Abstract
When fibroblasts are placed within a three-dimensional collagen matrix, cell locomotion results in translocation of the flexible collagen fibrils of the matrix, a remodeling process that has been implicated in matrix morphogenesis during development and wound repair. In the current experiments, we studied formation and maturation of cell–matrix interactions under conditions in which we could distinguish local from global matrix remodeling. Local remodeling was measured by the movement of collagen-embedded beads towards the cells. Global remodeling was measured by matrix contraction. Our observations show that no direct relationship occurs between protrusion and retraction of cell extensions and collagen matrix remodeling. As fibroblasts globally remodel the collagen matrix, however, their overall morphology changes from dendritic to stellate/bipolar, and cell–matrix interactions mature from punctate to focal adhesion organization. The less well organized sites of cell–matrix interaction are sufficient for translocating collagen fibrils, and focal adhesions only form after a high degree of global remodeling occurs in the presence of growth factors. Rho kinase activity is required for maturation of fibroblast morphology and formation of focal adhesions but not for translocation of collagen fibrils.
INTRODUCTION
Form and function of multicellular organisms depends on tissue-specific programs of cell locomotion (Trinkaus, 1984). Much of what is known about cell locomotion comes from studies of cell migration, especially of fibroblastic cells, on rigid, planar substrata. Here, cell translocation occurs through lamellipodia extension and tail retraction coordinated with formation and turnover of cell adhesions (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996; Sheetz et al., 1999).
Morphologically, the most prominent sites of cell adhesion on planar surfaces are focal adhesions located beneath the cell's leading lamellipodia and in the tail region. Focal adhesions and the in vivo equivalent fibronexus junctions (Singer et al., 1984) are the paradigmatic example of integrin connection between the extracellular matrix and the internal cell cytoskeleton (Hynes, 1992; Burridge and Chrzanowska-Wodnicka, 1996). The precise role of focal adhesions in cell migration has been somewhat enigmatic, however, because their presence typically limits the capacity of cells to migrate (Burridge et al., 1988). Indeed, the tractional force for cell migration has been shown to be exerted at nascent adhesions (Galbraith and Sheetz, 1997; Oliver et al., 1999; Beningo et al., 2001). Nascent adhesions also can undergo tension-dependent strengthening (Wang and Ingber, 1994; Choquet et al., 1997) and mature into focal adhesions under the influence of the small G protein Rho (Clark et al., 1998; Rottner et al., 1999) and the Rho effector Rho kinase (Amano et al., 1997; Maekawa et al., 1999; Geiger and Bershadsky, 2001). In addition, focal adhesions have been shown to move, and their movement has been implicated both in regulation of cell migration (Smilenov et al., 1999) and in fibronectin fibril formation (Pankov et al., 2000; Zamir et al., 2000).
Compared with planar surfaces, much less is known about cell–matrix interactions in three dimensions. Fibroblasts incubated on top of three dimensional detergent-extracted embryo matrix were observed to move more rapidly in comparison with cells on planar substrata and were shown to form three-dimensional matrix adhesions whose molecular composition resembles focal adhesions with the major difference that paxillin but not focal adhesion kinase becomes phosphorylated (Cukierman et al., 2001).
When fibroblasts are placed within three-dimensional matrices such as native collagen, cell locomotion results in translocation of the flexible collagen fibrils of the matrix. This remodeling process resembles matrix morphogenesis during development and wound repair (Bell et al., 1979; Harris et al., 1981; Grinnell, 1994; Tomasek et al., 2002). Several different integrins can mediate collagen matrix remodeling, including α1β1 (Carver et al., 1995), α2β1 (Klein et al., 1991; Schiro et al., 1991), α11β1 (Tiger et al., 2001), and αvβ3 (Cooke et al., 2000). In addition, both cellular fibronectin (Yoshizato et al., 1999) and polymerized fibronectin (Hocking et al., 2000) enhance the remodeling process, although a direct role for α5β1 (fibronectin) receptors in remodeling has not been observed (Tomasek and Akiyama, 1992).
The mechanics of how cells exert force necessary for collagen matrix remodeling is unclear. Immediately after polymerization, the collagen matrix is highly pliable and cells remodel the matrix as they begin to spread (Grinnell, 1994; Eastwood et al., 1996; Freyman et al., 2002). As remodeling progresses, the overall mechanical properties of the matrix change, which in turn can influence the cells' tractional activity (Brown et al., 1998; Tranquillo, 1999; Grinnell, 2000; Shreiber et al., 2001).
Because cell tractional activity had been shown to change in response to mechanical properties of the matrix, we tested whether cell adhesion would change as remodeling of the collagen matrix progressed. To carry out these studies, conditions were established in which it was possible to distinguish local from global matrix remodeling. Our observations suggest that as fibroblasts encounter resistance in the matrices during global remodeling, their overall morphology changes from dendritic to stellate/bipolar, and cell–matrix interactions mature from punctate to focal adhesion organization. The less well organized sites of cell–matrix interaction are sufficient for translocating collagen fibrils. Rho kinase activity is required for maturation of fibroblast morphology and formation of focal adhesions but not for translocation of collagen fibrils.
MATERIALS AND METHODS
Growth Factors, Inhibitors, and Antibodies
Platelet-derived growth factor (PDGF-BB) was obtained from Upstate Biotechnology (Lake Placid, NY). Lysophosphatidic acid (LPA) and bovine serum albumin (BSA) fatty acid free were obtained from Sigma-Aldrich (St. Louis, MO). Rho kinase inhibitor Y27632 was a generous gift from Welfide (Osaka, Japan). Rat anti-β1 integrin (clone 9EG7) was purchased from BD PharMingen (San Diego, CA). Mouse anti-vinculin and α-actinin was purchase from Sigma-Aldrich. Alexa Fluor 488 goat anti-mouse and anti-rat IgG and rhodamine-conjugated phalloidin were obtained from Molecular Probes (Eugene, OR).
Cell Culture
All incubations with cells were carried out at 37°C in a humidified incubator with 5% CO2. Fibroblasts from human foreskin specimens (<10th passage) were maintained in 75-cm2 tissue culture flasks (Falcon Plastics, Oxnard, CA) in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Intergen, Purchase, NY). Fibroblasts to be used for time-lapse experiments were harvested by trypsinization, washed with DMEM/10% FBS, and incubated 30 min in DMEM/1% FBS with green fluorescent protein (GFP)-adenovirus (Ad5.CMV5-GFP; Q-Biogene, Carlsbad, CA) (5500 virus particles/cell). After infection, cells were cultured in DMEM/10% FBS overnight to allow GFP expression.
To prepare collagen matrices, cells were harvested from monolayer culture with 0.25% trypsin/EDTA (Invitrogen) and washed with DMEM/10% FBS followed by DMEM. Neutralized solutions of collagen (1.5 mg/ml) containing 105 cells/ml (low density, LD) or 106 cells/ml (high density, HD) were prewarmed to 37°C for 3–4 min and then 0.2-ml samples were placed in 24-well culture plates (Corning Glassworks, Corning, NY) for contraction assays or in 35-mm glass bottom No. 0 microwell dishes (MatTek, Ashland, MA) for time-lapse observations. In the culture dishes, each aliquot of collagen occupied an area outlined by a 12-mm-diameter circular score within a well. Matrices containing cells were incubated 60 min (zero time in the figures) after which 1 ml of basal medium (DMEM, 5 mg/ml BSA) was added with 10 μM LPA or 50 ng/ml PDGF added where indicated for the times shown.
Global Matrix Remodeling Measured by Contraction
At the end of the incubations, matrices in culture plates were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and washed with PBS. Matrix thickness was measured on an inverted microscope (Zeiss, Jena, Germany) equipped with dial test indicator (01–100 mm) (Mitutoyo) as described previously (Guidry and Grinnell, 1985). Data shown are averages and SDs from triplicate samples.
Local Matrix Remodeling Measured by Embedded Bead Movement
LD matrices were prepared containing GFP-expressing fibroblasts and fluorescent carboxylated modified microspheres (Molecular Probes) as a marker for the position of collagen fibrils (Roy et al., 1997), at a concentration of 4 × 107 beads/ml. After medium was added, microwell dishes were placed in an environmental chamber/air stream incubator with 37°C and 5% CO2. Cells surrounded by 10–15 beads in the visual field were selected to be observed. Five optical sections of the cell were made every 30 s by using a confocal laser-scanning inverted microscope (TCS-SP; Leica, Heidelberg, Germany). Laser power was selected based on preliminary experiments to avoid damaging the cells. Merging of optical sections, movie processing, and measurement of bead displacement were carried out using Openlab software (Improvision, Boston, MA).
To observe bead displacement, projections of optical sections that included the complete field recorded at different times, were artificially colored and superimposed, avoiding any shift of the image. To quantify bead displacement, the distance of the beads at each time, T1 and T2, was measured from the center of the frame, and the difference T1-T2 was calculated. Because the cell was in the center of the frame, local contraction was expected to result in centripetal movement of the beads toward the center of the field resulting in T2 < T1 everywhere in the visual field. On the other hand, a global shift in the matrix itself was expected to result in parallel translocation of the beads such that their relative movement with respect to the center of the visual field should average to zero. The appearance of halos surrounding some of the beads was a contrast artifact.
Fluorescence Microscopy
Samples were fixed for 30 min at room temperature with 3% paraformaldehyde in PBS, blocked with 1% glycine/1% BSA in PBS for 30 min. Samples to be stained for actin, α-actinin, or vinculin were permeabilized with 0.5% Triton X-100 in PBS for 10 min. To stain for actin, samples were incubated with rhodamine-conjugated phalloidin (8 U/ml) for 30 min followed by washes with PBS. Anti-α-actinin, anti-vinculin, and anti-β1 integrin were incubated with the samples for 60 min at room temperature, washed in PBS followed by 30-min incubation with Alexa-Fluor-488 anti-mouse or anti-rat IgG. After additional washes, samples were mounted on glass slides with Fluoromount G (Southern Biotechnology Associates, Birmingham, AL). Observations and micrographs were made using an inverted confocal laser-scanning microscope, and projections of optical sections were processed with TCS NT software (Leica).
RESULTS
Fibroblasts Remodel Collagen Matrices Locally or Globally Depending on Cell Number and Growth Factors
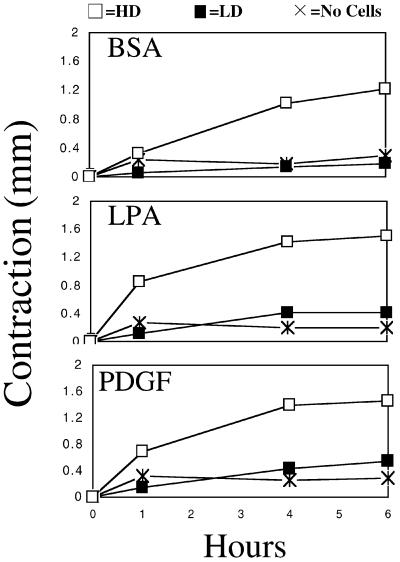
To observe changes in fibroblast adhesion as remodeling of the collagen matrix progressed, conditions were established in which the matrix remodeling process occurred at different rates. HD matrices containing 106 cells/ml or LD matrices containing 105 cells/ml were prepared and incubated in the presence of growth factors LPA or PDGF or basal medium (BSA). Global matrix remodeling, which results in matrix contraction and an increase in collagen density, was measured by decrease in matrix height (Guidry and Grinnell, 1985). Figure 1 shows that over a 6-h period, global matrix remodeling was evident in HD matrices in the presence of LPA or PDGF. In either case, contraction was ∼50% by 1 h and 80–90% by 6 h (starting height ∼1.8–1.9 mm). Remodeling also occurred in basal medium, but the rate was slower. The small change in height in the absence of cells is a consequence of adding medium to the polymerized matrices and after 1 h was no different from matrices containing cells but without added growth factors. Contraction independent of added growth factors could be observed by 4–6 h. Previously, it has been shown that this slower contraction in the absence of growth factors depends on an autocrine effect (Guidry and Grinnell, 1985).
Figure 1.
Global remodeling of collagen matrices. HD matrices (□), LD matrices (▪), or matrices without cells (×) were incubated for the times shown in basal medium (BSA) or medium containing LPA or PDGF. At the end of the incubations, contraction was determined by measuring reduction in cell height. In the presence of LPA or PDGF, matrix contraction was ∼50% by 1 h and 80–90% by 6 h (starting height ∼1.8–1.9 mm). In basal medium, the rate of contraction was slower with little contraction observed after 1 h and only 60–70% by 6 h. Studies were carried out in triplicate. SDs were smaller than the size of the points.
Figure 1 also shows that when the cell density was reduced 10-fold to 105 cells/ml (LD matrices), little if any global matrix remodeling was detected. Experiments then were carried out to determine whether cells in the LD matrices caused local remodeling even though there was no change in overall collagen organization. Local matrix remodeling was measured by embedding green fluorescent protein-expressing cells and fluorescent beads in the matrix and by using time-lapse laser-scanning confocal microscopy to track cell and bead movements (Roy et al., 1997, 1999).
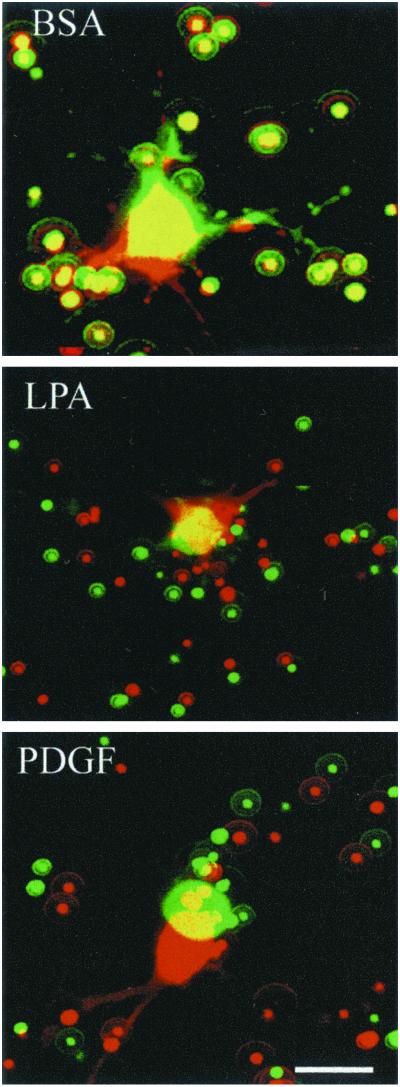
Figure 2 shows projections of optical sections that included the complete field recorded at 0 and 4 h, artificially colored green and red, respectively, and superimposed avoiding any shift of the image. Because the cell was in the center of the frame, local contraction was expected to result in centripetal movement of the beads toward the center of the field, whereas a global shift in the matrix itself was expected to result in parallel translocation of the beads.
Figure 2.
Local remodeling of collagen matrices. Images of cells from LD matrices at 1 h (green) and 4 h (red) in basal medium (BSA) or medium containing LPA or PDGF as indicated. Overlay shows displacement of beads during the incubations. Little bead displacement occurred in LD matrices in basal medium, although cell extensions were prominent and undergoing protrusion and withdrawal. Centripetal displacement of beads occurred in matrices in medium containing LPA or PDGF. Data shown are from representative films; two to four films were made for each condition. Bar, 20 μM.
In medium containing PDGF or LPA, red beads were closer than green beads to the center everywhere in the visual field, demonstrating a significant contraction of collagen toward the cell. Much less bead movement was seen, however, in DMEM/BSA medium, indicating that little collagen translocation occurred in the absence of growth factors. Cell bodies also showed a small degree of translocation during the incubation period, which tended to be greatest in PDGF-containing medium.
Visual observations were quantified by measuring the distances between the beads and the center of the visual field and calculating T1 (0 h) − T2 (4 h). Table 1 shows the results compared with no cell controls. Average bead movement toward the center of the field was ∼3.5 μm in LPA-containing medium and ∼5 μm in PDGF-containing medium, but close to zero in cell-free controls and in basal medium. These results were analogous to global remodeling of HD matrices after 1 h, in which case cell-dependent contraction occurred in LPA- or PDGF-containing medium but not in the absence of growth factors. An autocrine effect similar to that observed in HD matrices was not detected, probably because of the low cell number.
Table 1.
Bead displacement during local collagen matrix remodeling with different growth factors
| Treatment | Bead number | Bead displacement (μm)
|
p value |
|---|---|---|---|
| Average ± SD | |||
| Control (no cells) | 14 | 0.26 ± 1.45 | |
| BSA | 13 | −0.10 ± 0.26 | 0.186 |
| LPA | 15 | 3.48 ± 1.76 | <0.001 |
| PDGF | 11 | 4.86 ± 0.85 | <0.001 |
Measurements were made on images in Figure 2, as well as control matrices with no cells. Positive bead displacement reflects centripetal movement of the beads towards the center of the visual field during the 4-hr interval of the experiment. Student's t-Test was used for statistical comparison of each condition compared to no-cell controls.
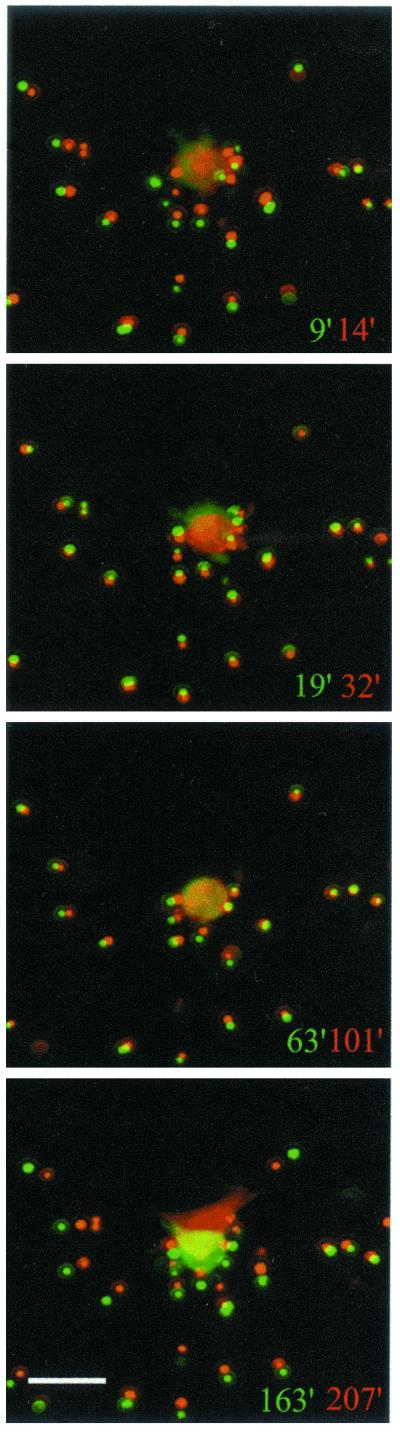
In the presence of PDGF, movement of beads toward the cells occurred in a unidirectional manner. In the presence of LPA, bead movement occurred in two waves, shown visually in Figure 3 and quantified in Table 2. The first wave occurred rapidly after addition of LPA; this corresponds to the time when the cells retract their dendritic network of extensions in response to LPA (Figure 4A). From 9′ to 14′, average bead displacement toward the cells was 1.5 μm. Subsequently (19′-32′), bead movement stopped or even reversed direction slightly. Bead displacement toward the cells began again around 1 h and averaged ∼1 μm from 63′ to 101′. During this time, the cells were just beginning to develop extensions. As new extensions formed, average bead displacement became larger, ∼2.4 μm from 163′ to 207′. These data indicated that there was no simple and direct relationship between formation or retraction of fibroblast extensions and the matrix-remodeling process.
Figure 3.
Time course of local remodeling of collagen matrices in LPA-containing medium. Images from LD matrices at the times shown. Overlaid images show displacement of beads during selected intervals. An initial period of centripetal bead displacement (9′ 14′) was followed by reversal (19′ 32′). During this time cells had retracted their extensions and started to bleb. After blebbing ceased, displacement of beads began again (63′ to 101′), which became more pronounced as the cells began to protrude new extensions (163′ to 207′). Data shown are from a representative film; three films were made. Bar, 14 μM.
Table 2.
Bead displacement during local remodeling of collagen matrices in LPA-containing medium
| Time interval (min) | Bead displacement (μm)
|
|---|---|
| Average ± SD | |
| 9–14 | 1.51 ± 0.66 |
| 19–32 | −0.63 ± 0.46 |
| 63–101 | 0.83 ± 0.30 |
| 163–207 | 2.37 ± 1.22 |
Measurements were made on images in Figure 3. Positive bead displacement reflects centripetal movement of the beads towards the center of the visual field during the time interval of the experiment. Number of beads measured at each time = 14.
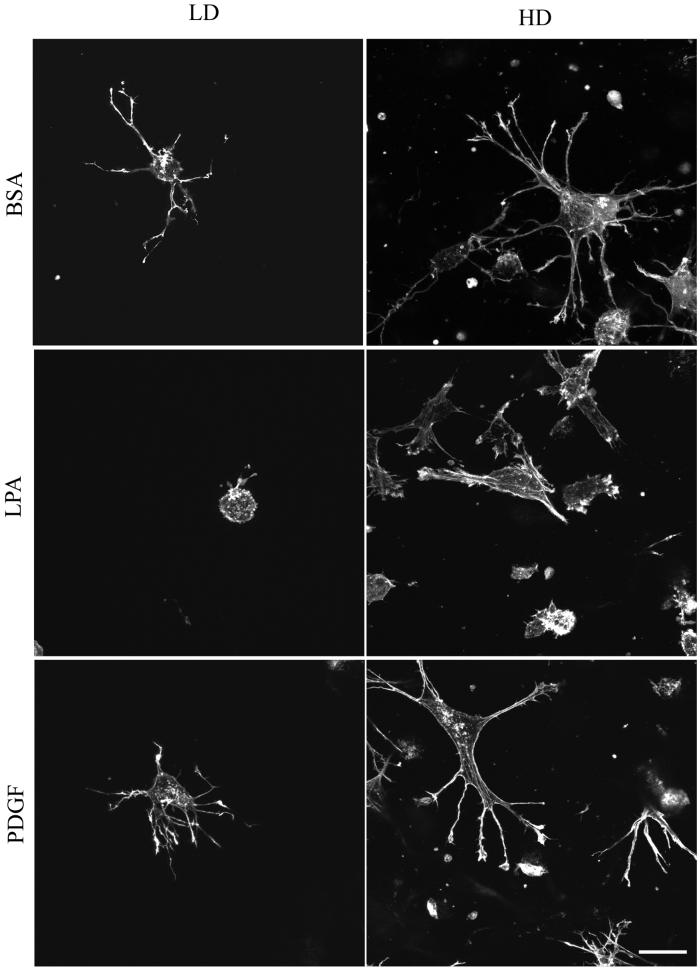
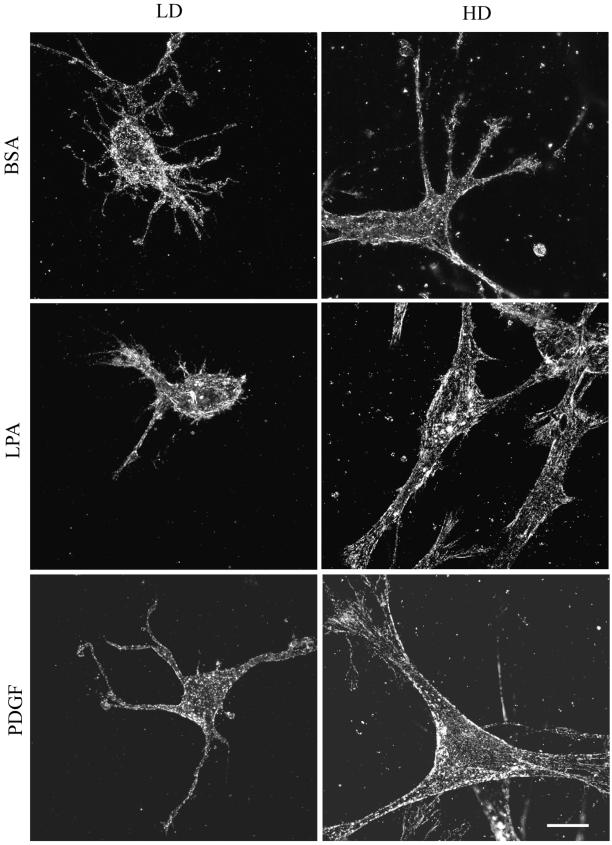
Figure 4.
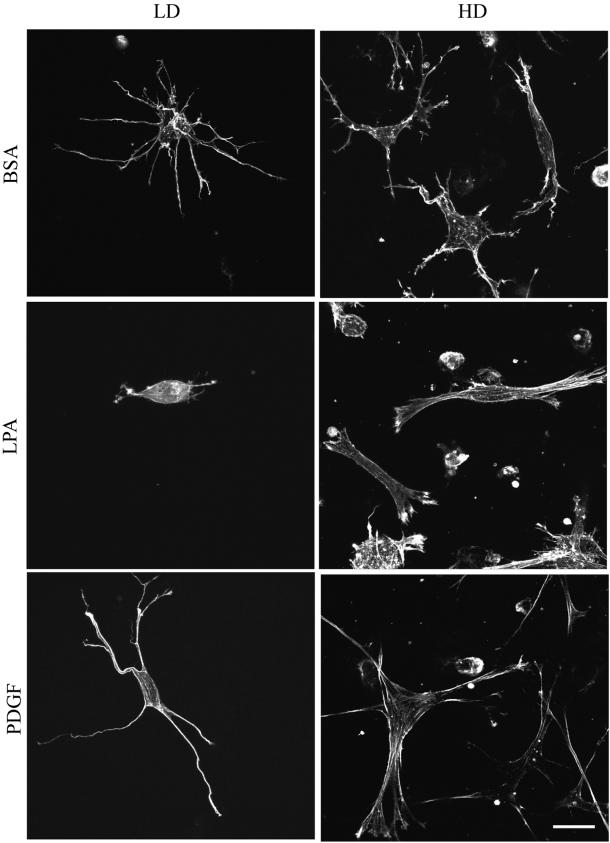
(A) Morphology of fibroblasts in LD and HD matrices after 1 h. LD matrices and HD matrices were incubated for 1 h in basal medium (BSA) or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for actin. Cells in matrices in basal medium or PDGF-containing medium formed a dendritic network of cell extensions. In LPA-containing medium these extensions were retracted completely in LD matrices and partly in HD matrices. In HD matrices and PDGF or LPA medium, actin stress fibers were evident. Bar, 17 μm. (B) Morphology of fibroblasts in LD and HD matrices after 4 h. LD matrices and HD matrices were incubated for 4 h in basal medium or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for actin. In LD matrices, cells in basal medium or PDGF-containing medium extended further the dendritic network of cell extensions. In LPA-containing medium, small extensions reappeared and tended to be organized in a bipolar manner in contrast to the circumferential distribution of extensions around cells in basal or PDGF medium. In HD matrices, cells became stellate or bipolar and contained stressed fibers in PDGF- and LPA-containing medium. Bar, 17 μm.
Cell Morphology Differs in LD and HD Matrices
The foregoing experiments established conditions in which fibroblasts remodeled collagen matrices either locally or globally depending on cell number and the presence of growth factors. Studies then were carried out to compare cell morphology and cell–matrix interactions under these different conditions. Figure 4A shows overall cell morphology after 1 h as visualized by phalloidin staining for actin. In LD matrices, cells in basal medium (DMEM/BSA) or PDGF-containing medium were surrounded by a dendritic network of cell extensions. The extensions were highly dynamic structures, observed by time-lapse microscopy to undergo continual protrusion and withdrawal through the matrix. Addition of LPA to cells in LD matrices, on the other hand, caused cells to retract their extensions. This retraction process, which depends on Rho and Rho kinase, is the subject of a different study (Grinnell et al., unpublished data). Figure 4B shows that after 4 h the network of extensions around cells in LD matrices had increased in size and complexity in basal medium (DMEM/BSA) or PDGF medium. In addition, short extensions reappeared in cells in LPA medium.
Figure 4, A and B, also show that fibroblasts in HD matrices, which had undergone substantial matrix remodeling and contraction by 1 h (Figure 1), developed more prominent extensions than cells in LD matrices. By 4 h, these extensions had thickened and simplified giving the cells a more stellate appearance in PDGF or basal medium or bipolar appearance in LPA-containing medium. Also, cells in PDGF- and LPA-containing medium began to develop actin stress fibers by 1 h, and these structures were found in most cells after 4 h (Figure 5B). These results suggested that as global matrix remodeling occurred, increased stiffness permitted the matrix to begin to resist the cells, in response to which the cells began to develop isometric tension and make the transition from dendritic to stellate/bipolar morphology. In contrast, cells in LD matrices, which do not undergo global remodeling, retain their dendritic appearance after 4 h and do not make the morphological transition.
Figure 5.
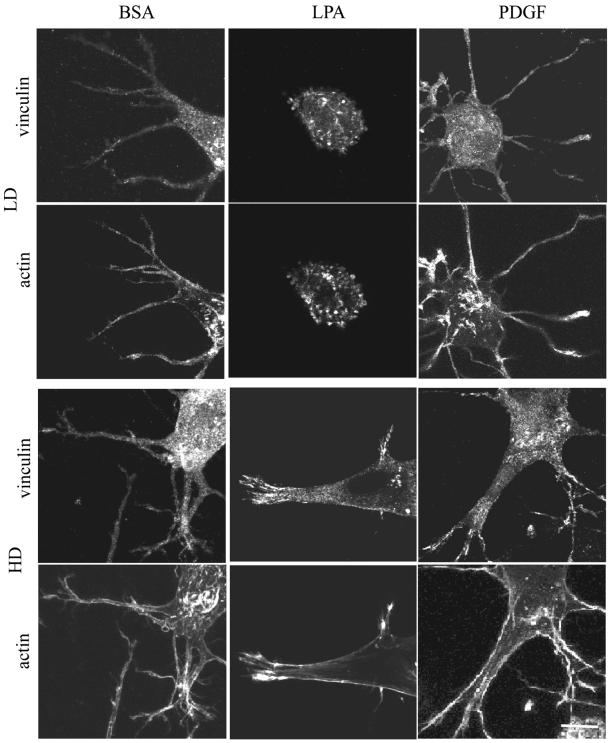
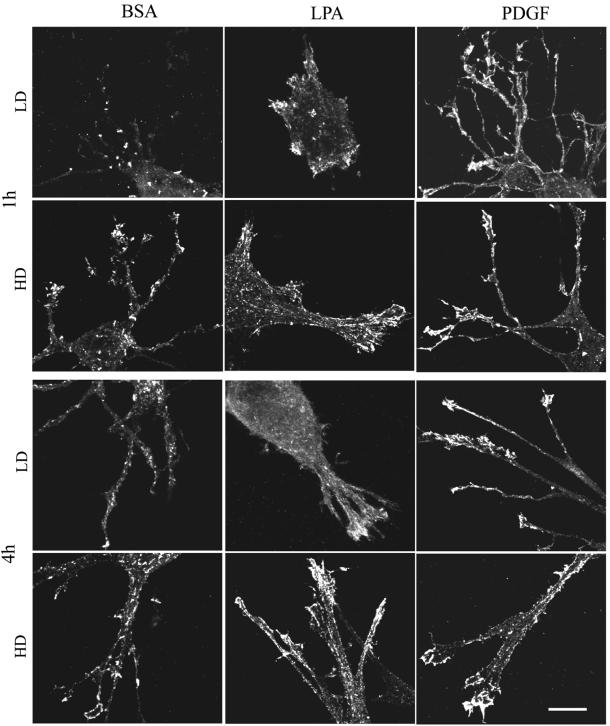
(A) Codistribution of vinculin and actin in fibroblasts in LD and HD matrices after 1 h. LD matrices and HD matrices were incubated for 1 h in basal medium (BSA) or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for actin and vinculin. Fibroblasts in LD matrices had diffuse vinculin staining. The large punctate spots of vinculin in cells stimulated by LPA might have been related to cell-blebbing activity. Actin stress fibers were undetectable. Cells in HD matrices had streaks of vinculin and short actin stress fibers at the tips of cell extensions in medium containing LPA or PDGF. Bar, 7 μm. (B) Codistribution of vinculin and actin in fibroblasts in LD and HD matrices after 4 h. LD matrices and HD matrices were incubated for 4 h in basal medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for actin and vinculin. In LD matrices after 1 h, vinculin staining was diffuse. In HD matrices and PDGF or LPA medium, vinculin streaks were prominent and widely distributed over the extensions, and actin stress fibers seemed to insert into the focal adhesions. Bar, 7 μm.
Focal Adhesions Develop within 4 h in Cells in HD Matrices in the Presence of Growth Factors
Experiments also were carried out to assess formation of focal adhesions in LD and HD matrices. Vinculin has been used as a marker for focal adhesion formation by fibroblasts in collagen matrices (Vaughan et al., 2000). The distribution of vinculin and actin in human fibroblasts in HD and LD matrices is shown in Figure 5, A (1 h) and B (4 h). Streaks of vinculin and short actin stress fibers were observed at the tips of cell extensions in HD matrices after 1 h in medium containing LPA or PDGF (Figure 5A). By 4 h, vinculin streaks were more prominent and widely distributed over the extensions (Figure 5B). In addition, actin stress fibers increased in length and seemed to insert into focal adhesions. In basal medium (DMEM/BSA), on the other hand, vinculin staining tended to be diffuse and no actin stress fibers were evident.
Rather than streaks, Figure 5, A and B, show that fibroblasts in LD matrices only had diffuse vinculin staining. The large punctuate spots of vinculin in LPA-stimulated cells (Figure 5A) might have been related to cell-blebbing activity, which was observed in the cells at this time. Actin stress fibers were undetectable in LD matrices after 1 h, but they did begin to develop after 4 h in medium containing LPA or PDGF. These results provided further evidence that the switch in fibroblast morphology during matrix remodeling reflected development of isometric tension within the cells. Significant matrix remodeling, however, seemed to be required before focal adhesions formed.
Ligand-occupied β1 Integrin Localizes around the Cell Body and along Cell Extensions
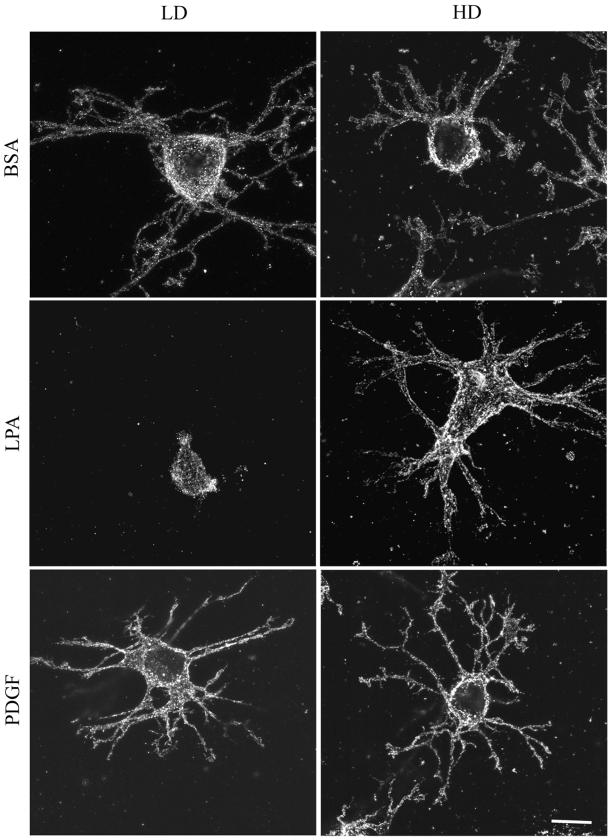
The distribution of β1 integrins on fibroblasts in HD and LD matrices was studied using antibody 9EG7, which is specific for the ligand-occupied receptor (Bazzoni et al., 1995). Staining was carried out using nonpermeabilized cells so that only cell surface receptors would be detected. Figure 6, A (1 h) and B (4 h) show that ligand-occupied β1 integrin could be detected on the cell body and extensions of fibroblasts in LD and HD matrices with or without growth factors. In LD matrices, the distribution was predominantly punctate after 1 h with some linear arrays evident by 4 h. Therefore, local matrix remodeling occurred under conditions when the punctate organization of β1 integrins predominated. Integrin occupancy alone, however, was not sufficient for remodeling because the punctate distribution of occupied integrins also occurred on cells in basal medium but there was much less matrix remodeling. In HD matrices, as was the case for actin and vinculin, linear arrays and streaks of integrins were visible by 4 h in LPA- or PDGF-containing medium, providing further evidence that focal adhesions formed under the latter conditions.
Figure 6.
(A) Distribution of β1 integrin in fibroblasts in LD and HD matrices after 1 h. LD matrices and HD matrices were incubated for 1 h in basal medium (BSA) or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for β1 integrin. Ligand-occupied β1 integrin could be detected in a punctate distribution on the cell body and extensions of fibroblasts in LD and HD matrices with or without growth factors. Bar, 10 μm. (B) Distribution of β1 integrin in fibroblasts in HD and LD matrices after 4 h. HD matrices and LD matrices were incubated for 4 h in basal medium or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for β1 integrin. In LD matrices, some linear arrays of β1 integrin were evident by 4 h. In HD matrices, linear arrays and streaks resembling focal adhesions occurred by 4 h in LPA- or PDGF-containing medium. Bar, 10 μm.
α-Actinin Localizes in a Punctate Distribution along Extensions of Cells in HD and LD Matrices and along Actin Stress Fibers of Cells in HD Matrices Stimulated with LPA or PDGF
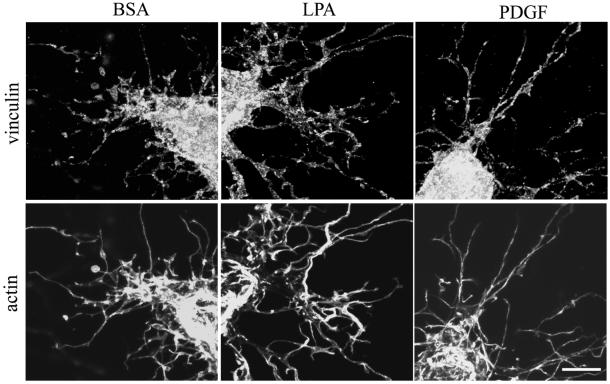
Studies also were carried out to determine the distribution of α-actinin, which can associate with both integrins and the actin cytoskeleton (Edlund et al., 2001; Laukaitis et al., 2001). Figure 7 shows that α-actinin staining accumulated at the tips of cell extensions in both LD and HD matrices, but staining was more evident in LPA or PDGF than basal medium (DMEM/BSA). In addition, in HD matrices, the α-actinin staining pattern of the extensions became organized into linear streaks after 4 h and localized along actin stress fibers (our unpublished data).
Figure 7.
Distribution of α-actinin in fibroblasts in LD and HD matrices after 1 or 4 h. HD matrices and LD matrices were incubated for 1 or 4 h in basal medium (BSA) or medium containing LPA or PDGF. At the end of the incubations, samples were fixed and stained for α-actinin. α-Actinin staining accumulated at the tips of cell extensions in both LD and HD matrices, but staining was more evident in LPA or PDGF than basal medium. In HD matrices, the α-actinin staining pattern of the extensions became organized into linear streaks after 4 h. Bar, 7 μm.
Blocking Rho Kinase Prevents Formation of Focal Adhesions and Stress Fibers but Only Partially Inhibits Global Remodeling of HD Matrices
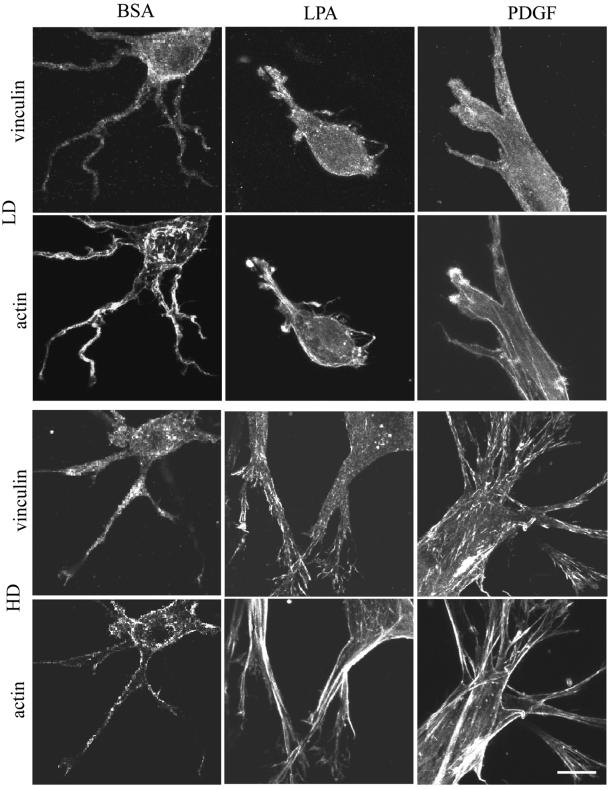
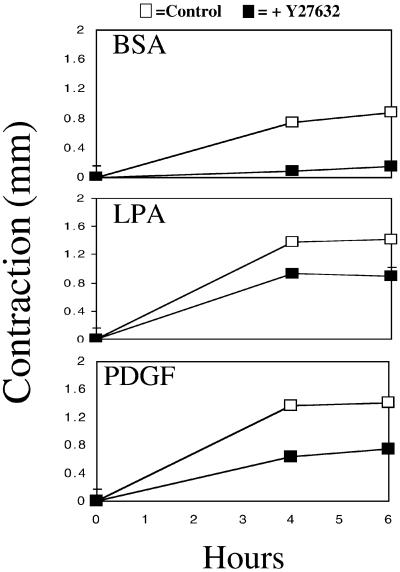
Finally, studies were carried out to determine the effects of the Rho kinase inhibitor Y27632 (Narumiya et al., 2000) on fibroblast morphology and adhesion in HD matrices as well as matrix remodeling. As mentioned in the INTRODUCTION, Rho kinase has been implicated in maturation of nascent cell adhesions into focal adhesions. Figure 8 shows that in the presence of the Rho kinase inhibitor, cells in HD matrices after 4 h were still surrounded by the dendritic network of cell extensions even in the presence of LPA. Actin stress fibers and streaks of vinculin staining were absent, indicating that both the stellate/bipolar morphology and formation of focal adhesions had been inhibited. On the other hand, as shown in Figure 9, substantial global matrix remodeling occurred with LPA- or PDGF-containing medium in the presence of the Rho kinase inhibitor. This finding provided direct evidence for previous observations that focal adhesions and stress fibers were a consequence and not a prerequisite for global matrix remodeling.
Figure 8.
Effect of Rho kinase inhibitor on codistribution of vinculin and actin in fibroblasts in HD matrices after 4 h. HD matrices were incubated for 4 h in basal medium (BSA) or medium containing LPA or PDGF in the presence of Rho kinase inhibitor Y27632 (10 μM). At the end of the incubations, samples were fixed and stained for actin and vinculin. Maturation of cells from the dendritic network of extensions to stellate/bipolar appearance was completely blocked and actin stress fibers and streaks of vinculin staining were absent. Bar, 7 μm.
Figure 9.
Effect of Rho kinase inhibitor on global remodeling of collagen matrices in the presence of Rho kinase inhibitor. HD matrices were incubated for the times shown in basal medium (BSA) or medium containing LPA or PDGF (□) or plus Rho kinase inhibitor Y27632 (10 μM) (▪). At the end of the incubations, contraction was determined by measuring cell height. Addition of Y27632 inhibited global remodeling in basal medium almost completely but only partly reduced remodeling in LPA and PDGF medium. Studies were carried out in triplicate. Data show averages and SDs (most of which were smaller than the size of the points).
DISCUSSION
The collagen matrix-remodeling process has been implicated in matrix morphogenesis during development and wound repair (Bell et al., 1979; Harris et al., 1981; Grinnell, 1994; Tomasek et al., 2002). Immediately after polymerization, the collagen matrix is highly pliable and cells remodel the matrix as they begin to spread (Grinnell, 1994; Eastwood et al., 1996; Freyman et al., 2002). As remodeling progresses, the overall mechanical properties of the matrix change, which in turn can influence the cells' tractional activity (Brown et al., 1998; Tranquillo, 1999; Grinnell, 2000; Shreiber et al., 2001). Because cell tractional activity had been shown to change in response to mechanical properties of the matrix, we tested whether cell adhesion would change as remodeling of the collagen matrix progressed.
Global remodeling of collagen matrices was quantified by measuring matrix height and occurred in HD matrices in a cell and growth factor-dependent manner, consistent with previous findings (Guidry and Grinnell, 1985). Slower remodeling of HD matrices observed after 4–6 h in the absence of added growth factors is thought to depend on cellular autocrine activity (Guidry and Grinnell, 1985). To assess local remodeling, observations were made on the movement of collagen-embedded beads, an adaptation of the method used to measure the ability of individual cells to exert force on collagen fibrils (Roy et al., 1997, 1999). LD matrices (10-fold reduced cell density compared with HD matrices) underwent local remodeling but little global remodeling. Like global matrix remodeling, local remodeling was cell and growth factor dependent, consistent with findings reported by others (Kolodney and Elson, 1993; Roy et al., 1999).
Analysis of cell morphology in LD matrices, which caused only local matrix remodeling, demonstrated that there was no direct relationship between local remodeling and protrusion or retraction of cell extensions. That is, bead translocation occurred when extensions were retracted as in the case of LPA or protruded as in the case of PDGF. On the other hand, protrusion and movement of the extensions alone, which was observed in basal medium, were not sufficient for remodeling. Moreover, in the absence of global matrix remodeling, cells in LD matrices did not undergo the transition from dendritic to stellate/bipolar shape or formation of focal adhesions.
In HD matrices, on the other hand, several changes occurred that could be attributed to global matrix remodeling. One of these was the overall increase in the size of cell extensions, which probably is the three-dimensional equivalent of the observation that on planar, flexible surfaces, fibroblasts spread more as the surface becomes less compliant (Pelham and Wang, 1997). Another was the different response by cells to the addition of LPA. Rather than retraction of extensions, increased resistance provided by the matrix resulted in stabilization of attached cell processes in the extended condition. This difference has been described in detail elsewhere (Grinnell et al., unpublished data). Finally, as discussed in more detail below, cells in HD matrices underwent a transition to stellate/bipolar morphology and developed stress fibers and focal adhesions.
In general, the extensions of dendritic fibroblasts in LD matrices or in HD matrices in the absence of growth factors were associated with an actin meshwork and punctate distribution of occupied β1 integrin receptors. Therefore, adhesive interactions of dendritic extensions seemed to be weak and under little tension (compared with focal adhesions). Nevertheless, these binding interactions were sufficient for local remodeling of collagen matrix as long as growth factors were present. It should be noted that several different β1 integrins have been implicated in matrix contraction (Klein et al., 1991; Schiro et al., 1991; Carver et al., 1995; Cooke et al., 2000). At present, we have no way of distinguishing which of the occupied β1 integrins observed in the punctate distribution was actually involved in remodeling. Integrin occupancy alone was not sufficient for remodeling, however, because we observed a punctate distribution of occupied integrins on cells in basal medium, but there was less matrix remodeling under these conditions. Staining of α-actinin, which can link integrins and the cell cytoskeleton (Edlund et al., 2001; Laukaitis et al., 2001; Rajfur et al., 2002), also was reduced in basal medium, so it may be that linkage between occupied integrins and components of cell cytoskeleton was compromised in the absence of growth factors.
In HD matrices, concomitant with global remodeling, fibroblast extensions seemed to reorganize and simplify over time. After 4 h, cells in PDGF medium appeared stellate, whereas cells in LPA medium tended to become bipolar. Previous studies on fibroblasts in collagen matrices often have emphasized the bipolar morphology of cells (Elsdale and Bard, 1972; Tomasek and Hay, 1984), which might be a response to the LPA in serum. In any case, the difference in cell shape in PDGF- compared with LPA-containing medium suggests that there is some type of topographic specificity in the cellular signaling systems responsive to these growth factors. In addition, along with the change in morphology, cell adhesion sites reorganized, resulting in the appearance of prominent focal adhesions containing vinculin and β1 integrins and associated actin stress fibers and α-actinin.
For focal adhesions to develop, substratum stiffness has to be able to resist the force that the cells exert. Therefore, the stiffness of newly polymerized collagen matrices was not sufficient to permit development of focal adhesions. Once sufficient collagen matrix remodeling occurred, however, it was possible to observe the transition from punctate to focal adhesions. Inhibiting Rho kinase blocked formation of focal adhesions by cells in collagen matrices and the cell transition from dendritic to stellate/bipolar morphology, consistent with the observation that tension-dependent adhesion strengthening resulting in formation of focal adhesions, depends on Rho kinase (Amano et al., 1997; Maekawa et al., 1999; Geiger and Bershadsky, 2001).
Despite preventing the transition in cell morphology and adhesion, inhibition of Rho kinase only partially prevented collagen matrix remodeling. Therefore, the question remains as to the identity of the motor mechanism required for collagen matrix remodeling. Fibroblasts can bend individual collagen fibrils bound to their surfaces, which has been postulated to occur by movement of integrin relative to each other (Lee and Loeser, 1999). Also, collagen beads bound to integrin receptors have been shown to translocate along the cell surface and eventually are phagocytosed (Lee et al., 1996). In neither case, however, has the motor mechanism responsible for translocation been identified. In fibroblasts and smooth muscle cells on planar surfaces, it has been reported that myosin phosphorylation and cell contractile activity can be independently stimulated by myosin light chain kinase and Rho kinase in different topographic regions of the cells (Totsukawa et al., 2000; Katoh et al., 2001; Miyazaki et al., 2002). Also, maturation of focal adhesions in size has been attributed in part to α-smooth muscle actin (Dugina et al., 2001). One possibility is that the cellular contractile activity required for collagen matrix remodeling switches from Rho kinase independent to Rho-kinase dependent as the stiffness of the matrix increases. This would explain why remodeling becomes completely dependent on Rho kinase activity in matrices that already have undergone sufficient remodeling so as to develop isometric tension (Parizi et al., 2000; Yanase et al., 2000).
In summary, our studies provide new evidence in support of the notion that the mechanism of force generation in collagen matrix remodeling varies with matrix resistance (Grinnell, 2000). The results suggest that as fibroblasts encounter resistance in the matrices, their morphology changes from dendritic to stellate/bipolar, and cell–matrix interactions mature from punctate to focal adhesion organization. The less well organized sites of cell–matrix interaction are sufficient for translocating collagen fibrils, and focal adhesions only form after a high degree of global matrix remodeling occurs in the presence of growth factors. Rho kinase activity is required for maturation of fibroblast morphology and formation of focal adhesions but not for translocation of collagen fibrils.
ACKNOWLEDGMENTS
We are grateful to Ross Payne for help with confocal microscope and to Drs. William Snell, Mathew Petroll, and James Jester for helpful comments. These studies were supported by grant GM-31321 from the National Institutes of Health.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0291. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0291.
REFERENCES
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Carver W, Molano I, Reaves TA, Borg TK, Terracio L. Role of the alpha 1 beta 1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol. 1995;165:425–437. doi: 10.1002/jcp.1041650224. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci. 2000;113:2375–2383. doi: 10.1242/jcs.113.13.2375. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- Eastwood M, Porter R, Khan U, McGrouther G, Brown R. Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J Cell Physiol. 1996;166:33–42. doi: 10.1002/(SICI)1097-4652(199601)166:1<33::AID-JCP4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Edlund M, Lotano MA, Otey CA. Dynamics of α-actinin in focal adhesions and stress fibers visualized with α-actinin-green fluorescent protein. Cell Motil Cytoskeleton. 2001;48:190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyman TM, Yannas IV, Yokoo R, Gibson LJ. Fibroblast contractile force is independent of the stiffness which resists the contraction. Exp Cell Res. 2002;272:153–162. doi: 10.1006/excr.2001.5408. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Sheetz MP. A micromachined device provides a new bend on fibroblast traction forces. Proc Natl Acad Sci USA. 1997;94:9114–9118. doi: 10.1073/pnas.94.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signaling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Guidry C, Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem. 2000;275:10673–10682. doi: 10.1074/jbc.275.14.10673. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am J Physiol Cell Physiol. 2001;280:C1669–C1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin α2β1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–23855. [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Loeser RF. Cell surface receptors transmit sufficient force to bend collagen fibrils. Exp Cell Res. 1999;248:294–305. doi: 10.1006/excr.1999.4418. [DOI] [PubMed] [Google Scholar]
- Lee W, Sodek J, McCulloch CA. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J Cell Physiol. 1996;168:695–704. doi: 10.1002/(SICI)1097-4652(199609)168:3<695::AID-JCP22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Yano T, Schmidt DJ, Tokui T, Shibata M, Lifshitz LM, Kimura S, Tuft RA, Ikebe M. Rho-dependent agonist-induced spatio-temporal change in myosin phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:725–734. doi: 10.1074/jbc.M108568200. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of α(5)β(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibers and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol. 2002;4:286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Roy P, Petroll WM, Cavanagh HD, Chuong CJ, Jester JV. An in vitro force measurement assay to study the early mechanical interaction between corneal fibroblasts and collagen matrix. Exp Cell Res. 1997;232:106–117. doi: 10.1006/excr.1997.3511. [DOI] [PubMed] [Google Scholar]
- Roy P, Petroll WM, Cavanagh HD, Jester JV. Exertion of tractional force requires the coordinated up-regulation of cell contractility and adhesion. Cell Motil Cytoskeleton. 1999;43:23–34. doi: 10.1002/(SICI)1097-0169(1999)43:1<23::AID-CM3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin α2β1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Felsenfeld D, Galbraith CG, Choquet D. Cell migration as a five-step cycle. Biochem Soc Symp. 1999;65:233–243. [PubMed] [Google Scholar]
- Shreiber DI, Enever PA, Tranquillo RT. Effects of pdgf-bb on rat dermal fibroblast behavior in mechanically stressed and unstressed collagen and fibrin gels. Exp Cell Res. 2001;266:155–166. doi: 10.1006/excr.2001.5208. [DOI] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Kazazis DM, Clark RA. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984;98:2091–106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration, and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Akiyama SK. Fibroblast-mediated collagen gel contraction does not require fibronectin-α5β1 integrin interaction. Anat Rec. 1992;234:153–160. doi: 10.1002/ar.1092340202. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J Cell Biol. 1984;99:536–549. doi: 10.1083/jcb.99.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquillo RT. Self-organization of tissue-equivalents: the nature and role of contact guidance. Biochem Soc Symp. 1999;65:27–42. [PubMed] [Google Scholar]
- Trinkaus J. Cells into Organs: The Forces that Shape the Embryo. Englewood Cliffs, NJ: Prentice-Hall; 1984. [Google Scholar]
- Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase M, et al. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277:72–78. doi: 10.1006/bbrc.2000.3634. [DOI] [PubMed] [Google Scholar]
- Yoshizato K, Tsukahara M, Oki T, Hayashi M, Obara M, Morpho Y. The interaction of cellular fibronectin with collagen during fibroblast-mediated contraction of collagen gels. J Invest Dermatol Symp Proc. 1999;4:190–195. doi: 10.1038/sj.jidsp.5640207. [DOI] [PubMed] [Google Scholar]
- Zamir E, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]