Abstract
Glycinebetaine is an osmoprotectant accumulated by barley (Hordeum vulgare) plants in response to high levels of NaCl, drought, and cold stress. Using barley seedlings in hydroponic culture, we characterized additional inducers of glycinebetaine accumulation. These included other inorganic salts (KCl, MgCl2, LiCl, and Na2SO4), oxidants (H2O2 and cumene hydroperoxide), and organic compounds (abscisic acid, polymixin B, n-butanol, salicylic acid, and aspirin). Stress symptoms brought on by high NaCl and other inducers, and not necessarily correlated with glycinebetaine accumulation, include wilting, loss of chlorophyll, and increase in thiobarbituric acid reacting substances. For NaCl, Ca2+ ions at 10 to 20 mm decrease these stress symptoms without diminishing, or even increasing, glycinebetaine induction. Abscisic acid induces glycinebetaine accumulation without causing any of the stress symptoms. NaCl, KCl, and H2O2 (but not other inducers) induce glycinebetaine at concentrations below those needed for the other stress symptoms. Mg2+ at 10 to 20 mm induces both stress symptoms and glycinebetaine, but only at low (0.2 mm) Ca2+. Although illumination is needed for optimal induction, a significant increase in the leaf glycinebetaine level is found in complete darkness, also.
For barley (Hordeum vulgare) plants, an important response to stress conditions is the induction of synthesis of the osmoprotectant glycinebetaine (Rhodes and Hanson, 1993). Its synthesis is induced by high salt (Arakawa et al., 1990, 1992), drought and water deficits (Hitz et al., 1982), and cold stress (Kishitani et al., 1994). Abscisic acid (ABA) was found to increase expression of betaine aldehyde dehydrogenase (BADH; Ishitani et al., 1995). In the current work, attention has been paid to interactions of the induction process with ionic conditions in the medium, and a number of alternative inducing compounds have been found.
Glycinebetaine is made from choline. Bacteria like Escherichia coli do not produce choline but can take it up effectively from the environment (Andresen et al., 1988). In E. coli the membrane-bound choline dehydrogenase encoded by betA oxidizes choline to glycinebetaine aldehyde and this aldehyde to glycinebetaine at about the same rate. In other bacteria (Arthrobacter globiformis), the enzyme choline oxidase converts choline in one step to glycinebetaine (Hayashi et al., 1997). In plants, glycinebetaine is made in two steps, with glycinebetaine aldehyde as intermediate. In spinach the first step is performed by an interesting, chloroplast-localized enzyme, choline monooxygenase, which uses electrons from photosystem I via ferredoxin to help do the oxidation (Brouquisse et al., 1989; Rathinasabapathi et al., 1997).
In barley, the BADH cDNA has been isolated and shown by northern-blot analysis to be induced to higher levels due to NaCl stress (Ishitani et al., 1995). Two genes for BADH have been found, one of them targeted to peroxisomes (Nakamura et al., 1997). Expression of the gene product not targeted to peroxisomes or chloroplasts is increased by salt stress (Nakamura et al., 1998, 2001). However, in cereals including barley, neither the enzyme oxidizing choline nor a cDNA homolog of the spinach choline monooxygenase has been found as of yet.
Unresolved questions about the induction of glycinebetaine in barley include the site and nature of the initial salt or osmotic stress receptor, the nature of the signal transduction pathway, and whether the osmoprotectant is made in chloroplasts or in the cytosol. In this work we present evidence that induction of glycinebetaine differs from that of other stress symptoms in that it is not brought about by an unfavorable K+ to Na+ ratio or by Na+ excluding Ca2+ from the root cell surface. Also, induction is found in the leaf due to oxidants applied to the roots. Induction by some organic compounds provides indirect evidence for the participation of protein kinase C and phospholipase D in the transduction pathway.
RESULTS
Stress Symptoms Due to High Salt
Exposing the roots of barley plants for 4 d to 100 to 300 mm NaCl caused the expected rise in glycinebetaine content, and also some other stress effects. The leaves wilted, and this condition persisted. In our measurements, it showed up as an increase in the percentage of water deficit (Table I). Little or no water loss occurred from the medium, indicating that the stomates remained mostly closed. The roots turned a partially brown color. Also, in many experiments, some chlorophyll degradation occurred. The level of sodium ions found in leaves increased directly with the NaCl concentration used in the medium (data not shown). Acid extracts of stressed leaves were often colored red; and the thiobarbituric acid assay showed an increase in unknown compounds, designated as thiobarbituric acid reaction substances (TBARS; see “Materials and Methods”).
Table I.
Leaf stress symptoms due to increasing NaCl in the medium
| NaCl | Glycinebetaine | TBARS | Water Deficit | Chlorophyll |
|---|---|---|---|---|
| mm | μmol g−1 | A450 g−1 | % | mg g−1 |
| 0 | .47 ± .07 | 6.17 ± .58 | 0.97 ± .44 | 1.51 ± .06 |
| 50 | .77 ± .09 | 4.67 ± .58 | 2.50 ± .35 | 1.24 ± .20 |
| 75 | 1.55 ± .05 | 6.14 ± .69 | 2.21 ± .70 | 1.30 ± .09 |
| 100 | 1.90 ± .25 | 6.61 ± .21 | 2.39 ± 1.26 | 1.34 ± .02 |
| 125 | 2.29 ± .16 | 5.96 ± .38 | 6.46 ± 1.18 | 1.24 ± .07 |
| 150 | 2.82 ± .27 | 6.54 ± .42 | 6.30 ± .06 | 1.15 ± .10 |
| 175 | 4.05 ± .16 | 8.59 ± .82 | 5.56 ± .64 | 0.96 ± .08 |
| 200 | 3.77 ± .78 | 12.74 ± 2.76 | 12.4 ± 5.85 | 0.67 ± .18 |
| 250 | 2.66 ± .10 | 18.03 ± 1.37 | 12.0 ± 3.92 | 0.70 ± .16 |
Normal nutrient medium used for growth. Eight-day-old plants had NaCl added to the growth medium on d 0, and the experimental medium was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. The numbers are the mean of three leaf samples ± se.
In a concentration series of NaCl (Table I), 100 and 150 mm in the medium provided strong accumulation of glycinebetaine in the leaves, but the TBARS level did not begin to rise until 200 mm. The percentage of water deficit showed an intermediate concentration dependence. By these indicators, the signal from NaCl to form glycinebetaine may be somewhat more specific than that needed to cause the more general stress condition.
Other Inorganic Salts
KCl induced glycinebetaine with a concentration curve identical to that of NaCl (data not shown). There was no loss of induction due to NaCl when the K+ concentration was increased from 1.5 to 7.5 mm, and at higher levels the induction by KCl itself supplemented that by NaCl. LiCl and Na2SO4 were also effective, with minor differences in their concentration dependence compared with NaCl (data not shown). Thus the induction is not due to the specific cation or anion.
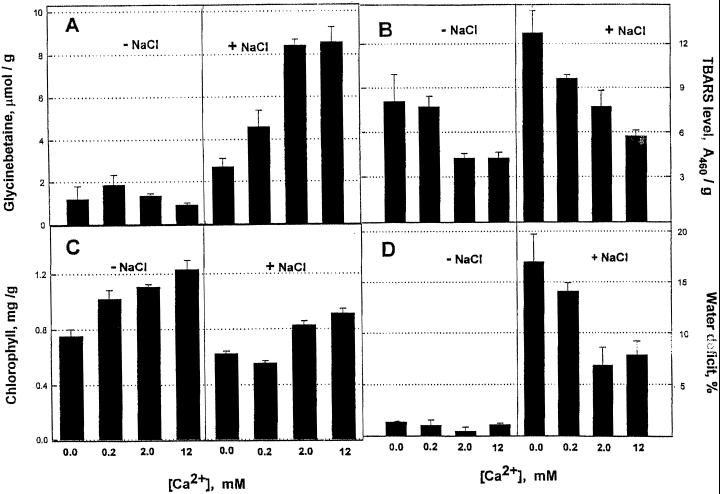
Ca2+ ions up to 10 or even 20 mm did not, in themselves, induce glycinebetaine. Figure 1 shows the effects of media with either 2 mm EGTA and divalent cations at 0.4 mm, i.e. with only a trace of Ca2+ in the medium, or no EGTA and CaCl2 added to 0.2, 2, or 12 mm. Clearly higher Ca2+ levels prevented water stress and chlorophyll loss, decreased the amount of TBARS, but increased the accumulation of glycinebetaine.
Figure 1.
NaCl stress effects at four levels of Ca2+ ions in the medium. NaCl was used at 225 mm. The “0” Ca2+ medium contained 0.2 mm each of Ca(NO3)2 and MgSO4 and 1.0 mm EGTA, with NaNO3 and Na2SO4 making up the sulfate and nitrate concentrations. Medium with 0.2 mm Ca(NO3)2 had 2.0 mm MgSO4; 2.0 mm was the normal concentration for Ca(NO3)2, and 12 mm was the same with addition of 10 mm CaCl2. All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. A, Glycinebetaine; B, TBARS; C, chlorophyll; and D, percent water deficit. Numbers are the mean of three samples ± se.
As reported earlier (Lauchli, 1990), Ca2+ imposes restraint on the uptake of Na+ and therefore maintains a more favorable K+ to Na+ ratio in the cell. We could confirm this in our barley leaves in which extra CaCl2 diminished the uptake of Na+ into the leaves (data not shown).
Lowering the Ca2+ in the medium from 2.0 down to 0.2 mm decreased glycinebetaine induction due to NaCl and KCl and possibly that due to ABA. However, other compounds able to induce glycinebetaine (see below) were not affected by lowering the Ca2+ concentration (Table II).
Table II.
Low (0.2 mm) vs. normal (2.0 mm) Ca2+ with eight inducers
| Inducer | Glycinebetainea 0.2 mm Ca2+ | Glycinebetaine 2.0 mm Ca2+ | Ratiob 0.2/2.0 mm Ca | ||
|---|---|---|---|---|---|
| μmol g−1 | |||||
| None | 0.90 | 1.14 | 0.82 | 1.17 | 1.02 |
| PEG | 4.60 | 4.42 | 4.12 | 4.48 | 1.04 |
| H2O2 | 2.86 | 2.62 | 2.68 | 2.49 | 0.93 |
| CuHP | 3.01 | 2.64 | 2.50 | 3.04 | 1.10 |
| ABA | 3.68 | 4.31 | 4.95 | 3.87 | 0.93 |
| Salicylate | 3.08 | 2.63 | 2.27 | 2.08 | 1.32 |
| MgCl2 | 4.62 | 5.25 | 1.40 | 1.64 | 3.22 |
| NaCl | 5.02 | 5.70 | 7.26 | 7.84 | 0.71 |
| KCl | 4.90 | 5.22 | 8.78 | 7.07 | 0.64 |
The concentrations were NaCl and KCl at 150 mm, MgCl2 at 15 mm, ABA at 50 μm, salicylate at 1.5 mm, CuHP at 0.5 mm, PEG at 15% (w/v), and H2O2 at 15 mM. All inducers were added to the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed.
Results with duplicate leaf samples shown.
Ratio, based on the average for the two determinations.
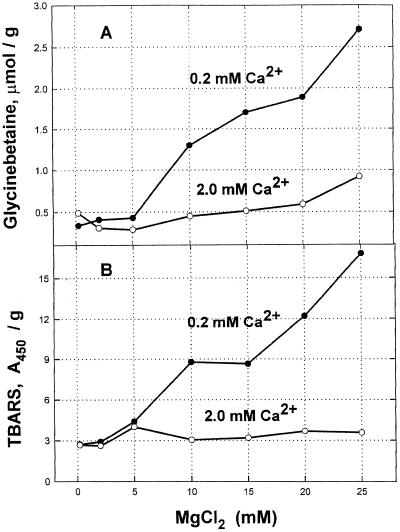
In testing whether the Ca2+ effect could be duplicated by higher Mg2+, we found that Mg ions are themselves very effective inducers of glycinebetaine. However induction by Mg2+ only occurs at very low Ca2+ levels, i.e. when the Mg to Ca ratio in the medium is 50:1 or more (Fig. 2). Unlike the case for Na+ and K+, induction of glycinebetaine by Mg2+ was closely correlated with a rise in TBARS. Thus there is no indication of a more specific receptor for Mg2+ ions in causing glycinebetaine induction.
Figure 2.
Glycinebetaine and TBARS rise caused by MgCl2 at 2 levels of Ca2+. Defined nutrient medium used, with NaNO3 substituted for Ca(NO3)2 and Na2SO4 for MgSO4 as needed. All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. A, Glycinebetaine; B, TBARS.
Compounds and Conditions Not Inducing Glycinebetaine
A number of potential growth or stress regulators and conditions were tested for induction of glycinebetaine or for an interaction with induction by NaCl. Of these, wounding (by rubbing part of the leaf or by puncture or partial laceration) had no effect. Gibberellin, methyl jasmonate, and brassinolide all had no effect on the observed induction process with NaCl, and had no effect by themselves (data not shown).
Effect of Oxidants
A number of stress conditions, high salt (Arakawa et al., 1990, 1992), cold (Kishitani et al., 1994), heat (T. Takabe, unpublished data), and drought by either withholding water (Hitz et al., 1982) or using polyethylene glycol (PEG; Arakawa et al., 1992), lead to induction of glycinebetaine. All of them will inhibit photosynthesis; and when photosynthesis is inhibited, excited-state chlorophylls cause the formation of reactive oxygen species (ROS). It was, therefore, of interest to test other, more direct producers of ROS for glycinebetaine induction. Of several potential donors tested, consistent induction effects were found with H2O2 and cumene hydroperoxide (CuHP; Table II, and below).
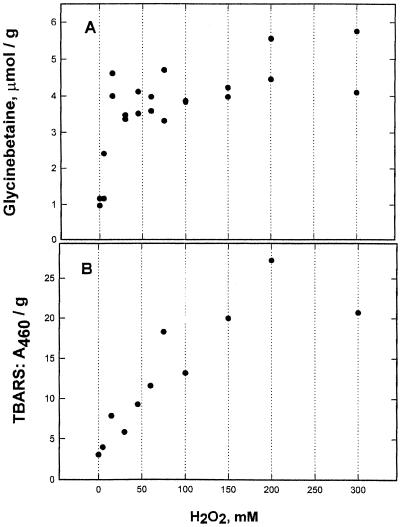
H2O2 has an observable effect at 5 mm and brings on almost maximal glycinebetaine accumulation at 25 mm (Fig. 3). Wilting and a higher percentage of water deficit did not occur until quite high concentrations (300–500 mm), where the leaf bases became white and completely lacking in turgor and the leaves fell over. A strong rise in TBARS also occurred, but only at concentrations higher than those bringing on the major amount of glycinebetaine.
Figure 3.
TBARS and glycinebetaine rise caused by H2O2. H2O2 concentrations calculated on the basis of 8.823 m for a 30% solution. All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. A, Glycinebetaine; B, TBARS from the same experiment. Numbers from replicate leaf samples are shown. In other experiments a maximum accumulation of glycinebetaine did not occur until 40 mm H2O2.
With H2O2, there is no need for 2 mm Ca2+ ions; as much induction occurs with 0.2 mm (Table II). The calcium chelator EGTA partially inhibited induction by all other inducers but consistently increased the induction caused by H2O2 (Table III and other data).
Table III.
EGTA effects with several inducers
| Inducer | Conc. | Glycinebetainea
|
Inducer Δ (− control)
|
EGTA effect | ||
|---|---|---|---|---|---|---|
| − EGTA | + EGTA | − EGTA | + EGTA | |||
| μmol g−1 | % | |||||
| None | 0.64 ± .06 | 1.02 ± .18 | – | – | ||
| PEG | 18% (w/v) | 3.90 ± .62 | 4.16 ± .84 | 3.26 | 3.14 | (−3.7)b |
| ABA | 50 μm | 3.04 ± .25 | 3.63 ± .17 | 2.40 | 2.16 | (−8.8)b |
| NaCl | 175 mm | 2.03 ± .46 | 1.70 ± .15 | 1.39 | 0.68 | −51 |
| CuHP | 0.5 mm | 3.37 ± .35 | 2.57 ± .07 | 2.73 | 1.55 | −43 |
| Salicylate | 5 mm | 1.65 ± .27 | 1.40 ± .69 | 1.01 | 0.38 | −62 |
| PmxB | 0.2 mg mL−1 | 2.70 ± .18 | 2.02 ± .08 | 2.06 | 1.00 | −51 |
| H2O2 | 30 mm | 1.64 ± .14 | 2.26 ± .17 | 1.00 | 1.24 | +24 |
EGTA was present at 1.0 mm where indicated. The growth medium to which inducers were added contained CaCl2 and MgCl2 at 0.2 mm each. The experimental media were changed on the 2nd d; leaves were harvested on d 4 and stored frozen until analyzed.
Average of three leaf samples ± se.
Not a significant difference.
The concentrations of H2O2 needed to induce glycinebetaine in the leaves were unphysiologically high, probably because most of it would be destroyed by catalase and other detoxifying enzymes in barley leaves. The roots, however, were white and limp with peroxide in the medium and did not show any induction of glycinebetaine. This was likely to be a consequence of the toxicity of H2O2, because roots did have increases in glycinebetaine content when exposed to NaCl, PEG, or ABA (data not shown).
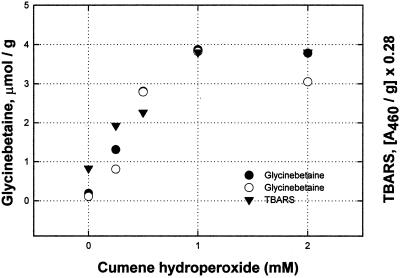
Another indication of oxidative stress was found in induction caused by CuHP (Fig. 4), which is effective at 0.25 to 1 mm, much below the concentrations needed with H2O2. Here, a rise in TBARS came at the same concentrations as those inducing glycinebetaine.
Figure 4.
Circles are for glycinebetaine (micromoles per gram) increase over the control level with no CuHP; triangles are for the increase in TBARS, normalized (multiplied by 0.28 to fit the same scale as glycinebetaine). All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed.
Other Compounds
ABA, previously shown to induce gene expression of BADH in barley (Ishitani et al., 1995), was found here to cause accumulation of the metabolic product, glycinebetaine. Compared with the other inducers studied here, it is unique in bringing on the induction without causing a rise in TBARS or wilting or an increase in the percentage of water deficit (Table IV).
Table IV.
Induction of glycinebetaine—without TBARS rise or loss of chlorophyll (ABA); without wilting (ABA, H2O2)
| Inducer | Glycinebetaine | TBARS | Water Deficit | Chlorophyll | ||||
|---|---|---|---|---|---|---|---|---|
| μmol g−1 | Δ | A450 g−1 | Δ | % | Δ | mg g−1 | % loss | |
| None | 0.72 ± .02 | 5.1 ± 0.2 | 1.4 ± .6 | 1.64 ± .08 | ||||
| NaCl | 2.61 ± .26 | 1.89 | 12.7 ± 0.2 | 7.6 | 17.7 ± .9 | 16.3 | 1.02 ± .06 | 38 |
| H2O2 50 mm | 2.74 ± .09 | 2.02 | 22.8 ± 2.2 | 17.7 | 1.0 ± .7 | – | 1.15 ± .04 | 30 |
| ABA | 2.68 ± .04 | 1.96 | 6.6 ± 0.7 | 1.5 | 0.5 ± .3 | – | 1.44 ± .04 | 12 |
All inducers were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. All numbers shown are the average of three samples ± se.
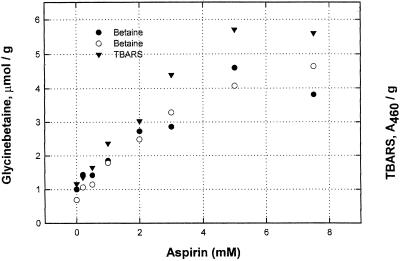
Salicylic acid and acetyl salicylic acid (aspirin) induced glycinebetaine accumulation in the range of 0.5 to 2.5 mm. Although Figure 5 shows data using aspirin, results with sodium salicylate were essentially identical. They both are active, producing maximum accumulation between 3 and 5 mm.
Figure 5.
Aspirin induces glycinebetaine accumulation and increases TBARS level at the same concentrations. Aspirin concentrations as shown. All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. Leaves harvested after 4 d. Circles, Glycinebetaine; triangles, TBARS level.
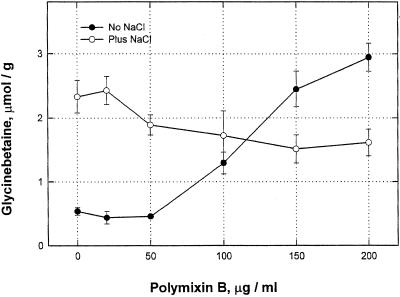
Polymixin B (PmxB), an inhibitor of protein kinase C (Schatzman et al., 1984), was a strong inducer of glycinebetaine at 0.20 mg/mL (Fig. 6). Its interaction with NaCl was unexpected; the two components seemed mutually antagonistic. At 0.05 mg/mL it decreased glycinebetaine induction by 150 mm NaCl, and the NaCl partially inhibited the PmxB effect. Trifluoperazine, a calmodulin antagonist, had essentially no effect (data not shown). n-Butanol at 5, 10, and 15 mm induced glycinebetaine accumulation, but neither i- nor t-butanols did so at the same concentrations (Table V).
Figure 6.
Induction by PmxB, and interaction with NaCl. NaCl used at 225 mm; PmxB as shown. All reagents were used in the growth medium, which was changed on d 2. Leaves were harvested on d 4 and stored frozen until analyzed. PmxB was added 1 d before the NaCl. Numbers are the mean of three samples ± se.
Table V.
Induction of glycinebetaine by n-butanol
| Conc. | n-Butanol | i-Butanol | t-Butanol |
|---|---|---|---|
| mm | μmol glycinebetaine/g | ||
| 0 | 0.69 ± .14 | 0.69 ± .14 | 0.69 ± .14 |
| 5 | 1.94 ± .14 | 0.48 ± .09 | 0.37 ± .04 |
| 10 | 4.20 ± .33 | 0.68 ± .04 | 0.43 ± .08 |
| 15 | 3.01 ± .33 | 0.65 ± .13 | 0.49 ± .07 |
The growth medium was used with the addition of PPM at 1:1,000. The media containing indicated butanols were changed on d 2; leaves harvested on d 4. Numbers are the mean of three samples ± se.
Requirement for Light
When plants were grown in the light but placed into complete darkness (except for 45 s per day while changing the medium), a much lower, but still significant, induction of glycinebetaine occurred due to NaCl (Table VI). The increase in glycinebetaine in the dark needed 2 mm Suc in the medium, made possible by use of the bacteriostatic plant protective medium (PPM) and with the medium changed daily. The requirement of light for optimal accumulation probably reflects the need for photosynthesis. In earlier experiments (before the use of Suc and PPM), 10 min of light twice a day did not lead to induction. Also red light was as effective as white light.
Table VI.
Induction of glycinebetaine synthesis in the dark
| Condition | Sucrose | Leavesa
|
NaCl Δ | Rootsb
|
NaCl Δ | ||
|---|---|---|---|---|---|---|---|
| − NaCl | + NaCl | − NaCl | + NaCl | ||||
| μmol glycinebetaine g−1 | % | μmol glycinebetaine g−1 | % | ||||
| Light | 0 | 0.62 ± .11 | 3.09 ± .32 | 398 | .38 ± .03 | .87 ± .07 | 129 |
| 2 mm | 0.80 ± .12 | 3.36 ± .42 | 320 | .21 ± .06 | .49 ± .07 | 133 | |
| Dark | 0 | 0.97 ± .11 | 1.19 ± .11 | 23 | .17 ± .02 | .39 ± .03 | 129 |
| 2 mm | 0.70 ± .25 | 1.81 ± .20 | 158 | .14 ± .002 | .39 ± .06 | 178 | |
| 10 mm | 0.79 ± .08 | 1.53 ± .18 | 94 | .17 ± .02 | .31 ± .03 | 182 | |
| 20 mm | 0.81 ± .02 | 1.64 ± .23 | 102 | .10 ± .015 | .22 ± .002 | 120 | |
The NaCl was used at 150 mm. All reagents were used in the growth medium, which was changed daily. Leaves were harvested on d 4 and stored frozen until analyzed. For dark treatment, plants were kept under an aluminum foil-covered box in the growth chamber, except for exposure to room light for approximately 45 s per day as the medium was changed.
Average of three leaf samples ± se.
Average of two root samples ± se.
DISCUSSION
Our results dissociate the system inducing glycinebetaine from some of the factors known to cause plant stress symptoms. For instance, a major factor in damage by Na+ ions is thought to be their antagonism versus K+ (Epstein, 1998). However, KCl is just as effective in NaCl in causing glycinebetaine induction. Also, increasing the K+ to Na+ ratio in the medium did not diminish induction of glycinebetaine, so it does not seem likely to be the controlling factor here. As induction was brought on by Na2SO4 and LiCl as well, osmotic or ionic strength must be the route by which salts exert their effect.
Other evidence (Lauchli, 1990) connected onset of stress symptoms in the plant with loss of Ca2+ from external sites on the roots. This was probably a major cause for the increase in TBARS level, water deficit, and loss of chlorophyll, where adding extra Ca2+ to the medium diminished or prevented these stress symptoms (Fig. 1). However loss of Ca2+ from the roots could not be the signal for induction of glycinebetaine synthesis, because adding extra Ca2+ to the medium never diminished glycinebetaine accumulation and sometimes even increased it. Also, addition of EGTA to chelate external Ca2+ decreased glycinebetaine accumulation (Table III).
It was shown previously that NaCl stress effects are decreased by high levels of Ca2+ ions in the soil or growth medium. This has been considered to be, at least in part, due to a favorable change in the K+ to Na+ uptake ratio (Lauchli, 1990; Liu and Zhu, 1997; Epstein, 1998). By contrast, glycinebetaine accumulation due to either Na+ or K+ was greater with 2 mm than with 0.2 mm Ca2+, although this was not true for other inducers (Table II). However, the total absence of Ca2+ due to adding EGTA to the medium (Table III) partially inhibited the effectiveness of all inducers except for ABA and PEG (whose water stress would cause internal production of ABA).
Cytosolic Ca2+ plays a significant role in most known plant signal transduction pathways (Reddy, 2001). A striking example with salt stress is the finding that the sequence of the SOS3 gene from Arabidopsis encodes a protein with strong homology to the yeast calcineurin regulatory subunit. Mutations in this gene cause hypersensitivity to NaCl and LiCl, but increasing Ca2+ to millimolar levels suppressed the mutant phenotype (Liu and Zhu, 1997, 1998).
Induction by Mg2+ ions is unique among those tested in requiring a low Ca2+ level in the medium (Fig. 2). It seemed to be inducing a general stress response, representing a confirmation of the well-known requirement for a balance of cations in plant nutrition. It is also likely that the 50- to 100-fold excess of Mg2+ in the medium caused a significant release of Ca2+ from the roots.
Major unsolved questions about high salt induction of glycinebetaine are the nature of the initial receptor and steps in the signal transduction pathway. The work reported here consists entirely of observations on the physiology of the induction. At this level the mechanisms in signal perception and transduction cannot be solved; however, our report may help point at significant areas to be investigated at a deeper level. We would also like to point out that none of the compounds we tested can be direct inducers of the genes for glycinebetaine synthesis. It is certain that different ones will affect the system at different points, and it is very likely that interacting pathways are involved.
ABA, which induces the biosynthesis of BADH (Ishitani et al., 1995) and of glycinebetaine itself (Tables II–IV), is probably a significant and late step in the signal transduction pathway. Both NaCl and dehydration stresses are known to cause a rise in the plants' ABA levels. Its closeness to the final step is supported by the fact that induction by ABA is not accompanied by induction of the other stress symptoms (TBARS rise, chlorophyll loss, wilting, and water deficit; Table IV) and by the lack of sensitivity to EGTA. However, in molecular studies of osmotic stress, both ABA-sensitive and -insensitive pathways have been found (Shinozaki and Yamaguchi-Shinozaki, 1997). Thus, it is not certain whether all of the conditions inducing glycinebetaine do so through ABA. Critical evidence for ABA as a part of the system for glycinebetaine synthesis by salt or the other compounds needs a study using mutants that either do not make or are not sensitive to ABA. It is unfortunate that no ABA-deficient or -insensitive mutants in barley are available as far as we know.
In an experiment using fluridone, an inhibitor of carotene synthesis and therefore of the formation of ABA, seedlings were grown in the dark (with 2 mm Suc) to avoid death from photodamage. The induction of a small amount of glycinebetaine under those conditions was not inhibited by fluridone at 3 μm (data not shown). It suggests that at least some of the induction caused by NaCl goes through a pathway not including ABA.
The induction of glycinebetaine by PmxB (Fig. 6) suggests the involvement of the (Ca2+-dependent, phospholipid-sensitive) protein kinase C (Schatzman et al., 1984). This effect would be consistent with a phosphorylated protein acting as a negative regulator of the induction, becoming inactive if it is not continually rephosphorylated (Sheen, 1996; D'Agostino and Kieber, 1999).
An effect of n-butanol not shared by i- or t-butanol is considered to show the involvement of phospholipase D in the signal pathway (Tobias et al., 1999). The specific induction of glycinebetaine by n-butanol (Table V) suggests the need for a particular phospholipid in induction, or a breakdown product as a negative regulatory element.
In spinach, the first step in glycinebetaine synthesis (oxidation of choline to betaine aldehyde) requires electrons from photosystem I (Brouquisse et al., 1989; Rathinasabapathi et al., 1997). Accumulation of some glycinebetaine in barley in the dark (Table VI) means that at least a part of the pathway, and possibly all of it, is not located inside chloroplasts. This would be consistent with the finding that the major barley BADH appears to be located in the cytosol (Nakamura et al., 1998, 2001).
Most stress conditions will inhibit photosynthesis, and when photosynthesis is inhibited, ROS are produced within chloroplasts (Asada, 1994; Moran et al., 1994; Halliwell and Gutteridge, 1999). For this reason, it seemed worthwhile to look for induction by oxidative stress. In a few experiments 3-(3,4-dichlorophenyl)-1,1-dimethylurea did induce glycinebetaine (data not shown); in others it did not at all. Similar variability was found in induction by methylviologen. It may be that induction by these photosynthesis inhibitors is subject to a delicate balance between an oxidative stress effect and the strong need for active photosynthesis (above).
However H2O2 at 5 to 300 mm (Fig. 3) and CuHP at 0.2 to 1.0 mm (Fig. 4) were consistent inducers of glycinebetaine. H2O2 has been found recently to be a possible signal for disease resistance gene induction in plants (Van Camp et al., 1998). It is possible that it serves in part of the signal pathway for glycinebetaine synthesis as well.
The effectiveness of salicylate and acetyl salicylate (aspirin) in induction (Fig. 5) is intriguing. Salicylate is a signal molecule for induction of systematic acquired resistance in part by binding to and inhibiting catalase, thereby causing increased levels of H202 in the cell (Chen et al., 1993). This could be an important part of the reason for its induction of glycinebetaine in barley. It has other effects as well; a recent report describes its induction of one and activation of another protein kinase when aroused by hyperosmotic stress (Hoyos and Zhang, 2000).
Although H2O2 caused large increases in the leaf TBARS level, its major inductive effect occurred at concentrations where only a small TBARS effect occurred (Fig. 3). This was also true for NaCl and the other monovalent inorganic salts (Table I). These concentration discrepancies and the effects of Ca2+ ions indicate that the mechanism(s) for inducing glycinebetaine differs from those causing other major stress symptoms. A suggestively similar finding was that the sos3 mutant in Arabidopsis made the plants hypersensitive to low concentrations of NaCl and LiCl (although not to KCl) but did not affect their sensitivity to osmotic stress due to mannitol or KCl (Liu and Zhu, 1997). Similarly, a distinction was found between salt and hydraulic (osmotic) effects in the induction of BADH mRNA in sugar beets (McCue and Hanson, 1992). With other inducers—PmxB, salicylates, PEG, and Mg2+—the induction of glycinebetaine and rise in TBARS occur at the same concentrations.
High NaCl concentrations have been shown to induce expression of a large number of genes (Shinozaki and Yamaguchi-Shinozaki, 1997). It may be useful to check their induction at different concentrations of salt to see which genes are similar to that for BADH in being activated at the lower, more specific salt level and which ones are only part of the general stress syndrome.
MATERIALS AND METHODS
Barley (Hordeum vulgare cv Haruna nijo) seeds were grown in unstirred hydroponic culture on top of one of three kinds of containers: either 1-L or 420-mL capacity plastic boxes or 50-mL conical bottom centrifuge tubes. Seeds and vermiculite were supported on either cheesecloth or a plastic mesh. The growth medium was a slightly modified version of that used by Haughn and Somerville (1986). The final composition included 2.0 mm Ca(NO3)2, 2 mm MgCl2, 2.5 mm sodium phosphate (pH 6), 4.5 mm KNO3, 0.5 mm NH4Cl, 0.05 mm FeEDTA, and micronutrients as described (Haughn and Somerville, 1986).
When an organic compound that could support the growth of bacteria (such as Suc or salicylate) was used, the medium included a 1:1,000 dilution of PPM, a bacteriostatic preparation (Plant Cell Technology, Inc., Washington, DC). Tests had shown no adverse effects of PPM on glycinebetaine induction with any of the several materials tested. Plants were grown for 7 to 8 d after seed imbibition and then exposed to the stress condition for 4 d. The stress medium was routinely changed on the 2nd d. When harvested, the fresh weight of leaves was measured. They were then immersed in de-ionized water for 3 to 5 h and blotted dry, and turgid weight was measured. The difference in weight divided by the turgid weight is reported as percent water deficit. All measurements (glycinebetaine, chlorophyll, TBARS, or Na+ content) are reported per gram turgid weight.
Preparation of leaf samples for glycinebetaine measurement went through several variations from the previously reported one (Arakawa et al., 1990). The method finally adopted consisted of chilling with liquid nitrogen approximately 0.5 g of frozen leaf sample in a centrifuge tube and then crumbling the leaves to small pieces with the help of a glass stirring rod with a button end and a spatula. Extraction proceeded overnight using 1.75 mL of 0.5 m H2SO4. The extracted samples were passed through a fiberglass filter, rinsed with two 0.5-mL portions of fresh H2SO4, and squeezed dry with the top end of a plastic syringe plunger. The clear fluid was collected in a 15-mL graduated, conical bottom plastic centrifuge tube. The volume was measured, and a 1.4-mL aliquot was transferred to a 2-mL Eppendorf tube to which 0.58 mL of I2-KI solution (1.2 m of each) was added. After 2 or more h at 0°C, the precipitated complexes of iodine with quaternary amines and other components were collected by centrifuging at 4°C. The supernatant solution was aspirated off and discarded, and the precipitates were dried for 1 h in a heating block at 65°C with a fan blowing air over their tops. The precipitates were resuspended in 200 μL of 2H2O and evaporated for 5 h at 65°C. The final precipitates were resuspended in 700 μL of 2H2O with 0.5 mm t-butanol as internal standard. After removing precipitates by centrifugation, the supernatants were transferred to tubes for measurement by integration of peak areas produced in a 500-mHz NMR machine.
For measurement of leaf chlorophyll, crumbled frozen leaves were extracted overnight with 100% methanol, and absorbance was measured at 652 and 665 nm (Porra et al., 1989).
Crumbled frozen leaves were extracted with 2 mL of 10% (w/v) trichloroacetic acid, 0.01% (w/v) butylated hydroxytoluene for measurement of lipid breakdown products with thiobarbituric acid (Moran et al., 1994). Aliquots (0.4 mL) were transferred to 12- × 75-mm glass tubes, and 0.4 mL of 0.5% (w/v) thiobarbituric acid in 10% (w/v) trichloroacetic acid was added and heated at 95°C for 30 min. After cooling, the solutions were extracted with 1.0 mL of n-butanol, and optical density was measured. The butanol extracts were perfectly clear, and there was no need to subtract the A600 to correct for “turbidity.” With stressed barley leaves, the peak at 532 nm due to the typical lipid breakdown product, malondialdehyde, was much smaller than major peaks (indicating unknown compounds) at 440 and 460 nm. It is interesting to note that with leaves stressed in darkness, only the 460-nm peak could be seen. These TBARS overwhelmed the 532-nm peak, which therefore could not be relied on. However, they did show a good correlation with increasing stress conditions for many of the inducers.
Sodium ions in the leaves were measured in an acid extract, using either a liquid ion chromatographic HPLC system or an inductively coupled argon plasma apparatus (Shimadzu, Columbia, MD).
ACKNOWLEDGMENT
We are grateful to Michael Rutzke at Cornell University for measurements of sodium ion with the ICP machine.
Footnotes
This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to T.T.), and by the Liberty Hyde Bailey Professorship (to A.T.J.) The faculty and chairman of the Bioscience Center, Nagoya University provided A.T.J. with a Visiting Research Fellowship during which time this work was initiated.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010392.
LITERATURE CITED
- Andresen PA, Kaasen I, Styrvold OB, Boulnois G, Strom AR. Molecular cloning, physical mapping and expression of bet genes governing the osmoregulatory choline-betaine pathway of Escherichia coli. J Gen Microbiol. 1988;134:1737–1746. doi: 10.1099/00221287-134-6-1737. [DOI] [PubMed] [Google Scholar]
- Arakawa K, Katayama M, Takabe T. Levels of glycinebetaine and glycinebetaine aldehyde dehydrogenase activity in the green leaves, and etiolated leaves and roots of barley. Plant Cell Physiol. 1990;31:797–803. [Google Scholar]
- Arakawa K, Mizuno K, Kishitani S, Takabe T. Immunological studies of betaine aldehyde dehydrogenase of barley. Plant Cell Physiol. 1992;33:833–840. [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense System in Plants. Orlando, FL: CRC Press; 1994. , pp77–104. [Google Scholar]
- Brouquisse R, Weigel P, Rhodes D, Yocum CF, Hanson AD. Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma. Plant Physiol. 1989;90:322–329. doi: 10.1104/pp.90.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- D'Agostino IB, Kieber JJ. Phosphorelay signal transduction: the emerging family of plant response regulators. TIBS. 1999;24:452–456. doi: 10.1016/s0968-0004(99)01465-6. [DOI] [PubMed] [Google Scholar]
- Epstein E. How calcium enhances plant salt tolerance. Science. 1998;280:1906–1907. doi: 10.1126/science.280.5371.1906. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. UK: Oxford University Press; 1999. [Google Scholar]
- Haughn GW, Somerville C. Sulfonyl-resistant mutant of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Hayashi H, Alia L, Mustardy L, Deshnium P, Ida M, Murata N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997;12:133–142. doi: 10.1046/j.1365-313x.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- Hoyos ME, Zhang S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000;122:1355–1363. doi: 10.1104/pp.122.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz WD, Ladyman JAR, Hanson AD. Betaine synthesis and accumulation in barley during field water stress. Crop Sci. 1982;22:47–54. [Google Scholar]
- Ishitani M, Nakamura T, Han SY, Takabe T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol Biol. 1995;27:307–315. doi: 10.1007/BF00020185. [DOI] [PubMed] [Google Scholar]
- Kishitani S, Watanabe K, Yasuda S, Arakawa K, Takabe T. Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Environ. 1994;17:89–95. [Google Scholar]
- Lauchli A. Calcium, salinity and the plasma membrane. In: Leonard RT, Hepler PK, editors. Calcium in Plant Growth and Development. The American Society of Plant Physiologists Symposium Series. Vol. 4. Rockville, MD: The American Society of Plant Physiologists; 1990. pp. 26–35. [Google Scholar]
- Liu J, Zhu JK. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA. 1997;94:14960–14964. doi: 10.1073/pnas.94.26.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu JK. A calcineurin sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- McCue KF, Hanson AD. Effects of soil salinity on the expression of betaine aldehyde dehydrogenase in leaves: investigation of hydraulic ionic and biochemical signals. Aust J Plant Physiol. 1992;19:555–564. [Google Scholar]
- Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta. 1994;194:346–352. [Google Scholar]
- Nakamura T, Nomura M, Ku MSB, Takabe T. Isolation of a barley gene encoding betaine aldehyde dehydrogenase that is not localized in microbody. In: Garab JG, editor. Photosynthesis: Mechanisms and Effects. Vol. 4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 3031–3034. [Google Scholar]
- Nakamura T, Nomura M, Mori H, Jagendorf AT, Ueda A, Takabe T. An isozyme of betaine aldehyde dehydrogenase in barley. Plant Cell Physiol. 2001;42:1088–1092. doi: 10.1093/pcp/pce136. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, Takabe T. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. Plant J. 1997;11:1115–1120. doi: 10.1046/j.1365-313x.1997.11051115.x. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Rathinasabapathi B, Burnet M, Russel BL, Gate DA, Liao PC, Nye GJ, Scott P, Golbeck JM, Hanson AD. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycinebetaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci USA. 1997;94:3454–3458. doi: 10.1073/pnas.94.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN. Calcium: silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Rhodes D, Hanson AD. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- Schatzman RC, Turner RF, Kuo JF. Phospholipid-sensitive Ca2+-dependent protein phosphorylation. In: Cheng WY, editor. Calcium and Cell Function. Vol. 5. New York: Academic Press; 1984. pp. 33–66. [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias J, Ritchie S, Assmann SM, Gilroy S. Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Van Montagu M, Inze D. H202 and NO: redox signals in disease resistance. Trends Plant Sci. 1998;3:330–334. [Google Scholar]