Abstract
Transforming growth factor-β (TGF-β) superfamily members regulate a wide range of biological processes by binding to two transmembrane serine/threonine kinase receptors, type I and type II. We have previously shown that the internalization of these receptors is inhibited by K+ depletion, cytosol acidification, or hypertonic medium, suggesting the involvement of clathrin-coated pits. However, the involvement of the clathrin-associated adaptor complex AP2 and the identity of the AP2 subunit that binds the receptors were not known. Herein, we have studied these issues by combining studies on intact cells with in vitro assays. Using fluorescence photobleaching recovery to measure the lateral mobility of the receptors on live cells (untreated or treated to alter their coated pit structure), we demonstrated that their mobility is restricted by interactions with coated pits. These interactions were transient and mediated through the receptors' cytoplasmic tails. To measure direct binding of the receptors to specific AP2 subunits, we used yeast two-hybrid screens and in vitro biochemical assays. In contrast to most other plasma membrane receptors that bind to AP2 via the μ2 subunit, AP2/TGF-β receptor binding was mediated by a direct interaction between the β2-adaptin N-terminal trunk domain and the cytoplasmic tails of the receptors; no binding was observed to the μ2, α, or ς2 subunits of AP2 or to μ1 of AP1. The data uniquely demonstrate both in vivo and in vitro the ability of β2-adaptin to directly couple TGF-β receptors to AP2 and to clathrin-coated pits, providing the first in vivo evidence for interactions of a transmembrane receptor with β2-adaptin.
INTRODUCTION
The transforming growth factor-β (TGF-β) superfamily mediates a wide range of biological processes (Massagué and Chen, 2000). TGF-β transduces signals through activation of two different serine/threonine kinases, known as the type I (TβRI) and type II (TβRII) receptors. TβRII is a constitutively active kinase that upon ligand binding recruits TβRI into a heteromeric complex. TβRII activates TβRI by transphosphorylating it in the glycine-serine–rich GS domain. Activated TβRI propagates the signal via phosphorylation of Smad proteins that translocate to the nucleus and modulate transcription of TGF-β–responsive genes (Massagué and Chen, 2000; ten Dijke et al., 2000; Wrana, 2000).
Because the cell surface expression of TGF-β receptors must be tightly regulated to prevent uncontrolled cell proliferation that may contribute to oncogenesis, defining the mechanisms of TGF-β receptor endocytosis is particularly relevant. However, the fact that 1) TβRI and TβRII comprise only a small fraction of the proteins that bind TGF-β; 2) TGF-β binding assays routinely have a high degree of nonspecific binding; and most importantly, 3) the identification of both heteromeric and homomeric TGF-β receptor complexes on the cell surface in the presence or absence of ligand (Gilboa et al., 1998) has made reliable endocytic studies using iodinated ligand problematic. A recent study where this approach was attempted reported very high and atypical internalization rates for TGF-β1 (Zwaagstra et al., 2001). This is in contrast to previous investigations (Koli and Arteaga, 1997) that showed a relatively slow TGF-β1 endocytosis rate and a high fraction of noninternalized ligand (in accord with its binding to additional sites on cells and the extracellular matrix). To overcome the multisite binding of TGF-β, chimeric TGF-β receptors were generated to examine defined TβRI and/or TβRII interactions (Anders et al., 1997, 1998; Doréet al., 2001). These studies suggested differential regulation of homomeric and heteromeric TGF-β receptor complexes, a requirement for TβRII kinase activity, and distinct mechanisms of endocytic control in epithelial vs. mesenchymal cells. Importantly, the internalization of chimeric TGF-β receptors whose extracellular domain was swapped with that of human granulocyte-macrophage colony-stimulating factor receptors was found to occur via clathrin-coated pits (Anders et al., 1997). This was validated for the native/full-length epitope-tagged TβRII and TβRI, along with the involvement of a di-leucine signal in the constitutive endocytosis of TβRII (Ehrlich et al., 2001; Ehrlich and Henis, unpublished observations). However, the mode of TGF-β receptor coupling to the clathrin-coated pit pathway was not known. Specifically, there was no information on 1) whether TβRII and TβRI were targeted to coated pits via binding to AP2 or clathrin; 2) the nature of such interactions (stable or transient); or 3) the identity of the AP2 subunit to which the receptors bind. The present manuscript was designed to address each of those issues.
Endocytosis via clathrin-coated pits requires interactions of receptor internalization signals with clathrin, usually via the clathrin-associated adaptor protein complex specific for the plasma membrane (AP2), with participation of additional proteins (Mellman, 1996; Schmid, 1997; Kirchhausen, 1999). There are three major groups of internalization signals: tyrosine based (YXXZ and NPXY, where X is any amino acid and Z is a hydrophobic amino acid), di-leucine based, and a less defined variable third group (Bonifacino and Dell'Angelica, 1999; Kirchhausen, 1999). These groups may also interact differently with clathrin-coated pits. YXXZ signals were reported to bind directly to AP2 via its μ2 subunit (see below) (Ohno et al., 1995; Boll et al., 1996; Bonifacino and Dell'Angelica, 1999). It is less clear which AP2 subunit binds di-leucine signals (Ohno et al., 1995; Hofmann et al., 1999); studies on the binding of peptides containing di-leucines to the μ chains of AP2 and AP1 yielded contradictory results, and it was reported that they could bind to the β subunits of AP (Ohno et al., 1995; Rapoport et al., 1998; Hofmann et al., 1999). On the other hand, NPXY signals were suggested to bind directly to clathrin (Kibbey et al., 1998).
AP2 consists of two large subunits, α and β2 (also called adaptins), a medium subunit (μ2), and a small subunit (ς2) (Schmid, 1997; Kirchhausen, 1999). Both α and β2 contain an N-terminal trunk domain, a proline-rich hinge domain, and a C-terminal appendage or ear domain (Kirchhausen, 1999). The N terminus of the α subunit (amino acids 130–330) is primarily responsible for targeting AP2 to the plasma membrane (Page and Robinson, 1995), whereas the C-terminal region (amino acids 695–938) regulates the binding of key accessory molecules involved in the assembly of clathrin-coated vesicles, such as Epsin, Eps15, AP180/CALM, and Amphyphysin I/II (Benmerah et al., 1996; Chen et al., 1998; Owen et al., 1999; Traub et al., 1999). The β2 subunit mediates the binding of AP2 to the clathrin triskelion and promotes clathrin cage assembly through a consensus motif (LLD/NLD) in the hinge region (Shih et al., 1995).
Herein, we investigated the mode of TGF-β receptor coupling to the clathrin-dependent endocytosis pathway in both intact cells and in vitro, combining biophysical experiments to characterize the interactions of TβRII and TβRI with coated pits at the surface of live cells with biochemical studies on their interactions with AP2. We show that the lateral diffusion rates of TβRI and TβRII at the cell surface are decreased by transient interactions with coated pits, as evidenced by the loss of the inhibitory interactions upon dissociation of AP2 from the plasma membrane and their enhancement after “freezing” of the coated pits by cytosol acidification. These findings are corroborated by both the colocalization of TGF-β receptor patches (induced by antibody cross-linking) with AP2 and the coimmunoprecipitation of AP2 with the receptors. Furthermore, yeast two-hybrid assays in combination with in vitro interaction studies identify the trunk domain of β2-adaptin as the specific binding partner for both TβRI and TβRII. These results have important implications for understanding the events involved in TGF-β receptor cell surface expression and endocytosis, and document that β2-adaptin can directly link cell surface receptors to the endocytic machinery.
MATERIALS AND METHODS
Materials and Cell Culture
Recombinant TGF-β1 was from R & D Systems (Minneapolis, MN) or Austral Biologicals (San Ramon, CA). 9E10 α-myc mouse ascites were from Harvard Monoclonals (Cambridge, MA); IgG and Fab′ fragments were prepared from these ascites as described previously (Henis et al., 1994). Chicken α-myc was from Chemicon International (Temecula, CA). AP.6 mouse IgG against the AP2 α chains were made using AP.6 hybridoma (CRL-2227; American Type Culture Collection, Manassas, VA). Fluorophore-labeled secondary IgGs were from Jackson Immunoresearch Laboratories (West Grove, PA), except GαM Alexa 546-F(ab′)2 (Molecular Probes, Eugene, OR). Fluorescent F(ab′)2 were converted to Fab′ as described previously (Gilboa et al., 1998). M2 mouse α-FLAG and M2-agarose α-FLAG affinity gel were from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibodies to β2-adaptin were from Transduction Laboratories (Lexington, KY), and X.22 mouse anti-clathrin heavy chain antibodies were from Covance Research (Denver, PA). Mouse anti-hemagglutinin (HA) tag (α-HA) antibodies and bovine serum albumin (BSA, fraction V) were from Roche Applied Science (Indianapolis, IN), and Mowiol was from Aventis (Strasbourg, France). Cell culture media were from Invitrogen (Carlsbad, CA) or Biological Industries (Beit Haemek, Israel) and fetal calf serum was from Summit Labs (Fort Collins, CO) or Biological Industries. All other reagents were from Sigma-Aldrich. Cos7 cells (CRL 1651; American Type Culture Collection) were grown in DMEM containing 10% (vol/vol) fetal calf serum and transiently transfected with the indicated constructs. Constructs encoding the cDNAs of AP2 and AP1 subunits and the TGN38 cytoplasmic tail in pACT-2 (Ohno et al., 1995) were generously provided by Dr. Juan Bonifacino (NIH, Bethesda, MD).
Fluorescence Photobleaching Recovery
Cos7 cells grown on glass coverslips were transiently transfected by DEAE dextran as described previously (Gilboa et al., 1998) by using pcDNA1 or pcDNA3 (Invitrogen) containing myc-TβRII (TβRII with a myc tag at the extracellular terminus) (Henis et al., 1994), myc-TβRI (Gilboa et al., 1998), or S199 (a truncation mutant of myc-TβRII) (Ehrlich et al., 2001). After 48 h, the cells were washed with cold Hanks' balanced salt solution containing 20 mM HEPES and 2% BSA, at pH 7.2; HBSS/HEPES/BSA) and labeled at 4°C with monovalent Fab′ (mouse α-myc Fab′ followed by Alexa 546-GαM Fab′, each at 50 μg/ml, 30 min). After three washes, the coverslips were mounted over a chamber containing buffer (cold HBSS/HEPES/BSA or one of the buffers used to alter coated pit structure). To minimize internalization, measurements were at 18°C, replacing samples within 15 min. Lateral diffusion was measured by fluorescence photobleaching recovery (FPR) (Axelrod et al., 1976; Koppel et al., 1976) with previously described instrumentation (Henis and Gutman, 1983). The monitoring laser beam (Coherent Innova 70 argon ion laser, 529.5 nm, 1 μW) was focused through a microscope (Zeiss, Jena, Germany) to a Gaussian radius of 0.85 μm by using a 63× oil immersion objective. A brief pulse (5 mW, 20 ms) bleached 60–75% of the fluorescence in the illuminated region. The fluorescence recovery was followed by the attenuated monitoring beam. The lateral diffusion coefficient (D) and the mobile fraction (RF) values were extracted from fluorescence recovery curves by nonlinear regression analysis (Petersen et al., 1986). Incomplete recovery was interpreted to represent fluorophores that are immobile on the FPR experimental time scale (D ≤ 5 × 10−12 cm2/s).
Treatments That Alter Coated Pit Structure
The treatments used were 1) incubation in hypertonic medium to disperse the clathrin lattices underlying coated pits (Heuser, 1989; Hansen et al., 1993); 2) acidification of the cytosol to block the pinching-off of clathrin-coated vesicles (Heuser, 1989; Hansen et al., 1993); or 3) incubation with the cationic amphiphilic drug chlorpromazine, which causes a redistribution of AP2 from the plasma membrane to endosomes (Wang et al., 1993). Hypertonic treatment was performed by a 15-min incubation (37°C) in HBSS/HEPES/BSA containing 0.45 M sucrose (hypertonic buffer) (Fire et al., 1995). The cells were kept in hypertonic medium during all labeling steps and the ensuing experiments. Cytosol acidification was performed as detailed previously (Fire et al., 1995), loading the cells with NH4Cl followed by 5 min (37°C) incubation in potassium-amiloride (KA) buffer (0.14 M KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM amiloride-HCl, 20 mM HEPES pH 7.2). The cells were washed with cold KA buffer containing 2% BSA, in which all the ensuing labeling steps and FPR or copatching measurements were carried out. Treatment with chlorpromazine was performed by incubating the cells with the drug (100 μM, 37°C, in DME) for 30 min. Chlorpromazine was maintained in the HBSS/HEPES/BSA buffer during all subsequent labeling steps and FPR experiments.
Immunofluorescence Copatching
To measure the association of AP2 with TGF-β receptors at the cell surface, we used immunofluorescence microscopy to detect the colocalization of AP2 with antibody-mediated patches of epitope-tagged TGF-β receptors at the cell surface. Briefly, Cos7 cells were transfected with myc-tagged TGF-β receptors. After 48 h, live cells (untreated or treated to alter coated pit structure) were incubated at 4°C (to ensure cell surface labeling and eliminate internalization) with chicken α-myc (20 μg/ml, 1 h, together with 200 μg/ml normal goat IgG for blocking) followed by Cy3-donkey anti-chicken (DαC) IgG (20 μg/ml, 30 min) to induce receptor clustering. After the patching step, the cells were washed and fixed/permeabilized at −20°C in methanol (5 min) followed by acetone (2 min). Intracellular α-adaptin was then labeled with mouse AP.6 IgG (20 μg/ml,1 h, 22°C, together with normal goat IgG) followed by fluorescein isothiocyanate (FITC)-GαM IgG (5 μg/ml, 30 min, 22°C). The Cells were mounted with Mowiol containing 29 mM n-propylgallate, and fluorescence images were acquired with a charge-coupled device camera as described previously (Keren et al., 2001). The FITC and Cy3 images were exported to Photoshop (Adobe Systems, Mountain View, CA) and superimposed. The numbers of red, green, and yellow (superimposed red and green) patches were counted on 20 × 20-μm2 flat cell regions; in each case, ∼100 patches were counted per cell on 10–15 cells.
Coimmunoprecipitation of AP2 and Clathrin with TGF-β Receptors
C-Terminally FLAG-tagged TβRI and TβRII were generated from the respective HA-tagged receptors (Wrana et al., 1994) by using polymerase chain reaction to swap the HA tag with FLAG. They were transiently expressed in Cos7 cells with LipofectAMINE 2000 (Invitrogen). Then 24 h posttransfection, cells were washed twice with phosphate-buffered saline (PBS) and placed in DMEM containing 0.2% serum at 37°C for 30 min. The plates were cooled at 4°C for 5 min, incubated with or without 10 ng/ml TGF-β1 (4°C, 1 h), and shifted to 37°C for 20 min. Cells were washed twice with PBS and lysed in 50 mM HEPES, pH 7.4, 0.5% Triton, 150 mM KCl, 1 mM sodium orthovanadate, 2 mM MgCl, 1 mM CaCl2, 10% glycerol in the presence of Complete protease inhibitors (Roche Applied Science). Lysates were precleared with protein A-agarose beads, normalized for equal protein amounts, and precipitated with M2-agarose anti-FLAG affinity gel at 4°C for 2 h. After washing 3× with lysis buffer, bound material was resolved by 8% SDS-PAGE followed by Western blotting with antibodies to β2-adaptin, the clathrin heavy chain or the FLAG epitope.
Yeast Two-Hybrid Analysis
The cytoplasmic domains of TβRI (amino acids 148–503) and TβRII (amino acids 190–565) as well as the truncated TβRII (S199, amino acids 1–199) and TGN38 cytoplasmic tail (amino acids 324–357) were generated by polymerase chain reaction downstream of the GAL4 DNA binding domain in pAS2–1 (MATCHMAKER GAL4; CLONTECH, Palo Alto, CA). The constructs were used to transform yeast strain Y190 and transformants were selected on Trp− plates. Clones expressing the fusion protein were verified by Western blotting and transformed with subunits of AP2 or AP1 fused to the GAL4 DNA activation domain in the pACT-2 vector. Cotransformants were selected on Trp−Leu− plates and protein interactions determined by β-galactosidase expression and growth on Trp−Leu−His− plates containing 25 μg/ml aminotriazole.
Construction and Purification of Glutathione S-Transferase (GST)-Fusion Protein
The AP2 β2 subunit was cloned into pGEX-4T-2 (Pharmacia, Peapack, NJ) and used to transform BL21(DE3) cells (Novogen, Madison, WI). Cultures were grown at 37°C to OD600 of ≈0.3, shifted to 30°C for continued growth to OD600 of ≈0.6–0.8, and induced with 0.1 mM isopropyl β-d-thiogalactoside (2 h, 30°C). Bacterial pellets were suspended in ice-cold STE buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA) containing 100 μg/ml lysozyme and incubated on ice for 15 min. After sonication, bacterial lysates were centrifuged at 10,000 × g for 30 min and supernatants were combined with glutathione-agarose beads (50% vol/vol in PBS). The mixtures were rocked at 4°C for 1 h and the beads washed with 40× bed volume of cold PBS. Fusion protein was eluted with 100 mM Tris-HCl, pH 8, 0.1% Triton, 150 mM NaCl, 15 mM glutathione. Eluates were concentrated and exchanged with storage buffer (20 mM Tris-HCl, pH 7.5, 20% glycerol, 150 mM KCl, 0.5 mM dithiothreitol in the presence of Complete protease inhibitors) in Centricon Plus-20 (Millipore, Bedford, MA).
GST Fusion Protein Binding to TGF-β Receptors
Two approaches were adopted to demonstrate receptor/AP2 subunit interactions. First, TβRI and TβRII (full length or cytoplasmic domains) were cloned into pGEM7Z(+) (Promega) under the control of the T7 promoter and translated in vitro by using a TNT Coupled Reticulocyte Lysate System (Promega) in the presence of EASY TAG EXPRESS [35S]methionine (PerkinElmer Life Sciences, Boston, MA). Aliquots of the labeled products were separated on SDS-PAGE followed by phosphorimaging analysis by using a GS363 molecular imager (Bio-Rad, Hercules, CA) with Molecular Analysis software. Equal amounts of the translated receptors were suspended in 400 μl of rabbit reticulocyte lysate containing an ATP-regenerating system (3 mM MgCl2, 10 mM phosphocreatin, 10 U of creatin phosphokinase, 5 mM ATP) or binding buffer (50 mM HEPES, pH 7.3, 0.05% Triton, 10% glycerol, 0.1% BSA, 100 mM KCl, 2 mM MgCl2, 1 mM CaCl2). Equal moles of GST alone or GST-β2 protein coupled to glutathione-agarose beads were added and the mixtures rocked at room temperature for 1 h. The beads were washed 5× with binding buffer and bound material was resolved by 8% SDS-PAGE and visualized by autoradiography. The second approach used Cos7 cells transfected with C-terminal HA-tagged TβRI and/or TβRII provided by Dr. J. Wrana (Wrana et al., 1994). Then 24 h after transfection, the cells were lysed in 50 mM HEPES, pH 7.4, 0.5% Triton, 150 mM KCl, 50 mM NaF, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM diothiothreitol, 10% glycerol in the presence of Complete protease inhibitors. Lysates expressing equal receptor levels were combined with 10 μg of GST-β2 fusion protein coupled to glutathione-agarose beads and rocked at 4°C for 2 h. After washing 4× with lysis buffer, bound material was separated on 8% SDS-PAGE followed by immunoblotting with α-HA to detect the TGF-β receptors.
RESULTS
Lateral Diffusion of TβRII and TβRI at Surface of Intact Cells Is Inhibited by Interactions with Coated Pits
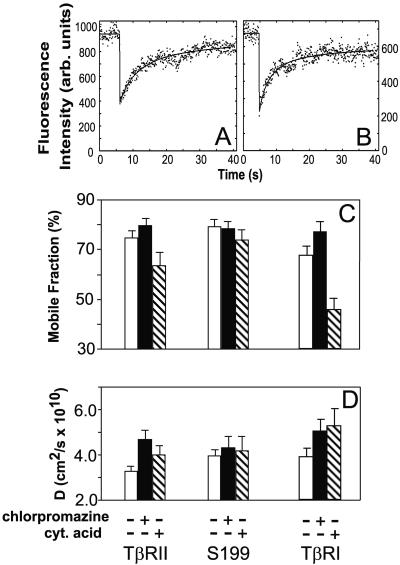
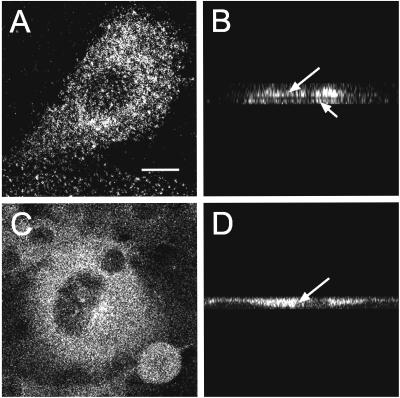
Interactions of membrane proteins with coated pits can retard their lateral diffusion. Therefore, studies on the effects of deleting or mutating internalization signals and/or altering the coated pit structure on the lateral mobility of a receptor can be used to characterize receptor/coated pit interactions in live cells (Fire et al., 1991, 1995). In the current study, we explored the interactions of TβRI and TβRII with coated pits by comparing the lateral mobilities of the full-length receptors, as well as of an endocytosis-impaired TβRII truncation mutant (Ehrlich et al., 2001), in cells with either intact or altered clathrin coat structures. The lateral mobility experiments were performed by fluorescence photobleaching recovery (FPR; see MATERIALS AND METHODS), by using live transiently transfected Cos7 cells expressing different myc-tagged TGF-β receptors (Henis et al., 1994; Gilboa et al., 1998; Ehrlich et al., 2001). The cell-surface receptors were sequentially labeled in the cold with 9E10 mouse anti-myc (α-myc) Fab′ followed by goat anti-mouse (GαM) Alexa 546-Fab′. The results of the FPR experiments (carried out at 18°C, to minimize internalization of endocytosis-competent receptors) are depicted in Figure 1. We first compared the lateral mobility parameters of full-length myc-TβRII and its endocytosis-incompetent mutant S199 (missing most of the cytoplasmic domain) in untreated cells (Figure 1, white bars). Although the RF values (mobile fraction) of the receptors were similar (p ≥ 0.05 according to Student's t test; Figure 1C), the D value of the S199 mutant was significantly higher than that of TβRII (p < 0.005). A reduction in D with no change in RF is typical of transient interactions with immobile structures (presumably coated pits), as explained in detail previously (Fire et al., 1991, 1995). This occurs because each receptor molecule will undergo several association-dissociation cycles with the immobile structures during the FPR measurement, spending some of the time bound to the immobile entity while being free to diffuse the rest of the time. On the other hand, stable association with the immobile entity for the entire duration of the FPR measurement (∼30 s) would reduce RF with no effect on D, because a molecule associated with the immobile structure remains bound for the entire duration of the measurement (Fire et al., 1991, 1995). To verify that the mobility retardation of TβRII is due to interactions with coated pits, we treated the cells with chlorpromazine, which causes a redistribution of AP2 from the plasma membrane to endosomes (Wang et al., 1993) and thereby eliminates any receptor-coated pit interaction. Before studying the effect of chlorpromazine on the lateral mobility of the TGF-β receptors (Figure 1), we tested the effectiveness of chlorpromazine treatment under our experimental conditions. To this end, we examined the effect of chlorpromazine on the association of AP2 with the plasma membrane. The α-adaptin subunits (specific to AP2) were labeled by fluorescent antibodies, and their localization was visualized by confocal microscopy. As shown in Figure 2, AP2 was mostly associated with the plasma membrane in untreated cells, but detached from the cell surface and shifted to a cytosolic distribution after chlorpromazine treatment. Figure 1 demonstrates that this treatment significantly increased D of myc-TβRII (p < 0.001), which became comparable with that of S199 (p > 0.1). D of S199 was not affected, in accord with the absence of an internalization signal in this mutant. These findings are in good correlation with our earlier endocytosis studies, which demonstrated that the endocytosis of this mutant is defective (Ehrlich et al., 2001).
Figure 1.
Treatments that alter the structure of coated pits affect the lateral diffusion of both TβRI and TβRII. Myc-TβRII or myc-TβRI in pcDNA1 or the S199 truncation mutant of myc-TβRII were transfected into Cos7 cells. After 48 h, the live cells were fluorescently labeled in the cold by using monovalent Fab′ fragments (see MATERIALS AND METHODS). Treated cells were preincubated with the buffers specific for each treatment and kept in them during all labeling and FPR measurement steps, conducted at 18°C (see MATERIALS AND METHODS). (A) Representative FPR curve depicting the lateral mobility of myc-TβRII at the surface of untreated cells. The lateral diffusion rate is slower than in B, which depicts a curve obtained on cells treated with chlorpromazine. The dots represent the fluorescence intensity; solid lines are the best fit to the lateral diffusion equation (Petersen et al., 1986). (C and D) Average RF and D values, respectively, derived from all the FPR measurements. Bars represent the mean values ± SEM of 40–60 measurements. White bars, untreated cells; black bars, cells treated with chlorpromazine; crossed bars, cells subjected to cytosol acidification.
Figure 2.
Confocal microscopy demonstrates a shift of AP2 from the plasma membrane to the cytoplasm after chlorpromazine treatment. Cos7 cells growing on glass coverslips were treated with 100 μM chlorpromazine for 30 min as described under MATERIALS AND METHODS. After fixation and permeabilization, intracellular α-adaptin was labeled for immunofluorescence as described for the copatching experiments (see MATERIALS AND METHODS). Fluorescence images were collected on the LSM 510 confocal microscope. Bar, 10 μm. (A and B) Untreated cells. (C and D) Cells treated with chlorpromazine. (B and D) z-Scan analysis of A and C, respectively. Although AP2 labeling of the top (long arrow) and bottom (short arrow) membrane surfaces is evident in untreated cells (B), the labeling shifts to a typical cytoplasmic pattern (arrow) after chlorpromazine treatment (D).
To further demonstrate the involvement of coated pits, we used cytosol acidification, a treatment that freezes the plasma membrane coated pits at an altered conformation (Heuser, 1989; Hansen et al., 1993) and which was shown to convert transient interactions of relatively weak internalization signals into stable ones (Fire et al., 1991, 1995). As shown in Figure 1, C and D, this treatment led to a significant decrease in the RF of myc-TβRII (p < 0.005), whereas RF of S199 was not affected significantly (p ≥ 0.05). Concomitantly, D of myc-TβRII was elevated significantly (p < 0.001), becoming similar to that of S199 (p > 0.05), which does not interact with coated pits. The dual effect of cytosol acidification on myc-TβRII mobility (reducing RF and increasing D) is exactly the outcome expected for a shift from transient interactions to stable entrapment in frozen coated pits. Under these conditions, the receptor molecules residing in coated pits become stably entrapped for the entire duration of the FPR measurement (reducing RF), whereas those located outside the pits are free to diffuse unperturbed (Fire et al., 1991, 1995) (see DISCUSSION).
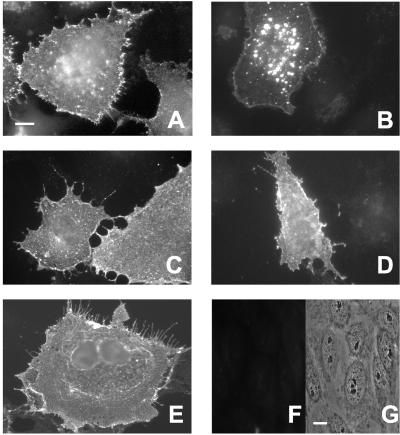
Examination of the lateral diffusion of TβRI in untreated vs. treated cells reveals an analogous picture (Figure 1, C and D). Removal of AP2 from the plasma membrane by chlorpromazine markedly increased the lateral diffusion rate of the receptor (p < 0.001), accompanied by a small increase in RF (p < 0.005). On the other hand, cytosol acidification caused a dramatic drop in RF (p < 0.001) of myc-TβRI with a concomitant increase in D (p < 0.001). These findings are supported by their correlation with the functional inhibition of the internalization of myc-TβRI by either treatment (Figure 3): in untreated cells, the fluorescent-labeled cell surface myc-TβRI shifted upon incubation at 37°C from a homogeneous distribution at the cell surface (Figure 3A) to a vesicular fluorescent pattern typical of endocytosis (Figure 3B), but failed to do so in the treated cells (Figure 3, C–E). Analogous findings demonstrating the effectiveness of these treatments in blocking TβRII endocytosis and showing no internalization of the S199 mutant were reported by us previously (Ehrlich et al., 2001; our unpublished data). Taken together, these results suggest that in untreated cells the lateral diffusion of TβRI and TβRII is inhibited by chlorpromazine-sensitive transient interactions with coated pits, which shift to stable entrapment upon alteration of the coated pit structure by cytosol acidification.
Figure 3.
Functional inhibition of TβRI internalization by treatments that block coated pit-mediated endocytosis. Cos7 cells were transfected with myc-TβRI as in Figure 1. The internalization protocol was as described by us previously (Ehrlich et al., 2001). Then 48 h posttransfection, the cell surface receptors were labeled at 4°C as in Figure 1, except that for the primary antibodies monoclonal α-myc IgG replaced the α-myc Fab′ fragment. (A) Untreated cells kept in the cold to avoid internalization. (B) Untreated cells, 20 min at 37°C; note the shift from a uniform to a vesicular endocytic staining pattern. (C) Chlorpromazine-treated cells, 20 min at 37°C. (D) Cells treated by hypertonic medium, 20 min at 37°C. (E) Cells subjected to cytosol acidification treatment, 20 min at 37°C. Note that all the treatments (C–E) blocked the shift to vesicular endocytic pattern. The labeling specificity is demonstrated by the absence of fluorescent staining (F) in untransfected cells labeled with the same antibodies; G is a phase contrast image of the same field. Bar, 10 μm.
AP2 Colocalization with Antibody-mediated Patches of TGF-β Receptors Depends on the Coated Pit Organization
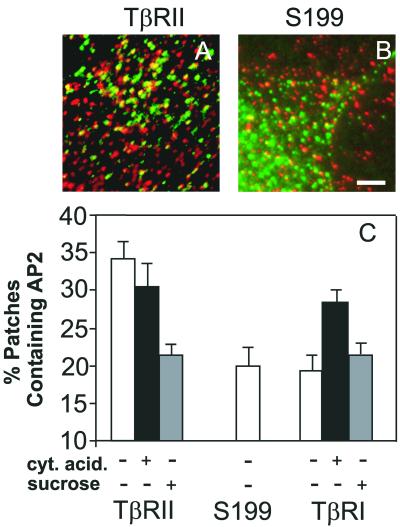
The FPR measurements (Figure 1) suggest transient interactions between TGF-β receptors and coated pits. To obtain direct evidence for interactions of the receptors with the endocytic machinery at the surface of live cells, we measured the degree of colocalization of the receptors with AP2, an essential component of the endocytic coat complex. To this end, we used a variation (Keren et al., 2001) of the immunofluorescence copatching method described by us in detail previously (Henis et al., 1994; Gilboa et al., 1998, 2000). Live Cos7 cells transiently expressing myc-tagged TGF-β receptors were labeled in the cold by chicken α-myc followed by Cy3-IgG, causing the formation of fluorescent patches of cell surface receptors. The cells were then fixed/permeabilized and the intracellular AP2 was labeled using antibodies to α-adaptin and FITC-GαM IgG. The results (Figure 4) show that although a relatively high percentage (33%) of the myc-TβRII patches also contained appreciable α-adaptin (specific to AP2) labeling, a significantly lower level of AP2 (19%, p < 0.001) colocalized with the S199 myc-TβRII truncation mutant. This low value, which is similar to that observed for the copatching of influenza hemagglutinin (a protein completely devoid of any internalization signals) with AP2 (Keren et al., 2001), represents the basal level of copatching due to the cumulative contribution of factors other than specific association (occasional overlap between densely located patches, nonspecific interactions, and possible formation of complexes of the endocytosis-defective mutants with endogenous wt TGF-β receptors expressed at low levels). Hypertonic treatment, which disperses the clathrin lattices underlying the membrane, significantly reduced the level of AP2/myc-TβRII colocalization (22%, p < 0.001), bringing it essentially to the basal level observed with the S199 mutant. This is in accord with the notion that in the absence of clathrin lattices, which are dispersed by the hypertonic treatment, the affinity of AP2 for internalization signals (and thus for the receptors) is reduced (Rapoport et al., 1997). On the other hand, cytosol acidification did not affect significantly AP2/myc-TβRII colocalization (p > 0.05). Because this treatment stabilizes the interactions of TβRII with coated pits (shifting them from transient to stable; Figure 1), these results suggest that the transient interactions in untreated cells are strong enough to allow copatching of TβRII within coated pits, even before stabilization by cytosol acidification. In contrast to myc-TβRII, only a basal level of myc-TβRI colocalization with AP2 (similar to that measured for the endocytosis-negative S199; p > 0.05) was observed on both untreated and hypertonically treated cells (Figure 4C). This indicates that in untreated cells the complexes of TβRI with coated pits are weaker than those of TβRII, enabling their dissociation during the patching step. This is in line with the higher D value of myc-TβRI relative to myc-TβRII (Figure 1D, white bars), as expected for weaker interactions with mobility-restricting structures. Interestingly, cytosol acidification resulted in a significant elevation (to 28%) in the level of AP2/myc-TβRI colocalization (p < 0.001), suggesting a stabilization of TGF-β receptor interactions with the frozen coated pit structures. This conclusion is supported by the strong reduction in RF of myc-TβRI after cytosol acidification (Figure 1C).
Figure 4.
Colocalization of AP2 complexes with antibody-mediated patches of TGF-β receptors at the cell surface. Cos7 cells expressing myc-TβRII, myc-TβRI, or S199 were either untreated or subjected to hypertonic or cytosol acidification treatments (see MATERIALS AND METHODS). The cell surface receptors were patched and labeled in the cold with chicken α-myc followed by Cy3-DαC IgG (red fluorescence; see MATERIALS AND METHODS). The cells were fixed/permeabilized and labeled for α-adaptin by AP.6 mouse IgG followed by FITC-GαM IgG (green fluorescence). The fluorescent images were taken by charge-coupled device by using selective filter sets for FITC and Cy3 and superimposed (see MATERIALS AND METHODS). (A) Representative cell expressing myc-TβRII. (B) Representative cell expressing the S199 endocytosis-negative myc-TβRII mutant. (C) Quantification of the percentage of myc-tagged TGF-β receptor patches that contain AP2. Superimposed red and green images were analyzed by counting the numbers of green (G), red (R), and yellow (Y) patches, counting around 100 patches/cell on 10–15 cells in each case. The bars are mean ± SEM of these measurements. The percentage of patches containing AP2 is given by 100 × Y/(Y + R).
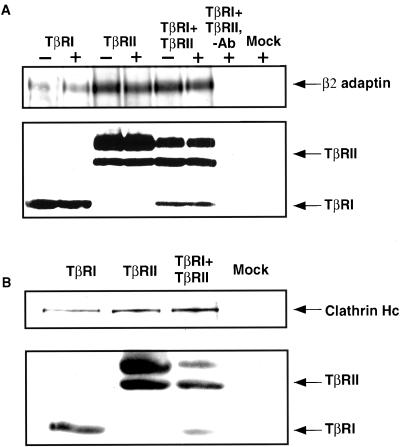
TGF-β Type I and Type II Receptors Coimmunoprecipitate with AP2 and Clathrin
The lateral mobility and copatching studies (Figures 1 and 4) suggested transient interactions between TGF-β receptors and clathrin coats/AP2 in live cells. To further investigate these interactions, we measured the coprecipitation of AP2 and clathrin with the receptors. Cos7 cells were transiently transfected with FLAG-tagged TβRI and/or TβRII, and incubated with or without 10 ng/ml TGF-β1 (1 h at 4°C followed by 20 min at 37°C). Cell lysates were then immunoprecipitated with M2-agarose α-FLAG. As shown in Figure 5, although AP2 and clathrin coprecipitated with either singly expressed TβRI or TβRII, their association with TβRI was weaker than with TβRII, in correlation with the observations made in the FPR and copatching studies (Figures 1 and 4). Interestingly, ligand binding had no appreciable effect on the binding of TβRI and/or TβRII to AP2 (Figure 5A) or clathrin (Figure 5B). Although this is distinct from what has been reported for EGF receptors (Sorkin and Carpenter, 1993), it is likely a reflection of the constitutive TGF-β receptor-recycling activity we have recently reported (Doréet al., 2001; Mitchell and Leof, unpublished data).
Figure 5.
TβRI and TβRII coprecipitate with the AP2 complex and clathrin. (A) Cos7 cells were transiently transfected with vector alone (Mock), FLAG-TβRI, and/or FLAG-TβRII. After 24 h the cells were treated with (+) or without (−) 10 ng/ml TGF-β1 and lysed as described in MATERIALS AND METHODS. The lysates were incubated with α-FLAG agarose affinity gel (4°C, 2 h) or with agarose alone (−antibody). Bound material was detected by Western blotting by using antibodies to β2-adaptin. Bottom, TGF-β receptor expression in the same blot after stripping and reprobing with α-FLAG. (B) Cos7 cells were treated as in A in the absence of ligand and lysates probed with anti-clathrin heavy chain antibody followed by reprobing with α-FLAG to detect TβRI and TβRII. Data shown are of a representative result of three experiments.
TGF-β Receptors Specifically Interact with β2 Subunit of AP2
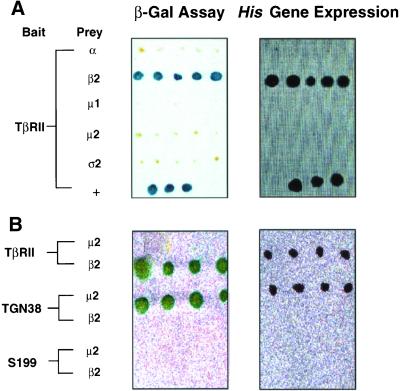
The above-mentioned studies (Figures 1–5) demonstrate interactions of TβRII and TβRI with AP2 and clathrin; however, they do not identify the AP2 subunit(s) with which the receptors interact. To determine whether the interaction of the TGF-β receptors with AP2 is direct and identify the specific AP2 subunit(s) that binds the receptors, we used the cytoplasmic domains of TβRI and TβRII as baits in yeast two-hybrid assays. Clones expressing relatively equal levels of the cytoplasmic domains were selected by Western blotting and independently transfected with the four AP2 subunits (α, β2, μ2, and ς2) or the medium chain of AP1 (μ1). Figure 6A shows that the cytoplasmic region of TβRII specifically interacts with the β2 subunit of AP2, but not the other subunits. To more critically define the specific association of β2-adaptin with the cytoplasmic tail of TβRII, the binding of S199 (a truncated mutant of TβRII with only ∼10 amino acids downstream of the transmembrane domain) or TGN38 to the AP2 β2 or μ2 chains was explored (Figure 6B). Consistent with that observed in Figures 1 and 4, the truncated S199 receptor did not bind either AP2 subunit. As expected, the cytoplasmic tail of TGN38 only associated with the μ2 chain. Thus, TβRII specifically binds AP2 through a defined interaction between the receptor's cytoplasmic tail and the β2 chain. No specific binding of the cytoplasmic domain of TβRI to any of the subunits tested could be detected (our unpublished data).
Figure 6.
Cytoplasmic domain of TβRII interacts with the β2 subunit of AP2. Yeast strain Y190 was transformed with bait plasmid consisting of the cytoplasmic domain of TβRII, the S199 TβRII mutant, or the TGN38 cytoplasmic tail fused downstream of the DNA binding domain of GAL4 (GAL4BD). Clones expressing bait proteins were selected on Trp− plates and transformed with the indicated AP subunits fused downstream of the activation domain of GAL4 (GAL4AD). Double transformants were tested for reporter gene expression. (A) Left, double transformants capable of growth on Trp−Leu− plates were lysed and β-galactosidase expression was determined by incubating with 80 μg/ml X-gal (Invitrogen) at 37°C for 2–6 h; blue colonies indicate protein interactions. Right, clones assessed in the left panel were streaked on Trp−Leu−His− plates; growth indicates protein interactions. Y190 cotransformed with GAL4BD-p53 and GAL4AD-TAg (large T antigen) was used as positive controls (+). (B) Studies similar to those described in A were performed using the indicated proteins as bait and prey.
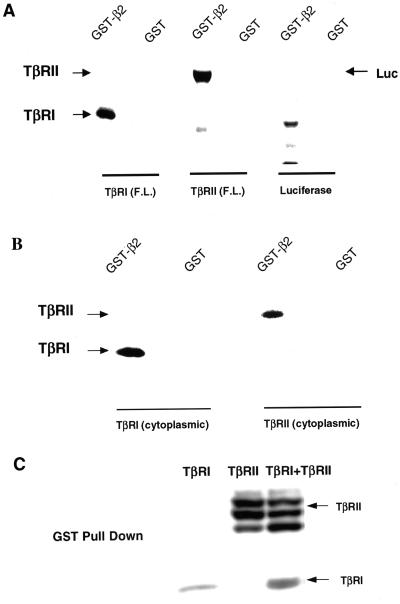
To further confirm the association of β2 with TβRII and to investigate whether the inability to detect AP2 subunit/TβRI interaction by two-hybrid analysis simply reflected the weaker TβRI/AP2 association observed previously (Figures 1, 4, and 5), we performed binding assays with GST-β2 subunit fusion protein and in vitro translated full-length (Figure 7A) or cytoplasmic domain alone (Figure 7B) TGF-β receptors. After incubation with the [35S]methionine-labeled receptors, TGF-β receptor complexes bound to the GST fusion proteins were isolated and visualized by autoradiography. To minimize the influences of salt and/or detergent on receptor/AP2 interactions, we also performed binding assays in an ATP-reconstituted rabbit reticulocyte lysate, with essentially similar results (Figure 7B; our unpublished data). Under both conditions, TβRI and TβRII (full-length and cytoplasmic domains alone) specifically interacted with the GST-β2 fusion protein but not with GST alone, whereas in vitro translated luciferase protein did not bind to GST-β2.
Figure 7.
Type I and type II TGF-β receptors bind the β2 subunit of AP2. (A) Full-length (F.L.) TβRI, TβRII or luciferase were in vitro translated in the presence of [35S]methionine. Equal molar amounts were suspended in 400 μl of binding buffer (see MATERIALS AND METHODS). Then 10 μg of GST alone or GST-β2 bound to glutathione-agarose beads was added and the mixture rocked 1 h at 22°C. After extensive washing, bound material was analyzed by SDS-PAGE and autoradiography (see MATERIALS AND METHODS). Analogous results were obtained when binding was performed in reticulocyte lysate (our unpublished data). (B) Studies identical to those described in A were performed using in vitro translated cytosolic domains of TβRI or TβRII in 400 μl of rabbit reticulocyte lysate supplemented with an ATP-regenerating system. Similar results (our unpublished data) were obtained when the binding was carried out in binding buffer as in A. (C) Cos7 cells were transiently transfected with HA-tagged TβRI and/or TβRII. After 24 h, cells were lysed as described under GST fusion protein binding (see MATERIALS AND METHODS). Lysates were normalized for equal receptor expression and incubated with 10 μg of GST-β2. After washing, bound material was separated by SDS-PAGE and analyzed by immunoblotting with α-HA. Each panel depicts a representative experiment of five.
To substantiate the interaction of β2-adaptin with TβRI and/or TβRII, GST-β2 fusion protein was incubated with lysates prepared from Cos7 cells transiently transfected with HA-tagged full-length TβRI and/or TβRII. Similar to the results described above, Figure 7C shows that GST-β2 interacts with both TβRI (lane 1) and TβRII (lane 2). Although coexpression of both receptors did not appreciably modulate the association with β2-adaptin (lane 3), the interaction with TβRII was more pronounced, in accord with the studies on live cells (Figures 1 and 4). These results (Figures 5–7) document specific in vivo and in vitro binding of TGF-β receptors to the β2 subunit of AP2.
TβRI and TβRII Bind Trunk Domain of β2-Adaptin
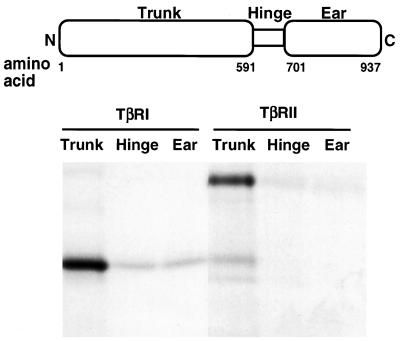
The two large subunits of AP1 (γ and β1) and AP2 (α and β2) can be divided into three structural domains: the N-terminal “trunk”; the C-terminal “ear”; and the Pro/Gly rich “hinge” region (Hirst and Robinson, 1998; Kirchhausen, 1999). To determine which domain(s) of β2-adaptin was responsible for mediating the interactions with TβRI and TβRII, GST fusion proteins of the trunk, hinge, and ear regions were purified. Equal molar concentrations of fusion protein were then incubated with in vitro translated full-length TβRI or TβRII. As shown in Figure 8, both receptors strongly interacted with the β2 trunk, but not with either the hinge or the ear region. These findings indicate that TβRI and TβRII bind to β2-adaptin at a site(s) distinct from that of clathrin and that the β2 trunk domain can directly link plasma membrane receptors with the endocytic machinery.
Figure 8.
TGF-β receptors bind the trunk domain of β2-adaptin. Full-length TβRI or TβRII was in vitro translated in the presence of [35S]methionine. Equivalent amounts of receptor protein were incubated (1 h, 22°C) with equal molar amounts of GST fusion proteins consisting of the β2 trunk (amino acids 1–591), hinge (amino acids 592–700), or ear (amino acids 701–937) domains. After washing, bound receptors were analyzed by SDS-PAGE and autoradiography (see MATERIALS AND METHODS). The data are representative of three independent experiments.
DISCUSSION
Clathrin-coated pit-mediated endocytosis is a major mechanism regulating the level of receptors at the plasma membrane (Sorkin and Waters, 1993; Mukherjee et al., 1997; Schmid, 1997). Several reports have demonstrated that the internalization of TβRII and TβRI proceeds via this pathway (Anders et al., 1997; Ehrlich et al., 2001). To explore the hitherto unknown mode of TGF-β receptor coupling to the endocytic pathway, we studied the interactions of the receptors with coated pits, with AP2 complexes, and with specific AP2 subunits and their domains. These issues were thoroughly investigated by combining biophysical and biochemical methods, enabling studies of the interactions both in vivo and in vitro with specific proteins and protein subdomains.
Because the binding of membrane proteins to structures that are laterally immobile on the time scale of the mobility measurements affects either their D or RF values, studies on their lateral mobility in cells with intact vs. disrupted coated pits can characterize their interactions with coated pit structures (Fire et al., 1991, 1995). The dissociation rate of the membrane protein from the immobile entity determines the nature of the effect: transient interactions (labile complexes) result in a reduction of D, whereas stable entrapment (duration of association longer than the characteristic lateral diffusion time) leads to a reduced RF (Fire et al., 1991, 1995). The data on the lateral mobility of myc-TβRII, myc-TβRI, and the S199 endocytosis-impaired TβRII mutant in untreated cells (Figure 1) clearly demonstrate a reduction in D of myc-TβRII (and to a lesser degree of myc-TβRI) relative to the endocytosis-negative S199. These findings are in accord with the notion that the cytoplasmic tail of full-length TGF-β receptors interact transiently with immobile structures, presumably coated pits (as confirmed by the biochemical experiments described below). The identification of these structures as coated pits is supported by the effects of two independent treatments known to affect the structure of clathrin-related endocytic complexes. The first, removal of AP2 from the plasma membrane by chlorpromazine (Wang et al., 1993) elevated D of full-length TβRII to the rate measured for S199, suggesting that AP2 removal abrogates the mobility-restricting interactions. The second treatment used cytosol acidification to freeze the coated pits in an altered conformation at the cell surface (Heuser, 1989; Hansen et al., 1993). This treatment led to a reduction in the RF values of both myc-TβRI and myc-TβRII (as expected for stable entrapment of the receptor subpopulation associated with the frozen coated pits), accompanied by an increase in D of the mobile subpopulation. These findings suggest a shift from transient interactions to stable entrapment in the altered coated pit structures after cytosol acidification, as we have demonstrated for other membrane proteins with transiently interacting internalization signals (Fire et al., 1991, 1995).
The studies on the colocalization of AP2 with antibody-mediated patches of TGF-β receptors complement the FPR studies and demonstrate the association of AP2 with the mobility-restricting structures (Figure 4). The results of the copatching experiments are in good correlation with the lateral mobility studies. In both experiments, myc-TβRII exhibited stronger association with coated pits/AP2 compared with myc-TβRI, whose interactions were significantly increased after cytosol acidification (Figures 1C and 4). These interactions seem to be stronger when AP2 is associated with clathrin, because disruption of the clathrin lattices by hypertonic treatment, which leaves AP2 associated with membrane proteins carrying strong internalization signals (Keren et al., 2001), reduced the copatching of AP2 with TGF-β receptors to the basal level measured for the S199 internalization-negative mutant (Figure 4). This notion is in line with the report (Rapoport et al., 1997) that the binding of signal peptides to AP2 is significantly stronger when AP2 is associated with clathrin.
To validate the association of the TGF-β receptors with clathrin-coated pit components, we further analyzed these interactions by coimmunoprecipitation of endogenous AP2 and clathrin with epitope-tagged TGF-β receptors in Cos7 cells (Figure 5). Both receptors were able to bind AP2 and clathrin when singly expressed; however, TβRII demonstrated stronger association with AP2 and clathrin than TβRI. This result correlates with the observations made in the FPR and copatching studies (Figures 1 and 4). Interestingly, TGF-β ligand did not enhance the binding of coexpressed TβRI and TβRII to AP2 complexes. This is distinct from that observed for EGF receptors, whose association with AP2 was augmented by ligand treatment due to exposure of receptor motifs that interact with AP2 upon ligand-stimulated autophosphorylation (Nesterov et al., 1995). Although this may reflect distinct mechanisms for various receptor families, it is also possible that because TβRII is constitutively autophosphorylated, the availability of the receptor regions that bind to AP2 does not depend on ligand stimulation. Moreover, phosphorylation or kinase activity of TβRI does not affect AP2 binding (our unpublished data). Thus, the results depicted in Figures 1 and 3–5 indicate that 1) TβRII and TβRI interact with coated pit structures; 2) disruption or alteration of coated pit structures by chlorpromazine, hypertonic medium, or cytosolic acidification prevents TβRI endocytosis, as demonstrated previously for TβRII; 3) AP2 complexes colocalize and coimmunoprecipitate with full-length TGF-β receptors; 4) TβRII binds AP2 with greater affinity than TβRI; and 5) coexpression of TβRI and TβRII or the addition of ligand do not significantly modulate receptor association with AP2.
The above-mentioned results (Figures 1–5) strongly suggest that TGF-β receptors interact transiently with clathrin-coated pit structures and AP2. Although these studies provided important information on the association of the receptors with the clathrin-based endocytic system, they could not determine the component(s) of this machinery that binds the receptors. In light of the variety of possible interactions between internalization signals and the endocytosis machinery (Ohno et al., 1995; Kibbey et al., 1998; Bonifacino and Dell'Angelica, 1999; Hofmann et al., 1999), we attempted to identify the interacting AP2 subunit(s). To this end, we used two independent methods: yeast two-hybrid assays by using the cytoplasmic receptor tails and the different AP2 subunits, and the binding of in vitro-translated TGF-β receptors to GST-β2 subunit fusion proteins. The yeast two-hybrid screens (Figure 6B) indicated that TβRII specifically interacted with the β2 subunit of AP2; no functional interaction was observed with any of the other AP2 subunits (α, μ2, or ς2) or the μ1 subunit of AP1. Moreover, β2-adaptin binding was only observed with TβRII, whereas TGN38 (as expected) showed no β2 binding but associated with the AP2 μ2 chain (Figure 6B). Last, in agreement with the FPR and colocalization studies (Figures 1 and 4), the cytoplasmic tail of TβRII was necessary for AP2 binding because the S199-truncated TβRII mutant was unable to interact with the β2 subunit (Figure 6B). These results were further supported by the finding that in vitro-translated TβRII (full-length or cytoplasmic domain) binds specifically to the GST-β2 fusion protein (Figure 7). Although TβRI failed to yield positive two-hybrid interactions with any of the AP2 subunits (our unpublished data), this seems to be due to weaker interactions that become undetectable under the conditions of the two-hybrid assay because in vitro-translated TβRII and TβRI could both bind to GST-β2 (Figure 7). This conclusion is supported by the studies on intact cells (FPR and copatching) as well as by the GST pulldown studies, which indicate that TβRI associates with AP2 albeit with lower affinity compared with TβRII (Figures 1, 4, 5, and 7). Although the best-established endocytic motif-adaptor interactions occur via the μ2 subunit, the binding of the TGF-β receptors to β2-adaptin is in line with reports that point to the existence of different saturable components involved in the recognition of distinct internalization signals (Marks et al., 1997; Warren et al., 1998).
It has been previously determined that clathrin association with AP2 occurs via binding to an LLD/NLD sequence in the hinge domain of the β2 subunit (Shih et al., 1995). To define the domain of β2-adaptin mediating TGF-β receptor interaction, the binding of in vitro translated TGF-β receptors to GST-fusion proteins encoding the trunk, hinge, or ear region of β2-adaptin was measured (Figure 8). Both receptors were found to bind to the trunk domain, in accord with the distinct functions proposed for the various domains of β2-adaptin (Owen et al., 2000).
The present study is the first thorough demonstration, encompassing information from studies in intact cells to direct in vitro binding studies, of the association between specific receptors (TβRI and TβRII) and the clathrin-based endocytic apparatus via binding to β2-adaptin. The application of biophysical experiments to live, intact cells allowed the first characterization of the transient mode of interactions of receptors from the TGF-β superfamily with the endocytic machinery. An additional unique feature of the present study is the identification of the molecular domains (the cytoplasmic domains of the receptors and the trunk domain of β2-adaptin) involved in this interaction. A recent report describing the role of β1- and β2-adaptin in the down-regulation of CD4 underscores the importance of defining interactions between an endocytic motif (a di-leucine motif in the cytoplasmic human immunodeficiency virus Nef protein) and β-adaptin (Greenberg et al., 1998). The present report demonstrates that such binding is not limited to cytoplasmic proteins. In light of the present results, the involvement of β2-adaptin in direct targeting of other membrane receptors for endocytosis should be considered.
ACKNOWLEDGMENTS
We thank Mark Wilkes for excellent technical assistance, Drs. Richard Pagano and Mark McNiven for helpful comments, Dr. Juan Bonifacino for the AP2 two-hybrid constructs, and Dr. Jeffrey Wrana for HA-tagged TGF-β receptor constructs. This work was supported in part by Public Health Service grants GM-54200 and GM-55816 from the National Institute of General Medical Science and the Mayo Foundation (to E.B.L.), and by grants from the Israel Science Foundation (grant 414/01) and the Israel Cancer Research Fund (to Y.I.H.).
Abbreviations used:
- α-FLAG
mouse monoclonal antibodies against the FLAG epitope tag
- α-HA
mouse monoclonal antibodies that recognize a specific epitope of the HA protein
- α-myc
antibodies recognizing a specific c-myc sequence
- DαC
donkey IgG anti-chicken IgG
- D
lateral diffusion coefficient
- FPR
fluorescence photobleaching recovery
- GαM
goat IgG anti-mouse IgG
- GST
glutathione S-transferase
- RF
mobile fraction
- TGF-β
transforming growth factor-β
- TβRI
TGF-β type I receptor
- TβRII
TGF-β type II receptor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–07–0104. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–07–0104.
REFERENCES
- Anders RA, Arline SL, Doré JJE, Jr, Leof EB. Distinct endocytic responses of heteromeric and homomeric transforming growth factor β receptors. Mol Biol Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Doré JJE, Jr, Arline SA, Garamszegi N, Leof EB. Differential requirement for type I and type II transforming growth factor β receptor kinase activity in ligand-mediated receptor endoctyosis. J Biol Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Axelrod D, Koppel DE, Schlessinger J, Elson EL, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of alpha-adpatin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular basis for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Doré JJE, Jr, Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB. Mechanisms of transforming growth factor-β receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol Biol Cell. 2001;12:675–684. doi: 10.1091/mbc.12.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Shmuely A, Henis YI. A single internalization signal from the di-leucine family is critical for constitutive endocytosis of the type II TGF-β receptor. J Cell Sci. 2001;114:1777–1786. doi: 10.1242/jcs.114.9.1777. [DOI] [PubMed] [Google Scholar]
- Fire E, Gutman O, Roth MG, Henis YI. Dynamic or stable interactions of influenza hemagglutinin mutants with coated pits: dependence on the internalization signal but not on aggregation. J Biol Chem. 1995;270:21075–21081. doi: 10.1074/jbc.270.36.21075. [DOI] [PubMed] [Google Scholar]
- Fire E, Zwart DE, Roth MG, Henis YI. Evidence from lateral mobility studies for dynamic interactions of a mutant influenza hemagglutinin with coated pits. J Cell Biol. 1991;115:1585–1594. doi: 10.1083/jcb.115.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P. Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol Biol Cell. 2000;11:1023–1035. doi: 10.1091/mbc.11.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI. Oligomeric structure of type I and type II transforming growth factor β receptors: homodimers form in the ER and persist at the plasma membrane. J Cell Biol. 1998;140:767–777. doi: 10.1083/jcb.140.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis YI, Gutman O. Lateral diffusion and patch formation of H-2Kk antigens on mouse spleen lymphocytes. Biochim Biophys Acta. 1983;762:281–288. doi: 10.1016/0167-4889(83)90082-4. [DOI] [PubMed] [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF. The types II and III transforming growth factor-β receptors form homo-oligomers. J Cell Biol. 1994;126:139–154. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989;108:401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Hofmann MW, Honing S, Rodinonov D, Dobberstein R, von Figura K, Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J Biol Chem. 1999;274:36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- Keren T, Roth MG, Henis YI. Internalization-competent influenza hemagglutinin mutants form complexes with clathrin-deficient multivalent AP-2 oligomers in live cells. J Biol Chem. 2001;276:28356–28363. doi: 10.1074/jbc.M102235200. [DOI] [PubMed] [Google Scholar]
- Kibbey RG, Rizo J, Gierasch LM, Anderson RG. The LDL receptor clustering motif interacts with the clathrin terminal domain in a reverse turn conformation. J Cell Biol. 1998;142:59–67. doi: 10.1083/jcb.142.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Koli KM, Arteaga CL. Processing of the transforming growth factor beta type I and II receptors: biosynthesis and ligand-induced regulation. J Biol Chem. 1997;272:6423–6427. doi: 10.1074/jbc.272.10.6423. [DOI] [PubMed] [Google Scholar]
- Koppel DE, Axelrod D, Schlessinger J, Elson EL, Webb WW. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976;16:1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen YG. Controlling TGFβ signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Kurten RC, Gill GN. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem. 1995;270:6320–6327. doi: 10.1074/jbc.270.11.6320. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Noble ME, Hunter JB, Dafforn TR, Evans PR, McMahon HT. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR. The structure and function of the β2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LJ, Robinson MS. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol. 1995;131:619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NO, Felder S, Elson EL. Measurement of lateral diffusion by fluorescence photobleaching recovery. In: Weir DM, Herzenberg LA, Blackwell CC, Herzenberg LA, editors. Handbook of Experimental Immunology. Edinburgh: Blackwell Scientific Publications; 1986. pp. 24.21–24.23. [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson S, Kirchhausen T. Dileucine-based sorting signals bind to the β chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T. A clathrin-binding site in the hinge of the β2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- Traub LM, Downs MA, Westrich JL, Fremont DH. Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc Natl Acad Sci USA. 1999;96:8907–8912. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RA, Green FA, Stenberg PE, Enns CA. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J Biol Chem. 1998;273:17056–17063. doi: 10.1074/jbc.273.27.17056. [DOI] [PubMed] [Google Scholar]
- Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Zwaagstra JC, El-Alfy M, O'Connor-McCourt MD. TGF-β1 internalization: modulation by ligand interaction with TGF-β receptors types I and II and a mechanism that is distinct from clathrin-mediated endocytosis. J Biol Chem. 2001;276:27237–27245. doi: 10.1074/jbc.M100033200. [DOI] [PubMed] [Google Scholar]