Abstract
Recently, we recognized two genes, gbpA and gbpB, encoding putative cGMP-binding proteins with a Zn2+-hydrolase domain and two cyclic nucleotide binding domains. The Zn2+-hydrolase domains belong to the superfamily of β-lactamases, also harboring a small family of class II phosphodiesterases from bacteria and lower eukaryotes. Gene inactivation and overexpression studies demonstrate that gbpA encodes the cGMP-stimulated cGMP-phosphodiesterase that was characterized biochemically previously and was shown to be involved in chemotaxis. cAMP neither activates nor is a substrate of GbpA. The gbpB gene is expressed mainly in the multicellular stage and seems to encode a dual specificity phosphodiesterase with preference for cAMP. The enzyme hydrolyses cAMP ∼9-fold faster than cGMP and is activated by cAMP and cGMP with a KA value of ∼0.7 and 2.3 μM, respectively. Cells with a deletion of the gbpB gene have increased basal and receptor stimulated cAMP levels and are sporogeneous. We propose that GbpA and GbpB hydrolyze the substrate in the Zn2+-hydrolase domain, whereas the cyclic nucleotide binding domains mediate activation. The human cGMP-stimulated cAMP/cGMP phosphodiesterase has similar biochemical properties, but a completely different topology: hydrolysis takes place by a class I catalytic domain and GAF domains mediate cGMP activation.
INTRODUCTION
cAMP and cGMP are important signaling molecules in prokaryotes and eukaryotes. These molecules are produced by cyclases, degraded by phosphodiesterases, and exert their functions by binding to specific proteins. In prokaryotes, cAMP regulates gene expression via binding to the cyclic nucleotide binding (cNB) domain of catabolic repressor transcription factors (Passner et al., 2000). In eukaryotes, cAMP and cGMP regulate enzyme and channel activity mainly through protein kinases, RapGEFs, or channels (Houslay and Milligan, 1997; Lohmann et al., 1997; De Rooij et al., 1998; Kraemer et al., 2001). In addition to this large family of cAMP/cGMP binding proteins, some phosphodiesterases contain a GAF domain, which is an unrelated cGMP-binding domain that regulates enzyme activity (Francis et al., 2000).
cAMP is probably present in all eukaryotes and cAMP-dependent protein kinase is a universal target even in primitive eukaryotes. Much less is known about the synthesis and function of cGMP in the lower eukaryotes. Yeast seems to lack cGMP, because the genome of Saccharomyces cerevisiae does not provide indications for putative guanylyl cyclases or cGMP-binding domains. Guanylyl cyclases have been identified in Paramecium, Tetrahymena, and Plasmodium, but the role of cGMP in these organisms is not yet resolved (Linder et al., 1999; Carucci et al., 2000).
In Dictyostelium, cAMP has an extracellular function as chemoattractant and an intracellular function as inducer of development (Reymond et al., 1995). Extracellular cAMP binds to G protein-coupled receptors, which results in the activation of several signaling systems, including adenylyl cyclase, guanylyl cyclase, phosphatidylinositol 3-kinase, and calcium channels (Van Haastert and Kuwayama, 1997; Parent and Devreotes, 1999; Chung et al., 2001). The produced intracellular cAMP is partly secreted where it activates neighboring cells. Intracellular cAMP may also bind to the regulatory subunit of cAMP-dependent protein kinase, mediating gene regulation and development. Eventually, cAMP is degraded by the extracellular phosphodiesterase PsdA (Lacombe et al., 1986) and by the intracellular phosphodiesterase RegA (Shaulsky et al., 1998; Thomason et al., 1998).
Activation of the cAMP receptor also results in the transient activation of guanylyl cyclases. The produced cGMP is rapidly degraded, mainly by a cGMP-stimulated cGMP-specific phosphodiesterase (Ross and Newell, 1981; Van Haastert et al., 1982b). As a consequence of the brief activation of guanylyl cyclases and the substrate stimulation of phosphodiesterase activity, the cGMP accumulation has the shape of a spike with a maximum at 10 s and recovery of basal levels after 30 s. The function of cGMP in Dictyostelium probably concentrates on chemotaxis and osmoregulation, as was suggested by mutants defective in cGMP metabolism (Kuwayama et al., 1993, 1996). Mutant stmF lacks the cGMP-stimulated phosphodiesterase (PDE) activity, whereas mutant KI8 shows very low levels of guanylyl cyclase activity. The genes defective in these mutants have not been identified.
To understand the function of cGMP in Dictyostelium it is essential to identify the genes that encode cGMP-metabolizing enzymes and cGMP target proteins. Recently, we characterized two unusual guanylyl cyclases in Dictyostelium, GCA and sGC, that are not related to vertebrate guanylyl cyclases, but are homologous to 12-transmembrane and soluble adenylyl cyclase, respectively (Roelofs et al., 2001a,b). In addition, four genes were identified, named gbpA-gbpD, which possess putative cNB domains (Goldberg et al., 2002). GbpC and GbpD are likely to mediate cGMP functions, because these proteins contain Ras, Kinase, and RasGEF domains besides the two putative cGMP-binding domains. Previous experiments have shown that Dictyostelium contains a cGMP-stimulated cGMP-phosphodiesterase (Van Haastert et al., 1982a; Coukell et al., 1984). We speculated that the cGMP-stimulated cGMP-phosphodiesterase is encoded by GbpA or GbpB, because these proteins contain a putative cGMP-binding domain and a Zn2+-binding hydrolase domain that is distantly related to a small family of class II phosphodiesterases (Carfi et al., 1995). We have inactivated the four gbp genes and analyzed the resulting cell lines for myosin phosphorylation and chemotaxis (Bosgraaf et al., 2002). The experiments identified a cGMP-signaling cascade in which G protein-coupled receptors stimulate two novel guanylyl cyclases. The produced cGMP is transduced via GbpC to regulate myosin phosphorylation and assembly in the cytoskeleton, which are critical for chemotaxis. GbpA and GbpB were shown to be involved in the degradation of cGMP (Bosgraaf et al., 2002). Herein, we report on the characterization of GbpA as the cGMP-stimulated cGMP-specific phosphodiesterase absent in mutant stmF, whereas GbpB seems to be a phosphodiesterase with dual specificity with respect to substrate and activation by both cAMP and cGMP.
MATERIALS AND METHODS
Strain and Culture Conditions
AX3 (“wild-type”), DH1 (an uracil auxotroph wild-type, kindly provided by P.N. Devreotes; Johns Hopkins Medical School, Baltimore, MD), and the mutant cell lines described below were grown in HG5 medium (HL5 with 10 g/l glucose) to a density of ∼2 × 106 cells/ml. When grown with selection, HG5 medium was supplemented with 10 μg/ml blasticidine S. Starved cells were obtained by shaking for 4–5 h in 10 mM phosphate buffer (PB), pH 6.5, at a density of 107 cells/ml. Tight aggregates were obtained by starving the cells on nonnutrient agar for ∼10 h; aggregates were collected in PB, washed by centrifugation, and disrupted to small cell clumps by passing the aggregates 10 times through a 0.5 × 16-mm needle.
Gene Disruption
The disruptant strains were obtained as described previously (Bosgraaf et al., 2002). Briefly, a 468-base pair genomic fragment of gbpA was obtained by polymerase chain reaction (PCR) by using primers TCATAGATCTAGAAGGTGATTATACAG and AGTTGGATCCATTGTTGCTAATTC. The PCR product was subcloned, and the Bsr selection cassette (Sutoh, 1993) was cloned into the MslI site of the genomic fragment. To disrupt the gbpB gene, a PCR product of 900 base pairs was amplified using the primers CCATTCTATGTGAAGTCAATC and AATTACTACTTACCAGCACC. The pyr5/6 cassette was cloned in the BclI restriction site. The selection cassette with gbp flanking sequences was amplified by PCR and ∼5 μg of the PCR product was used to transform Dictyostelium DH1 cells. To select for transformants with the bsr cassette, HG5 was supplemented with 10 μg/ml blasticidin, whereas transformants with the pyr5/6 cassette were selected using uracil-deficient FM medium (Bio 101, Vista, CA). Potential knockouts were screened by PCR and confirmed by Southern analysis.
Overexpression of GbpB in Dictyostelium
The full-length copy of gbpB without introns was obtained from cDNA fragments and PCR products. The gbpB sequence started with AGATCTAAAAATGAATTCTAAATAT (the BglII restriction site underlined and the start codon in bold), whereas the sequences had a BamHI restriction site engineered after the stop codon. The DNA was sequenced to verify the absence of mutations. The BglII/BamHI fragment of full-length gbpB was cloned in the BglII site of plasmid AH2 and transformed to gbpA−/gbpB− double-null cells. Plasmid AH2 is derivative of the extrachromosomal plasmid MB12neo (Heikoop et al., 1998), except that the Neo selection and gene expression cassettes contain the actin8 terminator.
Phosphodiesterase Assay of Dictyostelium Lysates
Cells were washed twice with PDE lysis buffer (40 mM HEPES/NaOH, pH 7.0, 0.5 mM EDTA) and resuspended at a density of 108 cells/ml in PDE lysis buffer supplemented with 0.25 M sucrose. Cells were lysed by passage through a 0.45-μm Nuclepore filter. The lysate was centrifuged for 2 min at 14,000 × g and the supernatant was used.
The PDE assay mixture (final concentrations) contained assay buffer (40 mM HEPES/NaOH, pH 7.0, 0.5 mM EDTA, 0.25 M sucrose, 5 mM MgCl2), 10 nM [3H]cAMP, or 10 nM [3H]cGMP as substrate, 5 mM dithiothreitol to inhibit the very active PDE1, and 30 μl of lysate in a total volume of 100 μl; the lysates were diluted to achieve between 10 and 30% hydrolysis of substrate. After incubation for 15 min at 22°C, reactions were terminated by boiling for 1 min. The product was dephosphorylated by calf intestine phosphatase (1 unit of enzyme in 100 μl of CIP buffer incubated for 1 h at 37°C). Finally, 300 μl of a 50% slurry of DOWEX AG1X2 was added to remove remaining substrate. After 15-min incubation at 22°C, samples were centrifuged for 2 min at 14,000 × g, and the radioactivity in 200 μl of the supernatant was determined.
cAMP and cGMP Responses
Cells were starved for 5 h in PB, washed, and resuspended in PB to a density of 108 cells/ml. For determination of the cGMP response, cells were stimulated with 0.1 μM cAMP and lysed at the times indicated by the addition of an equal volume of 3.5% (vol/vol) perchloric acid. Cells were stimulated with 10 μM 2′deoxy-cAMP and 10 mM dithiotreitol for induction of the cAMP response. Lysates were neutralized with KHCO3, and cGMP and cAMP levels were determined by isotope dilution assays by using a cGMP-specific antibody or the regulatory subunit of cAMP-dependent protein kinase, respectively.
Spore Formation
The assay for induction of spore formation is essentially as described previously (Shaulsky et al., 1998; Thomason et al., 1998). Cells were washed and resuspended to a density of 4 × 105 cells/ml in spore buffer (10 mM MES, 10 mM NaCl, 10 mM KCl, 1 mM CaCl2, 1 mM MgSO4, pH 6.5), and 500 μl of the suspension was added to a well of a 24-well plate, yielding a density of 105 cells/cm2. Cells were incubated in the absence or presence of 5 mM cAMP or 20 μM Sp-cAMPS. After 36 h, when some spore-like cells were observed in some incubations, the buffer was replaced by PB with 0.5% (vol/vol) NP-40 to kill remaining amoebae. After 15 min at 22°C, samples were centrifuged for 3 min at 1000 × g, the pellet was washed twice with PB, and resuspended in 100 μl of PB. The number of viable spores was determined by plating 2 μl of the suspension in association with Klepsiella aerogenes. The number of colonies was determined three days later, and could be maximally 4000 if all amoebae were retrieved and converted to viable spores.
RESULTS
Topology of GbpA and GbpB
GbpA and GbpB are both composed of two potential cNB domains and one Zn2+-binding domain (Figure 1). The alignment of the four cNB domains, together with the cNB domains of bacterial CAP protein, Drosophila protein kinase G (PKG), Dictyostelium protein kinase A (PKA), Caenorhabditis elegans cyclic nucleotide regulated channel, and Epac are presented in Figure 2A. The Dictyostelium cNB domains of GbpA and GbpB comply reasonably well with the consensus sequence, but are more divergent than for instance the cNB domains of Dictyostelium cAMP-dependent PKA. In the crystal structure of the CAP protein (Passner et al., 2000), cAMP interacts mainly with the amino acids IGEL and RSAxV (Figure 2A). In PKA, the amino acid at the position of the serine in RSA is an alanine, whereas in PKG this amino acid is a threonine and mutagenesis to alanine provides cAMP binding (Shabb et al., 1991). This region is relatively poorly conserved in GbpA and GbpB, especially in the second cNB domains. The first cNB domains of both GbpA and GbpB possess a serine at the position of RTA, which may suggest that the first cNB domains more likely bind cGMP than cAMP. However, the cNB domains of GbpA and GbpB are also homologous to CAP proteins, which bind cAMP and cGMP with similar affinity and contain a serine at this position.
Figure 1.
GbpA and GbpB. GbpA and GbpB have the same domain topology; a Zn2+-hydrolase putative catalytic domain and two cNB domains. The asterisks indicate the position of disruption in the knockout cell lines. The topology of the cGMP-stimulated cAMP/cGMP phosphodiesterase from human (HsPDE) is shown for comparison; this enzyme has the same biochemical properties as GbpA, but is composed of a class I PDE domain and two cGMP-binding GAF domains.
Figure 2.
Sequence alignment. (A) Alignment of the four cNB domains from GbpA and GbpB with the cNB domains from Drosophila PKG, Dictyostelium regulatory subunit of PKA, human Epac, Escherichia coli CAP, and a C. elegans cyclic nucleotide-gated channel. The consensus is based on the alignment of 140 cNB domains; a black background indicates amino acids conserved in >80% of the sequences and a gray background in >60% of the sequences. The consensus sequence refers to hydrophobic (h), polar (o), small (s), and negatively charged (−) amino acids, or to specific amino acids (capital letters). The asterisks (*) denote the amino acids that have been shown to interact with cAMP in the CAP crystal structure (Passner et al., 2000). (B) Alignment of the putative Zn2+-binding motifs of two Zn2+-hydrolase domains from GbpA and GbpB with those from Dictyostelium β-lactamase (Dd-BLA), Bacillus cereus β-lactamase (Bc-BLA), human glyoxalase (Hs-GLO2), Vibrio fischeri phosphodiesterase (Vf-PDE), Schizosaccharomyces pombe phosphodiesterase (Sp-PDE), and Dictyostelium phosphodiesterase (Dd-PDE1). The consensus is based on 98 sequences with gray scales indicating conserved amino acids in >95% (black) or >80% (gray) of the sequences. The asterisks (*) indicates amino acids that are conserved in class II PDEs but are variant in GbpA and GbpB.
The Zn2+-binding domains of GbpA and GbpB show a high degree of identity to each other (44% identity) and belong to the superfamily of β-lactamases with a metal-dependent hydrolase fold (Figure 2B). This domain is characterized by conserved histidines and aspartates that are also present in GbpA and GbpB. The superfamily of Zn2+-binding domains contains many hydrolases such as β-lactamases, glyoxalases, and class II cyclic nucleotide phosphodiesterases (Carfi et al., 1995). SMART and Pfam programs recognize the Zn2+-binding domains of GbpA and GbpB as β-lactamases, but not as class II phosphodiesterases. The alignment reveals several amino acids that are conserved in class II phosphodiesterases, but not in GbpA and GbpB (Figure 2B, asterisks). Also phylogenetic analysis indicates that the Zn2+-binding domains of GbpA and GbpB are more closely related to the β-lactamases than to the monophyletic group of class II phosphodiesterases (our unpublished data).
GbpA Encodes a cGMP-stimulated cGMP-specific Phosphodiesterase
To investigate the function of GbpA and GbpB, Dictyostelium cells were transformed with knockout constructs. Clones were screened by PCR for putative knockout strains and confirmed by Southern blots (data not shown). In this way, three cell lines were obtained with single and double knockouts of the gbp genes. The expression of gbpA and gbpB in knockout strains was investigated using Northern blots, demonstrating the absence of expression of even a truncated messenger in the knockout strains (data not shown).
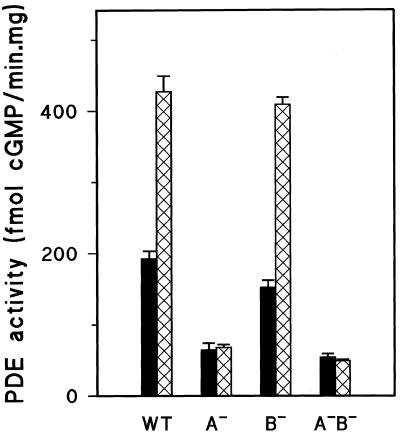
The main cGMP-phosphodiesterase activity in Dictyostelium can be stimulated by the analog 8-bromo-cGMP (Van Haastert et al., 1982b). To test whether the cGMP-stimulated cGMP-phosphodiesterase is encoded by gbpA and/or gbpB, we measured cGMP-phosphodiesterase activity in the absence and presence of 8-bromo-cGMP in the gbp− null strains. High levels of cGMP-PDE activity were found in wild-type cells, and this activity was stimulated two- to threefold by 8-bromo-cGMP (Figure 3). This enzyme activity was also present at high levels in gbpB− null cells, indicating that gbpB does not encode the enzyme. In contrast, cGMP-phosphodiesterase activity was very low in gbpA− null cells, and this small activity was not stimulated by 8-bromo-cGMP. The residual activity in gbpA− cells had the kinetic properties of DdPDE3 (low KM value and inhibition by isobutylmethylxanthine; our unpublished data; Kuwayama et al., 2001). The double mutant gbpA−/gbpB− had a similar low cGMP-phosphodiesterase activity as gbpA−. These results indicate that gbpA encodes the well-characterized cGMP-stimulated cGMP-phosphodiesterase activity in Dictyostelium and that gbpB may encode a phosphodiesterase with different biochemical properties.
Figure 3.
cGMP-PDE activity in vegetative gbp− null cells. The hydrolysis of 10 nM [3H]cGMP in the absence (black bar) or presence of 1 μM 8-bromo-cGMP (hatched bar) was measured in the supernatant of a lysate prepared from 2-h starved wild-type DH1 cells (WT), gbpA− cells (A−), gbpB− cells (B−), and the double-null gbpA−/gbpB− cells (A−B−). The results shown are the means and SDs of three independent experiments with triplicate determinations and reveal a loss of 8-bromo-cGMP–stimulated phosphodiesterase activity in gbpA− cells.
GbpB May Encode a Dual Specificity Phosphodiesterase Stimulated by cGMP and cAMP
Northern blots reveal that gbpB is expressed maximally in the multicellular stage (Goldberg et al., 2002). Therefore, we measured phosphodiesterase activity in lysates prepared from tight aggregates. Phosphodiesterase activity of gbpA− null cells is composed of several (partly unknown) phosphodiesterases except GbpA, whereas gbpA−/gbpB− cells possess the same mixture of enzymes except GbpA and GbpB. Thus, by subtracting the activity of gbpA−/gbpB− lysates from gbpA− lysates, information on GbpB is obtained. Similarly, the difference of enzyme activity between gbpB− and gbpA−/gbpB− yields the biochemical properties of GbpA. Assays were conducted with [3H]cAMP or [3H]cGMP as substrate in the absence or presence of 8-bromo-cAMP or 8-bromo-cGMP as activators (Figure 4).
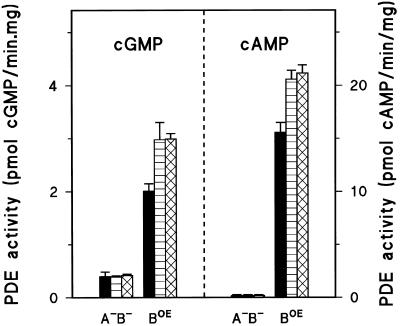
Figure 4.
cGMP and cAMP PDE activity in tight aggregate gbp− null. Cells were starved and developed on nonnutrient agar until the tight aggregate stage, collected, dissociated, and lysed. The hydrolysis of 10 nM [3H]cGMP or [3H]cAMP was measured in the absence (black bar) or presence of 1 μM 8-bromo-cGMP (cross-hatched bar) or 8-bromo-cAMP (striped bar) in the supernatant of a lysate prepared from gbpA− cells (A−), gbpB− cells (B−), and the double-null gbpA−/gbpB− cells (A−B−). The difference in activities between A−B− and A− characterizes GbpB, whereas the difference between A−B− and B− provides information of GbpA. The results confirm the observations in Figure 3 that GbpA encodes a cGMP-stimulated cGMP-PDE, and suggest that GbpB hydrolyzes cAMP and perhaps cGMP and is activated by both 8-bromo-cAMP and 8-bromo-cGMP.
The lysates prepared from tight aggregates of gbpA−/gbpB− double null cells contain a small cGMP- and cAMP-hydrolyzing activity that is not affected by 8-bromo-cAMP or 8-bromo-cGMP. GbpA is characterized by the additional activity in gbpB− cells, demonstrating cGMP-hydrolyzing activity that is stimulated fourfold by 8-bromo-cGMP; 8-bromo-cAMP has no effect, and cAMP is not a substrate. These deduced properties of GbpA in tight aggregates are essentially identical to those of GbpA in aggregation-competent cells described above. GbpB was characterized using gbpA− cells, showing a small cGMP- and a larger cAMP-hydrolyzing activity on top of the cGMP- and cAMP-hydrolyzing activity of gbpA−/gbpB− double-null cells. This activity is stimulated by both 8-bromo-cAMP and 8-bromo-cGMP. These findings suggest that GbpB might be a dual specificity phosphodiesterase, both in respect to the substrate as well as the activator. However, the activity is rather low for a full biochemical characterization of GbpB, and therefore we overexpressed GbpB in Dictyostelium.
Overexpression of GbpB in Dictyostelium gbpA−/gbpB− Cells
GbpB was expressed in growing cells from a strong actin promoter by using the extrachromosomal expression vector AH2. We used the double null gbpA−/gbpB− as host to have a null background of GbpA and GbpB enzyme activity. The lysates of gbpA−/gbpB−/GbpBOE cells contain cAMP- and cGMP-hydrolyzing activity that is much higher that the activity observed in lysates from gbpA−/gbpB− cells (Figure 5). The increase of cAMP-hydrolyzing activity is 15 pmol/min/mg protein, which is ∼60-fold higher than the estimated endogenous GbpB activity of wild-type cells at 10 nM cAMP (∼0.26 pmol/min/mg protein; Table 2). Overexpression of GbpB provides a much smaller increase of cGMP-hydrolyzing activity (1.6 pmol/min/mg protein above the activity in gbpA−/gbpB− cells), indicating that GbpB is ∼9-fold more active toward cAMP than toward cGMP. Both the cAMP- and cGMP-hydrolyzing activity are stimulated ∼1.5-fold by 1 μM 8-bromo-cGMP and 8-bromo-cAMP. These data on overexpressed GbpB confirm the provisional conclusions on phosphodiesterase activity in tight aggregates of gbpA− that GbpB is a dual-specificity phosphodiesterase with preference for cAMP.
Figure 5.
Overexpression of GbpB in gbpA−/gbpB− cells The hydrolysis of 10 nM [3H]cGMP or [3H]cAMP was measured in the absence (black bar) or presence of 1 μM 8-bromo-cGMP (cross-hatched bar) or 8-bromo-cAMP (striped bar) in the supernatant of a lysate prepared from gbpA−/gbpB− cells (A−B−) and from gbpA−/gbpB−/GbpBOE (BOE). The results demonstrate that GbpB hydrolyses cAMP ∼8-fold faster than cGMP; both 8-bromo-cAMP and 8-bromo-cGMP activate the enzyme.
Table 2.
Properties of six Dictyostelium PDEs
| Phosphodiesterase | PDE1 | PDE2 | PDE3 | PDE4* | PDE5 | PDE6 |
|---|---|---|---|---|---|---|
| Name of gene | psdA | regA | DdPDE3 | DdPDE4 | gbpA | gbpB |
| Localization | Cell surface | Cytosol | Cytosol | Cell surface | Cytosol | Cytosol |
| Class | II | I | I | I | II | II |
| cAMP/cGMP selectivity | 3 | >200 | ∼0.0015 | Unknown | <0.003 | 9 |
| cAMP hydrolysis | ||||||
| KM (μM) | 0.8 | 5 | >100 | Unknown | >500 | 200 |
| (150) | (1800) | |||||
| VMAX (pmol/min/mg) | 700 | 50 | – | Unknown | – | 5200 |
| KA (μM) | – | – | – | Unknown | >300 | 0.7 |
| AMAX | – | – | – | – | – | 1.47 |
| cGMP hydrolysis | ||||||
| KM (μM) | 1.8 | >1000 | 0.22 | –* | 5.2–20 | 800 |
| VMAX (pmol/min/mg) | 490 | – | 2 | –* | 390 | 2400 |
| KA (μM) | – | – | – | –* | 0.16 | 2.3 |
| AMAX | – | – | – | – | 2.40 | 1.86 |
| Intracellular cAMP degradation (pmol/min/mg) | ||||||
| 0.1 μM | – | 1 | – | –* | – | 2.6 |
| 1 μM | – | 8 | – | –* | – | 26 |
| 5 μM | – | 25 | – | –* | – | 127 |
| Intracellular cGMP degradation (pmol/min/mg) | ||||||
| 0.1 μM | – | – | 0.6 | –* | 3.3 | 0.3 |
| 1 μM | – | – | 1.6 | –* | 48 | 3.0 |
| 5 μM | – | – | 1.9 | –* | 163 | 15 |
PDE4
, the enzyme has not been characterized biochemically; sequence data suggest that the enzyme is cAMP specific and has a signal sequence and two transmembrane segments predicting the catalytic domain to be extracellular. The cAMP/cGMP selectivity refers to the calculated VMAX/KM for cAMP divided by the VMAX/KM for cGMP. The data for the KM in parentheses refer to the cAMP concentration inducing half-maximal inhibition of the hydrolysis of cGMP. The kinetic constants were derived from Van Haastert et al. (1983) for PDE1; from Shaulsky et al. (1998) and Thomason et al. (1998) for PDE2, and from Figure 4 to calculate the VMAX in vivo, assuming that cAMP hydrolysis in gbpA−/gbpB− is derived from RegA; for PDE6 from Kuwayama et al. (2001) for PDE3; from Figure 7 for PDE5; and from Figure 6 for PDE6. The rates of intracellular degradation were calculated using the obtained kinetic constants and are presented for three concentrations representing basal levels (0.1 μM), maximal levels in wild-type cells (1 μM), and maximal levels in some deletion mutants (5 μM).
Biochemical Properties of GbpA and GbpB
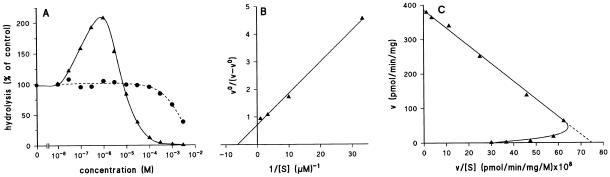
The biochemical properties of GbpB were determined using the gbpA−/gbpB−/GbpBOE cells (Figure 6). The hydrolysis of 10 nM [3H]cAMP or 10 nM [3H]cGMP was measured in the absence or presence of different concentrations of cAMP or cGMP, respectively. Figure 6A demonstrates that low concentrations of cAMP stimulate the hydrolysis of 10 nM [3H]cAMP, whereas concentrations above 10 μM cAMP inhibit the hydrolysis of [3H]cAMP. For the hydrolysis of 10 nM [3H]cGMP we observed similar properties: stimulation at low concentrations of cGMP and inhibition at high cGMP concentrations. These data were used to obtain the activation constant KA, the Michaelis-Menten constant KM, and the VMAX of GbpB. Figure 6B demonstrates that cAMP and cGMP stimulate the enzyme maximally 1.5- and 1.9-fold, respectively. The KA value is 0.71 μM for cAMP and 2.3 μM for cGMP. The data on the hydrolysis of cAMP and cGMP are presented as Eady-Hofstee plot in Figure 6C, demonstrating activation at low concentrations and linear curves at higher concentrations. The slopes of the linear parts yield a KM value of 200 μM for cAMP and 800 μM for cGMP, whereas the intercepts with the abscissa yield a VMAX value of 650 and 300 nmol/min/mg protein for cAMP and cGMP, respectively.
Figure 6.
Characterization of GbpB. A lysate was prepared from gbpA−/gbpB−/GbpBOE cells and incubated with 10 nM [3H]cGMP or [3H]cAMP in the presence of different concentrations of unlabeled cGMP or cAMP, respectively. The effect of unlabeled cAMP (●) or cGMP (▴) on the hydrolysis of the radioactive tracer is presented in A. These data are converted in panel B (see Van Haastert and Van Lookeren Campagne, 1984); vo is the hydrolysis in the absence and v in the presence of the indicated concentrations of unlabeled substrate [S]. The plot allows the determination of the activation constant KA (intercept abscissa is −1/KA) and the maximal fold activation AMAX (intercept ordinate is 1/(AMAX − 1)). The data of A were also used for an Eady-Hofstee plot (C) to calculate the KM value of the activated enzyme (slope is −1/KM) and VMAX (intercept ordinate). The obtained kinetic data are listed in Table 2.
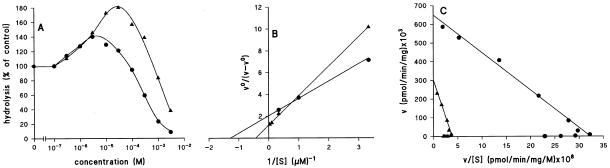
The biochemical properties of GbpA were derived from a partially purified enzyme from wild-type cells (Van Haastert and Van Lookeren Campagne, 1984) by using the same analysis as for GbpB. The enzyme preparation does not show hydrolysis of [3H]cAMP, indicating that cAMP hydrolysis is at least 100-fold slower than cGMP. Figure 7A reveals that low concentrations of cGMP stimulate the hydrolysis of [3H]cGMP, whereas concentrations above 1 μM inhibit the hydrolysis of [3H]cGMP; cAMP does not activate the hydrolysis of [3H]cGMP but inhibits at very high concentrations with a KI value of 1.8 mM. The activation constant KA of GbpA for cGMP is 0.16 μM, and the enzyme is activated maximally 2.4-fold (Figure 7B). The Eady-Hofstee plot reveals a Michaelis-Menten constant KM value for cGMP of 5.2 μM.
Figure 7.
Characterization of GbpA. A partially purified preparation of GbpA was prepared from wild-type cells and incubated with 10 nM [3H]cGMP in the presence of different concentrations of unlabeled cGMP (▴) or cAMP (●). The enzyme preparation does not show detectable hydrolysis of [3H]cAMP, indicating that cAMP is hydrolyzed at least 100-fold slower than cGMP. The kinetic constants for cGMP were derived from the plots in B and C, as described in Figure 6, and are listed in Table 2.
In summary, GbpA and GbpB are novel cyclic nucleotide stimulated cyclic nucleotide phosphodiesterases. GbpA is a cGMP-specific enzyme, whereas GbpB is a dual specificity enzyme with preference for cAMP. Activation of GbpB occurs at higher cGMP concentrations than activation of GbpA and does not discriminate between cAMP and cGMP; in contrast, activation of GbpA is at least 300-fold more specific for cGMP than for cAMP.
cGMP Response in gbpA and gbpB Mutants
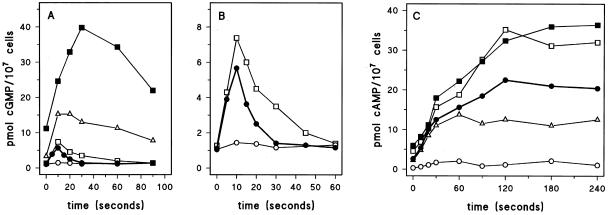
The consequences of deletion of gbpA and gbpB on basal cGMP levels and on the cAMP-mediated cGMP response of 5-h starved cells are presented in Figure 8A. Basal cGMP levels in wild-type cells are ∼1 pmol/107 cells that increase to 6 pmol/107 cells upon stimulation with cAMP; maximal levels are obtained after 10 s, and basal levels are recovered after ∼30 s. Deletion of the cGMP-stimulated cGMP-PDE in gbpA− cells leads to an increase of basal cGMP levels from 1 to 3 pmol/107 cells. The cAMP-mediated cGMP response is enlarged from 6 to ∼15 pmol/107 cells; the cGMP accumulation continues and persists for a longer period than in wild-type cells, causing the cGMP peak to occur at 20 s; basal levels are recovered after ∼120 s. The altered cGMP response in gbpA− cells is essentially identical to the cGMP response in the mutant stmF, which also lacks the cGMP-stimulated cGMP-PDE (Van Haastert et al., 1982a).
Figure 8.
cGMP and cAMP responses in gbp− mutant cells. The cell were starved for 5 h followed by stimulation with 0.1 μM cAMP for the cGMP response (A and B) and with 10 μM 2′-deoxy-cAMP and 10 mM dithiotreitol for the cAMP response (C). The symbols refer to wild-type DH1 (●); gbpA− (▵); gbpB− (□); gbpA−/gbpB− (▪), and gbpA−/gbpB−/GbpBOE (○). Identical data are presented for wild-type DH1, gbpB−, and gbpA−/gbpB−/GbpBOE in A and B. The results shown are the means of triplicate determinations from a typical experiment repeated once. The data in panel A were derived, in part, from Bosgraaf et al. (2002).
Disruption of gbpB has only a small effect on cGMP levels (Figure 8B); basal levels and the cGMP response are increased by ∼25% relative to wild-type cells, confirming the relatively small contribution of GbpB to the total cGMP-PDE activity in vivo. The potential cGMP-hydrolyzing activity of GbpB can be demonstrated in a gbpA− null cells, which lack the major cGMP-PDE activity. Disruption of gbpB in a gbpA− background results in a further increase of basal cGMP levels from 3 pmol/107 cells in gbpA− to 12 pmol/107 cells in gbpA−/gbpB−. The cAMP-induced cGMP response is also substantially enhanced and prolonged from a maximum of 15 pmol/107cells at 20 s after stimulation in gbpA− to 40 pmol/107 cells at 30 s after stimulation in the gbpA−/gbpB− strain; basal levels were reached after ∼3–4 min (Figure 8A). These results demonstrate that the low cGMP-PDE activity of GbpB becomes functionally significant when the much more active cGMP-PDE activity of GbpA is deleted.
Overexpression of GbpB in gbpA−/gbpB− cells whips out the dramatic cGMP response seen in the gbpA−/gbpB− cells (Figure 8B). Basal cGMP levels are ∼1.1 pmol/107 cells in the overexpressor strain relative to 12 pmol/107 cells in the parental gbpA−/gbpB− cells and 1 pmol/107 cells in wild-type cells. The cGMP response is also substantially reduced to 1.5 pmol/107 cells, which is even much lower than the cGMP response of wild-type cells.
cAMP Response in gbpA and gbpB Mutants
Stimulation of aggregation-competent cells with cAMP induces a transient accumulation of intracellular cAMP. In wild-type cells ∼50% of the produced cAMP is secreted and ∼50% is degraded intracellularly (Dinauer et al., 1980). We stimulated cells with 2′-deoxy-cAMP and dithiotreitol and measured the accumulation of cAMP in the cell suspension. The analog 2′-deoxy-cAMP binds to surface cAMP receptor with high affinity but does not interfere with the determination of cAMP levels. Dithiotreitol inhibits the surface and extracellular PDE activity encoded by the psdA gene, but has no effect on GbpA or GbpB (our unpublished data). Thus, in this experiment we detect receptor-stimulated cAMP formation that eventually accumulates in the extracellular medium; the data below are presented for 107 cells. Basal cAMP levels of wild-type cells is ∼2.6 ± 0.5 pmol (Figure 8C); 2′-deoxy-cAMP induces the accumulation of cAMP at an initial rate of ∼0.35 ± 0.06 pmol/s, and the final increase of (extracellular) cAMP is 18.7 ± 1.1 pmol above basal levels. Deletion of the cAMP-PDE in gbpB− cells leads to an increase of basal cAMP levels to 4.6 ± 0.7 pmol. The 2′-deoxy-cAMP–mediated increase of cAMP levels shows approximately the same initial rate as in wild-type cells (0.38 ± 0.10 pmol/s), but continues for a longer period by which eventually ∼1.6-fold more cAMP accumulates in the extracellular medium (28.2 ± 4.4 pmol). Basal cAMP levels and the cAMP response in gbpA−/gbpB− cells are essentially identical to the response seen in gbpB− cells. The normal initial cAMP accumulation rate in gbpB− and gbpA−/gbpB− cells strongly suggests that the receptor-stimulated production of cAMP is not altered in the mutants. The increased accumulation of extracellular cAMP indicates that, by deleting the cAMP-PDE activity of GbpB, intracellular cAMP is not effectively degraded and more cAMP is available for secretion. Because in wild-type cells ∼50% of the produced cAMP is degraded intracellularly, complete inhibition of this degradation would induce not more than a twofold increase of the cAMP response.
GbpA does not hydrolyze cAMP, but may affect the cAMP response indirectly, because the enzyme regulates cGMP levels, and cGMP activates the cAMP-PDE activity of GbpB. Consistent with this notion, we observed that the extracellular cAMP accumulation in gbpA− cells is reduced ∼50% relative to cAMP accumulation in wild-type cells. The initial cAMP accumulation rate is unaffected (0.33 ± 0.09 pmol/s), but the accumulation plateaus to a lower level (9.5 ± 1.1 pmol), suggesting that the same amount of cAMP is produced but less cAMP is available for secretion.
Overexpression of GbpB leads to a very strong reduction of the cAMP response, basal levels are decreased to 0.3 ± 0.2 pmol, ∼12% from wild-type cells, and the cAMP accumulation is only 1.2 ± 0.2 pmol, which is only 6% of the response seen in wild-type cells. The results suggest that GbpB is an important PDE to modulate intracellular cAMP levels. Null cells show increased cAMP levels, whereas overexpression leads to a strong reduction of cAMP.
Phenotypes of gbpA and gbpB Mutants
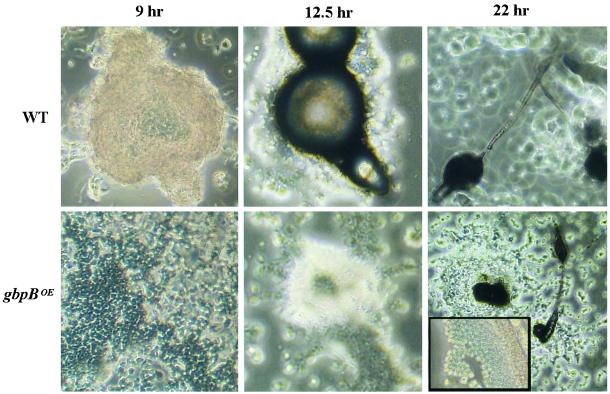
Cell aggregation of gbpA− cells, gbpB− cells, and gbpA−/gbpB− cells is normal compared with wild-type cells (our unpublished data). The aggregation time is not different from wild-type cells, and fruiting bodies have a relatively normal size. Overexpression of GbpB (gbpA−/gbpB−/gbpBOE cells) leads to very slow and poor aggregation (Figure 9). Cell aggregation in wild type starts at 8 h and fruiting body formation is completed after ∼20 h. The gbpA−B−/gbpBOE cells start to aggregate at ∼12 h after the onset of starvation, and slugs are first visible after 15 h, which is at least 4 h later than in wild-type cells. Eventually, fruiting bodies are formed after 27 h, but many cells do not participate in multicellular development.
Figure 9.
Phenotype of GbpBOE mutant cells. GbpB was overexpressed in gbpA−/gbpB− cells reaching an activity that was ∼50-fold higher than GbpB activity of wild-type cells. Photographs show the phenotypes at different times after starvation; the inset shows normal spore formation after 27 h in gbpA−/gbpB−/gbpBOE cells.
The phenotype of GbpB overexpression is similar to the phenotype of overexpression of RegA, the first characterized intracellular cAMP-PDE in Dictyostelium (Shaulsky et al., 1998; Thomason et al., 1998). Deletion of the regA gene has been shown to lead to increased intracellular cAMP levels, leading to a sporogenous phenotype: spores are formed in the multicellular stage earlier than in the wild type, and extracellular cAMP can induce spore formation in monolayers of regA− cells under buffer. We tested whether deletion of GbpB, the second cAMP-PDE in addition to regA, also leads to a sporogeneous phenotype (Table 1). Wild-type cells did not form spores when incubated with cAMP in submerged conditions. In contrast, a significant fraction of gbpB− and gbpA−/gbpB− cells had form spores. This was not observed for the gbpA− cells, indicating that the sporogenous phenotype is specific for deletion of a cAMP-PDE activity.
Table 1.
Spore formation in gbp mutants
| Strain | No. of viable spores | |
|---|---|---|
| Wild type | 0 | 0 |
| gbpA− | 0 | 1 |
| gbpB− | 28 | 47 |
| gbpA−/gbpB− | 30 | 46 |
Cells were incubated under buffer with cAMP for 36 h, treated with 0.5% NP-40 to kill amoebae, and plated in association with bacteria. The figures refer to the number of colonies obtained in two experiments, each derived from the original 4000 amoebae.
DISCUSSION
Two families of cyclic nucleotide phosphodiesterases have been recognized in eukaryotes, the ubiquitous class I phosphodiesterases present in essentially all eukaryotes and the small family of class II enzymes found in some bacteria, several yeast species, and Dictyostelium (the surface cAMP-phosphodiesterase PsdA). The class II enzymes belong to the superfamily of proteins with a Zn2+-binding hydrolase fold that also includes β-lactamases, glyoxylases, and arylsulfatases (Carfi et al., 1995). The GbpA and GbpB enzymes described in this report are phosphodiesterases and members of the superfamily, but sequence alignment and phylogeny suggest that they are not very closely related to the subfamily of class II phosphodiesterases (Goldberg et al., 2002; our unpublished data). The domain programs SMART and Pfam support this notion, because they recognize GbpA and GbpB as β-lactamases, but not as class II phosphodiesterases.
Inactivation and overexpression of the gbpA and gbpB genes indicate that gbpA encodes the cGMP-stimulated cGMP-specific phosphodiesterase characterized previously at a biochemical level (Van Haastert and Van Lookeren Campagne, 1984), whereas gbpB encodes a novel cAMP/cGMP-stimulated dual-specificity enzyme. Using about 20 cGMP analogs to characterize GbpA, it was demonstrated that the cyclic nucleotide specificity for activation and hydrolysis are very different, which was regarded as strong evidence that the enzyme possesses different cGMP-binding sites for activation and catalysis (Kesbeke et al., 1985). The domain structure of GbpA supports this hypothesis, because the enzyme is composed of a Zn2+-binding hydrolase fold, likely mediating hydrolysis of cGMP, and two cNB domains of which one is predicted to be a cGMP-binding regulatory domain. The kinetic properties of GbpA are summarized in Table 2, showing half-maximal activation at 0.16 μM cGMP, and a KM value of 5–20 μM cGMP for the cGMP-activated and -nonactivated enzyme, respectively. GbpA does not hydrolyze cAMP and is not stimulated by cAMP.
The biochemical phenotype of the gbpA− cells is very similar to that of the chemically mutated Dictyostelium stmF cell line, which both lacks the same cGMP-stimulated PDE activity (Ross and Newell, 1981; Van Haastert et al., 1982b). Two alleles of stmF are known, NP368 that lacks all GbpA-PDE activity, and NP377 that shows ∼5% of wild-type activity with altered KM for cGMP and altered KA for 8-bromo-cGMP (Van Haastert et al., 1982b; Coukell and Cameron, 1986). Therefore, we expected a severe mutation in NP368 leading to the absence of GbpA-PDE activity and a more subtle mutation in the open reading frame of NP377, leading to reduced and altered activity. Unexpectedly, we and Meima et al. (2002) have not been able to identify a DNA mutation in the gbpA gene of NP368 and NP377, respectively. NP368 shows normal mRNA levels for gbpA. The 5′-untranslated region of gbpA from NP368 was cloned between the actin promotor and GFP and did not reduce the expression of green fluorescent protein. The complete genomic copy of gbpA from NP368 was amplified and sequenced but did not reveal a mutation that would lead to inactivation of the expressed enzyme (such as stop codons or mutations in the proposed metal-binding catalytic site). We observed a Gly-to-Asp mutation at position 69, far before the proposed catalytic site; at this position the corresponding GbpB sequence has an Asp (L.B., H.R., and P.V.H., unpublished observations). Meima et al. (2002) observed reduced gbpA transcript levels in NP377 but could not detect any mutations in the promoter sequence.
The stmF mutants were originally isolated as “streamers,” making large streams of aggregating cells. However, revertants of the streamer phenotype have been shown still to be defective in cGMP-PDE activity, indicating that the streamer properties of stmF can be segregated from its altered cGMP-PDE activity (Coukell and Cameron, 1986). Consistent with this genetic analysis we did not observe a streamer phenotype in the cGMP-PDE–defective gbpA− cells. StmF mutants show an altered chemotaxis response during cell aggregation (Ross and Newell, 1981). However, wild-type cells mixed with a large portion of stmF mutant cells chemotax as mutant cells, whereas stmF cells mixed with a large portion of wild-type cells behave essentially as wild-type cells (Chandrasekhar et al., 1995). This suggests that the altered aggregation behavior of stmF is due to an altered chemotaxis signal rather than to a modified chemotaxis response. The reduced cAMP relay in gbpA− cells could explain this altered aggregation of stmF mutants.
GbpB is characterized as a dual-specificity enzyme with preference for cAMP; the enzyme is half-maximally activated by 2.3 μM cGMP and 0.7 μM cAMP, and hydrolyses cGMP ∼9-fold slower than cAMP. The catalytic site of GbpA shows high affinity and high selectivity for cGMP (KM = 5 μM cGMP and KI = 1800 μM cAMP), whereas GbpB has a much lower affinity and selectivity (KM = 800 μM cGMP and 200 μM cAMP). Experimental observations with cGMP analogs have demonstrated that cGMP is bound in the catalytic site of GbpA through a hydrogen bond to C6O, which cannot be formed with cAMP (Kesbeke et al., 1985). It is conceivable that this hydrogen bond potential is absent in GbpB by which the affinity for cGMP is low and no strong discrimination between cAMP and cGMP is possible in the catalytic site. Similar differences in the activator sites of GbpA and GbpB may explain the high affinity and selectivity of GbpA for cGMP relative to the nonspecific activation of GbpB.
GbpA and GbpB are the fifth and sixth PDE enzymes cloned in Dictyostelium. Therefore, these proteins may also be addressed as DdPDE5 and DdPDE6, respectively1. The Dictyostelium genome has been sequenced to >97% completion, suggesting that these six genes encode all phosphodiesterases in Dictyostelium, and we can begin with a detailed analysis of the relative contribution and function of the enzymes in modulating cAMP and cGMP levels in Dictyostelium (Table 2). PDE1, encoded by the psdA gene, is a class II nonselective enzyme located on the cell surface and in the extracellular medium (Lacombe et al., 1986). PDE2, encoded by the regA gene, is a cAMP-specific class I phosphodiesterase. The enzyme is located in the cytosol and is regulated by a histidine kinase and cAMP-dependent protein kinase (Shaulsky et al., 1998; Thomason et al., 1998). PDE3 is a high-affinity, cGMP-specific enzyme located in the cytosol (Kuwayama et al., 2001). PDE4 has not been characterized biochemically, but the primary sequence predicts the enzyme to be cAMP specific; furthermore, a putative signal sequence and two transmembrane segments are strongly indicated by structure prediction programs, suggesting that the enzyme is located at the plasma membrane with the catalytic domain in the extracellular medium (S.B. and P.V.H., unpublished observations). The six phosphodiesterases can be divided in three class I enzymes (PDE2, 3, and 4) and three class II enzymes (PDE1, 5, and 6). It is intriguing that GbpA shows similar biochemical properties as mammalian cGMP-stimulated phosphodiesterase, although the protein sequences are completely different. The catalytic domain of mammalian cGMP-stimulated phosphodiesterase belongs to the large family of PDE class I enzymes, and the cGMP-binding regulatory domain is unrelated to the cNB domain of GbpA but belongs to the group of GAF domains (Francis et al., 2000).
The cellular localization and cAMP/cGMP specificity of the six Dictyostelium phosphodiesterases suggest three functional groups: degradation of extracellular cAMP by PDE1 and PDE4, degradation of intracellular cAMP by PDE2 and PDE6, and degradation of intracellular cGMP by PDE3, PDE5, and PDE6. The estimated activities toward these substrates in vivo may provide information on the relative importance and functions of these enzymes. Because the biochemical properties of PDE4 have not been determined yet, the contribution of PDE4 in degradation of extracellular cAMP is unknown.
Intracellular cAMP is degraded by the basal activity of PDE2/RegA and PDE6/GbpB at approximately equal rates, suggesting that both enzymes are important. This hypothesis is supported by the observation that both regA− and gbpB− null cells are sporogenous. The finding that RegA is activated by cAMP-dependent protein kinase in vitro allows strong modulation of RegA phosphodiesterase activity by intracellular cAMP in vivo. Such modulation is also predicted for GbpB, which is activated by cAMP binding to its activating cNB domain. The regA− null cells may have a stronger phenotype than gbpB− null cells: regA− aggregates form multiple tips, whereas gbpB− aggregates are as in wild-type, and cAMP induces spore formation in ∼10% of regA− null cells vs. ∼1% of gbpB− null cells. It is conceivable that in vivo the activation of RegA by histidine kinase and cAMP-dependent protein kinase is stronger than the activation of GbpB by cAMP. In conclusion, intracellular cAMP is degraded by two complex phosphodiesterases that belong to different classes of enzymes and show entirely different mechanisms of regulation by cAMP.
Three enzymes participate in the degradation of intracellular cGMP. The relative affinities and capacities clearly demonstrate that PDE5/GbpA is the major cGMP-degrading enzyme in vivo. The high affinity but low capacity of PDE3 predicts that this enzyme mainly participates in modulating low cGMP concentrations. In agreement with this notion, we observed previously that PDE3 activity affects basal cGMP levels but does not contribute much to the degradation of the high cGMP levels that arise during stimulation (Kuwayama et al., 2001). The cGMP-PDE activity of PDE6/GbpB is also much smaller than the cGMP-PDE activity of PDE5/GbpA. These relative cGMP-PDE activities easily explain the effect of deletions of the three enzymes on basal cGMP levels. Single knockouts of the small PDE3 and PDE6 activities have little effect, whereas inactivation of the high PDE5/GbpA activity strongly affects cGMP levels. In a background of gbpA− cells, PDE3 and PDE6 are the only enzymes degrading cGMP, and have approximately equal activity. Therefore, deletion of gbpB in a gbpA− null background strongly increases cGMP levels. The function of cGMP is closely associated with chemotaxis via regulation of myosin II phosphorylation and myosin filament formation (de la Roche and Cote, 2001). We have elaborated on a large study toward the function of cGMP in myosin II regulation and chemotaxis by using cGMP phosphodiesterase mutants, also including double knockouts of the two guanylyl cyclases GCA and sGC and double knockouts of the two cGMP targets proteins GbpC and GbpD (Bosgraaf et al., 2002). The results demonstrate enhanced myosin II phosphorylation and filament formation in the gbpA−/gbpB− mutant with elevated cGMP levels; this increased myosin phosphorylation is associated with improved chemotaxis due to the suppression of lateral pseudopodia. This phenotype of the gbpA−/gbpB− mutant is consistent with the myosin and chemotaxis phenotype of mutant stmF that also has elevated cGMP levels (Liu and Newell, 1993; de la Roche and Cote, 2001).
In conclusion, we have identified novel cGMP- and cAMP-regulated phosphodiesterases with a combination of Zn2+-hydrolase and cNB domains not observed before. GbpA is a cGMP-stimulated cGMP-specific phosphodiesterase modulating cGMP levels, whereas GbpB is a dual-specificity phosphodiesterase with preference for cAMP modulating intracellular cAMP levels involved in multicellular development.
ACKNOWLEDGMENTS
We thank Janet Smith, Marcel Meima, and Pauline Schaap for stimulating discussions on the gbpA gene in stmF. This research was supported by the Netherlands Organization of Scientific Research.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0302. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0302.
The four previously recognized PDEs in Dictyostelium are as follows: PDE1, the class II enzyme encoded by the psdA gene (Lacombe et al., 1986); PDE2, the class I cAMP-specific enzyme encoded by the regA gene (Shaulsky et al., 1998; Thomason et al., 1998); PDE3, the class I cGMP-specific enzyme (Kuwayama et al., 2001); and PDE4, a sequence recognized in the database (clone JAX4b25f06.r1) coding for a class I putative phosphodiesterase; the cGMP-stimulated cGMP-PDE activity is not affected by disruption of the PDE4 gene (S.B. and P.V.H., unpublished data).
REFERENCES
- Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, Van Haastert PJM. A novel cGMP-signaling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002;21:4560–4570. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfi A, Pares S, Duee E, Galleni M, Duez C, Frere JM, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carucci DJ, Witney AA, Muhia DK, Warhurst DC, Schaap P, Meima M, Li JL, Taylor MC, Kelly JM, Baker DA. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J Biol Chem. 2000;275:22147–22156. doi: 10.1074/jbc.M001021200. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A, Wessels D, Soll DR. A mutation that depresses cGMP phosphodiesterase activity in Dictyostelium affects cell motility through an altered chemotactic signal. Dev Biol. 1995;169:109–122. doi: 10.1006/dbio.1995.1131. [DOI] [PubMed] [Google Scholar]
- Chung CY, Funamoto S, Firtel RA. Signaling pathways controlling cell polarity, and chemotaxis. Trends Biochem Sci. 2001;26:557–566. doi: 10.1016/s0968-0004(01)01934-x. [DOI] [PubMed] [Google Scholar]
- Coukell MB, Cameron AM, Pitre CM, Mee JD. Developmental regulation and properties of the cGMP-specific phosphodiesterase in Dictyostelium discoideum. Dev Biol. 1984;103:246–257. doi: 10.1016/0012-1606(84)90026-5. [DOI] [PubMed] [Google Scholar]
- Coukell MB, Cameron AM. Characterization of revertants of stmF mutants of Dictyostelium discoideum: evidence that stmF is the structural gene of the cGMP-specific phosphodiesterase. Dev Genet. 1986;6:163–177. doi: 10.1002/dvg.1020060303. [DOI] [PubMed] [Google Scholar]
- de la Roche MA, Cote GP. Regulation of Dictyostelium myosin I, and II. Biochim Biophys Acta. 2001;1525:245–261. doi: 10.1016/s0304-4165(01)00110-6. [DOI] [PubMed] [Google Scholar]
- De Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature, 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dinauer MC, MacKay SA, Devreotes PN. Cyclic 3′,5′-AMP relay in Dictyostelium discoideum. III. The relationship of cAMP synthesis and secretion during the cAMP signaling response. J Cell Biol. 1980;86:537–544. doi: 10.1083/jcb.86.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases. relating structure and function. Prog Nucleic Acid Res Mol Biol. 2000;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Bosgraaf L, Van Haastert PJM, Smith L. Identification of four candidate cGMP targets in Dictyostelium. Proc Natl Acad Sci USA. 2002;99:6749–6754. doi: 10.1073/pnas.102167299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikoop JC, Grootenhuis PD, Blaauw M, Veldema JS, Van Haastert PJM, Linskens MH. Expression of a bioactive, single-chain choriogonadotropin in Dictyostelium discoideum. Eur J Biochem. 1998;256:359–363. doi: 10.1046/j.1432-1327.1998.2560359.x. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Milligan G. Tailoring cAMP-signaling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–224. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- Kesbeke F, Baraniak J, Bulgakov R, Jastorff B, Morr M, Petridis G, Stec WJ, Seela F, Van Haastert PJM. Cyclic nucleotide specificity of the activator and catalytic sites of a cGMP-stimulated cGMP phosphodiesterase from Dictyostelium discoideum. Eur J Biochem. 1985;151:179–186. doi: 10.1111/j.1432-1033.1985.tb09083.x. [DOI] [PubMed] [Google Scholar]
- Kraemer A, Rehmann HR, Cool RH, Theiss C, de Rooij J, Bos JL, Wittinghofer A. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J Mol Biol. 2001;306:1167–1177. doi: 10.1006/jmbi.2001.4444. [DOI] [PubMed] [Google Scholar]
- Kuwayama H, Ecke M, Gerisch G, Van Haastert PJM. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- Kuwayama H, Ishida S, Van Haastert PJM. Non-chemotactic Dictyostelium discoideum mutants with altered cGMP signal transduction. J Cell Biol. 1993;123:1453–1462. doi: 10.1083/jcb.123.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H, Snippe H, Derks M, Roelofs J, Van Haastert PJM. Identification, and characterization of DdPDE3, a cGMP-selective phosphodiesterase from Dictyostelium. Biochem J. 2001;353:635–644. doi: 10.1042/0264-6021:3530635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe ML, Podgorski GJ, Franke J, Kessin RH. Molecular cloning and developmental expression of the cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum. J Biol Chem. 1986;261:16811–16817. [PubMed] [Google Scholar]
- Linder JU, Engel P, Reimer A, Kruger T, Plattner H, Schultz A, Schultz JE. Guanylyl cyclases with the topology of mammalian adenylyl cyclases and an N-terminal P-type ATPase-like domain in Paramecium, Tetrahymena and Plasmodium. EMBO J. 1999;18:4222–4232. doi: 10.1093/emboj/18.15.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Newell PC. Role of cyclic GMP in signal transduction to cytoskeletal myosin. Symp Soc Exp Biol. 1993;47:283–295. [PubMed] [Google Scholar]
- Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- Meima, M.E., Biondi, R.M., and Schaap, P. (2002). Identification of a novel cGMP phosphodiesterase that is defective in the chemotactic stmF mutants. Mol. Biol. Cell (in press). [DOI] [PMC free article] [PubMed]
- Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Passner JM, Schultz SC, Steitz TA. Modeling the cAMP-induced allosteric transition using the crystal structure of CAP-cAMP at 2.1 A resolution. J Mol Biol. 2000;304:847–859. doi: 10.1006/jmbi.2000.4231. [DOI] [PubMed] [Google Scholar]
- Reymond CD, Schaap P, Veron M, Williams JG. Dual role of cAMP during Dictyostelium development. Experientia. 1995;51:1166–1174. doi: 10.1007/BF01944734. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Meima M, Schaap P, Van Haastert PJM. The Dictyostelium homologue of mammalian soluble adenylyl cyclase encodes a guanylyl cyclase. EMBO J. 2001a;20:4341–4348. doi: 10.1093/emboj/20.16.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs J, Snippe H, Kleineidam RG, Van Haastert PJM. Guanylate cyclase in Dictyostelium discoideum with the topology of mammalian adenylate cyclase. Biochem J. 2001b;354:697–706. doi: 10.1042/0264-6021:3540697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FM, Newell PC. Streamers: chemotactic mutants of Dictyostelium discoideum with altered cyclic GMP metabolism. J Gen Microbiol. 1981;127:339–350. doi: 10.1099/00221287-127-2-339. [DOI] [PubMed] [Google Scholar]
- Shabb JB, Buzzeo BD, Ng L, Corbin JD. Mutating protein kinase cAMP-binding sites into cGMP-binding sites. Mechanism of cGMP selectivity. J Biol Chem. 1991;266:24320–24326. [PubMed] [Google Scholar]
- Shaulsky G, Fuller D, Loomis WF. A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development. 1998;125:691–699. doi: 10.1242/dev.125.4.691. [DOI] [PubMed] [Google Scholar]
- Sutoh K. A transformation vector for Dictyostelium discoideum with a new selectable marker bsr. Plasmid. 1993;30:150–154. doi: 10.1006/plas.1993.1042. [DOI] [PubMed] [Google Scholar]
- Thomason PA, Traynor D, Cavet G, Chang WT, Harwood AJ, Kay RR. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17:2838–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJM, Dijkgraaf PA, Konijn TM, Abbad EG, Petridis G, Jastorff B. Substrate specificity of cyclic nucleotide phosphodiesterase from beef heart and from Dictyostelium discoideum. Eur J Biochem. 1983;131:659–666. doi: 10.1111/j.1432-1033.1983.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJM, Kuwayama H. cGMP as second messenger during Dictyostelium chemotaxis. FEBS Lett. 1997;410:25–28. doi: 10.1016/s0014-5793(97)00416-x. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJM, Van Lookeren Campagne MM. Transient kinetics of a cGMP-dependent cGMP-specific phosphodiesterase from Dictyostelium discoideum. J Cell Biol. 1984;98:709–716. doi: 10.1083/jcb.98.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJM, Van Lookeren Campagne MM, Ross FM. Altered cGMP-phosphodiesterase activity in chemotactic mutants of Dictyostelium discoideum. FEBS Lett. 1982a;147:149–152. doi: 10.1016/0014-5793(82)81029-6. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJM, Van Walsum H, Van der Meer RC, Bulgakov R, Konijn TM. Specificity of the cyclic GMP-binding activity and of a cyclic GMP-dependent cyclic GMP phosphodiesterase in Dictyostelium discoideum. Mol Cell Endocrinol. 1982b;25:171–182. doi: 10.1016/0303-7207(82)90050-8. [DOI] [PubMed] [Google Scholar]