Abstract
The phytochrome photoreceptors and the circadian clock control many of the same developmental processes, in all organs and throughout the growth of Arabidopsis plants. Phytochrome A (phyA) provides light input signals to entrain the circadian clock. The clock is known to rhythmically regulate its light input pathway, so we tested rhythmic regulation of phyA, using transgenic plants carrying a PHYA promoter fusion to the luciferase reporter (PHYA:LUC). We provide the first images of LUC activity with subcellular resolution in intact tissue. PHYA transcription and the accumulation of all three PHYA mRNAs were indeed clock controlled. PHYA is expressed throughout the seedling, so we tested whether circadian rhythms were observed in all PHYA-expressing organs and whether the rhythms were autonomously controlled by each organ. In contrast to our previous results using other clock controlled genes, the rhythmic pattern of PHYA expression varied markedly among isolated organs and between isolated organs and intact plants. High-amplitude rhythms were maintained for many days in isolated leaves in darkness, whereas the leaves of intact plants rapidly lost rhythmicity. Wounding the leaves of intact plants had no effect. The rhythmic pattern of PHYA expression is not organ autonomous but depends upon the physical continuity or isolation of the rhythmic tissues, consistent with the presence of a transmitted signal that controls the overt expression of circadian rhythms without necessarily affecting the underlying clock. A circadian system might be present in most, if not all, plant cells, but its effect on intracellular rhythms can be controlled by supracellular signaling.
Organisms throughout nature have evolved endogenous circadian clocks to allow the synchronization of internal events with daily changes in the external environment. Under constant environmental conditions, the circadian clock drives biological rhythms with a period of about 24 h (rhythms that peak about once every 24 h). For a circadian clock to function correctly, however, it must not only have a 24-h period but also be entrained (set) in the correct relationship to the local day/night cycle. Light signals at dawn and dusk are most important in entraining circadian clocks, though temperature cycles also contribute (for review, see Lumsden and Millar, 1998). In plants the entraining light signals are transduced by “light input pathways” involving at least two classes of photoreceptor, which absorb red light (RL)/far RL (phytochromes) and blue light (cryptochromes; Somers et al., 1998a; Devlin and Kay, 2000).
Phytochromes are a major photoreceptor family in plants. They play a critical role regulating the photomorphogenic development of the plant (for review, see Kendrick and Kronenberg, 1994). In Arabidopsis there are five phytochrome genes, PHYA through PHYE (Sharrock and Quail, 1989; Clack et al., 1994). Phytochrome A (phyA) is the most abundant phytochrome in etiolated seedlings. Exposure to light converts the inactive phyA Pr to the active phyA Pfr form, which is rapidly degraded to a low steady-state level (Clough and Vierstra, 1997). The PHYA promoter in Arabidopsis has three transcription start sites; in the light, PHYA expression is negatively regulated by phyA and phyB (Canton and Quail, 1999). phyA plays a role in the light promotion of germination (Shinomura et al., 1994) and de-etiolation (Nagatani et al., 1993; Whitelam et al., 1993). It is also involved in shade avoidance (Johnson et al., 1994), entrainment of the circadian clock (Somers et al., 1998a), and the control of flowering time (Johnson et al., 1994; Reed et al., 1994).
The circadian clock regulates many processes during the plant's development including leaf movement (Engelmann et al., 1992), hypocotyl elongation (Dowson-Day and Millar, 1999), cytosolic [Ca +2] (Johnson et al., 1995; Sai and Johnson, 1999), and stomatal opening (Somers et al., 1998b). The circadian clock also regulates the expression of multiple genes involved in photosynthesis, metabolism, development, and UV protection (Harmer et al., 2000; Schaffer et al., 2001), including genes that encode chlorophyll a/b-binding proteins (CAB or LHCB genes). We have shown that rhythmic output signals from the clock feed back on the light input pathway, rhythmically controlling the expression of PHYB and also the function of the light input pathway to the clock (Bognar et al., 1999; McWatters et al., 2000). Multiple photoreceptors mediate light input (Somers et al., 1998a; Devlin and Kay, 2000), however, and it is unclear how many of these photoreceptors are regulated by the clock.
One of the hallmarks of circadian rhythms is their persistence in constant conditions. The observed rhythms are not insensitive to the environment, however. Their characteristics are often affected by the lighting conditions. The rhythmic expression of many plant genes becomes arrhythmic upon transfer to constant darkness, for example, going to a constant level within two or three cycles. Such rhythms are said to “damp.” Damping describes a decrease in the amplitude of the rhythm (the difference in level between the peak and the trough of a wave). Formally, damping in darkness could occur because light affects the clock via the light input pathways, because many rhythmic processes are regulated by light (independently of the clock), or because light affects the coupling between the clock and some target processes. For CAB expression, transfer to darkness leads to a rapid decrease in amplitude and expression level. The circadian clock probably does not damp in this way, because the expression rhythms of other genes can persist for many days in darkness (for example, Zhong et al., 1997). CAB expression is strongly regulated by light, via phytochrome. Treatment with far-RL prior to darkness results in more rapid damping of CAB expression, for example, whereas overexpression of PHYA prevents or delays damping (Nagy et al., 1988; Kay et al., 1989). These and other results indicate that rapid damping correlates with low levels of Pfr. CAT3 expression rhythms, unlike CAB expression, damp to a high level of expression in darkness. An Arabidopsis double mutant combination of phyA and cry1 defects prevents the damping of the CAT3 rhythm of expression, suggesting that the photoreceptor proteins are required for damping, by a mechanism that is not understood (Zhong et al., 1997).
We have previously shown that excised leaves have a robust circadian rhythm of CAB expression in constant light, which can be re-entrained by light-dark cycles (Thain et al., 2000). All CAB-expressing organs behaved alike in these experiments. A similar maintenance of rhythmicity and entrainability has been described for isolated fruit fly (Drosophila melanogaster) and zebrafish (Danio rerio) organs (Plautz et al., 1997a; Whitmore et al., 2000). This observation provides evidence for multiple copies of self-sustained and entrainable clocks in both plants and animals. The clocks can be desynchronized experimentally. Separate halves of a single leaf can be entrained to opposite light-dark cycles and subsequently oscillate in opposite phases, indicating that there is no communication of timing information or systemic light input signals within the leaf (Thain et al., 2000). The multiple copies of the clock can therefore function autonomously in vivo in plants as in the fruit fly (Giebultowicz et al., 2000) and probably in rodents (Yamazaki et al., 2000).
To investigate the circadian regulation of other photoreceptors we have fused the PHYA promoter to the firefly luciferase gene. PHYA:LUC activity reports the spatial pattern of PHYA expression with cellular resolution. We show that PHYA expression is coupled to the clock under RL and rhythmic PHYA expression rapidly damps to a high level in the dark. The damping is dependent upon the tissue connection to the plant, because in isolated leaves, the PHYA rhythm does not damp. Wounding intact leaves has no effect on rhythmic expression. These results indicate that a nonautonomous mechanism controls rhythmic gene expression patterns in the intact plant.
RESULTS
The Spatial Expression Pattern of PHYA
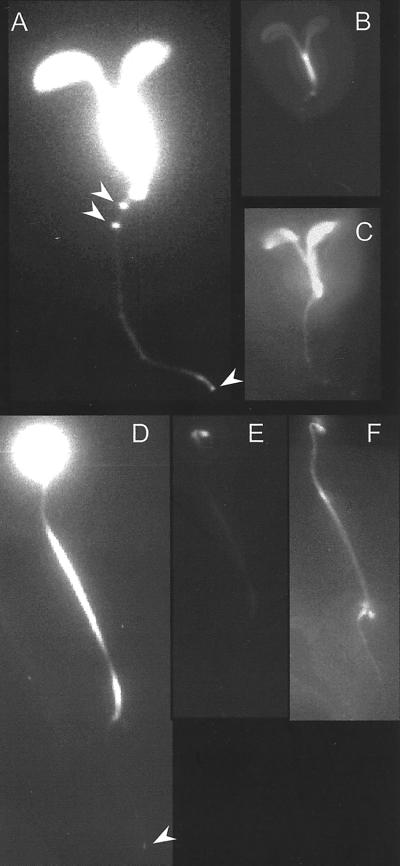
The bioluminescence expression patterns of Arabidopsis plants carrying the PHYA:LUC reporter were characterized by in vivo imaging of plants grown for 7 d in 12-h light/12-h dark cycles. PHYA was expressed throughout the light-grown seedling, with the strongest expression in the hypocotyl (Fig. 1, A and B). It is clear from the overexposed image that PHYA was also highly expressed in the primary and lateral root tips. In etiolated seedlings strong expression of PHYA was detected in the closed cotyledon and the apical hook (Fig. 1, D and E). A lower level of expression was detected throughout the hypocotyl and in the root tip (Fig. 1D).
Figure 1.
Patterns of luminescence in transgenic plants transformed with the PHYA:LUC construct. A through C, Images from a 7-d-old light-grown seedling. A, Overexposed luminescence image, the arrows point at the lateral root and primary root tip. B, Luminescence image. C, Reflected light image. D through F, Images from a 7-d-old dark-grown seedling. D, Overexposed luminescence image, the arrow points to the root tip. E, Luminescence image. F, Reflected light image.
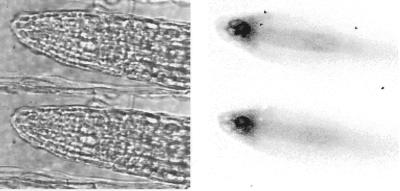
To test PHYA:LUC expression at higher spatial resolution, we coupled a cryogenically cooled CCD camera to a microscope that allowed high light transmission. LUC expression has previously been imaged at such resolution only in dissociated plant cells, following transient transfection (Gallie et al., 1989; Kost et al., 1995). We obtained images of the root tips of dark-adapted PHYA:LUC plants (Fig. 2). The bio-luminescence signal identified the outlines of individual cells in the columella root cap, providing the first images of LUC in intact tissue at cellular resolution. Exposure times were short (1–4 min), so stacks of images could be automatically acquired at a range of focal planes, allowing the removal of some unfocussed signal by image processing. The modified luciferase, LUC+, was critical to these experiments because this commercially available clone yielded at least 10-fold higher signals than wild-type LUC in transgenic Arabidopsis, without altering the dynamic properties of the reporter (A. Hall, L. Kozma-Bognár, F. Nagy, and A.J. Millar, unpublished data).
Figure 2.
Expression of PHYA in the root tip. Luminescence images (left) and corresponding bright-field images (right) of the root tip of dark-adapted PHYA:LUC plants. Gray levels of luminescence images are inverted (black represents the strongest signal), showing strongest expression in root cap cells near the meristem and weaker expression in the developing vasculature. Upper and lower panels show adjacent focal planes, which emphasize the lower signals from the older root cap cells.
The Expression of PHYA Is Coupled to the Circadian Clock
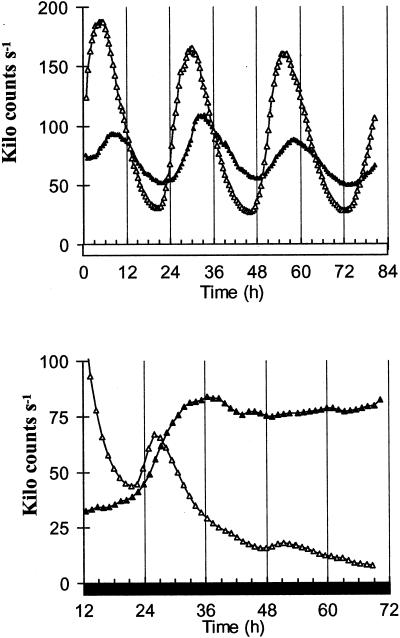
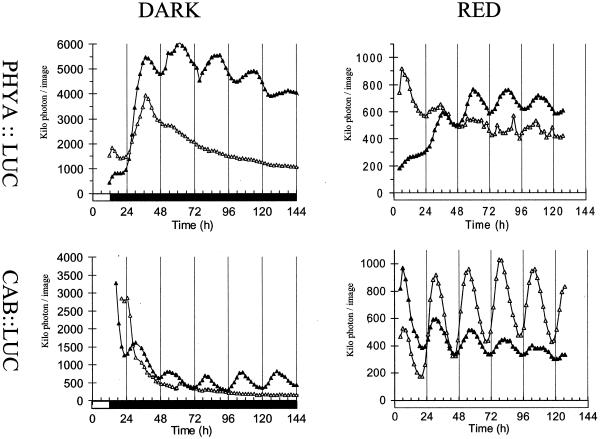
We have previously shown that the expression of PHYB is rhythmically regulated by the circadian clock (Bognar et al., 1999). Both CAB and PHYB expression rhythms peak at approximately the same time, 4 to 6 h after dawn. To test for circadian control of PHYA expression, seedlings grown for seven 12-h light/12-h dark cycles were assayed for PHYA:LUC expression under constant conditions. When entrained plants were transferred to constant RL the expression of PHYA had a robust circadian oscillation (high amplitude rhythms and constant period of approximately 24 h) in multiple transgenic lines, with a mean level similar to the level of CAB2:LUC expression but a lower rhythmic amplitude (Fig. 3A). Unlike CAB and PHYB expression, the expression of PHYA peaked in the late afternoon. Identical regulation of PHYA expression was observed in transformants of the Landsberg erecta ecotype (data not shown). In constant darkness the oscillation of PHYA rapidly damped, after about 24 h, to a high level of expression, approximately 4-fold higher than that of CAB2. This increase in PHYA expression is consistent with previous data (Somers and Quail, 1995) and is not unique to PHYA; the rhythmic expression of CAT3 and PHYB similarly damps to a high level of expression in the dark (Zhong et al., 1997; Bognar et al., 1999). Our data clearly identify PHYA transcription as being regulated by the circadian clock in Arabidopsis.
Figure 3.
The expression of PHYA is coupled to the circadian clock. Seedlings were grown for 7 d under 12-h light/12-h dark cycles the luminescence rhythms were then assayed under constant. A, Expression patterns of PHYA (black triangles) and CAB (white triangles) under constant RL. B, Expression patterns of PHYA (black triangles) and CAB (white triangles) in constant darkness. The values for PHYA:LUC luminescence have been divided by 4.
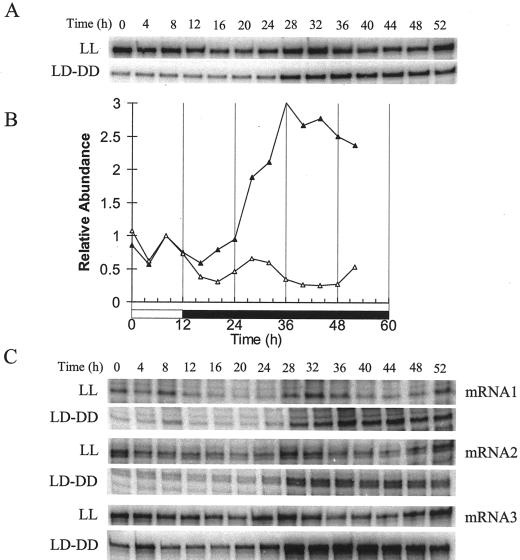
Similar circadian regulation was observed for PHYA RNA accumulation in plants harvested under constant light or darkness. The RNA accumulation rhythm had a low amplitude and peaked at a similar time to PHYA:LUC activity (Fig. 4, A and B). The RNA accumulation rhythm has been tested previously but not detected, either because the time points tested flanked the peak (Clack et al., 1994) or perhaps because of its low amplitude (Harmer et al., 2000). PHYA transcription is initiated from multiple sites in Arabidopsis, producing transcripts of three different sizes (Canton and Quail, 1999). Interestingly, all three of these transcripts show rhythmic transcription in constant light (Fig. 4C). The low amplitude rhythm is not due to a single arrhythmic RNA, as was the case for CAB (Millar and Kay, 1991). The accumulation pattern of total PHYA RNA in constant darkness (DD) was also consistent with the PHYA:LUC activity pattern. All three PHYA transcripts showed a similar damping expression rhythm (Fig. 4C).
Figure 4.
Rhythmic accumulation of total PHYA RNA and of all three PHYA transcripts. Seedlings were entrained for 2 weeks under 12-h light/12-h dark cycles. Tissue was harvested every 4 h following transfer to constant white light (white symbols) or during a further day of entrainment followed by darkness (black symbols). RNA was isolated and either total PHYA RNA or the three specific transcripts were measured by RNase protection assays. A, Accumulation of total PHYA RNA. B, The data for total PHYA RNA were normalized to ubiquitin RNA measurements; normalized values are plotted relative to the value at 8 h, when the samples are biologically equivalent. C, Accumulation of each of the PHYA RNA transcripts.
PHYA Expression Rhythms in Intact and Isolated Organs
The widespread expression of PHYA:LUC gave us the opportunity to investigate tissue-specific effects on circadian rhythms, using older plants. We measured PHYA:LUC activity in 3-week-old plants by close-up video imaging and analyzed luminescence signals from leaves and roots separately. The primary leaves of intact plants had circadian PHYA regulation broadly similar to that in seedlings. PHYA expression oscillated under constant RL but with lower amplitude than in seedlings (Fig. 5; compare with Fig. 3A). Supporting this conclusion, biomathematical analysis (see “Materials and Methods”) found a circadian period in 20 of 26 expression traces for intact PHYA:LUC plants under RL, compared with 24 of 25 traces for excised leaves and 47 of 48 profiles for seedlings (Fig. 3A). In the dark the circadian rhythm damped rapidly; the high level of expression reached in the first 48 h slowly decreased over the time course (Fig. 5). Very similar, low-amplitude circadian regulation of PHYA was observed in intact roots in the light; the rhythm damped rapidly in darkness, as in the leaves (data not shown).
Figure 5.
Leaf excision prevents damping of PHYA and CAB expression in the dark. Seedlings were germinated and grown for 3 weeks under 12-h light/12-h dark cycles. Leaves were excised (black symbols) or collars were placed around the leaves of intact plants (white symbols) 6 h before the luminescence assays began. Top left, PHYA:LUC luminescence in constant dark. Bottom left, CAB2:LUC luminescence in constant dark. Top right, PHYA:LUC luminescence in constant RL. Bottom right, CAB2:LUC luminescence in constant RL.
We have previously shown that excised organs maintain rhythmic CAB:LUC activity (Thain et al., 2000). Due to the pattern of CAB gene expression, we could only test aerial organs. We therefore examined PHYA regulation in leaf explants and found that both PHYA and CAB2 expression continued to oscillate robustly under constant light (Fig. 5). Interestingly, the rhythm of PHYA expression in explants appeared to be more robust (more regular and of higher amplitude) than in the leaves of intact plants of the same age. This is similar to observations of rhythmic period expression in excised fruit fly organs (Plautz et al., 1997a), though the reasons for the greater robustness are unclear.
Rhythms of PHYA and CAB2 Expression Do Not Damp in Tissue Explants
Damping of circadian rhythms in the dark is a common characteristic of clock-regulated genes in plants, but this feature of PHYA:LUC regulation was strikingly altered in explants. The circadian expression of PHYA:LUC damped rapidly in the leaves of intact 3-week-old plants (Fig. 5). When such leaves were excised the expression of PHYA remained robustly rhythmic in darkness, similar to the oscillations maintained in constant light (Fig. 5). Rhythmic CAB:LUC expression was also maintained in leaf explants in darkness, though at a lower amplitude than in the leaves of intact CAB:LUC plants in the light. This low amplitude presumably reflects control of CAB by the circadian rhythm alone, without the rhythmically regulated light induction that increases peak amplitude in the light (McWatters et al., 2000). The light-regulated increase in mean PHYA expression level and decrease in mean CAB expression level were not affected by the excision.
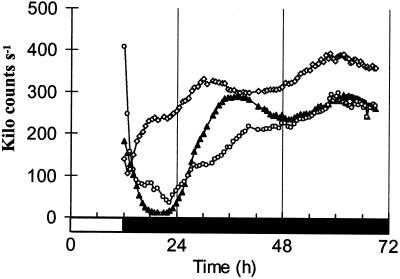
Comparing PHYA regulation among organ explants revealed significant differences. PHYA expression was very weakly rhythmic or arrhythmic in excised hypocotyl segments, although these had substantial expression levels (Fig. 6). Excised roots maintained only a low-amplitude oscillation of PHYA expression (Fig. 6). This was more robust than the rhythm in intact roots, which damped rapidly in the dark (data not shown), but less robust than the rhythms of excised leaves. Interestingly, the peak of PHYA expression was 3 to 4 h earlier in the root explants than in excised leaves. These differences in circadian timing among organs are reminiscent of the differences in [Ca+2] rhythms observed in various tissues of intact plants (Wood et al., 2001).
Figure 6.
Expression of PHYA in excised roots and hypocotyl segments in darkness. Seedlings germinated and grown for 3 weeks under 12-h light/12-h dark cycles. Organs were excised 6 h prior to transfer to constant dark. Luminescence of PHY:LUC was assayed under constant dark. Black triangles, Excised leaves. White diamonds, Excised roots. White circles, Excised hypocotyls. Hypocotyl and root values have been multiplied by 50.
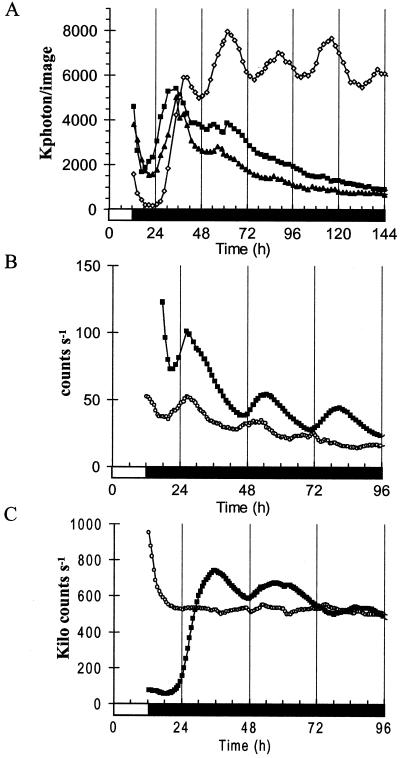
The fact that the amplitude of PHYA:LUC and CAB:LUC rhythms increased in the leaf explants indicates that this was not due to senescence: senescent leaves have very low-amplitude rhythms of CAB:LUC gene expression (S. Thain, A. Hall, and A.J. Millar, unpublished data). Several studies have suggested a role for phytochrome in the regulation of damping (Nagy et al., 1988; Kay et al., 1989; Zhong et al., 1997). It has been reported that light signaling pathways can be induced by stimuli other than light, such as pathogen challenges (Schenk et al., 2000). To test whether the prevention of damping in explants was a response to wounding, leaves on an intact plant were wounded and the rhythmic expression of PHYA was assayed. The rhythm in wounded intact leaves damped identically to the unwounded controls (Fig. 7A). This indicates that the lack of damping in isolated leaves is not due to the bypass or non-specific induction of light signaling by a wounding response. The lack of damping cannot be explained by callus formation in the excised tissues. Callus tissue can support circadian rhythms of CAB:LUC expression (Fig. 7, B and C; Sai and Johnson, 1999) but callus carrying the PHYA:LUC construct failed to show any rhythmicity (Fig. 7B). These results indicate that, although the expression of PHYA can be controlled by the circadian clock, a regulatory mechanism that depends upon an intact petiole connection rapidly suppresses the circadian rhythm in the intact plant in darkness.
Figure 7.
Neither wounding or callus formation prevents damping. A, Seedlings were entrained for 3 weeks under 12-h light/12-h dark cycles. Leaves were excised as described, and intact leaves and wounded leaves were placed in collars. PHYA:LUC luminescence was assayed in constant darkness. Black triangles, intact leaves. Black squares, wounded leaves. White diamonds, excised leaves. B and C, Calli were produced from excised leaves of plants transformed with the CAB:LUC (B) and PHYA:LUC (C) constructs. The calli were entrained for 3 d under 12-h light/12-h dark cycles, and luciferase activity was assayed in constant darkness. White circles, Callus. Black square, Excised leaves. CAB:LUC values for excised leaves were divided by 50 to bring them within the range of the calli.
DISCUSSION
Spatial Pattern of PHYA Gene Expression
We constructed transgenic Arabidopsis plants carrying a fusion of the Arabidopsis PHYA promoter to the firefly luciferase gene. The PHYA gene is widely expressed in Arabidopsis seedlings grown in light or darkness (Fig. 1), consistent with previous reports (Somers and Quail, 1995; Adam et al., 1996). PHYA:LUC expression was detected in individual cells of the columella root cap (Fig. 2), showing that luciferase can be used as a cell-level reporter. PHYA expression levels varied among organs: light-grown seedlings had particularly strong expression in the hypocotyl, for example. phyA function is related to gene dosage (Whitelam et al., 1993), so absolute levels of phyA signaling are expected to vary among cell types. Such quantitative differences in photoreceptor gene expression could therefore contribute to the differential light responses of various tissues, including differential regulation of circadian rhythms (A. Hall, L. Kozma-Bognar, R.M. Bastow, F. Nagy, and A.J. Millar, unpublished data; S. Thain, G. Murtas, and A.J. Millar, unpublished data; Lumsden and Millar, 1998).
Circadian Control of PHYA Gene Expression
Phytochrome photoreceptors have been directly implicated in providing light input signals to the circadian system in higher plants (for example, Devlin and Kay, 2001; Satter et al., 1974). We demonstrate that the circadian clock regulates the Arabidopsis PHYA promoter in transgenic PHYA:LUC Arabidopsis seedlings that were grown under light/dark cycles and transferred to constant light or to constant darkness (Figs. 3 and 5). The observed PHYA:LUC rhythm has a lower amplitude than the CAB2:LUC rhythm in constant light. The CAB2:LUC rhythm damps to low expression levels in darkness (Millar et al., 1992a, 1995). The rhythmicity of PHYA:LUC expression damps even more rapidly though expression levels remain high (Figs. 3 and 5).
PHYA RNA accumulation patterns have been reported for plants grown in light/dark cycles with variable results, some of which are likely due to differences among species (Adam et al., 1994; Clack et al., 1994; Hauser et al., 1998). However, microarray assays of Arabidopsis RNA have also scored PHYA as rhythmic (Schaffer et al., 2001) or not (Harmer et al., 2000). The luciferase reporter assay reproducibly revealed the low-amplitude regulation. We show that all three transcripts of Arabidopsis PHYA (Canton and Quail, 1999) are rhythmic at low amplitude in constant light (Fig. 4). The circadian clock therefore has the potential to regulate phyA function, creating an “outer loop” from circadian output to a circadian input pathway. Rhythmic regulation of the input pathway has been observed in many species and can have profound effects upon circadian rhythms (Lakin-Thomas, 2000; McWatters et al., 2000). In addition to rhythmic PHYA gene expression, the circadian clock might also affect post-translational mechanisms such as kinase activity (Fankhauser et al., 1999) and/or nuclear translocation (Kim et al., 2000).
Conditional Expression of PHYA Rhythmicity
We have previously shown that the circadian clock does not control PHYA expression in some conditions: Neither PHYA mRNA levels (Adam et al., 1994) nor the activity of the NtPHYA:LUC transgene (Kolar et al., 1998) were clock-regulated in tobacco seedlings. PHYA expression was therefore disconnected from the well-established rhythms of other circadian markers in this material (Millar et al., 1992; Kolar et al., 1995, 1998). NtPHYA:LUC activity showed a strong diurnal rhythm in adult tobacco plants under light-dark cycles but was not clock-regulated under constant conditions, in either intact or excised leaves (A. Hall, L. Kozma-Bognár, F. Nagy, and A.J. Millar, unpublished data). These and other results (Wildermann et al., 1978; Hauser et al., 1998) indicate that PHYA expression is not regulated by the circadian clock in all species. Our results show that the amplitude and damping of PHYA expression rhythms in light-grown Arabidopsis plants is variable, depending upon the type and age of tissue tested (Figs. 3, 5, 6, and 7). The rhythms of PHYA:LUC activity in intact plants rapidly damped to arrhythmia in darkness, as did the rhythms of all three PHYA transcripts: the transcripts are coordinately regulated by the clock, in contrast to their differential regulation by light (Canton and Quail, 1999). PHYA:LUC activity increased in darkness, reflecting the negative light regulation of PHYA transcription (Canton and Quail, 1999). Imaging individual organs of intact plants revealed similar, rapid damping of PHYA expression rhythms in leaves and roots. Most strikingly, this damping was prevented by excising the organs (Fig. 5 and 6).
Excised leaves had robust rhythms of PHYA expression in DD, roots showed lower-amplitude rhythms and hypocotyl segments were virtually arrhythmic (Fig. 6). The circadian system that controls PHYA expression is therefore present in multiple copies in the plant, at least in the leaves and roots, similar to the results from studies of CAB and PHYB rhythms (Thain et al., 2000). In contrast to previous results, the rhythmic pattern of PHYA:LUC activity was specific to the organ type, differing in the rate of damping and, in roots, also in the time of peak expression. The molecular basis for these differences is not yet clear but they underline the responsiveness of circadian regulation to developmentally programmed stimuli.
Leaves and roots had very similar, rapidly damped rhythms in the intact plant in darkness. The remaining parts of donor plants after organ excision exhibited damping like intact controls, so the persistent rhythms were specific to the excised roots and leaves (data not shown). The maintained rhythms were not due to de-differentiation, callus formation, or wounding effects in the excised tissue, because CAB expression in callus was rhythmic but PHYA expression was arrhythmic (Fig. 7B; Sai and Johnson, 1999). Furthermore, wounded leaves of otherwise intact plants showed damped PHYA expression rhythms, like unwounded controls (Fig. 7A). These results suggest that an interaction between organs caused the damping of rhythmic PHYA expression in the leaves and roots of intact plants, presumably via a transmitted signal or signals.
The mechanism that leads to the damping of circadian rhythms in Arabidopsis plants is not yet clear. Damping is not due to complete arrhythmia during dark adaptation; the rhythmic expression of genes such as CCR2 persists with high amplitude for many days (Kreps and Simon, 1997). It is formally possible that the multiple copies of the plant circadian clock (Thain et al., 2000) have radically different properties, such that the clocks controlling CAB and PHYA arrest in darkness but separate copies of the clock mechanism control CCR2 and these do not arrest. There is more evidence to suggest that damping affects the targets of clock output, rather than the clock mechanism. Damping results from the regulation of normally rhythmic genes by non-rhythmic pathways (most likely the light signaling pathways) that mask ongoing oscillation of the clock. A signal transmitted to the leaf of an intact plant might therefore lead to damping of a specific rhythm(s), whether or not the signal affected the circadian clock. Damping of CAB rhythms is strongly correlated with the decline of phytochrome signaling in darkness (A. Hall, L. Kozma-Bognar, R.M. Bastow, F. Nagy, and A.J. Millar, unpublished data; Nagy et al., 1988; Kay et al., 1989); the same decline is thought to up-regulate PHYA in DD (Canton and Quail, 1999). Leaf excision prevents the damping of both PHYA and CAB expression rhythms (Fig. 5), indicating that excision in darkness mimics an effect of light. The expansion of excised Arabidopsis cotyledons was previously shown to be independent of cry1, whereas intact plants required cry1 for full cotyledon expansion; again, excision appeared to mimic an effect of light signaling, bypassing the requirement for cry1 (Blum et al., 1994). Damping of rhythmic CAT3 gene expression in DD was prevented in phyA;cry1 double photoreceptor mutants, suggesting that these photoreceptor proteins were required for damping, rather than for maintained rhythmicity (Zhong et al., 1997). We and others have previously shown that light signaling pathways can initiate transmitted signals in intact plants, for example (Bischoff et al., 1997). The disruption of such transmitted light signals could account for the observed effects of organ excision, consistent with the involvement of phototransduction pathways in damping.
The expression of both PHYA and CAB promoters is controlled in a plastic manner by a network of endogenous and environmental factors, which presumably contributes to the adaptive regulation of light perception and light capture, respectively. The properties of the plant circadian clock likewise appear to be plastic. The phase of a single biological rhythm can vary among tissues in Arabidopsis (Wood et al., 2001), as we found in excised roots, and different rhythmic markers can exhibit different periods (Hennessey and Field, 1992; Park et al., 1999; A. Hall, L. Kozma-Bognar, R.M. Bastow, F. Nagy, and A.J. Millar, unpublished data; S. Thain, G. Murtas, and A.J. Millar, unpublished data). We have shown that rhythmic amplitude is also subject to complex regulation that differs among tissues, using the rhythms of PHYA gene expression as a marker. Since photoreceptors regulate the circadian clock, differential photoreceptor gene expression could partly underlie the observed flexibility of the plant circadian system.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, Wounding, and Callus Production
Arabidopsis seedlings were grown in sterile culture as described (Millar et al., 1992b), in temperature controlled rooms at 80 μmol m−2 s−1 fluorescent white light. The PHYA:LUC construct was built from a 2277-bp fragment of the Arabidopsis PHYA promoter (chromosome I BAC clone F14J9, 80,835–83,092 bp). The promoter was fused to the LUC+ gene (Promega, Madison, WI) with an NOS terminator. The PHYA:LUC construct was transformed into the Landsberg erecta and Wassilewskija ecotypes of Arabidopsis. The CAB2:LUC construct used here includes the same promoter region as has been described (Millar et al., 1992a), with the original LUC gene replaced by the LUC+ gene. The CAB2:LUC+ construct was transformed into Arabidopsis ecotype Wassilewskija.
Calli (Fig. 7) were produced from excised leaves of CAB:LUC+ or PHYA:LUC plants as described (Clarke et al., 1992). In wounding experiments (Fig. 7), a single leaf of each 3-week-old plant was wounded either by crushing with forceps or by making an incision parallel to the midrib with dissecting scissors. The results were identical; Figure 7 shows data from crushed leaves.
High-Resolution Luminescence Imaging
PHYA:LUC plants were grown for 14 d on solid agar medium under light:dark cycles, then transferred to darkness. Plants were removed from the agar and placed on microscope slides in a thin bed of the growth medium. Luciferin (5 mm) was added to the medium, and the coverslip was applied. While the luciferin diffused throughout the root (5–10 min), the sample was visually brought into focus. Control experiments indicated that total luminescence varied by less than 15% over the next 30 min. Luminescence (1-min integration in liquid N-cooled camera, see above) images were obtained at a range of focal planes under the control of MetaMorph software (Universal Imaging Corp., Downingtown, PA) to locate luminescent cells. An image stack was collected, comprising first a luminescence (4 min integration) then a bright field image at each focal position. Luminescence image stacks were processed with a haze removal algorithm (Universal Imaging). For bright field images, only the look-up table was adjusted to enhance contrast for printing. The motorized microscope (Axioplan 2) and objectives (Fluar 5×, 10×, and 20×) were from Zeiss (Jena, Germany).
Imaging of Seedlings and Adult Plants
The luciferase luminescence of seedlings (Figs. 3, 6, and 7) was measured by counting in an automated luminometer (Topcount, Packard, Meriden, CT) as described (McWatters et al., 2000). The Topcount plants were illuminated with RL at 1.5 μmol m−2 s−1. For luminescence measurements in adult plants, multiple plants were imaged on solid medium in 10-cm tissue culture dishes. Opaque collars were placed around leaves that remained attached to the plant to exclude luminescence scattering from the other organs and to allow analysis of organ-specific luminescence; excised organs were imaged simultaneously in the same dish (Figs. 5 and 6). RL at 10 μmol m−2 s−1 (Fig. 5) was provided by light-emitting diode arrays (Optimum Vision Ltd., Petersfield, UK) and luminescence was measured by low-light video imaging (Figs. 5 and 7), using intensified (Hamamatsu VIM, Hamamatsu City, Japan) and liquid-nitrogen cooled (LN/CCD-512-TKB, Princeton Instruments, Trenton, NJ) cameras essentially as described (Millar et al., 1992b; Michelet and Chua, 1996). Due to differences in the optical setup, absolute count levels are not directly comparable between cameras. The luminescence data shown is representative of three to four replicate experiments, incorporating at least two independently transformed lines, all of which gave very similar results. For example, period estimates for excised PHYA:LUC leaves in DD (Fig. 5) were always tightly clustered, as expected for robust rhythms, with a sd between 0.91 and 1.15 h in three independent experiments. The range of individual periods was 26.3 to 29.4 h. Testing attached leaves in DD yielded traces to which the FFT-NLLS period fitting software was either unable to fit rhythms or fitted rhythms with high relative amplitude error (a measure of the robustness of a rhythm; Plautz et al., 1997b) and period estimates with sds between 3.62 and 4.76 h in three experiments. The wide range of periods is typical of weakly rhythmic data (Dowson-Day and Millar, 1999). Some traces with widely differing luminescence levels were normalized to facilitate comparison, as indicated in the figure legends.
Rhythm Data Analysis
Luminescence levels were quantified and analyzed essentially as described (McWatters et al., 2000; Thain et al., 2000), using the software packages MetaMorph (Universal Imaging Corp.), I&A and TopTemp macro suites for Microsoft Excel (http://www.scripps.edu/cb/kay/shareware/; A. Hall, unpublished data) and FFT-NLLS (Plautz et al., 1997b). Rhythmic traces were scored as described (Dowson-Day and Millar, 1999).
RNAse Protection Assays
Total RNA was extracted as described (Adam et al., 1994). The PHYA RNase protection was performed as described (Adam et al., 1996) using 30 mg of total RNA per lane. For the detection of total PHYA RNA, a 239-bp HindII-XbaI fragment of Arabidopsis PHYA gene was used as a probe; for UBQ10 a 143-bp SalI-SacI fragment of the Arabidopsis UBQ10 gene was used. The abundance of each of the three PHYA transcripts was assayed using RNase protection as described (Canton and Quail, 1999). The RNA signals from replicate gels were quantified using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). PHYA-specific signals were normalized to the ubiquitin signal for each time point, to control for variation in gel loading. To correct for probe differences among experiments, normalized signals for constant light and light-dark-DD time courses are expressed relative to the peak signal 8 h after lights-on.
ACKNOWLEDGMENTS
We are grateful to members of the Millar and Nagy laboratories for helpful discussions and to V. Ravenscroft and P. Goode for expert technical assistance.
Footnotes
This work was supported by the Biotechnology and Biological Science Research Council (BBSRC; grants nos. G08667 and BI11209 to A.J.M.), the Human Frontier Science Program Organisation (to A.J.M. and F.N.), the Howard Hughes Medical Institute (grant no. 75185–542401), and by the Országos Tudományos Kutatási Alapprogramok (grant no. t012127 to F.N.). The luminescence imaging facility at Warwick is supported by the BBSRC, Gatsby Charitable Foundation, and Royal Society funding (to A.J.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010294.
LITERATURE CITED
- Adam E, Kozma-Bognar L, Kolar C, Schafer E, Nagy F. The tissue-specific expression of a tobacco phytochrome-b gene. Plant Physiol. 1996;110:1081–1088. doi: 10.1104/pp.110.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam E, Szell M, Szekeres M, Schafer E, Nagy F. The developmental and tissue-specific expression of tobacco phytochrome-A genes. Plant J. 1994;6:283–293. [Google Scholar]
- Bischoff F, Millar AJ, Kay SA, Furuya M. Phytochrome-induced intercellular signalling activates cab::luciferase gene expression. Plant J. 1997;12:839–849. [Google Scholar]
- Blum DE, Neff MM, Van Volkenburgh E. Light-stimulated cotyledon expansion in the blu3 and hy4 mutants of Arabidopsis thaliana. Plant Physiol. 1994;105:1433–1436. doi: 10.1104/pp.105.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bognar LK, Hall A, Adam E, Thain SC, Nagy F, Millar AJ. The circadian clock controls the expression pattern of the circadian input photoreceptor, phytochrome B. Proc Natl Acad Sci USA. 1999;96:14652–14657. doi: 10.1073/pnas.96.25.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton FR, Quail PH. Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 1999;121:1207–1215. doi: 10.1104/pp.121.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Wei W, Lindsey K. High-frequency transformation of Arabidopsis thaliana by Agrobacterium tumefaciens. Plant Mol Biol Rep. 1992;10:178–189. [Google Scholar]
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Devlin PF, Kay SA. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Circadian photoperception. Annu Rev Physiol. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- Engelmann W, Simon K, Phen CJ. Leaf movement rhythm in Arabidopsis thaliana. Z Naturforschung. 1992;47:925–928. [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science. 1999;284:1539–1541. doi: 10.1126/science.284.5419.1539. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Lucas WJ, Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989;1:301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz JM, Stanewsky R, Hall JC, Hege DM. Transplanted Drosophila excretory tubules maintain circadian clock cycling out of phase with the host. Curr Biol. 2000;10:107–110. doi: 10.1016/s0960-9822(00)00299-2. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Hauser BA, Cordonnier-Pratt MM, Pratt LH. Temporal and photoregulated expression of five tomato phytochrome genes. Plant J. 1998;14:431–439. doi: 10.1046/j.1365-313x.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- Hennessey TL, Field CB. Evidence of multiple circadian oscillators in bean plants. J Biol Rhythms. 1992;7:105–113. doi: 10.1177/074873049200700202. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- Johnson E, Bradley M, Harberd NP, Whitelam GC. Photoresponses of light-grown phya mutants of Arabidopsis: Phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SA, Nagatani A, Keith B, Deak M, Furuya M, Chua N-H. Rice phytochrome is biologically active in transgenic tobacco. Plant Cell. 1989;1:775–782. doi: 10.1105/tpc.1.8.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick RE, Kronenberg GHM. Photomorphogenesis in Plants. Vol. 1. Dordrecht, The Netherlands: Martinus Nijhoff; 1994. [Google Scholar]
- Kim L, Kircher S, Toth R, Adam E, Schafer E, Nagy F. Light-induced nuclear import of phytochrome-A:GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 2000;22:125–133. doi: 10.1046/j.1365-313x.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- Kolar C, Adam E, Schafer E, Nagy F. Expression of tobacco genes for light-harvesting chlorophyll a/b binding-proteins of photosystem-II is controlled by two circadian oscillators in a developmentally-regulated fashion. Proc Natl Acad Sci USA. 1995;92:2174–2178. doi: 10.1073/pnas.92.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar C, Fejes E, Adam E, Schafer E, Kay S, Nagy F. Transcription of Arabidopsis and wheat Cab genes in single tobacco transgenic seedlings exhibits independent rhythms in a developmentally regulated fashion. Plant J. 1998;13:563–569. doi: 10.1046/j.1365-313x.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Kost B, Schnorf M, Potrykus I, Neuhaus G. Non-destructive detection of firefly luciferase (LUC) activity in single plant cells using a cooled, slow-scan CCD camera and an optimized assay. Plant J. 1995;8:155–166. [Google Scholar]
- Kreps JA, Simon AE. Environmental and genetic effects on circadian clock-regulated gene expression in Arabidopsis. Plant Cell. 1997;9:297–304. doi: 10.1105/tpc.9.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Circadian rhythms: new functions for old clock genes? Trends Genet. 2000;16:135–142. doi: 10.1016/s0168-9525(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Lumsden PJ, Millar AJ. Biological Rhythms and Photoperiodism in Plants. Oxford: BIOS Scientific; 1998. [Google Scholar]
- McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–719. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- Michelet B, Chua NH. Improvement of Arabidopsis mutant screens based on luciferase imaging in planta. Plant Mol Biol Rep. 1996;14:320–329. [Google Scholar]
- Millar AJ, Kay SA. Circadian control of cab gene transcription and mRNA accumulation in Arabidopsis. Plant Cell. 1991;3:541–550. doi: 10.1105/tpc.3.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Chua NH, Kay SA. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell. 1992a;4:1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Hiratsuka K, Chua N-H, Kay SA. Firefly luciferase as a reporter of regulated gene expression in higher plants. Plant Mol Biol Rep. 1992b;10:324–337. [Google Scholar]
- Millar AJ, Straume M, Chory J, Chua N-H, Kay SA. The regulation of circadian period by phototransduction pathways in Arabidopsis. Science. 1995;267:1163–1166. doi: 10.1126/science.7855596. [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua N-H. A circadian clock regulates transcription of the wheat Cab-1 gene. Genes Dev. 1988;2:376–382. [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285:1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997a;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms. 1997b;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai J, Johnson CH. Different circadian oscillators control Ca2+ fluxes and Lhcb gene expression. Proc Natl Acad Sci USA. 1999;96:11659–11663. doi: 10.1073/pnas.96.20.11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter RL, Geballe GT, Galston AW. Potassium flux and leaf movement in Samanea saman. II. Phytochrome controlled movement. J Gen Physiol. 1974;64:431–442. doi: 10.1085/jgp.64.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Landgraf J, Accerbi M, Simon VV, Larson M, Wisman E. Microarray analysis of diurnal and circadian-regulated Genes in Arabidopsis. Plant Cell. 2001;13:113–123. doi: 10.1105/tpc.13.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville S, C, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed-germination in Arabidopsis thaliana is regulated principally by phytochrome-b and secondarily by phytochrome-a. Plant Physiol. 1994;105:773. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Devlin PF, Kay SA. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science. 1998a;282:1488–1490. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- Somers DE, Quail PH. Phytochrome-mediated light regulation of PHYA- and PHYB-GUS transgenes in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:523–534. doi: 10.1104/pp.107.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Webb AAR, Pearson M, Kay SA. The short-period mutant, toc1–1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998b;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- Thain S, Hall A, Millar A. Functional independence of multiple circadian clocks that regulate plant gene expression. Curr Biol. 2000;10:951–956. doi: 10.1016/s0960-9822(00)00630-8. [DOI] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, SassoneCorsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Wildermann A, Drumm H, Schafer E, Mohr H. Control by light of hypocotyl growth in de-etiolated mustard seedlings. I. Phytochrome as the only photoreceptor pigment. Planta. 1978;141:211–216. doi: 10.1007/BF00387891. [DOI] [PubMed] [Google Scholar]
- Wood NW, Haley A, Viry-Mousssaid M, Johnson CH, Van der Luit AH, Trewavas A. The circadian rhythms of different cell types oscillate with different circadian phases. Plant Physiol. 2001;125:787–796. doi: 10.1104/pp.125.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zhong HH, Resnick AS, Straume M, McClung CR. Effects of synergistic signaling by phytochrome A and cryptochrome1 on circadian clock-regulated catalase expression. Plant Cell. 1997;9:947–955. doi: 10.1105/tpc.9.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]