Abstract
Al toxicity is a severe impediment to production of many crops in acid soil. Toxicity can be reduced through lime application to raise soil pH, however this amendment does not remedy subsoil acidity, and liming may not always be practical or cost-effective. Addition of organic acids to plant nutrient solutions alleviates phytotoxic Al effects, presumably by chelating Al and rendering it less toxic. In an effort to increase organic acid secretion and thereby enhance Al tolerance in alfalfa (Medicago sativa), we produced transgenic plants using nodule-enhanced forms of malate dehydrogenase and phosphoenolpyruvate carboxylase cDNAs under the control of the constitutive cauliflower mosaic virus 35S promoter. We report that a 1.6-fold increase in malate dehydrogenase enzyme specific activity in root tips of selected transgenic alfalfa led to a 4.2-fold increase in root concentration as well as a 7.1-fold increase in root exudation of citrate, oxalate, malate, succinate, and acetate compared with untransformed control alfalfa plants. Overexpression of phosphoenolpyruvate carboxylase enzyme specific activity in transgenic alfalfa did not result in increased root exudation of organic acids. The degree of Al tolerance by transformed plants in hydroponic solutions and in naturally acid soil corresponded with their patterns of organic acid exudation and supports the concept that enhancing organic acid synthesis in plants may be an effective strategy to cope with soil acidity and Al toxicity.
Al makes up about 7% of the Earth's crust (Delhazie and Ryan, 1995). The solubility of Al in neutral and alkaline soils is too low to be toxic to plants, but in acid soils, which make up about 40% of the world's arable land, Al becomes soluble and readily enters roots where it inhibits root growth and development (Delhazie and Ryan, 1995; Kochian, 1995). As a result, the root system becomes inefficient in water and nutrient uptake, leading to lower crop yields (Delhazie and Ryan, 1995; Kochian, 1995). A common agricultural practice for acid soils is to apply lime to raise soil pH. However, farmers in developing countries frequently have neither access to the infrastructure required nor capital available to remedy acid soil and Al toxicity via lime application (Devine et al., 1990). In addition, liming acid soils does not remedy acidity in the subsoil layer. An alternative is to tailor plants to suit acid soils by identifying and/or developing plants with improved tolerance to Al in acid soils.
Al-tolerant maize and snapbean genotypes release more citrate into the rhizosphere in response to Al, whereas Al-tolerant wheat genotypes release more malate and succinate in response to Al (Miyasaka et al., 1991; Delhazie and Ryan, 1995; Pellet et al., 1996). The amount of organic acid exudation depends on both the amount of Al in the growth medium and the duration of exposure (Delhazie and Ryan, 1995). The main organic acids known to effectively detoxify Al are citrate, oxalate, and tartarate followed by malate, malonate, succinate, and acetate (Hue et al., 1986; Ginting et al., 1998). In research to increase organic acid production in plants, a citrate synthase gene from Pseudomonas aeruginosa controlled by the cauliflower mosaic virus (CaMV) 35S promoter was used to produce transgenic tobacco (Nicotiana tabacum) and papaya (Carica papaya) (de la Fuente et al., 1997). These plants were reported to have an increased internal citrate concentration, secrete up to 4-fold more citrate than control plants, and have enhanced Al tolerance. However, a recent study has shown that the increased citrate synthase activity in those same transgenic tobacco plants and in additional transgenic tobacco and alfalfa (Medicago sativa) did not correlate with increased citrate production nor was Al tolerance enhanced in transgenic plants (Delhazie et al., 2001). In the present study we explored the possibility that modification of expression of specific plant genes controlling other steps in organic acid biosynthesis might increase organic acid synthesis and Al tolerance in alfalfa.
Malate is a key metabolite in plants. It is involved in numerous processes, including C4 and Crassulacean acid metabolism photosynthesis, stomatal and pulvinual movement, nutrient uptake, respiration, nitrogen assimilation, fatty acid oxidation, and providing energy to bacteroids in root nodules (Vance and Heichel, 1991; Vance, 1997; Miller et al., 1998). Malate is synthesized via the concerted action of phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31), which catalyzes conversion of phosphoenolpyruvate to oxaloacetate, and by malate dehydrogenase (MDH; EC 1.1.1.37), which causes oxidation of oxaloacetate to form malate (Vance, 1997). Plants contain several isoforms of MDH (Miller et al., 1998) and PEPC (Chollet et al., 1996), and their expression is dependent upon function and tissue (Vance et al., 1994; Vance, 1997).
Legume root nodules contain specific forms of MDH and PEPC genes that are expressed 5- to 15-fold higher than in other tissues (Vance et al., 1994; Miller et al., 1998). These non-photosynthetic isoforms play a crucial role in providing the large amount of malate required by bacteroids to fix N2 and by the plant for assimilation of N2 (Vance, 1997). A particularly novel form of MDH (noduleenhanced; neMDH) has been isolated from alfalfa nodules (Miller et al., 1998). This isoform has unusual kinetic properties that drive the reaction toward the production of large quantities of malate in nodules (Miller et al., 1998). We hypothesized that over-expression of neMDH and PEPC would result in increased organic acid biosynthesis and Al tolerance. Thus, our objectives were to constitutively over-express neMDH and PEPC enzymes in alfalfa using the CaMV 35S as a promoter, to test whether such transgenic plants had enhanced malate synthesis and excretion, and to evaluate whether selected transgenic plants had increased Al tolerance.
RESULTS
Transgenic Alfalfa with Increased Expression of neMDH or PEPC
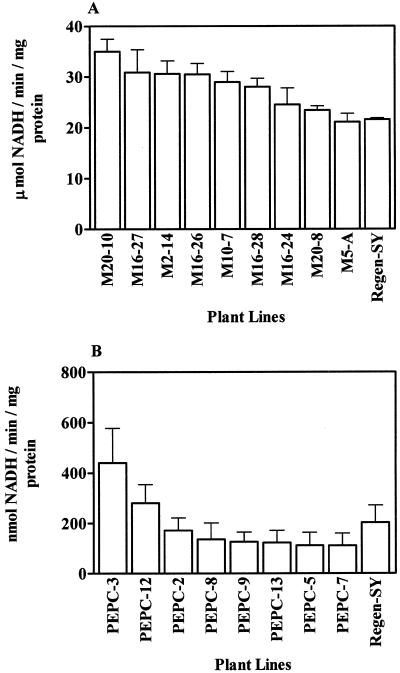
We first analyzed enzyme specific activities in root tips of transgenic plants in comparison with the untransformed parent (M. sativa cv Regen-SY). As shown in Figure 1, nine transgenic lines with the 35S::neMDH transgene showed up to a 1.6-fold increase in MDH specific activity, ranging from 23.4 to 35.0 μmol of NADH min−1 mg−1 protein (Fig. 1A). Six neMDH transgenic lines (M20-10, M16-27, M2-14, M16-26, M10-7, and M16-28) had significantly higher enzyme activity levels than the untransformed control (P < 0.05). By contrast, two transgenic lines with the 35S::PEPC transgene showed an increase in PEPC-specific activity (Fig. 1B), and only PEPC-3 had significantly higher enzyme activity than untransformed alfalfa cv Regen-SY (P < 0.05).
Figure 1.
Enzyme specific activities from root-tips of transgenic and untransformed alfalfa lines. A, MDH-specific activity; B, PEPC-specific activity. Bars = means + se (n = 5).
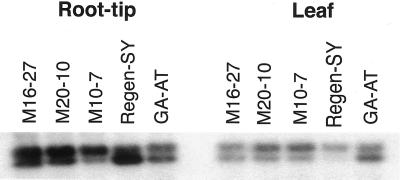
Transcript levels of neMDH and PEPC mRNA were analyzed in selected transgenic and alfalfa cv Regen-SY plants by quantitative reverse transcription (RT)-PCR as well as RNA blotting using neMDH cDNA as a probe. The neMDH cDNA probe specifically hybridizes to neMDH mRNA (S. Miller, personal communication). In addition, plants of an alfalfa germplasm, GA-AT, previously selected for acid soil tolerance (Bouton, 1996), were analyzed for comparison. The RT-PCR results were similar to northern-blot results (data not shown). Root tips of transgenic plants transformed with the 35S::neMDH transgene showed up to a 4.1-fold increase in neMDH transcript accumulation compared with root-tips of untransformed alfalfa cv Regen-SY plants (Fig. 2). Leaf samples of transgenic plants showed up to 60% reduction in the amount of neMDH transcripts compared with untransformed alfalfa cv Regen-SY plants. GA-AT plants also showed a 1.8-fold greater neMDH transcript accumulation than alfalfa cv Regen-SY in root-tips but not in leaf samples. In contrast, there was no detectable change in PEPC transcript levels from root-tip samples between PEPC transformants and untransformed plants (data not shown). Variation in the amounts of neMDH and PEPC transcripts was observed between different transformants, and this variation appeared to reflect differences in enzyme specific activities.
Figure 2.
Relative RT-PCR analysis of neMDH mRNA expression in selected transgenic and untransformed alfalfa lines. Relative expression of neMDH mRNA (upper band) was estimated after normalization with signals from the 18S transcript levels (lower band) used as an endogenous control in a multiplex RT-PCR reaction. Hybridization signals were analyzed using a Storm 840 PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
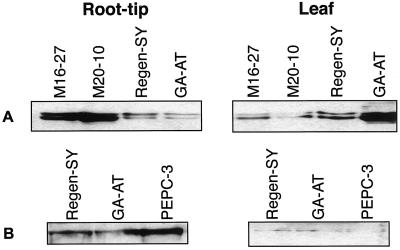
Monospecific antisera against alfalfa neMDH and PEPC were used to analyze expression of these proteins in transgenic, alfalfa cv Regen-SY, and GA-AT plants by western blotting. Using 10% (w/v) SDS- polyacrylamide gels, antisera to neMDH recognizes two bands of approximately 34 kD (S. Miller and C.P. Vance, unpublished data). Amounts of neMDH polypeptides in root-tip samples of selected neMDH transgenic lines were 4.2- to 4.7-fold greater than in root tips of the untransformed lines (alfalfa cv Regen-SY and GA-AT; Fig. 3A). At comparable total soluble protein loadings on SDS-PAGE, neMDH proteins were 6.7-fold higher in root tips of transgenic plants than in leaf samples (Fig. 3A). Consistent with the mRNA expression pattern, neMDH proteins in leaf samples of transgenic plants were reduced to about 50% of the amount in alfalfa cv Regen-SY. Compared with the amount of alfalfa cv Regen-SY protein, neMDH protein expression in GA-AT plants was reduced in root-tip samples but greater by 2.7-fold in leaf samples. The amount of PEPC protein in root-tip samples of PEPC-3 plants was 1.5-fold greater than amounts in control root tips (Fig. 3B). Also, the amount of PEPC polypeptide was elevated in root tips of PEPC-3 when compared with leaf samples (Fig. 3B).
Figure 3.
Immunoblot analysis of selected transgenic and untransformed alfalfa lines. Total soluble protein (10 μg/lane) from root-tips and leaf samples was separated by SDS-PAGE, blotted, and probed with neMDH (A) and PEPC (B) monospecific antibodies.
Over-Expression of neMDH or PEPC in Alfalfa Leads to Altered Organic Acid Production
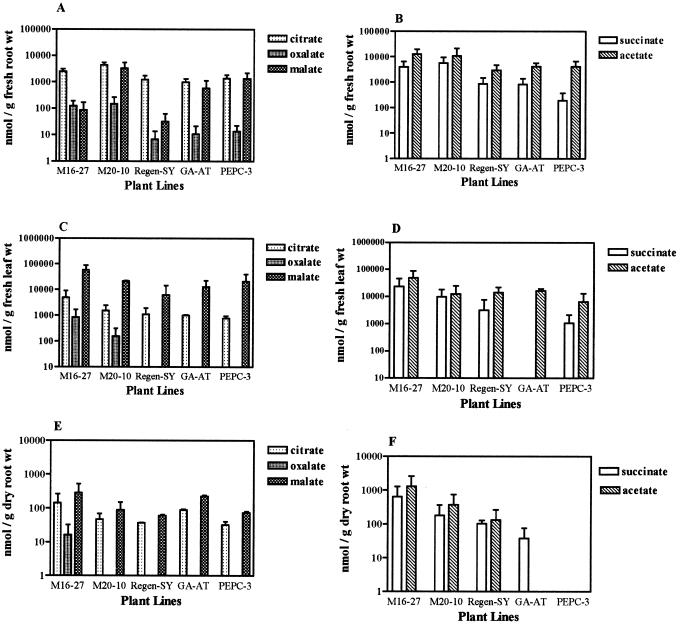
Organic acid content in roots and leaves and exudation of organic acids from roots into the rhizosphere by selected transgenic plants were compared with that of alfalfa cv Regen-SY and GA-AT plants using HPLC. Low amounts of malate were detected in root samples of M16-27 and alfalfa cv Regen-SY plants, although root tissues from line M20-10 showed considerably higher concentration of malate (Fig. 4A). Both transgenic lines evaluated (M16-27 and M20-10) containing the 35S::neMDH transgene, showed significantly elevated levels of citrate, oxalate, succinate, and acetate in root tissues compared with alfalfa cv Regen-SY plants (Fig. 4, A and B). Leaves from plants containing the 35S::neMDH transgene also showed increased organic acid concentrations compared with leaves from alfalfa cv Regen-SY and GA-AT plants (Fig. 4, C and D). Malate accumulation in leaves of M16-27 and M20-10 was 4- to 10-fold greater compared with alfalfa cv Regen-SY and GA-AT plants (Fig. 4C). Citrate and oxalate concentrations in root and leaf tissues of PEPC-3 plants were not significantly different from root and leaf concentrations in alfalfa cv Regen-SY and GA-AT plants (Fig. 4, A and C). Succinate was depressed in root samples of PEPC-3 when compared with alfalfa cv Regen-SY and GA-AT (Fig. 4B). Amounts of citrate, oxalate, succinate, and acetate were similar between root tissues of GA-AT and alfalfa cv Regen-SY (Fig. 4, A and B).
Figure 4.
Oraganic acid content and exudation in transgenic and untransformed alfalfa lines under neutral pH growth conditions. A and B, Root organic acid content (n = 7); C and D, leaf organic acid content (n = 2); and E and F, organic acid exudation into the rhizosphere (n = 4). Bars = means + se. Note that the figures are drawn to different log scales.
We investigated whether overexpression of neMDH or PEPC would increase the amounts of organic acids exuded from roots under neutral pH conditions. Over a 24-h period, M16-27 plants exuded significantly more citrate, oxalate, malate, succinate, and acetate into the rhizosphere than did alfalfa cv Regen-SY plants (Fig. 4, E and F). Similar amounts of citrate and malate were measured in root exudates of PEPC-3 and alfalfa cv Regen-SY plants (Fig. 4E). There was no detectable amount of oxalate in root exudates of M20-10, alfalfa cv Regen-SY, GA-AT, or PEPC-3 plants. PEPC-3 plants did not show detectable amounts of succinate or acetate in their root exudates. Amounts of citrate and malate released by roots of the conventionally bred acid soil tolerant alfalfa, GA-AT, were significantly higher than that of alfalfa cv Regen-SY (Fig. 4E). In contrast, exudation of succinate by roots of GA-AT plants was reduced from alfalfa cv Regen-SY levels (Fig. 4F).
Transgenic Plants Show Increased Al Tolerance in Hydroponic Solution and Acid Soil
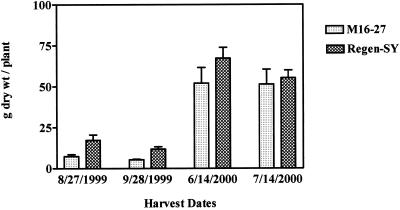
Plants of the transgenic lines and untransformed controls had similar leaf morphology and flowering patterns. After 30 d of growth in neutral pH (soil:sand, 1:1, v/v) under greenhouse conditions, however, alfalfa cv Regen-SY plants had accumulated more dry matter than did transgenic plants (data not shown). A reduced biomass accumulation by transgenic plants was observed during the 1st year of growth in field plots with soil pH 7.25 (Fig. 5). However, there was no significant difference in shoot dry matter accumulation in the following year (Fig. 5).
Figure 5.
Shoot biomass accumulation in transgenic and untransformed alfalfa lines grown in field plots with soil pH 7.25. Plants were transplanted on June 2, 1999, and aerial biomass was cut by hand on the dates shown. Bars = means + se (n = 3 with up to 10 plants in each replicate plot).
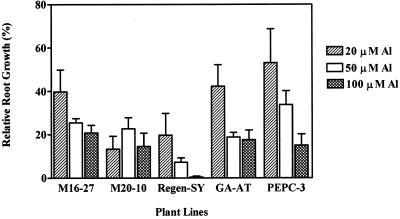
We evaluated root elongation of transgenic and untransformed control plants under acidic conditions minus Al in hydroponic culture. Root elongation of all plants occurred at pH 4.3, but root elongation of the untransformed alfalfa cv Regen-SY plants was significantly faster than that of any of the transgenic lines tested (Fig. 6). A slower rate of root growth by transgenic lines is consistent with the trends seen in shoot biomass accumulation of field-grown alfalfa plants (pH 7.25) during the 1st year of growth (Fig. 5). PEPC-3 plants showed the slowest root growth rate in acidic nutrient solutions without the addition of Al.
Figure 6.
Influence of Al on root elongation of transgenic and untransformed alfalfa lines. Relative root growth = (root elongation with Al/root elongation without Al) * 100. Plants were preconditioned in hydroponic solution containing 0.5 mm CaCl2 in deionized water, pH 4.3. After 3 d, Al was added to fresh preconditioning medium, and root length was measured before and after 24 h of growth. Bars = means + se (n = 10). Root elongation in the preconditioning medium without the addition of Al during the same 24 h period was: M16-27 = 14.9 mm, M20-10 = 11.0 mm, alfalfa cv Regen-SY = 23.6 mm, GA-AT = 16.5 mm, and PEPC-3 = 8.0 mm.
The primary response of plants to Al toxicity is the inhibition of root elongation, which is observed as early as 1 h after Al exposure (Pellet et al., 1996). Relative root growth, expressed as a percentage of the root elongation in an Al treatment compared with root elongation without Al treatment, is considered to be a reliable screening measure for Al tolerance in plants (Howeler, 1991). The root elongation rates of transgenic, untransformed alfalfa cv Regen-SY and GA-AT plants were evaluated in pH 4.3 hydroponic solutions containing 20, 50, or 100 μm Al. Compared with elongation in the absence of Al, the addition of 20, 50, or 100 μm Al inhibited root elongation of all plant lines (Fig. 6). Addition of Al at 20 μm resulted in a 40% to 80% root growth inhibition in transgenic and untransformed GA-AT and alfalfa cv Regen-SY plants (Fig. 6). The transgenic lines and GA-AT plants showed enhanced tolerance at the higher Al concentrations. The relative root growth at 100 μm Al was on the order of M16-27 ≥ GA-AT > M20-10 ≥ PEPC-3 > alfalfa cv Regen-SY plants (Fig. 6).
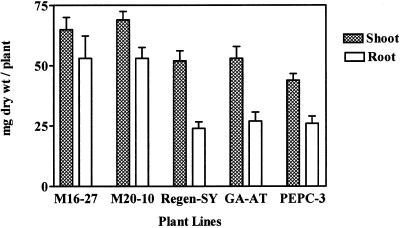
We evaluated the dry matter yield of alfalfa plants grown on naturally acidic, high-Al-containing soil. Biomass accumulation (shoot and root) of 35S::neMDH transgenic plants (lines M16-27 and M20-10) was significantly greater than that of GA-AT, alfalfa cv Regen-SY, and PEPC-3 plants (P < 0.01; Fig. 7). After 15 d of growth, neMDH transgenic lines produced 1.6-fold more biomass than alfalfa cv Regen-SY plants, although Al stress-like symptoms occurred in all plants.
Figure 7.
Biomass accumulation of transgenic and untransformed alfalfa lines. Plants were grown for 15 d in acid soil (pHH2O = 4.0, AlKCl = 71 μg mL−1). Bars = means + se (n = 10).
The amount of Al and P accumulated by alfalfa plants grown on naturally acid soil was evaluated. Consistent with increased biomass accumulation, M16-27 and M20-10 showed significantly higher Al content in roots and shoots compared with untransformed plants grown in the same soil (Table I). The content of Al in shoot tissue was significantly lower than the content in root tissue. P content in shoot and root tissues of M16-27 and M20-10 plants was up to 2.5-fold greater than P content in alfalfa cv Regen-SY and GA-AT plants (Table I). Root and shoot P content in PEPC-3 plants did not differ significantly from untransformed alfalfa cv Regen-SY and GA-AT levels (Table I). In contrast to the pattern of Al accumulation, P content was considerably higher in shoots than in roots.
Table I.
Shoot and root Al and P content of transgenic and untransformed alfalfa lines as determined by inductively coupled plasma–atomic emission spectroscopy analysis (n = 10)
| Plant Lines | Shoot Al | Root Al | Shoot P | Root P | ||||
|---|---|---|---|---|---|---|---|---|
| μg plant−1 | μg mg−1 | μg plant−1 | μg mg−1 | μg plant−1 | μg mg−1 | μg plant−1 | μg mg−1 | |
| M16-27 | 20.8a | 0.32 | 168.2a | 3.17 | 136.3a | 2.10 | 85.2a | 1.61 |
| M20-10 | 20.6a | 0.30 | 128.0a | 2.14 | 141.0a | 2.04 | 83.4a | 1.57 |
| Regen-SY | 15.2b | 0.29 | 52.0b | 2.17 | 96.7b | 1.86 | 34.1b | 1.42 |
| GA-AT | 10.5c | 0.20 | 54.6b | 2.02 | 103.8b | 1.96 | 43.0b | 1.59 |
| PEPC-3 | 14.4b | 0.33 | 58.9b | 2.27 | 86.5b | 1.97 | 34.1b | 1.31 |
Al or P contents (μg plant−1) were derived from dry matter yield (mg dry tissue plant−1) × Al or P concentrations (μg mg dry tissue−1). Means with different superscript letters in columns are significantly different at P < 0.05.
DISCUSSION
The present study showed that overexpression of neMDH cDNA in alfalfa resulted in modest but significant increases in malate concentration in tissues of some transgenic lines. Plants with overexpression of neMDH cDNA also had enhanced concentration of other organic acids in roots and leaves, increased organic acid exudation from roots, and a concomitant increase in tolerance to Al. By contrast, over-expression of the PEPC cDNA in alfalfa resulted in a reduction of organic acid content in roots and root exudates, although PEPC-3 plants displayed an increased malate concentration in leaf tissues. Increased root concentration of organic acids evidently does not necessarily correspond with increased exudation by roots.
The relative reduction in biomass accumulation of neMDH transgenic lines compared with untransformed alfalfa cv Regen-SY plants grown in soil of neutral pH during the early stages of plant growth may be due to the increased exudation of citrate and malate by these plants. Root exudation represents an appreciable drain on plant resources, thereby imposing an energy cost to plants (Smucker, 1984). When plants were grown in Al-containing acid culture, however, transgenic plants, especially those with the 35S::neMDH transgene, showed greater root elongation rates and higher biomass accumulation than did untransformed alfalfa cv Regen-SY plants, indicating that transgenic plants are much more tolerant to Al. The amounts of organic acids in roots and leaves and the exudation profiles for M20-10 plants were consistently different from that of M16-27 plants. The reason for this is unclear, because MDH-specific activity of roots in M20-10 plants was higher than in M16-27 plants. Although we have no direct evidence regarding the mechanism of Al tolerance in the transgenic alfalfa, the enhanced Al tolerance by 35S::neMDH transgenic lines and the poor growth of untransformed alfalfa cv Regen-SY plants in Al-containing hydroponic solution or soil culture corresponded with the organic acid synthesis and exudation patterns of these plants. For instance, M16-27 plants showed 4.2-fold more citrate, oxalate, malate, succinate, and acetate in roots and exuded 7.1-fold greater amounts of these organic acids into the rhizosphere compared with alfalfa cv Regen-SY plants. From the enhanced exudation of organic acids by roots of the transgenic M16-27 line, for example, it is appealing to speculate that Al-tolerance by this transgenic line may be in part due to chelation of Al in the rhizosphere. The observation that transgenic alfalfa with overexpression of neMDH also had increased P uptake when grown in high-Al-containing acid soil supports the idea that Al is complexed by organic acids released by the plant roots. Release of organic acids has been shown to improve P availability in acid soils by chelating Al from Al-P complexes (Fox et al., 1990).
Considering the constitutive nature of the 35S promoter, the decreased amount of neMDH transcript or protein in leaf samples of M16-27 and M20-10 plants was unexpected, and the reasons are not clear. Nevertheless, the 35S promoter in transgenic alfalfa leaves has been found to be affected by environmental conditions (D.A. Samac, unpublished data), which may be reflected in mRNA and protein accumulation of cloned genes driven by this promoter. Because the root apex is the site of Al toxicity (Kochian, 1995), targeting increased synthesis of organic acids to root tips may significantly improve Al tolerance and/or P uptake. To test this, additional transgenic alfalfa plants are being produced with the neMDH cDNA driven by promoters that are active in root meristematic tissues. This strategy may minimize the metabolic drain that appears to be associated with overexpression of organic acids in neMDH transgenic alfalfa.
Results from a number of laboratories have provided strong evidence for the role of organic acids in Al tolerance. Addition of organic acids to nutrient solutions alleviates phytotoxic Al effects, presumably by chelating Al and lowering the activity of free Al in the rhizosphere (Hue et al., 1986). In hydroponic systems an equimolar amount of citrate is needed to significantly reduce Al toxicity, whereas a 3-fold excess of malate and a 2-fold excess of oxalate relieves Al toxicity (Ryan et al., 1995; Ginting et al., 1998; Zheng et al., 1998). Al in the growing medium induces exudation of malate, citrate, succinate, or oxalate in many Al-tolerant plants (Miyasaka et al., 1991; Delhazie and Ryan, 1995; Pellet et al., 1996; Ma et al., 1997).
The transgenic plants over-expressing neMDH in the present study showed increased Al content in roots and shoots compared with untransformed plants grown in high-Al-containing acid soil. The increase in Al content was largely a reflection of a significantly greater biomass accumulation of M16-27 and M20-10 plants compared with dry matter yield of untransformed plants in the same soil. Increased Al uptake by tolerant plants was reported previously for Al-tolerant buckwheat (Ma et al., 1997) and alfalfa (Campbell, 1999). Recent studies show that a large fraction of the Al in hydroponic culture enters the root system fairly rapidly (Lazof et al., 1994; Vázquex et al., 1999). Therefore, an additional Al tolerance mechanism(s) appears to be active in tolerant varieties in which Al accumulates in the symplasm. An internal detoxification of Al by forming an Al-oxalate complex was used to explain Al tolerance of buckwheat that had accumulated high levels of Al in root tissues (Ma et al., 1997). Magnetic resonance imaging of roots of resistant and sensitive clones of alfalfa indicated that Al is complexed within roots of resistant clones and remains free in Al-sensitive clones (Campbell, 1999). Although it is unclear where complexing might have occurred, it is possible that increased organic acids in root and shoot tissue of M16-27 and M20-10 plants might have resulted in internal Al-complexing, rendering Al less toxic. Although there is strong evidence for a central role of organic acids in Al tolerance by crops, other suggested mechanisms of Al tolerance include (a) H+ influx at root tips resulting in a net increase in the root surface pH (Degenhardt et al., 1998), (b) binding of Al by excreted proteins (Basu et al., 1999), (c) localized excretion of phosphate at the root apex to precipitate Al (Pellet et al., 1996), (d) sequestration within the vacuole, and (e) presence of metal-binding and/or Al-tolerant enzymes (Taylor, 1991; Vázquex et al., 1999). The alfalfa germplasm GA-AT was developed through recurrent selection in acid soil for Al tolerance (Bouton, 1996). In hydroponic experiments, GA-AT plants demonstrated a level of Al tolerance similar to M16-27. The root organic acid synthesis and exudation pattern of GA-AT plants were different from M16-27, suggesting that an additional Al tolerance mechanism may be operating in GA-AT plants. Because organic acid exudation was measured in alfalfa plants under neutral conditions in the absence of Al, the profile of organic acids exuded by GA-AT plants may be altered by exposure to Al. We have made crosses of M16-27 with GA-AT plants and have found cosegregation of the transgene and enhanced organic acid synthesis. These crosses will allow us to test whether Al tolerance is subject to additive or synergetic effects related to germplasm source.
MATERIALS AND METHODS
Alfalfa Transformation and Plant Culture
The neMDH cDNA and PEPC cDNA isolated from alfalfa (Medicago sativa) nodule libraries were characterized previously (Pathirana et al., 1992; Miller et al., 1998). Full-length cDNAs were cloned separately in a polylinker between the CaMV 35S promoter and nopaline synthase 3′ terminator. Standard molecular techniques were used for DNA manipulation (Maniatis et al., 1992). The chimeric genes were transferred into Agrobacterium tumefaciens LBA 4404 by triparental mating (Horsch and Klee, 1986). Leaf pieces from a highly regenerable clone of M. sativa cv Regen-SY (Bingham, 1991) were transformed with A. tumefaciens as previously described (Austin et al., 1995). Transgenic plants were grown in sand:soil (1:1, v/v) in a greenhouse. Plants were watered daily with tap water and fertilized with water soluble fertilizer (20:10:20, v/v; N:P:K) every 2 weeks.
Nine alfalfa lines with the neMDH transgene and eight alfalfa lines containing the PEPC construct were confirmed by PCR to carry the NPTII selectable marker gene (data not shown). Transgenic plants were also verified by PCR analyses using primer sequences specific for the 35S promoter and the transgene(s) (data not shown). Because of the out-crossing nature of alfalfa, all experiments were performed with primary transformants (T0) propagated from stem cuttings in vermiculite in a greenhouse. All experiments using alfalfa cv Regen-SY and GA-AT plants were also from propagated cuttings. Unless otherwise stated, experiments were conducted in a growth chamber set at 21°C/19°C, day/night temperature and 16-h light at 500 μmol m−2 s−1.
Enzyme-Specific Activities and Immunoblotting
Root tip samples (approximately the distal 20 mm of root) and leaf samples (150 mg) were ground in liquid nitrogen and total soluble proteins were extracted as described (Gantt et al., 1992). Protein in the tissue supernatant was estimated using the Bio-Rad protein reagent (Bio-Rad, Hercules, CA; Bradford, 1976).
In vitro MDH or PEPC activity was measured spectrophotometerically as described previously (Egli et al., 1989). For western blotting, soluble proteins were separated on 10% (w/v) SDS-polyacrylamide gels, electroblotted to nitrocellulose membranes (Amersham, Buckinghamshire, UK), probed with rabbit anti-neMDH (Miller et al., 1998) or anti-PEPC (Pathirana et al., 1992), and visualized with a perioxidase-coupled secondary antibody and enhanced chemiluminescence western-blot detection reagent (Amersham). Cross-reacting protein levels were quantitated by densitometry using an AMBIS imaging system (Scananalytics, Billerica, MA).
RT-PCR Analysis
Root tips (400 mg) and leaf samples (200 mg) were ground in liquid nitrogen, and total RNA was extracted using the Trizol reagent (Life Technologies, Cleveland) according to the manufacturer's instructions. To remove contaminating DNA, 15 μg of RNA was incubated with 1 unit of RQ1 RNase-free DNase (Promega, Madison, WI) for 1 h at 37°C. RNA samples were purified with the RNeasy Plant Mini Kit (Qiagen USA, Madison, WI) and used as a template in a multiplex RT-PCR reaction using the access RT-PCR system (Promega). Primer sequences were M11F (5′-GACCTGCATCTCTATGATATCG-3′), MRTR (5′-CAACAACTGGAACATCCACATC-3′), and the universal 18S primer pairs and competimers (Ambion, Austin, TX) for amplifying an internal control. The M11F and MDHR primers correspond to positions 384–405 and 801–822 of the M. sativa neMDH cDNA sequence (GenBank accession no. AF020273). The multiplex RT-PCR amplification reactions (50 μL) consisted of 5 units of AMV Reverse Transcriptase, 5 units of Tfl DNA Polymerase, 1× AMV/Tfl reaction buffer, 1 mm MgSO4, 250 μm dNTPs, 20 pmol each of M11F and MRTR primers, 15 nm universal 18S primers, 35 nm competimers, and 250 ng of total RNA. Amplification conditions were one cycle of 48°C for 45 min, one cycle of 94°C for 2 min, 40 cycles of 94°C for 30 s, 53°C for 1 min, and 68°C for 2 min followed by a final extension at 68°C for 7 min. The PCR products were electrophoresed on 1% (w/v) agarose gel, transferred to Zetaprobe membrane (Bio-Rad), prehybridized, hybridized, and washed under highly stringent conditions. Probes were a 32P-labeled 1.65-kb neMDH cDNA fragment obtained by BamHI-NotI digestion of the pMDH-11 cDNA clone (Miller et al., 1998) and a 0.3-kb 18S cDNA obtained by RT-PCR amplification of total RNA from an alfalfa root tissue. Probes were labeled using a random priming kit (Amersham). Hybridization signals were analyzed using a storm 840 PhosphorImager and ImageQuant software (Molecular Dynamics). Relative transcript levels of neMDH were estimated after normalization with signals from the 18S transcript levels.
Organic Acid Extraction
Roots were washed gently to remove vermiculite and blotted dry with paper towels. About 0.3 to 0.5 g of root tips and leaves was collected, frozen in liquid nitrogen, and stored at −80°C until extracted. Organic acid extraction methods were essentially as described previously (Johnson et al., 1996). Acidic fractions were concentrated to dryness in a speed-vac (Savant Instruments, Holbrook, NY), redissolved in 4 mm sulfuric acid, filtered through a 0.45-μm syringe filter, and stored at −20°C until analysis. A 20-μL sample was analyzed by HPLC (Spectra Physics, San Jose, CA) equipped with an Aminex HPX-87H 300- × 7.8-mm column (Bio-Rad) and an organic acid guard column (Bio-Rad). The mobile phase was 4 mm sulfuric acid with a flow rate of 0.6 mL/min at 25°C. Individual components were detected at 210 nm on a UV/VIS detector (Spectroflow 757, ABI Analytical, Sunnyvale, CA).
To evaluate organic acid exudation, 3-week-old alfalfa cuttings were kept for 24 h in plastic pots filled with industrial quartz (Unimin Corporation, New Cannan, CT) containing 10 mL of deionized water. Plants were placed in a growth chamber and exudates were collected after rinsing roots and quartz with approximately 25 mL of sterile deionized water. Washes were filtered through Whatman paper (Clifton, NJ) and mixed with 1 volume of ethanol. Organic acids were extracted and analyzed as described above.
Alumimum Tolerance Experiments
Two-week-old cuttings were placed in a hydroponic chamber filled with a preconditioning solution consisting of 12 L of 0.5 mm CaCl2 in deionized water, pH 4.3. pH was adjusted by adding HCl. After 3 d of growth in the preconditioning solution, root length was measured, and the solution was replaced with 12 L of preconditioning solution containing 0, 20, 50, or 100 μm AlCl3 at pH 4.3. pH was adjusted by adding NaOH or HCl. Root length was measured before and after 24 h of growth in Al treatments.
To evaluate Al tolerance in soil, cuttings trimmed to 1 shoot node were planted in plastic pots filled with 300 g (dry soil weight) of a highly weathered unlimed soil: pHH2O = 4.0, AlKCl = 71 μg g−1, PBray = 40 μg g−1, Total n = 0.1%, NNO3 = 60.7 μg g−1, NNH4 = 8.2 μg g−1, K = 332 μg g−1, OM = 2.3%. The soil was obtained from Garden Plain, Sedgwick County in central Kansas. Growth experiments were conducted in a growth chamber set as described above. The soil in each pot was watered daily with deionized water. At harvest, plants were excavated from soil, roots were washed, plants were partitioned into shoots and roots, and dry matter yield was determined. Al and P concentrations in plant tissues were measured using ARL 3560 inductively coupled plasma–atomic emission spectroscopy by the Research Analytical Laboratory (University of Minnesota).
ACKNOWLEDGMENTS
We thank Susan Miller for assistance in protein work and Drs. Howard Rines and Richard Zeyen for critical reading of the manuscript. We thank Drs. Paul St. Amand and Daniel Skinner for supplying soil.
Footnotes
This work was funded by the North Central Biotechnological Initiative (Purdue grant no. 593–0244–05/U.S. Department of Agriculture [USDA] grant no. 97–34340–3987). This paper is a joint contribution from the Plant Science Research Unit, USDA, Agricultural Research Service, and the Minnesota Agricultural Experiment Station. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products and vendors that might also be suitable.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010376.
LITERATURE CITED
- Austin S, Bingham ET, Mathews DE, Shahan MN, Will J, Burgess RR. Production and field performance of transgenic alfalfa (Medicago sativa L.) expressing α-amylase and manganese-dependent lignin peroxidase. Euphytica. 1995;85:381–393. [Google Scholar]
- Basu U, Good AG, Aung T, Slaski J, Basu A, Briggs KG, Taylor GJ. A 23-kDa root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant. 1999;106:53–61. [Google Scholar]
- Bingham ET. Registration of alfalfa hybrid Regen SY germplasm for tissue culture and transformation research. Crop Sci. 1991;31:1098. [Google Scholar]
- Bouton JH. Screening the alfalfa core collection for acid soil tolerance. Crop Sci. 1996;36:198–200. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;54:484–489. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell TA. Magnetic resonance imaging of absorbed aluminum in alfalfa roots. J Plant Nutr. 1999;22:827–834. [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase, a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian L. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente JM, Ramirez-Rodrigues V, Cabreta-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhazie E, Hebb DM, Ryan PP. Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol. 2001;125:2059–2067. doi: 10.1104/pp.125.4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhazie E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine TE, Bouton JH, Mabrahtu T. Legume genetics and breeding for stress tolerance and nutrient efficiency. In: Baligar VC, Duncan RR, editors. Crops as Enhancers of Nutrient Use. Boston: Academic Press; 1990. pp. 211–252. [Google Scholar]
- Egli MA, Griffith SM, Miller SS, Anderson MP, Vance CP. Nitrogen assimilating enzyme activities and enzyme protein in effective and plant controlled ineffective alfalfa genotypes. Plant Physiol. 1989;91:898–904. doi: 10.1104/pp.91.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TR, Comerford NB, McFee WW. Phosphorous and aluminum release from a spodic horizon mediated by organic acids. Soil Sci Soc Am J. 1990;54:1763–1767. [Google Scholar]
- Gantt SJ, Larson RJ, Farnham MW, Pathirana SM, Miller SS, Vance CP. Aspartate aminotransferase in effective and ineffective alfalfa nodules: cloning of a cDNA and determination of enzyme activity, protein and mRNA levels. Plant Physiol. 1992;98:868–878. doi: 10.1104/pp.98.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginting S, Johnson BB, Wilkens S. Alleviation of aluminum pytotoxicity on soybean growth by organic anions in nutrient solutions. Aust J Plant Physiol. 1998;25:901–908. [Google Scholar]
- Horsch RB, Klee HJ. Rapid assay of foreign gene expression in leaf discs transformed by Agrobacterium tumefaciens: role of the T-DNA borders in the transfer process. Proc Natl Acad Sci USA. 1986;83:4428–4432. doi: 10.1073/pnas.83.12.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howeler RH. Identifying plants adaptable to low pH conditions. In: Wright RJ, Baligar VC, Murrmann RP, editors. Plant-Soil Interactions at Low pH. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 885–904. [Google Scholar]
- Hue NV, Craddock GR, Adams F. Effect of organic acids on aluminum toxicity in subsoil. Soil Sci Soc Am J. 1986;50:28–34. [Google Scholar]
- Johnson JF, Allan DA, Vance CP, Weiblen G. Root carbon dioxide fixation by phosphorus-deficient Lupinus albus: contribution to organic acid exudation by proteoid roots. Plant Physiol. 1996;112:19–30. doi: 10.1104/pp.112.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian KV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Mol Biol. 1995;46:237–260. [Google Scholar]
- Lazof DB, Goldsmith JG, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips: a microanalytical study using secondary ion mass spectrometry imaging. Plant Physiol. 1994;112:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Detoxifying aluminum with buckwheat. Nature. 1997;390:569–570. [Google Scholar]
- Maniatis TA, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP. Alfalfa malate dehydrogenaase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998;15:173–184. doi: 10.1046/j.1365-313x.1998.00192.x. [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirana SM, Vance CP, Miller SS, Gantt JS. Alfalfa root nodule phosphoenolpyruvate carboxylase, characterization of the cDNA and expression in effective and plant-controlled ineffective nodules. Plant Mol Biol. 1992;20:437–450. doi: 10.1007/BF00040603. [DOI] [PubMed] [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhazie E, Randal PJ. Characterization of Al-stimulated efflux of malate from apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Smucker AJM. Carbon utilization and losses by plant root systems. In: Barber SA, Bouldin DR, editors. Roots, Nutrients and Water Influx, and Plant Growth. Madison, WI: Soil Science Society of America; 1984. pp. 27–63. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. Curr Topics Plant Biochem Physiol. 1991;10:57–93. [Google Scholar]
- Vance CP. The molecular biology of N metabolism. In: Dennis DT, Turpin DH, Lefebrre DD, Layzell DB, editors. Plant Metabolism. Ed 2. London: Longman Scientific; 1997. pp. 449–477. [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzymes involved. Plant Sci. 1994;101:51–64. [Google Scholar]
- Vance CP, Heichel GH. Carbon in N2 fixation: limitation or exquisite adaptation. Annu Rev Plant Physiol. 1991;42:373–392. [Google Scholar]
- Vázquex MD, Poschenrieder C, Corrales I, Barcelo J. Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol. 1999;119:435–444. doi: 10.1104/pp.119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminum stress. Physiol Planta. 1998;103:209–214. [Google Scholar]