The varitint-waddler (Va) mutant mouse must be the prettiest mouse I have ever seen. The mutation shows semidominant inheritance, and mice carrying one copy of the Va mutation are tricolored, as its name suggests, with variable patches of fur in white, pale gray, and the rich chestnut color agouti (1). It is also deaf and has the classic head-bobbing and circling behavior typical of mice with defects of the vestibular (balance) part of the inner ear (2, 3). Now the mutated gene has been identified by Di Palma et al. (4) in this issue of PNAS, and it turns out to be Mcoln3, encoding a previously uncharacterized mucolipin member of the transient receptor potential (TRP)-channel superfamily.
Mcoln3 shows sequence similarity to other members of the TRP-channel superfamily including six predicted transmembrane domains (4). The Va mutation is an alanine-to-proline missense mutation within the fifth transmembrane domain, a region likely to be involved in determining the physiological properties of the putative Mcoln3 channel. A second mutation, VaJ, arose in the original varitint-waddler stock and seems to ameliorate the phenotypic effects of the Va mutation. This was identified as a second missense mutation in addition to the original Va mutation, this time substituting a threonine for an isoleucine residue in the second extracellular loop. The second mutation found in the VaJ allele seems to act as a partial intragenic suppressor of the original Va mutation. The Mcoln3 protein was detected in the cochlea of VaJ homozygotes, which together with the semidominant mode of inheritance suggests that the mutations may cause gain-of-function or dominant-negative effects on the protein (4).
It is not surprising that channels are important to hearing. Sensory hair cells in the cochlea are responsible for detecting minute sound vibrations not much larger than Brownian motion at the threshold of hearing. Channels in hair cells, along with many other types of molecules, maintain and rapidly restore their homeostatic state, allowing them to respond to and amplify these tiny mechanical stimuli (5–8). Each hair cell in the inner ear has a set of actin-filled projections called stereocilia arranged in a regular array in rows of graded height (illustrated in Figs. 1 and 2). Stereocilia are linked to each other by several types of extracellular crosslinks and by tip links (ref. 9; Fig. 1). When the bundle of stereocilia is deflected by sound toward the tallest row, the tip links are pulled taut and directly open transduction channels located at the end of the link (5–8). This opening allows cations to flood into the cell, leading to depolarization of the hair cell, release of synaptic vesicles, and initiation of an action potential in the associated neurons. The transduction channel is relatively nonselective for different cations, and the major current through the channel is thought to be carried by potassium and calcium ions. An influx of calcium will have many immediate effects, probably including changing the conductance of the transduction channel itself (7), and the plasma membrane covering stereocilia is packed with calcium pumps (encoded by Atp2b2) thought to control local calcium levels by pumping it straight out again (10). The influx of potassium more likely is removed through the basolateral membrane of the hair cell and recycled through a network of cells to the stria vascularis, a structure that is vital for maintaining electrochemical homeostasis within the cochlear duct (11–13). The stria vascularis secretes endolymph, an unusual fluid bathing the tops of hair cells, which has a high-potassium, low-sodium concentration and is maintained at a high positive resting potential, the endocochlear potential (discussed further below). The endocochlear potential enhances the potential difference across the hair-cell transduction channels, helping to drive cations into the negative interior of the cell when the channel is opened.
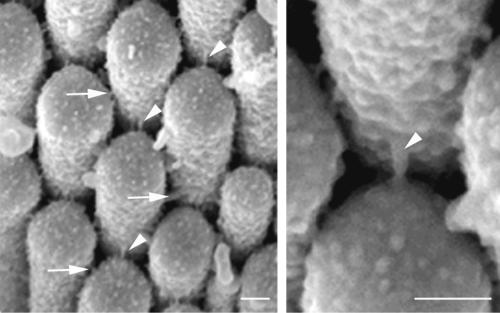
Fig 1.
(Left) Tip links (arrowheads) and crosslinks (arrows) between adjacent stereocilia of an adult mouse hair cell in the utriculus of the vestibular part of the inner ear. (Right) Higher magnification to show a tip link. The transduction channel is thought to be located at one end of the tip link. (Bars, 100 nm.) The figure was kindly provided by Elizabeth Quint (Nottingham, U.K.).
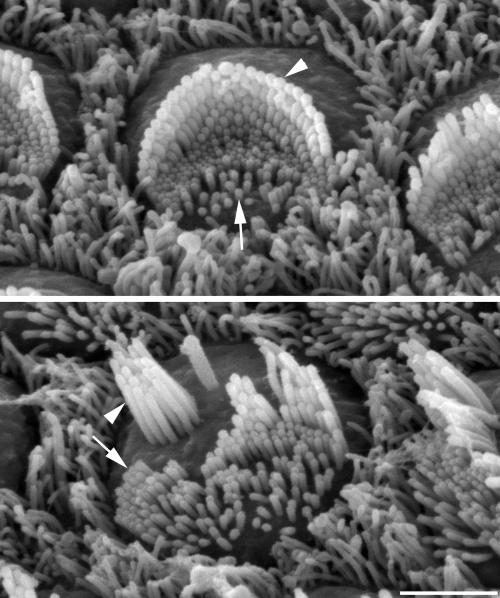
Fig 2.
(Upper) Hair bundle of a normal, immature (postnatal day 4) mouse outer hair cell showing the V-shaped array of stereocilia (arrowhead) on the outer side arranged in staircase-like rows of graded heights and microvilli (arrow) on the inner side that will be resorbed later. (Lower) Top of an outer hair cell from a postnatal day-4 waltzer mutant (Cdh23v2J/Cdh23v2J) showing that stereocilia (arrowhead) have grown tall and formed clusters with rows of graded heights, whereas microvilli (arrow) are present but will be resorbed later. Both hair cells are from the middle row of the three rows of outer hair cells and from a region 20% of the distance from the apex of the cochlear duct. (Bar, 2 μm.) The figure was kindly provided by Ralph Holme (Nottingham, U.K.).
How do the Mcoln3 mutations lead to deafness? The answer is not clear yet, but the phenotype of the varitint-waddler mutants gives some clues. Di Palma et al. (4) describe progressive disorganization of the bundle of stereocilia on cochlear hair cells during their early development. A growing number of deaf mouse mutants have been shown to have specific developmental defects of the stereocilia (or hair) bundle, emphasizing the importance of the integrity of these bundles to auditory function. These deaf mice include mutants with short stereocilia such as shaker2 (14) and whirler (15), mutants with thin stereocilia such as Tasmanian devils (16), mutants with rapid onset of stereocilia fusion as seen in Snell's waltzer mutants (17), and finally a group with progressive disorganization of the array of stereocilia including shaker1 (18), waltzer (19), Ames waltzer (20), tailchaser (21), and varitint-waddler (4). Ultimately, all these stereocilia defects lead to degeneration of the hair cells.
Normally, stereocilia develop from the microvilli that cover the apical surface of hair cells early in development. Microvilli on the outer (lateral) side of the hair cells grow longer and develop the characteristic features of stereocilia, whereas microvilli on the inner side of the hair cell are resorbed. In the group of mutants with progressive disorganization of stereocilia, there seem to be some common features. Stereocilia start to grow on the lateral edge, suggesting that planar polarity is established normally (although it may not be maintained). Individual microvilli grow and develop into normal-looking stereocilia, and they can form staircase-like rows of graded heights (Fig. 2). Crosslinks and tip links can be observed between adjacent stereocilia in at least one of this group of mutants, shaker1 (22). Hair cells can transduce and depolarize, measured either directly from single hair cells as in shaker1 (22) or indirectly as shown by summating potentials in varitint-waddler (3). However, the normal V-shaped arrangement of stereocilia is not maintained during development of these mutants, and clumps of stereocilia seem to drift apart, leading to the disorganized appearance. An example from the waltzer mutant is shown in Fig. 2.
One possible explanation for the progressive disorganization of hair bundles in varitint-waddler is that Mcoln3 channels are required for maintenance of local homeostasis within stereocilia, and that abnormal channel function leads to loss of at least some types of crosslinks, allowing stereocilia to drift apart. This is supported by the localization of Mcoln3 protein in stereocilia (4), and higher resolution study of varitint-waddler hair bundles might indicate whether this is a useful hypothesis to pursue. A second explanation is that stereocilia drift apart because of abnormal control of membrane turnover at the apical surface of the hair cell. The apical surface of hair cells is peppered with endocytotic pits, and the cytoplasm just below is very rich in vesicles (23, 24), suggesting that active membrane turnover occurs, and it is easy to imagine that quite small anomalies in turnover could lead to disruption of the hair bundle. This explanation is supported by the involvement of other members of the TRP-channel superfamily including the first mucolipin gene found, MCOLN1, in vesicle disorders leading to lysosomal storage defects (25–27; see ref. 4 for further references). Interestingly, another mouse mutant, mocha, shows hearing and balance defects and diluted coat color associated with lysosomal defects due to a mutation in Ap3d, which encodes a protein present in coated vesicles (28).
What of the pigmentation abnormalities in varitint-waddler? How do mutations in Mcoln3 lead to a tricolored coat? The answer is not known. In other mouse mutants with white spots on the coat such as mice with Kit, Kitl, or Mitf mutations, the white spots show no sign of the melanocytes that give the hairs their pigmentation (29, 30). In other mutants, diluted coat color results from abnormal distribution of the melanosomes (vesicle-derived, melanin-packed organelles) within melanocytes or abnormal melanosome development (29). If Mcoln3 is involved in vesicle formation or maintenance, this could affect melanosomes, leading to diluted patches, and could also affect viability of the melanocyte itself, leading to gray or white patches where some or all melanocytes have died. It is more difficult to explain how patches of normally pigmented (agouti) fur arise if Mcoln3 function is critical for melanocyte function or survival. Nonetheless, identification of Mcoln3 as the mutated gene in varitint-waddler will provide a key tool for understanding the cellular basis of coat color in these mutants.
Melanocytes not only provide pigmentation to the coat but also have a vital role in hearing. Melanocytes populate the stria vascularis, the structure responsible for generating the endolymph in the cochlea, and mutant mice with no melanocytes in the stria vascularis have no recordable endocochlear potential and consequently have severe hearing impairment (31, 32). This role of melanocytes in the cochlea explains the long-established link between white spotting of the coat and deafness observed in many mammals, including Dalmatian dogs, deaf white cats, and several mouse mutants: mutations that affect melanocyte development leading to a lack of coat melanocytes also lead to a lack of melanocytes in the stria vascularis. The molecular basis of the requirement for melanocytes has been discovered recently, because melanocytes are the only cell type in the stria to express the Kir4.1 potassium channel, and knockout of the gene encoding this channel, Kcnj10, abolishes the endocochlear potential and reduces endolymph potassium concentration (33). The Kir4.1 channel seems to be a limiting step in the passage of potassium through the stria vascularis ready for pumping out into the endolymph. In varitint-waddler mutants, there is patchy distribution of melanocytes in the strias, and some cochleas have no detectable melanocytes, which is associated with a reduced or absent endocochlear potential (3). The reason for the patchy distribution of strial melanocytes in varitint-waddler is not known, just as it is not known for the coat melanocytes.
Thus, Mcoln3 seems to be vital for at least two different functions in the cochlea, maintenance of hair bundles and generation of endocochlear potential. The two functions seem independent of each other, dealing a double blow to hearing in varitint-waddler mutants. There is clearly much to be done to understand the role of Mcoln3. For example, does Mcoln3 encode a functional channel, and how do the varitint-waddler mutations affect its function? Is the gene expressed in melanocytes, and how does it control melanocyte survival? How would loss of function of the protein, as in a knockout, affect hearing and pigmentation? Is the gene product essential for hair-cell transduction in vitro? Is it the elusive transduction channel itself? The identity of the transduction channel is still unknown but is keenly sought as one of the key molecules in sensory hair-cell function. Any new channel involved with hearing impairment, especially one such as the predicted Mcoln3 channel that is expressed in stereocilia, is a candidate for consideration, but this one is particularly interesting, because the transduction channel quite likely belongs to the TRP-channel superfamily (8). Once again, mouse mutants are providing us with invaluable tools to gain access to the molecular basis of hearing and deafness, but the mutants are just the beginning.
See companion article on page 14994.
References
- 1.Cloudman A. M. & Bunker, L. E. (1945) J. Hered. 36, 259-263. [Google Scholar]
- 2.Deol M. S. (1954) J. Genet. 52, 562-588. [Google Scholar]
- 3.Cable J. & Steel, K. P. (1998) Hear. Res. 123, 125-136. [DOI] [PubMed] [Google Scholar]
- 4.Di Palma F., Belyantseva, I. A., Kim, H. J., Vogt, T. F., Kachar, B. & Noben-Trauth, K. (2002) Proc. Natl. Acad. Sci. USA 99, 14994-14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie P. G. & Walker, R. G. (2001) Nature 413, 194-202. [DOI] [PubMed] [Google Scholar]
- 6.Holt J. R. & Corey, D. P. (2000) Proc. Natl. Acad. Sci. USA 97, 11730-11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard J. & Hudspeth, A. J. (1988) Neuron 1, 189-199. [DOI] [PubMed] [Google Scholar]
- 8.Strassmaier M. & Gillespie, P. G. (2002) Curr. Opin. Neurobiol. 12, 380-386. [DOI] [PubMed] [Google Scholar]
- 9.Goodyear R. & Richardson, G. P. (1992) J. Comp. Neurol. 325, 243-256. [DOI] [PubMed] [Google Scholar]
- 10.Yamoah E. N., Lumpkin, E. A., Dumont, R. A., Smith, P. J., Hudspeth, A. J. & Gillespie, P. G. (1998) J. Neurosci. 18, 610-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steel K. P. & Kros, C. J. (2001) Nat. Genet. 27, 143-149. [DOI] [PubMed] [Google Scholar]
- 12.Wangemann P. (2002) Hear. Res. 165, 1-9. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi T., Kimura, R. S., Paul, D. L. & Adams, J. C. (1995) Anat. Embryol. 191, 101-118. [DOI] [PubMed] [Google Scholar]
- 14.Probst F. J, Fridell, R. A., Raphael, Y., Saunders, T. L., Wang, A., Liang, Y., Morell, R. J., Touchman, J. W., Lyons, R. H., Noben-Trauth, K., et al. (1998) Science 280, 1403-1447. [DOI] [PubMed] [Google Scholar]
- 15.Holme R. H., Kiernan, B. W., Brown, S. D. M. & Steel, K. P. (2002) J. Comp. Neurol. 450, 94-102. [DOI] [PubMed] [Google Scholar]
- 16.Erven, A., Skynner, M. J., Okumura, K., Takebayashi, S., Brown, S. D. M., Steel, K. P. & Allen, N. D. (2002) Eur. J. Neurosci., in press. [DOI] [PubMed]
- 17.Self T., Sobe, T., Copeland, N. G., Jenkins, N. A., Avraham, K. B. & Steel, K. P. (1999) Dev. Biol. 214, 331-334. [DOI] [PubMed] [Google Scholar]
- 18.Self T., Mahony, M., Fleming, J., Walsh, J., Brown, S. D. M. & Steel, K. P. (1998) Development (Cambridge, U.K.) 125, 557-566. [DOI] [PubMed] [Google Scholar]
- 19.Di Palma F., Holme, R. H., Bryda, E. C., Belyantseva, I. A., Pellegrino, R., Kachar, B., Steel, K. P. & Noben-Trauth, K. (2001) Nat. Genet. 27, 103-107. [DOI] [PubMed] [Google Scholar]
- 20.Alagramam K. N., Murcia, C. L., Kwon, H. Y., Pawlowski, K. S., Wright, C. G. & Woychik, R. P. (2001) Nat. Genet. 27, 99-102. [DOI] [PubMed] [Google Scholar]
- 21.Kiernan A. E., Zalzman, M., Fuchs, H., Hrabé de Angelis, M., Balling, R., Steel, K. P. & Avraham, K. B. (1999) J. Neurocytol. 28, 969-985. [DOI] [PubMed] [Google Scholar]
- 22.Kros C. J., Marcotti, W., van Netten, S. M., Self, T. J., Libby, R. T., Brown, S. D. M., Richardson, G. P. & Steel, K. P. (2002) Nat. Neurosci. 5, 41-47. [DOI] [PubMed] [Google Scholar]
- 23.Richardson G. P., Forge, A., Kros, C. J., Fleming, J., Brown, S. D. M. & Steel, K. P. (1997) J. Neurosci. 17, 9506-9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kachar B., Battaglia, A. & Fex, J. (1997) Hear. Res. 107, 102-112. [DOI] [PubMed] [Google Scholar]
- 25.Fares H. & Greenwald, I. (2001) Nat. Genet. 28, 64-68. [DOI] [PubMed] [Google Scholar]
- 26.Sun M., Goldin, E., Stahl, S., Falardeau, J. L., Kennedy, J. C., Acierno, J. S., Bove, C., Kaneski, C. R., Nagle, J., Bromley, M. C., et al. (2000) Hum. Mol. Genet. 9, 2471-2478. [DOI] [PubMed] [Google Scholar]
- 27.Bargal R., Avidan, N., Ben-Asher, E., Olender, Z., Ziegler, M., Frumkin, A., Raas-Rothschild, A., Glusman, G., Lancet, D. & Bach, G. (2000) Nat. Genet. 26, 118-121. [DOI] [PubMed] [Google Scholar]
- 28.Kantheti P., Qiao, X., Diaz, M. E., Peden, A. A., Meyer, G. E., Carskadon, S. L., Kapfhamer, D., Sufalko, D., Robinson, M. S., Noebels, J. L. & Burmeister, M. (1998) Neuron 21, 111-122. [DOI] [PubMed] [Google Scholar]
- 29.Silvers W. K., (1979) The Coat Colors of Mice (Springer, New York).
- 30.Cable J., Jackson, I. J. & Steel, K. P. (1995) Mech. Dev. 50, 139-150. [DOI] [PubMed] [Google Scholar]
- 31.Steel K. P. & Barkway, C. (1989) Development (Cambridge, U.K.) 107, 453-463. [DOI] [PubMed] [Google Scholar]
- 32.Cable J., Barkway, C. & Steel, K. P. (1992) Hear. Res. 64, 6-20. [DOI] [PubMed] [Google Scholar]
- 33.Marcus D. C., Wu, T., Wangemann, P. & Kofuji, P. (2002) Am. J. Physiol. 282, C403-C407. [DOI] [PubMed] [Google Scholar]