Abstract
We proposed that spindle morphogenesis in Drosophila embryos involves progression through four transient isometric structures in which a constant spacing of the spindle poles is maintained by a balance of forces generated by multiple microtubule (MT) motors and that tipping this balance drives pole-pole separation. Here we used fluorescent speckle microscopy to evaluate the influence of MT dynamics on the isometric state that persists through metaphase and anaphase A and on pole-pole separation in anaphase B. During metaphase and anaphase A, fluorescent punctae on kinetochore and interpolar MTs flux toward the poles at 0.03 μm/s, too slow to drive chromatid-to-pole motion at 0.11 μm/s, and during anaphase B, fluorescent punctae on interpolar MTs move away from the spindle equator at the same rate as the poles, consistent with MT-MT sliding. Loss of Ncd, a candidate flux motor or brake, did not affect flux in the metaphase/anaphase A isometric state or MT sliding in anaphase B but decreased the duration of the isometric state. Our results suggest that, throughout this isometric state, an outward force exerted on the spindle poles by MT sliding motors is balanced by flux, and that suppression of flux could tip the balance of forces at the onset of anaphase B, allowing MT sliding and polymerization to push the poles apart.
INTRODUCTION
Mitosis depends upon the action of the mitotic spindle, a bipolar protein machine that uses nucleotide hydrolysis to assemble itself and to segregate sister chromatids. The mechanical properties of the spindle depend upon microtubules (MTs) and MT-based motor proteins, but the precise mechanisms by which these components act to coordinate spindle morphogenesis and chromosome movements remain unclear (Karsenti and Vernos, 2001; Mitchison and Salmon, 2001; Wittman et al., 2001).
Several properties of the spindle machinery are well established. First, the structure and polarity patterns of MTs within many mitotic spindles are known (McIntosh and McDonald, 1989). Second, several MT-based motors are proposed to slide spindle MTs in relation to adjacent MTs or to other spindle structures, thus generating forces for positioning spindle poles and chromosomes (McIntosh et al., 1969; Sharp et al., 1999a, 2000b). Third, sets of mitotic motors generate counterbalancing forces (Hoyt and Geiser, 1996). Finally, spindle MTs are dynamic and the polymerization-depolymerization of MTs exert pushing and pulling forces that may help position spindle poles and chromosomes (Mitchison, 1989; Inoue and Salmon, 1995; Waters et al., 1996).
The notion that multiple MT cross-linking and sliding motors generate counterbalancing forces within the spindle was a key feature of our recent model, which attempts to explain the morphogenesis of mitotic spindles in Drosophila embryos (Sharp et al., 2000a, 2000b). Based on real time observations of spindle pole separation, we proposed that the spindle passes through a series of four transient isometric structures, characterized by a constant spacing between the spindle poles, which we previously referred to as steady state structures. These isometric structures were proposed to be generated when the outward and inward forces exerted on the poles by multiple MT sliding motors balance one another, and the transitions between them, during which the spindle poles move further apart, result from tipping this balance.
However, our model is incomplete because it has been known since the classic studies of Inoue and Sato (1967) that agents that enhance MT polymerization tend to make spindles larger, with poles spaced further apart, whereas agents that drive MT depolymerization cause spindle pole spacing to decrease, and this issue was not addressed (Sharp et al., 2000a, 2000b). Here, we have used fluorescent speckle microscopy (FSM; Waterman-Storer et al., 1999) to address the relationship between MT dynamics, and motor function during mitosis in Drosophila embryos.
The dynamic properties of spindle MTs, coupled to motor-dependent poleward MT translocation, underlie flux, the movement of tubulin subunits from MT plus ends facing the spindle equator to the MT minus ends facing the poles (Mitchison, 1989; Mitchison and Salmon, 1992). Flux was proposed to drive chromatid-to-pole movement during anaphase A (Desai et al., 1998; LaFountain et al., 2001), although in some systems it can only contribute part of the motion (Mitchison and Salmon, 1992; Zhai et al., 1995). In Drosophila embryos previous studies showed that cytoplasmic dynein is required for chromosome-to-pole movement (Sharp et al., 2000c), but whether flux also contributed to anaphase A was not investigated.
One candidate for driving the poleward MT translocation associated with flux is the C-terminal kinesin Ncd, which has mitotic as well as meiotic functions (Endow et al., 1994a). For example, Ncd mutants display centrosome and spindle pole defects in early embryonic mitotic spindles, leading to the proposal that Ncd cross-links interpolar MTs (ipMTs) to kinetochore MTs (kMTs), allowing it to attach centrosomes to spindle poles and to mediate the poleward MT translocation associated with flux in kinetochore fibers (Endow et al., 1994a). In addition, the loss of Ncd function causes spindle poles to separate faster than usual and suppresses the spindle collapse caused by inhibiting the bipolar kinesin, KLP61F suggesting that Ncd may cross-link and slide interpolar MTs exerting an inward force on the spindle poles (Sharp et al., 1999).
In this study we used FSM to observe MT flux and MT sliding in spindle morphogenesis and anaphase A in wild-type and Ncd null mutant embryos. We found that MT flux persists in both kMTs and ipMTs throughout the metaphase/anaphase A isometric state, but is too slow to account for chromatid-to-pole movement during anaphase A, and that a suppression of flux in ipMTs occurs at the onset of pole-pole separation in anaphase B. We observed that Ncd appears to stabilize the metaphase/anaphase A isometric state, possibly by restraining the sliding apart of antiparallel interpolar MTs, but loss of its activity does not affect the rate of flux or the rate of MT-MT sliding during anaphase B. We propose that flux, involving MT-polymerization at the equator and MT-depolymerization at the poles, balances motor-generated forces during the metaphase/anaphase A isometric state and that a suppression of MT depolymerization at the poles tips the balance of forces, allowing MT polymerization at the equator and motor-generated sliding forces to drive anaphase B.
MATERIALS AND METHODS
Fly Stocks and Embryo Collection
Flies were maintained and embryos were collected as described previously (Sharp et al., 1999b). Experiments were performed on embryos expressing GFP::tubulin (provided by Dr. Allan Spradling, Carnegie Institute, Washington) or GFP::CID, the Drosophila CENPA homolog (Henikoff et al., 2000), which is a stable and specific kinetochore marker (provided by Dr. Steven Henikoff, Fred Hutchinson Cancer Research), and on Claret nondisjunctional (cand) mutant embryos. Ncd null embryos were generated by crossing homozygous cand females with heterozygous or homozygous cand males.
Fluorescent Speckle Microscopy
Drosophila embryos were injected with a low concentration of rhodamine tubulin (Cytoskeleton, Denver, CO). Time lapse confocal images were acquired on an Olympus microscope equipped with an Ultra View spinning disk confocal head (Perkin Elmer-Cetus Wallac, Inc., Gaithersburg, MD) with a 100× 1.4 NA objective with a time interval of 1.5–3.5 s. Images were analyzed using MetaMorph Imaging software (Universal Imaging Corporation, West Chester, PA). First, a background image was subtracted, and then the No Neighbors Deconvolution and Low Pass Filter commands were applied. Speckle movement was quantified using kymograph analysis. In the absence of spindle movement, we could use MetaMorph's kymograph command to obtain an image of a microtubule bundle as a function of time during the metaphase/anaphase A isometric state. To analyze periods of spindle elongation and spindles that move on the embryo's surface due to cortical contractions, we manually followed a MT bundle and created a “manual kymograph.” For each image in the stack, a line scan was obtained for the MT bundle of interest and logged into Excel. If a microtubule bundle changed its length over time, we aligned the centers of all the line scans. Using Matlab (The MathWorks, Inc., Natick, MA), we converted these pixel values into an image of the MT bundle as a function of time. In the kymographs, moving speckles appeared as oblique lines, whose slope corresponds to their rate of movement. To obtain the rate of flux, i.e., the velocity of speckles toward the pole, the movement of the pole was subtracted from that of the speckle. All calculations and statistical analyses were done on Microsoft Excel, graphs were plotted with Kaleidagraph (Synergy Software, Reading, PA).
Pole-to-Pole Spacing and Kinetochore-to-Pole Movement
Drosophila embryos were injected with rhodamine tubulin. Time lapse confocal images were acquired as explained above. The positions of the poles and of kinetochores, in GFP::CID-expressing embryos, were logged, and the distances between poles, between sister kinetochores and between kinetochore and corresponding pole were calculated in Excel and plotted as a function of time. The rates of anaphase B and chromatid-to-pole movement and the persistence of isometric states were calculated from these graphs.
RESULTS
To analyze the relationship between MT dynamics and spindle morphogenesis in Drosophila embryos, we microinjected substoichiometric levels of fluorescent tubulin and monitored the behavior of the resulting fluorescent speckles (Waterman-Storer et al., 1999) by time-lapse confocal microscopy. During metaphase and most of anaphase A the poles are maintained at a constant spacing, characteristic of the metaphase/anaphase A isometric state. The observation that pole-to-pole spacing is maintained as chromatids move to the poles and kinetochore MT (kMT) bundles disassemble makes it unlikely that kMT forces act on the poles during anaphase A. The observation is more consistent with the hypothesis that the forces responsible for maintaining the spacing of the spindle poles act on interpolar MT (ipMT) bundles and astral MTs rather than kMT bundles (Sharp et al., 2000a, 2000b). It also assisted us in distinguishing ipMT bundles from kMT bundles (see below).
Speckle Movement along MTs Reveals Flux throughout the Metaphase/Anaphase A Isometric State
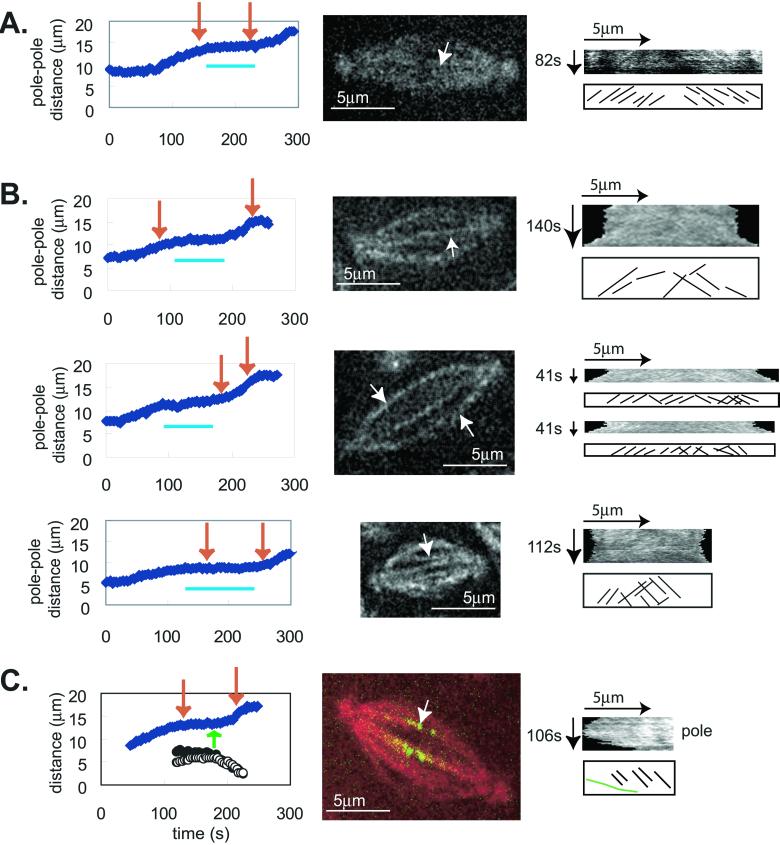
During metaphase and anaphase A, speckles of fluorescent tubulin move from the spindle equator toward the pole at 0.032 ± 0.013 μm/s (Figure 1; Table 1), presumably due to tubulin polymerization at the spindle equator and depolymerization at the poles (Mitchison, 1989). We measured speckle flux along individual MT bundles that could be distinguished as either ipMT or kMT bundles. For example, in embryos expressing GFP::CID (Henikoff et al., 2000), a stable and specific kinetochore marker, we could monitor MT bundles that ended in a bright spot of CID, indicating that they were kMTs (Figure 1C). In contrast, ipMT bundles were identified as those running from pole to pole without any GFP::CID along their length and persisting throughout anaphase B, unlike kMTs, which were mostly disassembled by the onset of anaphase B. Of 177 MT bundles analyzed, 28 were positively identified as kMTs and 25 as ipMTs (the remainder were not classified as they were not imaged in GFP::CID embryos so the kinetochore marker was not present). There was no significant difference in the rate of flux within ipMT and kMT bundles, suggesting that tubulin fluxes from the equator toward the pole along both ipMT and kMT bundles during the metaphase/anaphase A isometric state at 0.03 μm/s.
Figure 1.
Speckles flux toward the poles at 0.03 μm/s during the metaphase/anaphase A isometric state, but during anaphase B flux stops and speckles move at the same rate as the poles. Speckle movement was obtained from time lapse confocal images of Drosophila embryos injected with a low concentration of rhodamine labeled tubulin. For each spindle (center panels) we obtained the pole-pole distance as a function of time (graph at left) and followed an individual microtubule bundle to obtain the kymograph for the period of time during which it was clearly visible (shown by red arrows on graph). The horizontal blue lines under the data plots denote the metaphase/anaphase A isometric states. We measured speckle movement using both automatic and manual kymographs (see MATERIALS AND METHODS), which yielded the same results. The kymographs are shown in the right hand panels and the graph below each kymograph shows the trajectories of individual speckles. It should be noted that the printed images are not as clear as the original images analyzed in MetaMorph, from which the data were obtained, because the images were converted from 12 to 8 bits during figure preparation. (A) This kymograph was obtained using MetaMorph's function on a stationary spindle during the metaphase/anaphase A isometric state. (B) These kymographs were obtained manually (see MATERIALS AND METHODS). (C) Manual kymograph for a MT bundle going to a kinetochore. The graph on the left shows the distance between poles (solid blue diamonds) and the distance from two sister kinetochores to the poles (circles). The green arrow shows the onset of anaphase A. In the middle panel red shows tubulin and green shows GFP::CID, a kinetochore marker. The kymograph was obtained for the microtubule bundle going from the pole to the kinetochore (at the equatorial end of the bundle). The green line in the bottom sketch represents the movement of the kinetochore.
Table 1.
Kinetochore to pole velocity and metaphase/anaphase A flux
| Rate (μm/s) | |
|---|---|
| Kinetochore to pole | 0.106 ± 0.019 (15/3)a |
| Flux during the metaphase/ Anaphase A isometric stateb | 0.032 ± 0.013 (30/12) |
The numbers in parentheses are the number of spindles and the number of embryos analyzed.
The metaphase/anaphase A isometric states are shown by the blue lines in Figure 1.
Anaphase A Chromosome Movement
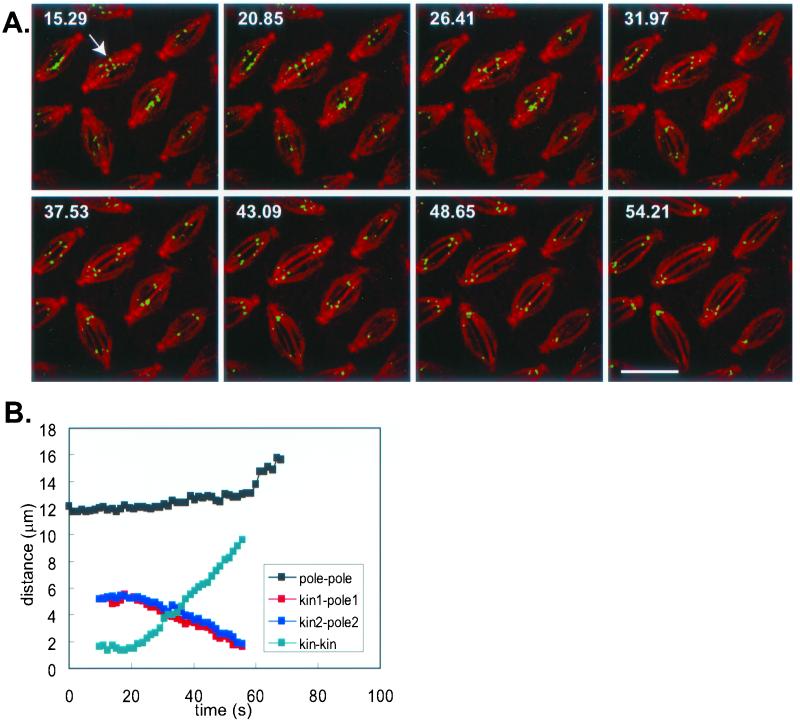
We measured the rate of kinetochore-to-pole motion in transgenic GFP::CID-expressing embryos microinjected with rhodamine tubulin (Figure 2). Simultaneous visualization of kinetochores and MTs allowed us to determine pole-to-pole, pole-to-kinetochore and kinetochore-to-kinetochore distances as functions of time (Figure 2). As noted above, we found that anaphase A chromosome movement was mostly (half to two thirds) complete during the metaphase/anaphase A isometric state, whereas the poles are maintained at a constant spacing, and we observed that kinetochores move toward the poles at rates of 0.106 ± 0.019 μm/s, about three times faster than flux (0.03 μm/s). In some cases we measured the rate of speckle flux and the rate of chromatid-to-pole movement on the same spindles (Figure 1C) and found the same result. Our data suggest that flux alone cannot drive anaphase A in Drosophila embryos, that it can only contribute up to 30% of the movement, and that kMTs depolymerize at both the minus and the plus ends during anaphase A.
Figure 2.
Kinetochores move toward the poles at 0.1 μm/s. Kinetochore-to-pole movement was calculated from time lapse confocal images of Drosophila embryos expressing GFP::CID injected with rhodamine tubulin (top). Time is given in seconds in each frame; scale bar, 10 μm. Bottom: the distance between the poles, between two sister kinetochores, and between a kinetochore and the corresponding pole were calculated from the time lapse images (shown here for the spindle marked by the arrow). Anaphase B begins after anaphase A is almost complete. GFP::CID is detectable from prometaphase to anaphase B.
FSM Reveals a Switch from MT Flux to MT Sliding at the Onset of Anaphase B
At the onset of anaphase B, we observed a striking change in the pattern of movement of tubulin speckles. As noted above, during the metaphase/anaphase A isometric state, speckles move toward the stationary poles at 0.03 μm/s, but during anaphase B the speckles move away from the equator at the same rate as the poles (Figure 1B), and consequently they do not move toward the pole (Table 2), consistent with net MT-MT sliding. This could be driven by motors in the interzone cross-linking and sliding MTs apart and/or by cortical dynein pulling astral MTs associated with the poles outward (Sharp et al., 2000a). Anaphase B occurs at 0.074 ± 0.015 μm/s (Table 2), about twice the rate of flux, which together with the observation that speckles move at the same rate as the poles, suggests that suppression of MT flux due to an inhibition of MT depolymerization at the poles could lead to net MT-MT sliding and anaphase B (discussed below).
Table 2.
Flux and anaphase B rate of sliding is not significantly different in Ncd null embryos, but the persistence of isometric states is decreased
| Metaphase/ anaphase A flux (μm/s) | Anaphase B flux (μm/s) | Anaphase B sliding (μm/s) | Persistence of the prometaphase isometric state (s)a | Persistence of the metaphase/ anaphase A isometric state (s) | |
|---|---|---|---|---|---|
| Wild-type | 0.032 ± 0.013 | −0.002 ± 0.019 | 0.074 ± 0.015 | 83 ± 10 | 78 ± 9 |
| (30/12)b | (12/8) | (13/2) | (13/2) | (13/2) | |
| Ncd | 0.041 ± 0.021 | 0.006 ± 0.015 | 0.066 ± 0.014 | 27 ± 12 | 44 ± 24 |
| (14/5) | (14/5) | (10/2) | (10/2) | (10/2) |
The isometric states are horizontal bars under the data plots in Figure 3A.
The numbers in parentheses are the number of spindles and the number of embryos analyzed.
Influence of Ncd on Flux and Sliding during the Metaphase/Anaphase A Isometric State and Anaphase B
During mitosis, Ncd is found on spindle fibers and spindle poles (Endow and Komma, 1996) and has been proposed to function as a brake that restrains pole-pole separation before anaphase B onset (Sharp et al., 2000a), to attach centrosomes to spindle poles and to drive poleward MT translocation associated with flux (Endow et al., 1994a). To contribute to flux, Ncd could transport MTs bound as cargo to its tail (McDonald et al., 1990; Karabay and Walker, 1999) along MT tracks whose minus ends face the spindle poles (Endow et al., 1994), similar to dynein in frog extracts (Heald et al., 1997). Thus, in the absence of Ncd function, flux would be suppressed. Ncd could function as a brake either by cross-linking antiparallel MTs and restraining outward sliding by motors such as KLP61F or by modulating MT dynamics, for example, enhancing MT depolymerization at the poles, suppressing MT polymerization at the interzone, or suppressing flux by binding directly to tubulin subunits along the polymer lattice. In Saccharomyces cerevisiae, the Ncd homolog Kar3p has MT depolymerizing activity (Endow et al., 1994b), and if Ncd were required for MT depolymerization at the spindle poles as proposed for Kar3p (Saunders et al., 1997), its downregulation at anaphase B onset could lead to the switch from flux to sliding noted above.
To refine our understanding of the role of Ncd, we used FSM to compare flux and sliding in wild-type and Ncd null mutant embryos. Ncd was suitable for these studies because the complete loss-of-Ncd function (in the cand mutant) does not lead to a mitotic arrest or spindle collapse phenotype, unlike, for example, dynein or KLP61F (Sharp et al., 2000a). Instead, in the absence of Ncd function, mitosis is completed and thus we were able to study Ncd's role during phases of mitosis subsequent to prophase by measuring both spindle length and flux.
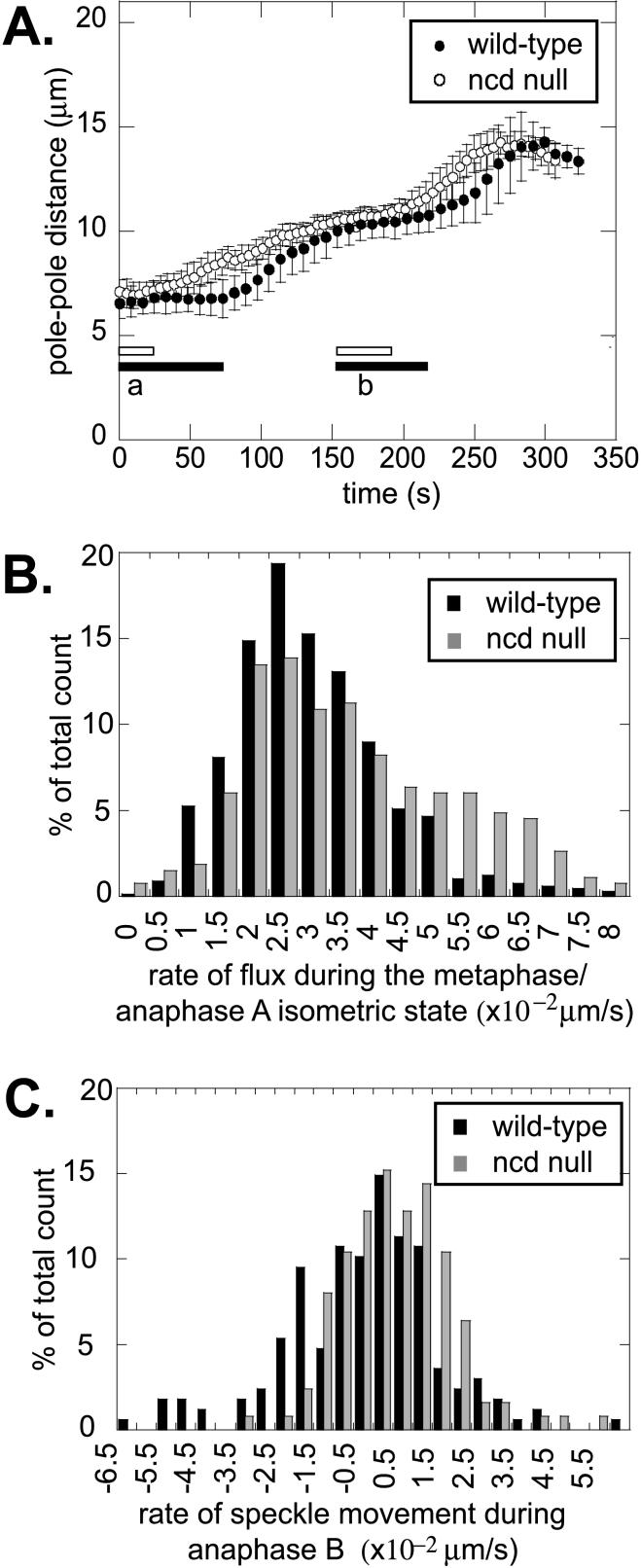
The rate of flux during metaphase and anaphase A was 0.041 ± 0.021 μm/s in Ncd null mutants, compared with 0.032 ± 0.013 μm/s for wild-type embryos (Table 2, Figure 3B). Given the large SDs in these values, they are not significantly different from one another, suggesting that Ncd activity is not essential for flux to occur, a result inconsistent with the idea that Ncd drives the poleward translocation of MTs associated with flux and with the notion that its activity is required for MT depolymerization at the spindle poles. During anaphase B, flux in both Ncd null mutant and wild-type embryos decreased to about zero (Table 2, Figure 3C), and there was no significant difference in the speed of net sliding (Table 2), indicating that the loss-of-Ncd function does not prevent the switch from flux to net sliding that accompanies anaphase B onset or affect the speed of MT-MT sliding associated with anaphase B.
Figure 3.
Flux and sliding in Ncd null mutant embryos. (A) Pole-pole distance as a function of time for wild-type (●) and Ncd null (○) embryos during cycle 12. The bars under the data plots represent the prometaphase isometric state (a) and the metaphase/anaphase A isometric state (b) for wild-type (closed bars) and Ncd null (open bars) embryos. In the Ncd mutants, isometric states are shortened and mitosis proceeds faster. (B) Histogram for the rate of flux during the metaphase/anaphase A isometric state for wild-type (black bars) and Ncd null (gray bars) embryos. The number of counts was normalized to the total number (667 for wild-type and 267 for Ncd null). The average is not significantly different. (C) Histogram for the rate of speckle movement during anaphase B for wild-type (black bars) and Ncd null (gray bars) embryos. The number of counts was normalized to the total number (168 for wild-type and 125 for Ncd null). Both are centered around 0, consistent with MT-MT sliding during anaphase B.
However, we did observe that the persistence of the prometaphase and the metaphase/anaphase A isometric states is decreased significantly in Ncd null embryos (Figure 3A, Table 2). As a result, spindle poles separate more steadily, the quiescent pauses characteristic of wild-type spindles are greatly reduced, and the outward MT-MT sliding that pushes the spindle poles apart during anaphase B is initiated earlier than normal. These results suggest that Ncd could serve to cross-link MTs within interpolar MT bundles to exert an inward force on spindle poles that restrains the rate of pole-pole separation specifically during the isometric states and plays an important role in maintaining the isometric spindle structures.
DISCUSSION
Our previous analysis of spindle morphogenesis led us to propose how multiple MT-sliding motors cooperate to drive spindle morphogenesis in Drosophila embryos, but this model is incomplete because it ignores the role of MT polymerization-depolymerization (Sharp et al., 2000a, 2000b). Here, we have begun to investigate how the dynamic properties of MTs might cooperate with MT sliding motors during spindle morphogenesis and anaphase A chromosome movement using FSM.
Flux Alone Cannot Drive Anaphase A
We found that during anaphase A, chromosomes move poleward at 0.1 μm/s. This fast rate is consistent with our previous measurements (Sharp et al., 2000c). We also found that throughout metaphase and anaphase A, MTs flux toward the pole along both ipMT and kMT bundles at 0.03 μm/s. This implies that flux alone is too slow to account for anaphase A chromatid-to-pole motion. These data are inconsistent with the report that both flux and kinetochore-to-pole movement occurred at the same rate of 0.05 μm/s in Drosophila early embryos (Desai, A.B., Maddox, P.S., Oegema, K.F., Field, C.M., Kapoor, T.M., Mitchison, T.J., and Salmon, E.D. American Society for Cell Biology Annual Meeting, 2000, abstract 2943), but the reason for this discrepancy is unclear at present.
Our results suggest that in addition to flux other factors are involved in anaphase A, at least in Drosophila embryos. Cytoplasmic dynein is a candidate factor, based on observations that inhibition of its function significantly reduces poleward tension on kinetochores and slows chromatid-to-pole motion (Savoian et al., 2000; Sharp et al., 2000c; Howell et al., 2001). However, whether dynein acts as a chromatid-to-pole translocator, as a component of the checkpoint control apparatus, or both, remains to be resolved. On dynein inhibition, anaphase A velocity was reduced 33% in one study (Howell et al., 2001) and 75% in others (Savoian et al., 2000; Sharp et al., 2000c), the latter value being consistent with the hypothesis that flux could account for up to 30% of the motion. This suggests that anaphase A is driven by the cooperative action of MT dynamics, cytoplasmic dynein, and possibly other MT motors as proposed for spindle pole separation (Sharp et al., 2000b). For example, motors moving chromatids poleward at 0.07 μm/s along kMT tracks that flux poleward at 0.03 μm/s could produce poleward chromatid motion at the net rate of 0.1 μm/s.
Anaphase B MT-MT Sliding
At the onset of anaphase B, when chromatids have reached the poles and consequently the kMT bundles have depolymerized, we observed a cessation of poleward flux. Speckles move outward with the poles, providing evidence for MT-MT sliding with no polymerization or depolymerization at the poles. A switch from fast microtubule turnover during metaphase to sliding of stable microtubules at the onset of anaphase B was observed by Fluorescence Photobleaching Recovery experiments in Schizosaccharomyces pombe (Mallavarapu et al., 1999). In that study there was no evidence of flux during metaphase, but it could have been obscured by the rapid turnover of tubulin and by the small size of the S. pombe spindle. MT-MT sliding was also visualized by Fluorescence Photobleaching Recovery experiments in PtK1 cells during late anaphase B and telophase (Saxton and McIntosh, 1987), but in those experiments the relationship between sliding and flux was not investigated. An abrupt decrease in flux within kMTs at the onset of anaphase has been observed in PtK1 cells (Zhai et al., 1995), but the suppression of flux that we observe in ipMTs at the onset of anaphase B has not been reported before.
Ncd Maintains the Prometaphase and the Metaphase/Anaphase A Isometric States, But Is Not Required for Flux or MT Depolymerization at Spindle Poles
MT flux in the spindle requires that MT polymerization at the equator and MT depolymerization at the poles be coupled to the translocation of the MT polymer lattice toward the spindle pole (Mitchison, 1989; Mitchison and Sawin, 1990). The motor(s) responsible for MT translocation could be plus end-directed MT motors located on the spindle poles or spindle interzone that “reel in” MTs or push apart MTs with their minus ends leading, or minus end-directed motors that translocate fluxing MTs poleward with their minus ends leading along adjacent MT tracks. The Ncd motor has MT-binding sites in its tail that could bind MTs as cargo (McDonald et al., 1990; Karabay and Walker, 1999) and thus it could, in principle, drive the poleward translocation of kMTs associated with flux as proposed by Endow et al. (1994), in a manner similar to dynein in spindles within frog extracts (Heald et al., 1997). Furthermore, it is also possible that Ncd could contribute to flux by depolymerizing MTs at the poles as proposed for Kar3p (Saunders et al., 1997) and that the suppression of this activity at the onset of anaphase B could trigger a switch from flux to sliding. However, our observation that flux persists in Ncd null mutant embryos that are totally lacking Ncd function shows that Ncd is not essential for flux and is not required for MT depolymerization at the poles. This suggests that motors other than Ncd drive the MT translocation that contributes to flux and that other factors depolymerize MTs at the poles.
We previously showed that the Ncd motor restrains progression through mitosis (Sharp et al., 2000a). Ncd could plausibly act as a brake that exerts an inward force on spindle poles by affecting MT dynamics within ipMT bundles, for example, by suppressing MT polymerization at plus ends, by enhancing MT depolymerization at minus ends, or by binding to the walls of spindle MTs to suppress flux directly. These effects might be revealed as changes in the rate of flux, but the lack of effect of loss of Ncd function on MT flux is more consistent with the idea that this motor serves as a brake by cross-linking antiparallel MTs within ipMT bundles, thereby inhibiting the sliding apart of ipMTs driven by other motors (Sharp et al., 1999b).
Subsequent to nuclear envelope breakdown, Ncd is needed to stabilize and maintain isometric spindle states because in the absence of Ncd function, the persistence of these states is shorter, but the rate of MT-MT sliding is not increased, suggesting that Ncd acts as a brake specifically during the isometric states. Moreover, in the absence of Ncd function, outward MT-MT sliding and the associated pole-pole separation are initiated prematurely, in support of the aforementioned hypothesis that Ncd normally acts as a brake by restraining outward MT-MT sliding during the isometric state, but further work is required to test this hypothesis. Further work is also required to understand the significance of the isometric structures. They could represent pauses in the process of spindle morphogenesis during which the structural integrity of the spindle can be assessed in these rapidly dividing embryonic cells, thus complementing conventional spindle assembly checkpoints. It is also possible that the isometric states represent periods during which spindle pole separation is paused because of the braking action of Ncd, in order to allow ipMT growth to produce a robust overlap zone. Once the robust overlap region has formed, plus end-directed MT motors, for example, the bipolar kinesin KLP61F, acting in concert with cortical dynein, can slide the antiparallel MTs apart, thereby pushing the poles apart. This would explain why, in the absence of Ncd activity, the poles start moving apart earlier than normal, yet the rate of MT-MT sliding and pole-pole separation are not accelerated. In this scenario, the isometric state pause is shorter than normal, so that other motors can start pushing and pulling the poles apart before a robust interzone has formed. The rate of outward MT-MT sliding and pole-pole separation then occur at rates that are limited by the rate of growth of overlapping MTs in the spindle interzone.
The observation that Ncd increases the duration of the metaphase/anaphase A isometric state but does not influence the rate or extent of anaphase B is consistent with the idea that its “braking” effect is turned off to initiate anaphase B spindle elongation (Sharp et al., 2000a, 2000b) and with the hypothesis presented above that the suppression of flux initiates anaphase B.
MT Polymerization and Depolymerization Act Cooperatively with MT Motors during Spindle Morphogenesis
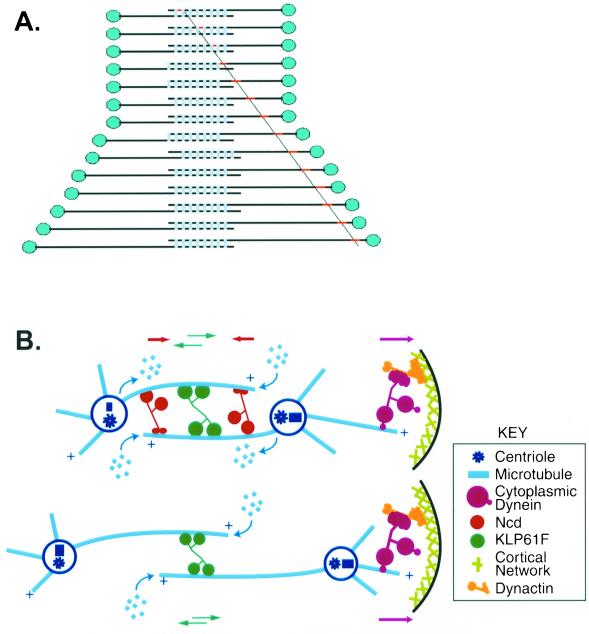
Our results (Figure 4A) indicate that changes in the assembly-disassembly properties of spindle MTs, associated with the transition from flux to sliding, contribute to tipping the balance of forces that positions the spindle poles and further suggest how the interplay between MT dynamics and MT-MT sliding can play important roles in spindle morphogenesis (Figure 4). We propose that during metaphase and anaphase A, the outward forces exerted by KLP61F and dynein exceed the inward force due to Ncd and tend to slide ipMTs outward, but the steady state positioning of the poles is maintained because the net outward sliding is balanced by MT polymerization in the overlap region at the equator combined with MT depolymerization at the poles, thus producing a “treadmill” (Figure 4B, top). However, this balance could be tipped by the suppression of MT depolymerization at the poles, by tipping the balance of motor dependent forces, e.g., by downregulating Ncd (Sharp et al., 2000a, 2000b), or both, allowing outward MT-MT sliding driven by the motors coupled with MT polymerization at the equator to exert a net outward force on the spindle poles that could drive anaphase B spindle elongation (Figure 4B, bottom).
Figure 4.
Flux is converted to sliding at the onset of anaphase B. (A) Behavior of speckles on ipMTs. During the metaphase/anaphase A isometric state, flux (red dot tracked by diagonal line) is due to subunit addition at plus ends of antiparallel MTs within ipMT bundles (over-lapping horizontal lines) and subunit loss at minus ends coupled to the sliding apart of ipMTs driven by MT sliding motors (light blue bars) so the poles (blue dots) maintain a constant spacing. At anaphase B onset, subunit loss at the poles stops while subunit addition at plus ends and MT-MT sliding continue. This converts flux to net sliding and consequently the poles are pushed apart. (B) Detailed schematic of the metaphase/anaphase A spindle (top) and anaphase B spindle (bottom), showing the three motors previously shown to be involved in mitosis (Sharp et al., 2000a) and sites of tubulin subunit addition and loss within ipMT bundles. During the metaphase/anaphase A isometric state, the action of sliding motors coupled to flux generates the balance of forces that maintains a constant spacing between the spindle poles. Tipping this balance by suppression of depolymerization at the poles leads to anaphase B, which may depend on KLP61F in the spindle interzone driving MT-MT sliding coupled to MT polymerization at the spindle equator (with cortical dynein augmenting pole-to-pole separation late in anaphase B; Sharp et al., 2000a).
It is striking that the rate of anaphase B sliding is about twice that of flux (Table 2), suggesting that a suppression of depolymerization at the poles is sufficient to tip the balance, allowing MT polymerization at the equator and motor generated sliding forces to drive anaphase B sliding. It is also about twice the rate of in vitro MT sliding driven by the bipolar kinesin KLP61F (0.04 μm/s; Cole et al., 1994), a motor required for anaphase B (Sharp et al., 2000a). This suggests that MT-MT sliding driven by the bipolar kinesin KLP61F coupled with polymerization of MT plus ends in the overlap zone may govern the rate of anaphase B (Figure 4B, bottom).
Further work will be required to test specific aspects of this model, for example, to examine the precise contributions of the suppression of flux and the downregulation of Ncd to the initiation of anaphase B as well as the contributions of equatorial MT polymerization and bipolar kinesin-driven MT-MT sliding to anaphase B, although this will be technically challenging. The studies reported here provide insights into the relationships that exist between MT dynamics and MT sliding motors during spindle morphogenesis in Drosophila embryos and suggest that further exploration of this problem in the fruit fly embryo will yield additional insights into the mechanism of mitosis.
Note added in proof. Maddox et al. (Curr. Biol. [2002] 12, 1670–1674) now report that, at 24°C, kinetochore-to-pole motion occur at 0.11 μm · s−1 (similar to our results) while flux occurs more slowly, at 0.087 μm · s−1 (faster than we observe). Thus, both groups agree that flux contributes to anaphase A, but differ over the extent of its contribution.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Allan Spradling and Dr. Tom Kaufman for GFP::tubulin embryos and Dr. Steven Henikoff for GFP::CID embryos. We thank Dr. Frank McNally, Dr. David Sharp, Dr. Greg Rogers, Mijung Kwon, and other members of the Scholey laboratory for discussion and Kristine Adjemian for technical support. This work was supported by National Institutes of Health (NIH) grant GM55507 to J.M.S. and NIH postdoctoral fellowship F2GM20776A to I.B.-M.
Abbreviations used:
- CID
centromere identifier
- FSM
fluorescent speckle microscopy
- GFP
green fluorescent protein
- ipMT
interpolar MT
- kMT
kinetochore MT
- MT
microtubule
- Ncd
nonclaret disjunctional
Footnotes
Online version contains video materials for Figures 1 and 2. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–05–0069. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–05–0069.
REFERENCES
- Cole DG, Saxton WM, Sheehan KB, Scholey JM. A “slow” homotetrameric kinesin-related motor protein purified from Drosophila embryos. J Biol Chem. 1994;269:22913–22916. [PMC free article] [PubMed] [Google Scholar]
- Desai A, Maddox PS, Mitchison TJ, Salmon ED. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA, Chandra R, Komma DJ, Yamamoto AH, Salmon ED. Mutants of the Drosophila Ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J Cell Sci. 1994a;107:859–867. doi: 10.1242/jcs.107.4.859. [DOI] [PubMed] [Google Scholar]
- Endow SA, Kang SJ, Satterwhite LL, Rose MD, Skeen VP, Salmon ED. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. EMBO J. 1994b;13:2708–2713. doi: 10.1002/j.1460-2075.1994.tb06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA, Komma DJ. Centrosome and spindle function of the Drosophila Ncd motor protein visualized in live embryos using Ncd-GFP fusion proteins. J Cell Sci. 1996;109:2429–2442. doi: 10.1242/jcs.109.10.2429. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Haberman A, Karsenti E, Hyman A. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K, Platero JS, van Steensel B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc Natl Acad Sci USA. 2000;97:716–721. doi: 10.1073/pnas.97.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Geiser JR. Genetic analysis of the mitotic spindle. Annu Rev Genet. 1996;30:7–33. doi: 10.1146/annurev.genet.30.1.7. [DOI] [PubMed] [Google Scholar]
- Inoue S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967;50(suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Karabay A, Walker RA. Identification of microtubule binding sites in the Ncd tail domain. Biochemistry. 1999;38:1838–1849. doi: 10.1021/bi981850i. [DOI] [PubMed] [Google Scholar]
- Karsenti E, Vernos I. The mitotic spindle, a self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- LaFountain JR, Oldenbourg R, Cole RW, Rieder CL. Microtubule flux mediates poleward motion of acentric chromosome fragments during meiosis in insect spermatocytes. Mol Biol Cell. 2001;12:4054–4065. doi: 10.1091/mbc.12.12.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavarapu A, Sawin K, Mitchison T. A switch in microtubule dynamics at the onset of anaphase B in the mitotic spindle of Schizosaccharomyces pombe. Curr Biol. 1999;9:1423–1426. doi: 10.1016/s0960-9822(00)80090-1. [DOI] [PubMed] [Google Scholar]
- McDonald HB, Stewart RJ, Goldstein LSB. The kinesin-like Ncd protein of Drosophila is a minus-end-directed microtubule motor. Cell. 1990;63:1159–1165. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Hepler PK, Van Wie DG. Model for mitosis. Nature. 1969;224:659–663. [Google Scholar]
- McIntosh JR, McDonald KL. The mitotic spindle. Sci Am. 1989;261:48–56. doi: 10.1038/scientificamerican1089-48. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Salmon ED. Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol. 1992;119:569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Salmon ED. Mitosis: a history of division. Nat Cell Biol. 2001;3:E17–E21. doi: 10.1038/35050656. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Sawin KE. MT flux in the mitotic spindle: where does it come from, where is it going? Cell Motil Cytoskelet. 1990;16:93–98. doi: 10.1002/cm.970160202. [DOI] [PubMed] [Google Scholar]
- Saunders W, Hornack D, Lengyel V, Deng C. The Saccharomyces cerevisiae kinesin-related motor Kar3p acts at preanaphase spindle poles to limit the number and length of cytoplasmic microtubules. J Cell Biol. 1997;137:417–31. doi: 10.1083/jcb.137.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoian MS, Goldberg ML, Rieder CL. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat Cell Biol. 2000;2:948–952. doi: 10.1038/35046605. [DOI] [PubMed] [Google Scholar]
- Saxton WM, McIntosh JR. Interzone microtubule behavior in late anaphase and telophase spindles. J Cell Biol. 1987;105:875–886. doi: 10.1083/jcb.105.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, Mitchison TJ, Scholey JM. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J Cell Biol. 1999a;144:125–138. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu KR, Sisson JC, Sullivan W, Scholey JM. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat Cell Biol. 1999b;1:51–54. doi: 10.1038/9025. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Brown HM, Kwon M, Rogers GC, Holland G, Scholey JM. Functional coordination of three mitotic motors in Drosophila embryos. Mol Biol Cell. 2000a;11:241–253. doi: 10.1091/mbc.11.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000b;407:41–47. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat Cell Biol. 2000c;2:922–930. doi: 10.1038/35046574. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Desai A, Salmon ED. Fluorescent speckle microscopy of spindle microtubule assembly and motility in living cells. Methods Cell Biol. 1999;61:155–173. doi: 10.1016/s0091-679x(08)61980-9. [DOI] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittman T, Hyman A, Desai A. The spindle, a dynamic assembly of microtubules and motors. Nat Cell Biol. 2001;3:E28–E34. doi: 10.1038/35050669. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.