Abstract
A general gas chromatography/mass spectrometry (MS)-based screen was performed to identify catabolites and conjugates of indole-3-acetic acid (IAA) during vegetative growth of Arabidopsis. This experiment revealed the existence of two new conjugates: N-(indole-3-acetyl)-alfa-alanine (IA-Ala) and N-(indole-3-acetyl)-alfa-leucine (IA-Leu). A method for quantitative analysis of IAA metabolites in plant extracts by liquid chromatography-electrospray tandem MS has been developed. The accuracy and precision of the new method are better than 10% for standards close to the detection limit, and are between 6% and 16% for the entire protocol applied to plant extracts. The low detection limits, 0.02 to 0.1 pmol for the different metabolites, made it possible to use as little as 50 to 100 mg of tissue for quantitative analysis. The analysis was performed on different tissues of an Arabidopsis plant at two stages of development, using heavy labeled internal standards of the catabolite 2-oxoindole-3-acetic acid as well as IAA conjugated to amino acids: aspartate, glutamate, Ala, and Leu. Expanding leaves and roots that generally contain high amounts of the free hormone also contained the highest levels of IA-aspartate, IA-glutamate, and 2-oxoindole-3-acetic acid, supporting their role as irreversible catabolic products. The levels of IA-Leu and IA-Ala did not follow the general distribution of IAA. Interestingly, the level of IA-Leu was highest in roots and IA-Ala in the aerial tissues.
Indole-3-acetic acid (IAA, auxin) is an important plant hormone controlling a variety of developmental processes. Reliable and sensitive quantification methods for IAA have been developed over the last decade, and a significant amount of information is available on the levels of free hormone in plants (Edlund et al., 1995; Ribnicky et al., 1998; Prinsen et al., 2000). Although the metabolism of IAA is relatively well investigated, the information on the levels and relative importance of its major metabolites is rare. IAA can be catabolized by several pathways including conjugation to sugars and amino acids and non-decarboxylative or decarboxylative oxidation (for review, see Normanly, 1997). Until recently, conjugated IAA species have been quantified indirectly, via hydrolysis to free hormone (Baldi et al., 1989; Bialek and Cohen, 1989). Ester conjugates can be hydrolyzed using mild alkaline conditions at room temperature, and amide conjugates require strong alkaline conditions and at least 100°C for hydrolysis. The latter approach is often called the total IAA method because under such conditions, both types of conjugated IAA are hydrolyzed and are subsequently measured as a sum of free IAA present in the sample before hydrolysis and IAA resulting from hydrolyzed conjugates. This relatively simple method has been favored by many researchers; however, for the biochemical studies on IAA metabolism, it is not specific or selective enough. Furthermore, some IAA-related indolic compounds have been reported to interfere with the assay. Indole-3-acetonitrile (IAN) can be converted to IAA during the strong alkaline hydrolysis, thus an appropriate correction involving measurement of IAN in the same samples has to be applied (Ilic et al., 1996). Müller and Weiler (2000) have recently published results where they suggested interference of indole-3-glycerol phosphate (IGP) with the total IAA method. One way to avoid such interference is to measure the individual conjugates directly. Suitable internal standards are not available commercially, however, the [indole-13C6]-labeled parent IAA molecule is, and a relatively easy protocol for synthesis of labeled amide conjugates has been published (Ilic et al., 1997).
Normanly and coworkers (Tam et al., 2000) have tested direct gas chromatography (GC)-selected ion monitoring-mass spectrometry (MS) measurement of one ester (IA-Glc) and two amide conjugates (IA-Asp and IA-Glu). Relatively high amounts of plant tissue had to be used to obtain measurable amounts of analytes, which required samples to be cleaned up with a complex procedure involving several preparative HPLC steps. The results have shown very low endogenous levels of all measured conjugates compared with total IAA results.
In this work, we performed a total screen to identify the IAA conjugates present in Arabidopsis tissues during vegetative growth. Thereafter, we developed a method with a relatively simple purification protocol for low amounts of plant tissues and a sensitive liquid chromatography (LC)-electrospray ionization (ESI)-MS/MS method for quantification of IAA metabolites. The developed method was used to describe the distribution of these metabolites in vegetative tissues of Arabidopsis.
RESULTS AND DISCUSSION
Screen for Amide Conjugates
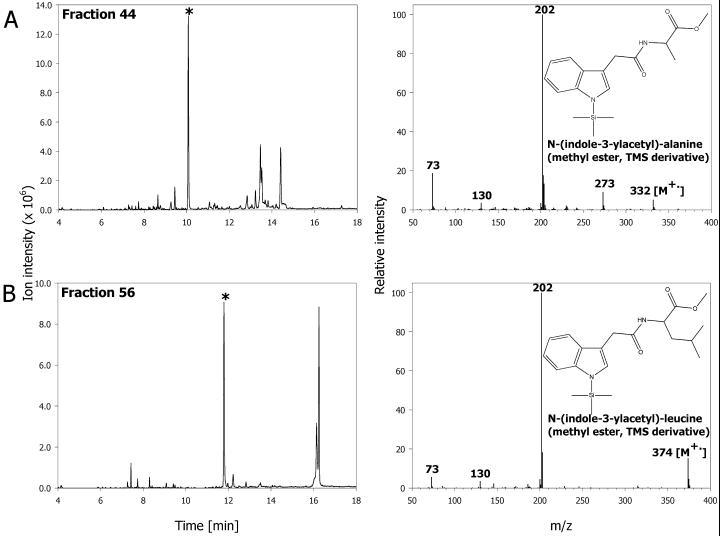
We have performed a total screen based on initial HPLC fractionation and subsequent GC/MS analysis to identify the IAA conjugates present in Arabidopsis tissues during vegetative growth. Plant extract was first fractionated into 65 samples by HPLC, and each individual sample was subjected to a full scan analysis. The generated full scan data served as a database to screen ions characteristic for indoles (Östin et al., 1998). Using this procedure, we confirmed the presence of endogenous metabolites previously identified in Arabidopsis (Östin et al., 1998; Barratt et al., 1999; Tam et al., 2000) such as IA-Asp, 2-oxoindole-3-acetic acid (OxIAA), and IA-Glu (data not shown), as well as identified two additional conjugates, N-(indole-3-acetyl)-α-Ala and N-(indole-3-acetyl)-Leu (Fig. 1). The ability of Arabidopsis tissues to synthesize IA-Leu was previously indicated using thin layer chromatography analysis (Sztein et al., 1995), but the compound was not conclusively identified as an endogenous IAA conjugate. Our identification based on mass spectrum, GC, and LC retention time is important because IA-Leu is proposed to be a key substrate for ILR1 hydrolase (Bartel and Fink, 1995). The evidence of IA-Leu being an endogenous constituent is also strong support for the suggested importance of this hydrolase in auxin homeostasis. The Ala conjugate of IAA has been identified in spruce (Picea abies; Östin et al., 1992) and tobacco (Nicotiana tabacum; M. Kowalczyk and G. Sandberg, unpublished data), but it has not been detected as an endogenous component of Arabidopsis. Interestingly, this compound is a high-specificity substrate for recently cloned IAR3 hydrolase (Davies et al., 1999). Formation of IA-Leu and IA-Ala was not observed in response to applied IAA (Östin et al., 1998; Barratt et al., 1999), which may show that their role in the hormone metabolism is different from the other conjugates. High concentrations of exogenous IAA stimulated the production of IA-Asp and IA-Glu, neither of which is a good substrate for ILR1/IAR3 proteins. This suggests that IA-Leu and IA-Ala may be used for hormone homeostasis and storage, whereas IAA conjugated to Asp (and possibly Glu) is a “sink” catabolite and a substrate for a further oxidative metabolism (Tuominen et al., 1994; Östin et al., 1998). This is also supported by the observation that IA-Ala is a good slow-release auxin source in the plant tissue cultures (Hangarter et al., 1980; Caruso, 1987; Magnus et al., 1992), whereas IA-Asp is only a mediocre auxin source.
Figure 1.
Ion chromatograms for m/z 202 (left) and mass spectra (right) of the new endogenous amide conjugates from Arabidopsis identified during a screen for indolic compounds in vegetative tissues. A, N-(indole-3-acetyl)-α-Ala from HPLC fraction 44; B, N-(indole-3-acetyl)-α-Leu from HPLC fraction 56. The asterisk on ion chromatogram indicates the peak from which spectrum was taken.
During the development of the multiple reaction monitoring analysis (MRM), we have obtained data indicating that IAA conjugated to β-Ala may be present in the samples from wild-type Arabidopsis plants, however, the full identification of this compound remains to be performed.
Although several other indolic compounds were picked up during the screen, we failed to find any other amide conjugates among them. Thus, it can be concluded that if such amide conjugates are present in vegetative tissues, their relative levels must be much lower than the ones we found. Alternatively, their chemical properties could prevent them from being analyzed by GC/MS, as for example, Nα-(indole-3-acetyl)-Gln (Barratt et al., 1999) or protein/peptide-linked IAA molecules (Bialek and Cohen, 1986).
Some authors have speculated that a large variety of amide conjugates must exist because of the variety and wide specificity of the conjugate-hydrolyzing enzymes (Tam et al., 2000). Based on data obtained from our screen, this seems not to be the case during the vegetative stage of growth. However, it may be completely different in other developmental stages, for example, during seed maturation and germination.
Method Optimizations and Choice of Diagnostic Transitions
Amide conjugates of IAA can be analyzed without any prior derivatization in positive or negative ESI modes. Our primary concerns were sensitivity and good chromatographic separation, and with such constraints, methylation of the carboxyl group is clearly an advantage.
In LC-ESI-MS, sensitivity depends upon the mobile phase composition and the nature of the analyte. It is generally accepted that mobile phase with lower concentrations of the electrolyte and higher composition of the organic solvent is more suitable to obtain good ionization efficiency. Methylated molecules elute from the column at higher concentrations of an organic phase, thus making the response higher because of an easier ion desorption process or because of a partitioning effect within ESI droplets (Cech et al., 2001). For a sensitive MRM method, stability of precursor and daughter ions are very important because the yield of a daughter ion determines total intensity of the signal (Guan et al., 1999). The optimal conditions for MRM are such that the precursor ion is stable enough to pass through the first quadrupole, yet fragile enough to fragment completely in the second quadrupole (collision-induced dissociation zone) and yield an abundant daughter ion that is monitored in the third quadrupole. We have investigated the fragmentation patterns for methyl esters and underivatized amide conjugates of IAA with no collision gas and collision energy set to 0 eV in ESI+ mode to evaluate the stability of [M+H]+ parent ions. The stability of parent ions for underivatized conjugates in ESI− mode was not tested, primarily because it required changes in pH of the mobile phase that resulted in poor chromatographic resolution. For methylated and underivatized compounds in “no collision” conditions, [M+H]+ ions were very intense, and practically no fragmentation was observed (data not shown). Introduction of collision-induced dissociation gas and setting collision energy to 18 eV usually caused (notably not for underivatized IA-Glu) complete breakage of the parent ions. Underivatized analytes yielded almost exclusively quinolinium/quinolonium ions (m/z 130 and 146) and amino acid side chain daughter ions, whereas methylated compounds produced some additional low-intensity fragments from the breakage of ester bonds. The signal strength was 50 to 100 times higher for methylated in comparison with underivatized conjugates. Prinsen and coworkers (Prinsen et al., 1997) also reported a similar increase in sensitivity and improvement in chromatography after methylation of the IAA carboxyl group.
Mass spectra for methylated amide conjugates were, in general, similar to these reported before using continuous flow fast-atom bombardment (Tuominen et al., 1994). In both types of ionization, the major fragment was a quinolinium ion at m/z 130 for amide conjugates and a quinolonium ion at m/z 146 for OxIAA. Transitions from the parent [M+H]+ ions to these most intense fragment ions were chosen for monitoring in MRM. Details of the mass spectra and diagnostic transitions are shown in Table I, and example standards and samples chromatograms in are shown in Figures 2 and 3, respectively.
Table I.
Diagnostic reactions and detection limits at signal to noise ratio (S/N) of three for the methyl esters of analyzed compounds and corresponding internal standards
| Compound | Reaction Monitored (Internal Standard Reaction) | Detection Limit |
|---|---|---|
| pg | ||
| OxIAA-Me | 206.08 → 146.06 | 3 |
| (212.10 → 152.08) | ||
| IAAla-Me | 261.12 → 130.07 | 1 |
| (267.14 → 136.09) | ||
| IAAsp-Me2 | 319.13 → 130.07 | 1.5 |
| (325.15 → 136.09) | ||
| IAGlu-Me2 | 333.15 → 130.07 | 1.25 |
| (339.17 → 136.09) | ||
| IALeu-Me | 303.17 → 130.07 | 0.5 |
| (309.19 → 136.09) | (S/N = 2.90) | |
| IAA-Me | 190.09 → 130.07 | 2 |
| (196.11 → 136.09) |
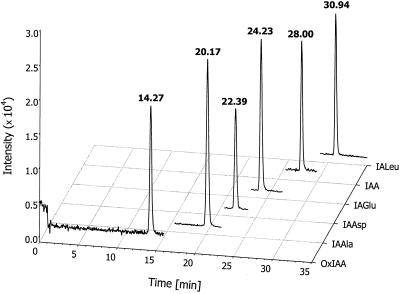
Figure 2.
Separation of the conjugates/catabolites standard mixture during LC-MRM-MS measurement. Each trace represents one transition from the pseudo-molecular parent ion to the most intense daughter ion (details shown in Table I). The mixture contained 50 pg of each metabolite.
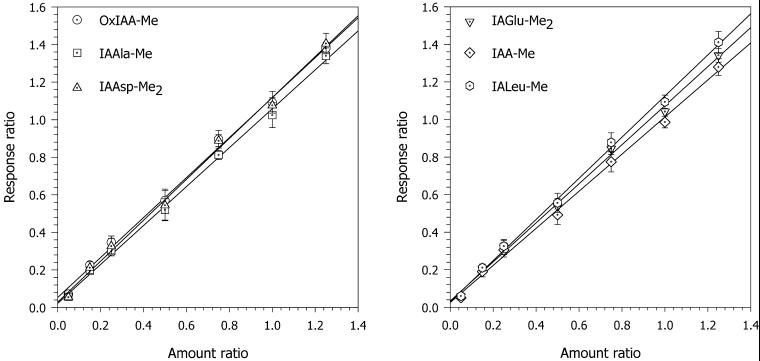
Figure 3.
Linearity and dynamic range of the response during LC-MRM-MS analysis of IAA conjugates/catabolites. Each data point on the calibration curves represents the mean of five independent samples, and the error bars represent the sd.
To avoid interference from other plant metabolites and to reduce the noise level, we decided to perform the analysis using groups (Fig. 2). Within the retention frame, the mass spectrometer was set to detect only two parent/daughter transitions: one for the endogenous compound eluting at that time frame and one for the added internal standard of the same or similar retention time. Although such an approach heavily depends on the retention time reproducibility, it usually produces very clean and interference-free results, making integration and quantification easier. In addition, using groups for the measurement allows increasing dwell time for each pair of ions, which may in turn increase sensitivity and selectivity.
Linearity, Accuracy, Precision, and Limits of Detection and Quantification
Linearity of the response was evaluated by analysis of several native standards replicates with [indole-13C6]-labeled internal standards. Response curves were found to be linear up to a ratio of 4 (linearity in the working range up to a ratio of 1.5 is shown in Fig. 4). With ratios twice as high, slight curvature was detected (data not shown). Because the internal standards have six 13C atoms, this curvature cannot be attributed to the effect of the natural isotope abundance and is caused by a fixed, independent-of-sample-concentration ion signal at higher analyte concentrations. Nevertheless, coefficients of determination (the square root of correlation coefficient) were between 0.998 and 0.999 for all calibration equations.
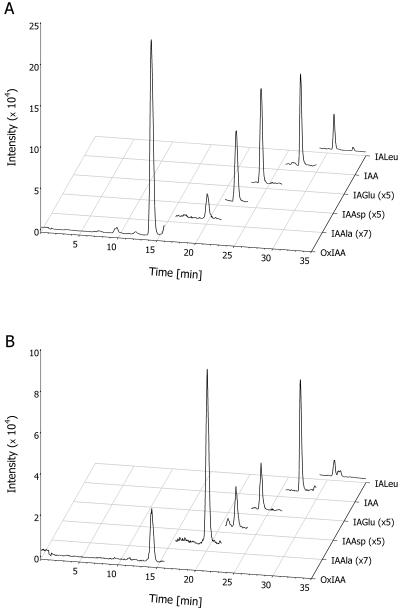
Figure 4.
LC-MRM-MS measurements of OxIAA and the conjugates in 100 mg of plant tissue. Endogenous metabolites of IAA in roots (A) and 3rd and 4th leaves (B) 10 d after germination (DAG). Traces for IA-Ala, IA-Asp, and IA-Glu are magnified to allow the same scaling for all chromatograms.
The reproducibility of the peak ratio response (Table II) for five replicate analyses corresponding to 5 and 200 pg were between 1.9% and 7.9%. Variation was higher for the 5-pg range, possibly because the analyses were performed very close to the detection limits. Due to the nature of the peak detection and integration algorithms in the software we have used, manual correction of the integration was usually required, probably introducing some additional errors. However, we found reproducibility of the peak ratio response to be in an acceptable range. The precision and accuracy was also estimated using plant extracts. Three purified plant samples were pooled and analyzed three times by MRM. Results obtained from the repetitions were almost identical for all the conjugates analyzed, with RSD of approximately 1.5% (Table III). The precision of purification was estimated using the extract from 1 g (fresh weight) of plant tissue subsequently divided into 10 samples purified and analyzed independently (Table III). For all the analytes, precision of purification was within the range of 15%. The least satisfactory results, 16.2% and 14.5%, were obtained for OxIAA and IA-Ala, possibly due to the difficulties in quantitative derivatization of these very polar compounds in the plant samples with ethereal diazomethane.
Table II.
Precision of response determination
| Compound | 5 pg %RSD (n = 6) | 200 pg %RSD (n = 4) |
|---|---|---|
| OxIAA-Me | 7.37 | 1.91 |
| IAAla-Me | 2.50 | 2.05 |
| IAAsp-Me2 | 3.22 | 1.99 |
| IAGlu-Me2 | 2.58 | 2.03 |
| IALeu-Me | 5.74 | 2.45 |
| IAA-Me | 7.66 | 3.07 |
Analysis was performed by coinjection of 5 and 200 pg of OxIAA and conjugates samples together with 50 pg of internal standards.
Table III.
Accuracy and precision of analysis and purification
| Compound | Accuracy of Analysis (n = 9) | Precision of Purification (n = 10) |
|---|---|---|
| % RSD | ||
| OxIAA-Me | 1.57 | 16.19 |
| IAAla-Me | 0.39 | 14.47 |
| IAAsp-Me2 | 1.27 | 9.79 |
| IAGlu-Me2 | 1.43 | 9.78 |
| IALeu-Me | 2.86 | 5.71 |
Accuracy of the analysis was estimated by three measurements of three Arabidopsis samples pulled together. Precision of purification was estimated by extracting IAA metabolites from 1 g of tissue. The extract was subsequently divided into 10 samples, which were purified and analyzed independently.
Detection limits at S/N = 3 were in range of 1 to 3 pg (Table I). The reason for these relatively high figures is that the noise from solvent in the mass region where parent ions are detected is rather high. However, detection limits of such range are rather typical for ESI+-based quantification methods. The method we developed should provide reliable quantification at 3 to 15 pg (S/N = 10) level, which is 2 to 10 times less than the expected levels of any IAA metabolite in 100 mg of Arabidopsis tissue. Estimated detection limits for underivatized analytes were around 10 to 20 times higher than those of methylated compounds (not shown).
Quantification of IAA Metabolites in Plant Tissues
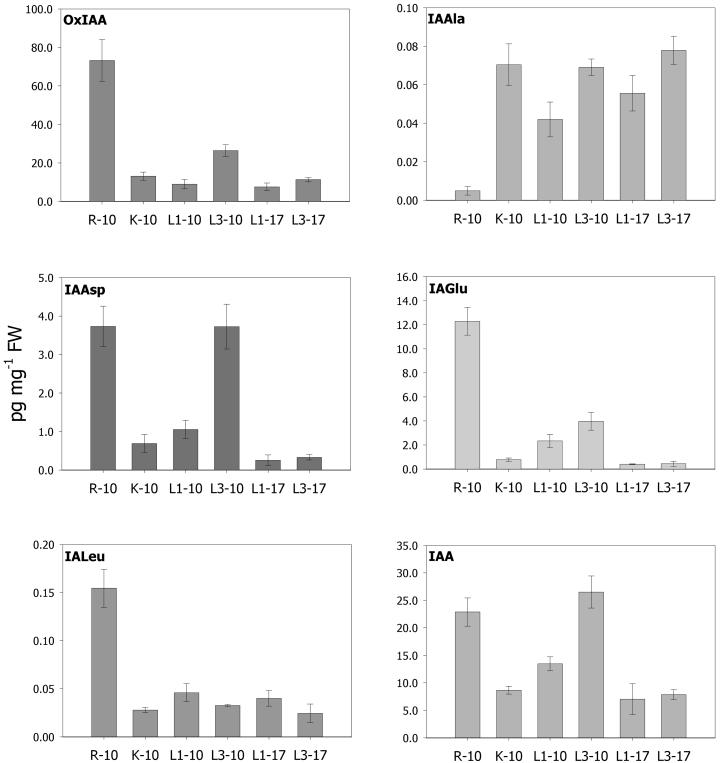
The method described in this paper was used to quantify IAA metabolites in various parts of the Arabidopsis plant during vegetative growth (Fig. 5). These results confirmed earlier findings from feeding experiments with low concentrations of exogenous IAA where the major products were OxIAA and IA-Asp (Östin et al., 1998). The most abundant metabolite in all analyzed tissues was OxIAA, with levels from 74 pg mg−1 of fresh weight in roots down to around 8 pg mg−1 of fresh weight in old leaves and cotyledons. The distribution pattern of OxIAA as well as IA-Asp and IA-Glu follows that of free IAA (Fig. 5), with high levels in leaves possessing cell division and elongation growth (leaves 3–4, 10 DAG). The IAA concentration drops when cell division ceases, as is shown by leaves 1 and 2 (10 DAG), which still undergo substantial elongation growth to reach relatively low IAA levels in leaves that have almost reached their final size (leaves 1st to 2nd and 3rd to 4th, 17 DAG). Interestingly, the close correlation between free IAA and its presumed catabolites, OxIAA, IA-Asp, and IA-Glu, observed in leaves also holds true for the root system. Our data showing high levels of free IAA in roots matched by high levels of the catabolites indicate very rapid turnover of the hormone in this tissue, which is in agreement with a recent finding that the root system has strong capacity for de novo synthesis of IAA (Ljung et al., 2001). The remaining two compounds measured did not follow the same distribution pattern. IA-Ala was distributed evenly at around 0.05 pg mg−1 fresh weight levels, except for roots, where it existed in very minute amounts close to the detection limit of the method. In contrast, IA-Leu was present at much higher levels in roots (around 0.15 pg mg−1 of fresh weight), and its levels in aerial parts were 60% lower. The total amount of amide-conjugated IAA in the tissues investigated 10 DAG was 26.1 pg mg−1 of fresh weight, almost 20 times less than usually obtained from the total IAA method (around 400 pg mg−1 of fresh weight for 12 DAG seedlings [Tam et al., 2000]). The pool susceptible to enzymatic hydrolysis (IA-Ala and IA-Leu) was never higher than of 0.1 pg mg−1 of fresh weight.
Figure 5.
Quantification of IAA metabolites in Arabidopsis. R-10, Roots 10 DAG; K-10, cotyledons 10 DAG; L1–10, 1st and 2nd leaves 10 DAG; L3–10, 3rd and 4th leaves 10 DAG; L1–17, 1st and 2nd leaves 17 DAG; L3–17, 3rd and 4th leaves 17 DAG. Error bars represent sd from three independent samples.
It is generally accepted that different conjugates play different roles in the IAA metabolism. Even within the chemically similar amide conjugate family, differences in function are likely. It has been postulated numerous times that IA-Asp could be an irreversible catabolite rather than a reversible storage compound (Östin et al., 1992; Riov and Bangerth, 1992; Sasaki et al., 1994; Tuominen et al., 1994; Östin et al., 1998). If this is the case in Arabidopsis, it would, from a physiological point of view, be misleading to treat IA-Asp (and possibly IA-Glu) equally to IA-Leu or IA-Ala because the latter conjugates most likely contribute back to the free IAA pool. Therefore, results of “total IAA” measurements would overestimate not only the levels of amide conjugates, but also their relative importance for hormone homeostasis. On the other hand, some evidence exists that IA-Asp may be hydrolyzed to the free hormone. Ludwig-Muller and coworkers (Ludwig-Muller et al., 1996) described a hydrolase from Chinese cabbage (Brassica campestris L. subsp. pekinensis) seedlings able to cleave IA-Asp. However, it was not the best substrate for this enzyme, and furthermore, significant hydrolysis of IA-Asp was only detected after the inoculation with Plasmodiophora brassicae. Unfortunately, specificity and origin of this highly active isoform were not established in the cited work. Another potential IA-Asp hydrolytic system was proposed by Oetiker and Aeschbacher (1997) in henbane. Although free hormone and IA-Asp could rescue the XIIB2 henbane mutant cells, suggesting release of free IAA from IA-Asp, this was not confirmed by a pulse-chase experiment. The authors also found that the major route of IA-Asp was oxidation rather than the hydrolysis to IAA.
It clearly appears beneficial to use a direct approach, yet this study, as well as the previous study by Tam and coworkers (Tam et al., 2000), reports very low amounts of conjugates present in the Arabidopsis, in contrast to the total IAA method. It is unlikely that the source of such differences are ester conjugates of IAA because application of IAA at low, close-to-physiological concentrations did not show any production of the ester conjugated hormone (Östin et al., 1998). This was also verified by Tam and coworkers (Tam et al., 2000), who confirmed that IA-Glc is not abundant during the vegetative stage. Therefore, it can be concluded that this discrepancy is caused by unknown amide conjugates or by interfering compounds.
The screen for amide-conjugated IAA performed by us revealed the existence of two new conjugates, IA-Ala and IA-Leu. Neither of the newly found compounds contributed significantly to the total IAA pool; in fact, their levels are much lower than that of IA-Asp and IA-Glu. Based on our screen for indolic compounds, we assume that other amide conjugates, if present in plants, would have even lower concentrations. If that is the case, they would not contribute more then 1 pg mg−1 of fresh weight to the total IAA pool. Alternatively, they may be not amenable to GC/MS analysis used for screening, like small peptides and IA-Gln, and should be screened using other techniques. The possibility that N-glycoside-linked IAA amide conjugates (Ljung et al., 2001) are present in the Arabidopsis should also not be excluded.
So far, two compounds that could potentially yield IAA in alkaline conditions and thus interfere with the total IAA method have been proposed. Correction for IAN interference, required for total IAA measurements in Arabidopsis (Ilic et al., 1996), is relatively easy to perform, but careful calibration is needed due to the fact that the available internal standard of IAN has only one 13C atom and the effect of natural 13C abundance is considerable. Ilic and coworkers (Ilic et al., 1996) have also observed that glucobrassicin, the parent indolic glucosinolate, is stable during high pH hydrolysis and does not yield IAN. This was further confirmed by experiments with synthetic glucobrassicin (Chevolleau et al., 1997). Although enzymatic breakdown of glucobrassicin at low pH produces IAN, it can be ignored if an IAN correction measurement is performed on the same sample prior to total IAA measurement or if conjugate extraction from the plant tissue involves organic solvent at the concentrations preventing enzymatic activities.
Müller and Weiler (2000) recently presented interesting results on interference of IGP with the total IAA method. The authors suggested that phosphatase- or plant protein extract-treated IGP broke down to indole-3-acetaldehyde and IAA at high pH. Conversion of IGP to IAA in alkaline conditions would obviously explain the difference between direct measurement and total IAA method, however, it seems impossible for such a reaction to occur only by thermal/pH catalysis.
In conclusion, we have developed a simple and sensitive method for direct quantification of selected IAA catabolites and conjugates in plant tissue. The method allowed us to analyze levels of four conjugates and one oxidative catabolite in Arabidopsis. The results obtained demonstrated that the distribution of OxIAA, IA-Asp, and IA-Glu follows the distribution of the free hormone in the plant, thereby showing that the tissues with high auxin levels also have rapid turnover of the hormone. We also confirmed the existence of two additional conjugates, IA-Ala and IA-Leu, in Arabidopsis. These two conjugates were not linked to the catabolism, and they showed tissue-specific distribution. It remains to be investigated whether they have a role in maintaining the auxin homeostasis in plant cells. Our data also positively verified the findings of Tam and coworkers (Tam et al., 2000) that major IAA conjugates found in feeding experiments exist naturally at very low levels. This raises a question about the source of large amounts of IAA detected with the total IAA measurements and, because this indirect method is commonly used and recommended (Prinsen et al., 2000), its further evaluation is necessary.
MATERIALS AND METHODS
Internal Standards and Reagents
[indole-13C6]-IAA was obtained from Cambridge Isotope Laboratories (Andover, MA). [indole-13C6] standards and unlabeled amide conjugates were synthesized according to Ilic et al. (1997), except that [indole-13C6]-labeled and “cold” IAA was used as a starting substrate, respectively. OxIAA and 2-oxo-[indole-13C6]-IAA were synthesized using a method modified from the protocol of van der Weet and coworkers (van de Weert et al., 1998). Approximately 200 μg of [indole-13C6]-IAA (or IAA for synthesis of unlabeled compound) was dissolved in 250 μL of a dimethyl sulfoxide:HCl:acetic acid (1:5:10, v/v) mixture and left in the open tube for 6 h at room temperature. The reaction mixture was then diluted with distilled, deionized water and applied to a SepPak C18 SPE column (Varian, Palo Alto, CA). After washing with two column volumes of 1% (v/v) acetic acid, the compound of interest was eluted with 60% (v/v) methanol/1% (v/v) acetic acid, concentrated in vacuum, and subsequently purified using the preparative HPLC system described below. The identity and purity of the compound was confirmed by GC/MS analysis. HPLC-grade solvents were from J.T. Baker (Phillipsburg, NJ), all other chemicals were from Sigma-Aldrich (St. Louis; unless stated otherwise).
Plant Material and Growth Conditions
Seeds of Arabidopsis ecotype Columbia were sterilized with 90% (v/v) ethanol for 2 min and dried on the sterile filter paper. Seeds were then germinated in petri dishes containing agar-solidified Murashige and Skoog medium and were grown for 14 d at 21°C in 16-h photoperiods. For indolic compounds screens, nonsterile greenhouse-grown plants were used.
Screen for the Amide Conjugates of IAA
Approximately 75 g of the entire 3-week-old Arabidopsis plants were frozen with liquid nitrogen, homogenized, and extracted with 80% (v/v) methanol containing 2.5 mm diethyl,dithio-carbamic acid. The extract was centrifuged (30 min, 18,500 rpm, 4°C), brought to a water phase in a rotary evaporator, and after the pH adjustment to 3.0, it was passed through an Env+ SPE column (Isolute Env+; International Sorbent Technology Ltd., Hengoed, Mid Glamorgan, UK). Absorbed material was subsequently eluted with 80% (v/v) methanol/1% (v/v) acetic acid, and eluate was concentrated under vacuum and dissolved in 1 mL of 5% (v/v) methanol/1% (v/v) acetic acid. The sample was next separated on a preparative HPLC system composed of a 600E controller and pump module (Waters, Milford, MA), a 996 diode-array detector (Waters), and a RediFrac100 fraction collector (Pharmacia, Piscataway, NJ). Linear gradient elution was used from 5% (v/v) methanol/1% (v/v) acetic acid to 90% (v/v) methanol/1% (v/v) acetic acid over 45 min, with a flow of 2.5 mL min−1. A regular preparative C18 column (ODS-2, 250 mm × 12 mm i.d.; Jones Chromatography Ltd., Hengoed, Mid Glamorgan, UK) was used. Sixty-five fractions of 2.5 mL each were collected and dried down. Samples were methylated with diazomethane, silylated with N,O-bis(trimethylsilyl)trifluoroacetamide/1% (v/v) trimethyl chlorosilane (Pierce, Rockford, IL) and then full scan GC/MS analysis in m/z range of 40 to 800 was performed in the standard conditions described before (Edlund et al., 1995). The search for typical fragments from indolic compounds was then performed on the obtained data using Xmass software from JEOL (Tokyo) and the custom spectra library.
LC-MRM-MS-Based Quantification
For LC-MS quantification, approximately 20 to 100 mg of the plant tissue was used. Internal standards concentrations used are summarized in Table I. After the extraction with 1 mL of 60% (v/v) 2-propanol and 2.5 mm diethyl,dithio-carbamic acid for 6 h at 4°C, samples were centrifuged, the organic solvent from supernatants was removed in vacuum, and pH was adjusted to 7.0. Next, samples were partitioned three times with 500 μL of diethyl ether. The water phase was acidified to pH 2.7 and was purified on the 50-mg Env+ SPE column (Isolute), dried, and methylated with diazomethane. The entire sample (35 μL in 5% [v/v] methanol/1% [v/v] acetic acid) was injected into a LC/MS system consisting of an Alliance HT module (Waters) and a Quatro Ultima mass spectrometer (Micromass International, Manchester, UK) operating in positive ESI mode. Compounds of interest were first absorbed on the SymetryShield C18 precolumn (10 × 2.1 mm i.d.; Waters) and washed with 10% (v/v) acetonitrile/1% (v/v) formic acid for 2 min at flow of 200 μL min−1. After that, the SymetryShield analytical column (150 × 2.1 mm i.d.; Waters) was connected via an automatic valve and exponential gradient elution from 10% to 80% (v/v) acetonitrile/1% (v/v) formic acid in 30 min at the same flow rate was started. Column effluent was introduced into the ESI ion source held at 100°C, and the capillary voltage was 3.22 kV, cone voltage was 48 V, and dissolvation gas temperature was 320°C. The mass spectrometer was operated in MRM mode with the collision energy set to 18eV. Table I summarizes monitored ions. Obtained data were processed with MassLynx 3.5 software (Micromass).
ACKNOWLEDGMENTS
The authors wish to thank Roger Granbom for technical assistance, as well as Anders Östin, Jan Eklöf, and Volker Magnus for discussions and critical reading of the manuscript.
Footnotes
This work was supported by the Swedish Research Council.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010525.
LITERATURE CITED
- Baldi BG, Maher BR, Cohen JD. Hydrolysis of indole-3-acetic-acid esters exposed to mild alkaline conditions. Plant Physiol. 1989;91:9–12. doi: 10.1104/pp.91.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt NM, Dong WQ, Gage DA, Magnus V, Town CD. Metabolism of exogenous auxin by Arabidopsis thaliana: identification of the conjugate N-α-(indol-3-ylacetyl)-glutamine and initiation of a mutant screen. Physiol Plant. 1999;105:207–217. [Google Scholar]
- Bartel B, Fink GR. ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science. 1995;268:1745–1748. doi: 10.1126/science.7792599. [DOI] [PubMed] [Google Scholar]
- Bialek K, Cohen DJ. Isolation and partial characterization of the major amide-linked conjugate of indole-3-acetic acid from Phaseolus vulgaris L. Plant Physiol. 1986;80:99–104. doi: 10.1104/pp.80.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K, Cohen JD. Quantitation of indoleacetic-acid conjugates in bean seeds by direct tissue hydrolysis. Plant Physiol. 1989;90:398–400. doi: 10.1104/pp.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso JL. The auxin conjugates. Hort Sci. 1987;22:1201–1204. [Google Scholar]
- Cech NB, Krone JR, Enke CG. Predicting electrospray response from chromatographic retention time. Anal Chem. 2001;73:208–213. doi: 10.1021/ac0006019. [DOI] [PubMed] [Google Scholar]
- Chevolleau S, Gasc N, Rollin P, Tulliez J. Enzymatic, chemical, and thermal breakdown of H-3-labeled glucobrassicin, the parent indole glucosinolate. J Agric Food Chem. 1997;45:4290–4296. doi: 10.1021/jf020125i. [DOI] [PubMed] [Google Scholar]
- Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B. IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell. 1999;11:365–376. doi: 10.1105/tpc.11.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Eklöf S, Sundberg B, Moritz T, Sandberg G. A microscale technique for gas-chromatography mass-spectrometry measurements of picogram amounts of indole-3-acetic-acid in plant tissues. Plant Physiol. 1995;108:1043–1047. doi: 10.1104/pp.108.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan FY, Ishii A, Seno H, Watanabe-Suzuki K, Kumazawa T, Suzuki O. Identification and quantification of cardiac glycosides in blood and urine samples by HPLC/MS/MS. Anal Chem. 1999;71:4034–4043. doi: 10.1021/ac990268c. [DOI] [PubMed] [Google Scholar]
- Hangarter RP, Peterson MD, Good NE. Biological activities of the indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980;68:761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic N, Magnus V, Östin A, Sandberg G. Stable-isotope labeled metabolites of the phytohormone, indole-3-acetic acid. J Label Compd Radiopharm. 1997;39:433–440. [Google Scholar]
- Ilic N, Normanly J, Cohen JD. Quantification of free plus conjugated indoleacetic acid in Arabidopsis requires correction for the nonenzymatic conversion of indolic nitriles. Plant Physiol. 1996;111:781–788. doi: 10.1104/pp.111.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001a;28:1–11. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- Ljung K, Östin A, Lioussanne L, Sandberg G. Developmental regulation of indole-3-acetic acid turnover in Scots pine seedlings. Plant Physiol. 2001b;125:464–475. doi: 10.1104/pp.125.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Muller J, Epstein E, Hilgenberg W. Auxin-conjugate hydrolysis in Chinese cabbage: characterization of an amidohydrolase and its role during infection with clubroot disease. Physiol Plant. 1996;97:627–637. [Google Scholar]
- Magnus V, Nigovic B, Hangarter RP, Good NE. N-(indol-3-ylacetyl)-amino acids as sources of auxin in plant tissue culture. J Plant Growth Regul. 1992;11:19–28. [Google Scholar]
- Müller A, Weiler EW. Indolic constituents and indole-3-acetic acid biosynthesis in the wild-type and a tryptophan auxotroph mutant of Arabidopsis thaliana. Planta. 2000;211:855–863. doi: 10.1007/s004250000353. [DOI] [PubMed] [Google Scholar]
- Normanly J. Auxin metabolism. Physiol Plant. 1997;100:431–442. [Google Scholar]
- Oetiker JH, Aeschbacher G. Temperature-sensitive plant cells with shunted indole-3-acetic acid conjugation. Plant Physiol. 1997;114:1385–1395. doi: 10.1104/pp.114.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A, Kowalczyk M, Bhalerao RP, Sandberg G. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 1998;118:285–296. doi: 10.1104/pp.118.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A, Monteiro AM, Crozier A, Jensen E, Sandberg G. Analysis of indole-3-acetic acid metabolites from Dalbergia dolichopetala by high-performance liquid-chromatography mass-spectrometry. Plant Physiol. 1992;100:63–68. doi: 10.1104/pp.100.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A, Moritz T, Sandberg G. Liquid-chromatography mass-spectrometry of conjugates and oxidative metabolites of indole-3-acetic acid. Biol Mass Spectrom. 1992;21:292–298. [Google Scholar]
- Prinsen E, Van Dongen W, Esmans EL, Van Onckelen H. HPLC linked electrospray tandem mass spectrometry: a rapid and reliable method to analyze indole-3-acetic acid metabolism in bacteria. J Mass Spectrom. 1997;32:12–22. [Google Scholar]
- Prinsen E, Van Laer S, Oden S, Van Onckelen H. Auxin analysis. Methods Mol Biol. 2000;141:49–65. doi: 10.1385/1-59259-067-5:49. [DOI] [PubMed] [Google Scholar]
- Ribnicky DM, Cooke TJ, Cohen JD. A microtechnique for the analysis of free and conjugated indole-3-acetic acid in milligram amounts of plant tissue using a benchtop gas chromatograph-mass spectrometer. Planta. 1998;204:1–7. doi: 10.1007/s004250050223. [DOI] [PubMed] [Google Scholar]
- Riov J, Bangerth F. Metabolism of auxin in tomato fruit tissue: formation of high-molecular weight conjugates of oxindole-3-acetic acid via the oxidation of indole-3-acetylaspartic acid. Plant Physiol. 1992;100:1396–1402. doi: 10.1104/pp.100.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Shimomura K, Kamada H, Harada H. IAA metabolism in embryogenic and nonembryogenic carrot cells. Plant Cell Physiol. 1994;35:1159–1164. [Google Scholar]
- Sztein AE, Cohen JD, Slovin JP, Cooke TJ. Auxin metabolism in representative land plants. Am J Bot. 1995;82:1514–1521. [Google Scholar]
- Tam YY, Epstein E, Normanly J. Characterization of auxin conjugates in Arabidopsis: low steady-state levels of indole-9-acetyl-aspartate, indole-3-acetyl-glutamate, and indole-3-acetyl-glucose. Plant Physiol. 2000;123:589–595. doi: 10.1104/pp.123.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Östin A, Sandberg G, Sundberg B. A novel metabolic pathway for indole-3-acetic-acid in apical shoots of Populus tremula (L.) × Populus tremuloides (Michx.) Plant Physiol. 1994;106:1511–1520. doi: 10.1104/pp.106.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weert M, Lagerwerf FM, Haverkamp J, Heerma W. Mass spectrometric analysis of oxidized tryptophan. J Mass Spectrom. 1998;33:884–891. [Google Scholar]