′ − Fs)/Fm′ = ΔF/Fm′) was calculated every hour during the ozone treatments. The electron transport rate was calculated by multiplying ΔF/Fm′ by the absorbed light intensity and assuming the light equally distributed between the two photosystems (Loreto et al., 1994). The non-photochemical quenching of fluorescence was calculated from the maximal and minimal fluorescence in dark-adapted and illuminated leaves, according to Van Kooten and Snel (1990).
Isoprene Measurements and Isoprene Inhibition by Fosmidomycin
Before reaching the infrared gas analyzer, an aliquot (36 mL) of the air exiting the cuvette was automatically injected every 3 min in a gas chromatograph (Syntech Spectras BTX Analyser GC 855, Syntech Spectras, Groningen, The Netherlands), and the amount of isoprene emitted by the leaf discs was detected by PID. Other details of this on-line system of isoprene measurements are reported in Loreto and Delfine (2000).
Isoprene emission was inhibited by adding fosmidomycin to the water in the vial and by allowing the compound to travel through the transpiration stream. We tested the effect of several concentrations of fosmidomycin (not shown) and used the minimal concentrations (20 μmol) at which the effect was complete (isoprene inhibition >90%).
Ozone Treatments
When photosynthesis and isoprene emission were stable, the leaf disc was fumigated with 300 nL L−1 of O3 for 3 h (short-term and acute treatment) or with 100 nL L−1 of O3 for 8 h (long-term or semi-chronic treatment). The ozone was formed from the oxygen of the air mixture by a voltaic arc that was generated applying a tension of 3000 V between two electrodes separated by inert gases (model 300, Ozonomatic, Roma, Italy). The O3 concentration inside the cuvette was continuously monitored with UV Photometric O3 analyzer (1108 Dasibi Environmental Corp., Glendale, CA) and adjusted to the desired concentration by mixing the air passing through and by-passing the ozone generator. Both the short- and long-term treatments were carried out in leaves emitting isoprene and in leaves on which isoprene emission was inhibited by fosmidomycin.
Determination of H2O2 Content
Hydrogen peroxide content in control leaves and in leaves exposed to fosmidomycin, ozone, and ozone + fosmidomycin was determined according to Velikova et al. (2000). Leaf tissues (0.07 g) were homogenized in an ice bath with 5 mL of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000g for 15 min and 0.5 mL of the supernatant was added to 0.5 mL of 10 mm potassium phosphate buffer (pH 7.0) and 1 mL of 1 m KI. The absorbance of the supernatant was measured at 390 nm. The content of H2O2 was calculated by comparison with a standard calibration curve previously made by using different concentrations of H2O2.
Determination of the MDA Content
For the measurements of lipid peroxidation in leaves, the thiobarbituric acid (TBA) test, which determines MDA as an end product of lipid peroxidation (Heath and Parker, 1968), was used. An aliquot (0.07 g) of control leaves and of leaves exposed to fosmidomycin, ozone, and ozone + fosmidomycin was homogenized in 5 mL of 0.1% (w/v) TCA solution. The homogenate was centrifuged at 12,000g for 15 min and 0.5 mL of the supernatant was added to 1 mL of 0.5% (w/v) TBA in 20% TCA. The mixture was incubated in boiling water for 30 min, and the reaction was stopped by placing the reaction tubes in an ice bath. Then the samples were centrifuged at 10,000g for 5 min, and the absorbance of the supernatant was measured at 532 nm, subtracting the value for non-specific absorption at 600 nm. The amount of MDA-TBA complex (red pigment) was calculated from the extinction coefficient 155 mm−1 cm−1.
Statistical Analysis
All results are presented as means ± se from three to six measurements for each experimental conditions. All parameters were considered as variables subjected to independent observations, and these observations were statistically treated with a series of univariate ANOVA tests. When comparing different experimental conditions, mean differences were statistically assessed at a 5% level by Tukey's test.
Footnotes
This work was supported by the European Union-Confirming the International Role of Community Research Program (project no. IC5–CT98–0102) and by the Consiglio Nazionale delle Ricerche-North Atlantic Treaty Organization (Outreach fellowship to V.V.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010497.
LITERATURE CITED
- Chameides WL. The chemistry of ozone deposition to plant leaves: role of ascorbic acid. Environ Sci Technol. 1989;23:595–600. [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Farage P, Long SP, Lechner EG, Baker NR. The sequence of change within the photosynthetic apparatus of wheat following short-term exposure to ozone. Plant Physiol. 1991;95:529–535. doi: 10.1104/pp.95.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Botteneheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A. Biogenic hydrocarbons in the atmosphere boundary layer: a review. Bull Am Met Soc. 2000;81:1537–1575. [Google Scholar]
- Gounaris K, Brain APR, Quinn PJ, Williams WP. Structural reorganization of chloroplast thylakoid membranes in response to heat stress. Biochim Biophys Acta. 1984;766:198–208. [Google Scholar]
- Heath RL. Possible mechanisms for the inhibition of photosynthesis by ozone. Photosynth Res. 1994;39:439–451. doi: 10.1007/BF00014597. [DOI] [PubMed] [Google Scholar]
- Heath RL, Parker L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hewitt CN, Kok GL, Fall R. Hydroperoxides in plants exposed to ozone mediate air pollution damage to alkene emitters. Nature. 1990;344:56–58. doi: 10.1038/344056a0. [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atm Chem. 1999;33:23–88. [Google Scholar]
- Lichtenthaler HK, Schwendler J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Logan BA, Anchordoquy TJ, Monson RK, Pan RS. The effect of isoprene on the properties of spinach thylakoids and phosphatidylcholine liposomes. Plant Biol. 1999;1:602–606. [Google Scholar]
- Logan BA, Monson RK. Thermotolerance of leaf discs from four isoprene-emitting species is not enhanced by exposure to exogenous isoprene. Plant Physiol. 1999;120:821–825. doi: 10.1104/pp.120.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Delfine S. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiol. 2000;123:1605–1610. doi: 10.1104/pp.123.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Di Marco G, Tricoli G, Sharkey TD. Measurements of mesophyll conductance, photosynthetic electron transport and alternative electron sinks of field grown wheat leaves. Photosynth Res. 1994;41:397–403. doi: 10.1007/BF02183042. [DOI] [PubMed] [Google Scholar]
- Loreto F, Förster A, Dürr M, Csiky O, Seufert G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998;21:101–107. [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S. Ozone quenching properties of isoprene and its antioxidant role in plants. Plant Physiol. 2001;126:1–8. doi: 10.1104/pp.126.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell EJ, Eckardt NA, Glick RE. Biochemical and molecular basis for impairment of photosynthetic potential. Photosynth Res. 1994;39:453–462. doi: 10.1007/BF00014598. [DOI] [PubMed] [Google Scholar]
- Pell EJ, Schlagnhaufer CD, Arteca RN. Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plant. 1997;100:264–273. [Google Scholar]
- Salter L, Hewitt CN. Ozone-hydrocarbon interactions in plants. Phytochemistry. 1992;31:4045–4050. [Google Scholar]
- Sauer F, Schafer C, Neeb P, Horie O, Moortgat GK. Formation of hydrogen peroxide in the ozonolysis of isoprene and simple alkenes under humid conditions. Atmos Env. 1999;33:229–241. [Google Scholar]
- Sharkey TD. Isoprene synthesis by plants and animals. Endeavor. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001;125:2001–2006. doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- Sharkey TD, Yeh S. Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD. The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ. 1998;21:1181–1188. [Google Scholar]
- Tennessen DJ, Singsaas EL, Sharkey TD. Light-emitting diodes as a light source for photosynthesis research. Photosynth Res. 1994;39:85–92. doi: 10.1007/BF00027146. [DOI] [PubMed] [Google Scholar]
- Van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyammines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Zeidler J, Schwender J, Müller C, Wiesner J, Weidemeyer C, Back E, Jomaa H, Lichtenthaler HK. Inhibition of the non-mevalonate 1-deoxy-d-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch. 1998;53:980–986. [Google Scholar]