Abstract
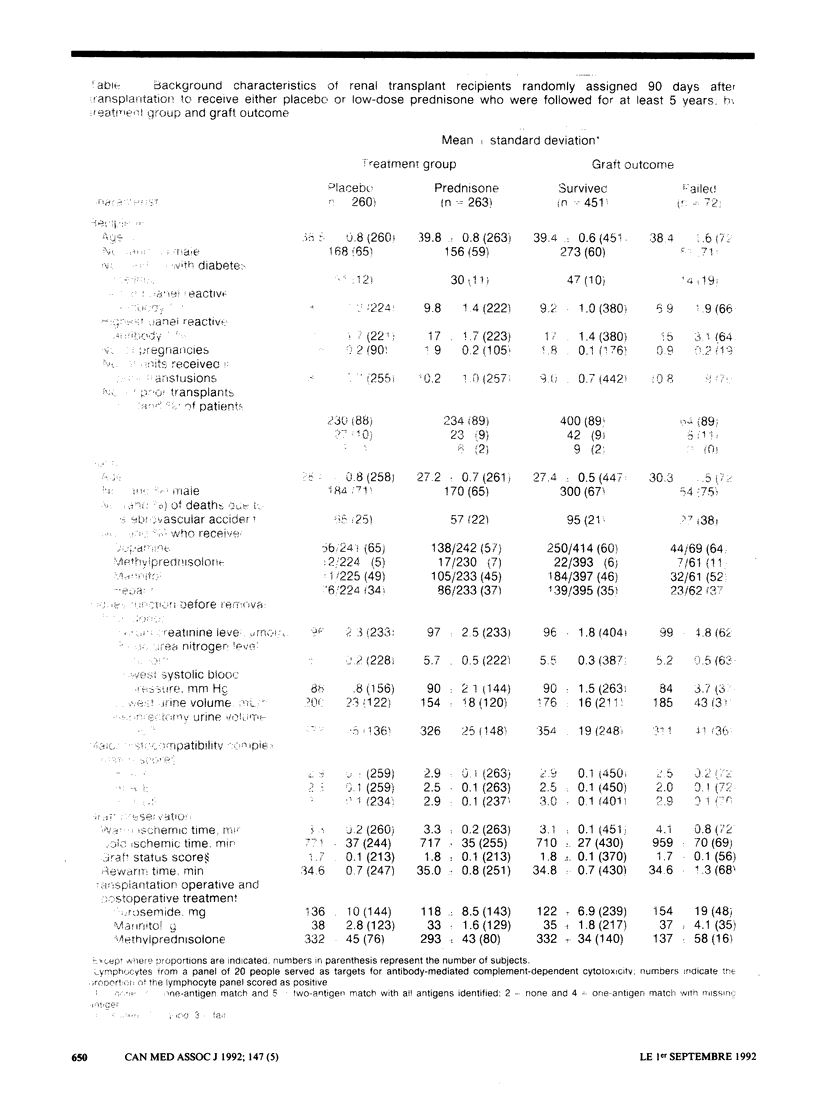
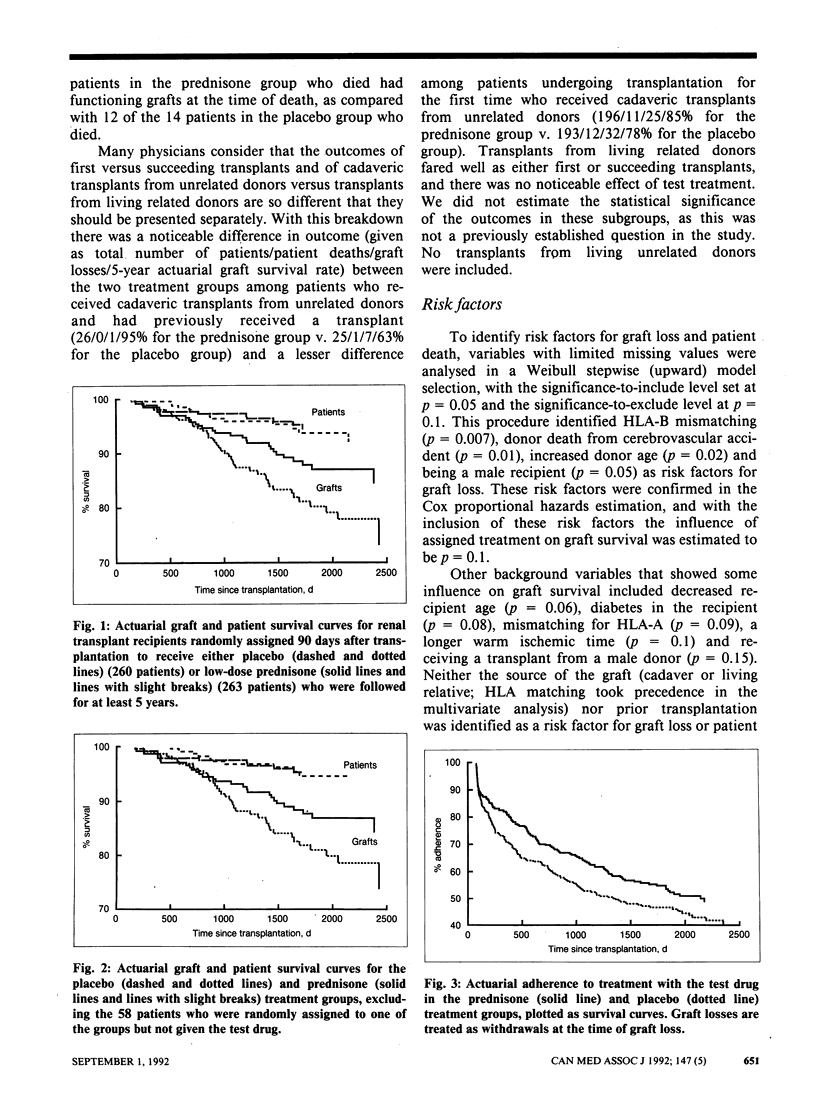
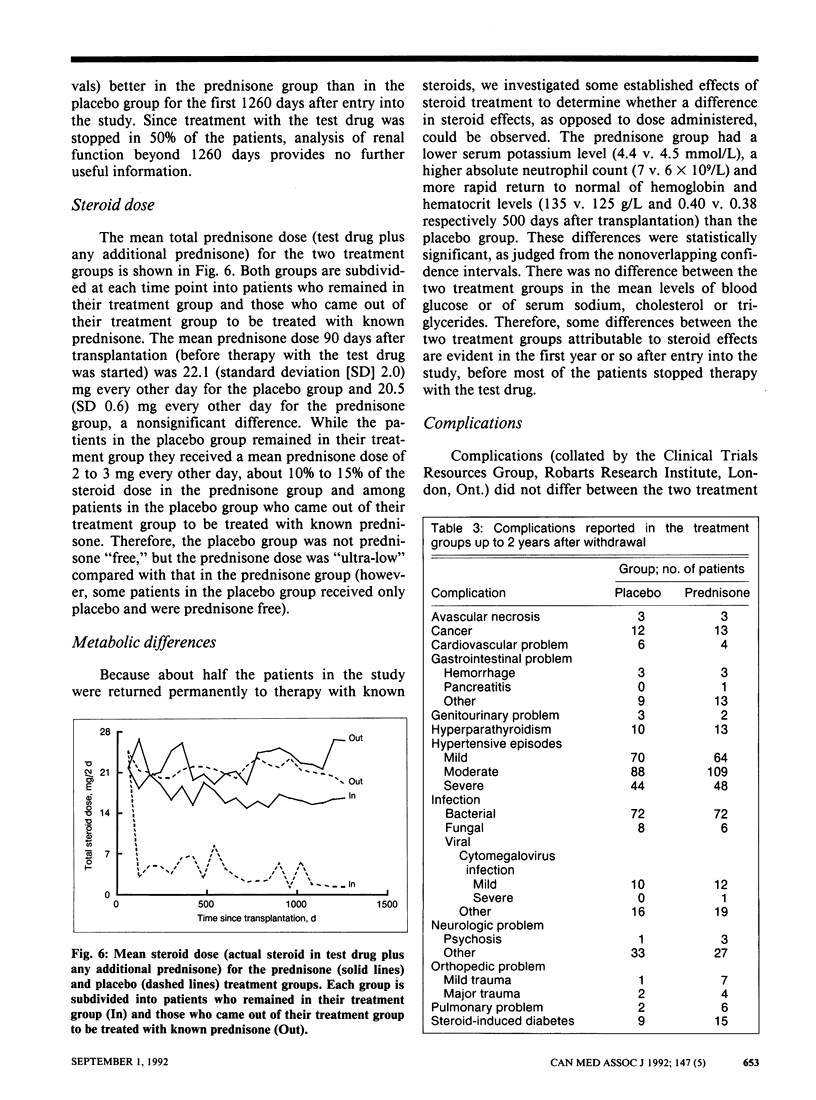
OBJECTIVE: Low-dose prednisone given on alternate days as a steroid adjunct to cyclosporine therapy was investigated primarily for its influence on kidney graft and patient survival and, secondarily, on renal function and complications. DESIGN: Multicentre randomized double-blind clinical trial. SETTING: Fourteen Canadian transplant centres. PATIENTS: A total of 523 patients with well-functioning renal transplants (cadaveric grafts or grafts from living related donors) and without active graft rejection reactions who were entered into the trial from 1982 to 1985. INTERVENTION: Patients were randomly assigned 90 days after transplantation to receive either placebo (260 patients) or low-dose prednisone (263 patients). MAIN OUTCOME MEASURES: Graft and patient survival. MAIN RESULTS: After at least 5 years of follow-up 50 patients assigned placebo had lost their graft and 17 had died; the corresponding figures for those assigned prednisone were 38 and 16. After an average interval of 1.4 years 143 patients in the placebo group and 123 patients in the prednisone group had stopped therapy with the test drug or had had their treatment group decoded or both. Patients were withdrawn from the study 2 years after stopping the test therapy. The actuarial 5-year graft survival rates were 73% and 85% in the placebo and prednisone groups respectively (p = 0.03), and the actuarial 5-year patient survival rates were 92% and 94% respectively (p = 0.6). This analysis included 43 and 29 graft losses and 14 and 12 deaths in the placebo and prednisone groups respectively. Weibull parametric modelling of graft survival identified the following variables as risk factors for graft loss: histocompatibility leukocyte antigen B (HLA-B) mismatching (p = 0.007), donor death from cerebrovascular accident (p = 0.01), increased donor age (p = 0.02) and being a male recipient (p = 0.05). When these factors were included in the Cox proportional hazards model, the influence of assigned treatment on graft survival was reduced to p = 0.1. Donor death from cerebrovascular accident (p = 0.002), diabetes mellitus in the recipient (p = 0.02) and increased recipient age (p = 0.05) were risk factors for patient death. Renal function and incidence of complications were similar in the treatment groups. CONCLUSIONS: Continued administration of low-dose prednisone on alternate days is advisable, particularly in patients with cadaveric grafts and those with previously failed transplants.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beschorner W. E., Ren H., Phillips J., Pulido H. B., Jr, Hruban R. H., Hess A. D. Prevention of syngeneic graft-versus-host disease by recovery of thymic microenvironment after cyclosporine. Transplantation. 1991 Oct;52(4):668–674. doi: 10.1097/00007890-199110000-00017. [DOI] [PubMed] [Google Scholar]
- Borel J. F. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology. 1976 Oct;31(4):631–641. [PMC free article] [PubMed] [Google Scholar]
- Brown M. W., Forwell M. A. Rejection reaction after stopping prednisolone in kidney-transplant recipients taking cyclosporine. N Engl J Med. 1986 Jan 16;314(3):183–183. doi: 10.1056/NEJM198601163140316. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Rolles K., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Henderson R. G., Aziz S. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979 Nov 17;2(8151):1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Cristinelli L., Brunori G., Setti G., Manganoni A., Manganoni A. M., Scolari F., Sandrini S., Scaini P. S., Savoldi S., Camerini C. Withdrawal of methylprednisolone at the sixth month in renal transplant recipients treated with cyclosporine. Transplant Proc. 1987 Feb;19(1 Pt 3):2021–2023. [PubMed] [Google Scholar]
- Donatsch P., Abisch E., Homberger M., Traber R., Trapp M., Voges R. A radioimmunoassay to measure cyclosporin A in plasma and serum samples. J Immunoassay. 1981;2(1):19–32. doi: 10.1080/01971528108062989. [DOI] [PubMed] [Google Scholar]
- Dunn J., Golden D., Van Buren C. T., Lewis R. M., Lawen J., Kahan B. D. Causes of graft loss beyond two years in the cyclosporine era. Transplantation. 1990 Feb;49(2):349–353. doi: 10.1097/00007890-199002000-00024. [DOI] [PubMed] [Google Scholar]
- Dunn J., Vathsala A., Golden D., Kerman R., Lawen J., Van Buren C. T., Lewis R., Kahan B. D. Impact of race on the outcome of renal transplantation under cyclosporine-prednisone. Transplant Proc. 1989 Dec;21(6):3946–3948. [PubMed] [Google Scholar]
- Dupont E., Wybran J., Toussaint C. Glucocorticosteroids and organ transplantation. Transplantation. 1984 Apr;37(4):331–335. doi: 10.1097/00007890-198404000-00002. [DOI] [PubMed] [Google Scholar]
- Griffin P. J., Da Costa C. A., Salaman J. R. A controlled trial of steroids in cyclosporine-treated renal transplant recipients. Transplantation. 1987 Apr;43(4):505–508. doi: 10.1097/00007890-198704000-00010. [DOI] [PubMed] [Google Scholar]
- Griño J. M., Alsina J., Castelao A. M., Sabate I., Mestre M., Gil-Vernet S., Andrés E., Sabater R. Low-dose cyclosporine, anti-lymphocyte globulin, and steroids in first cadaveric renal transplantation. Transplant Proc. 1988 Oct;20(5 Suppl 6):18–20. [PubMed] [Google Scholar]
- Häyry P., von Willebrand E., Ahonen J., Eklund B., Salmela K., Höckerstedt K., Pettersson E., Koskimies S. Effects of cyclosporine, azathioprine, and steroids on the renal transplant, on the cytologic patterns of intragraft inflammation, and on concomitant rejection-associated changes in recipient blood. Transplant Proc. 1988 Apr;20(2 Suppl 2):153–162. [PubMed] [Google Scholar]
- Häyry P., von Willebrand E. The influence of the pattern of inflammation and administration of steroids on class II MHC antigen expression in renal transplants. Transplantation. 1986 Oct;42(4):358–363. doi: 10.1097/00007890-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Iseki R., Mukai M., Iwata M. Regulation of T lymphocyte apoptosis. Signals for the antagonism between activation- and glucocorticoid-induced death. J Immunol. 1991 Dec 15;147(12):4286–4292. [PubMed] [Google Scholar]
- Ishigami T., Kim K. M., Horiguchi Y., Higaki Y., Hata D., Heike T., Katamura K., Mayumi M., Mikawa H. Anti-IgM antibody-induced cell death in a human B lymphoma cell line, B104, represents a novel programmed cell death. J Immunol. 1992 Jan 15;148(2):360–368. [PubMed] [Google Scholar]
- Jenkins M. K., Chen C. A., Jung G., Mueller D. L., Schwartz R. H. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990 Jan 1;144(1):16–22. [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H., Pardoll D. M. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988 Sep 23;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- Johnson R. W., Mallick N. P., Bakran A., Pearson R. C., Scott P. D., Dyer P., Donaghue D., Morris D., Gokal R. Cadaver renal transplantation without maintenance steroids. Transplant Proc. 1989 Feb;21(1 Pt 2):1581–1582. [PubMed] [Google Scholar]
- Jones R. J., Vogelsang G. B., Hess A. D., Farmer E. R., Mann R. B., Geller R. B., Piantadosi S., Santos G. W. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989 Apr 8;1(8641):754–757. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kahan B. D., Flechner S. M., Lorber M. I., Golden D., Conley S., Van Buren C. T. Complications of cyclosporine-prednisone immunosuppression in 402 renal allograft recipients exclusively followed at a single center for from one to five years. Transplantation. 1987 Feb;43(2):197–204. doi: 10.1097/00007890-198702000-00007. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Individualization of cyclosporine therapy using pharmacokinetic and pharmacodynamic parameters. Transplantation. 1985 Nov;40(5):457–476. doi: 10.1097/00007890-198511000-00001. [DOI] [PubMed] [Google Scholar]
- Keown P. A., Stiller C. R., Stawecki M., Freeman D. Pharmacokinetics of cyclosporine in solid organ transplantation. Transplant Proc. 1986 Dec;18(6 Suppl 5):160–164. [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A. S., Daloze P., Dandavino R., Jindal S., Bear L., Dossetor J. B., Klassen J., Stiller C. R., Lockwood B., Reeve C. E. A randomized study of cyclosporine with and without prednisone in renal allograft recipients. Canadian Transplant Group. Transplant Proc. 1987 Feb;19(1 Pt 3):1865–1866. [PubMed] [Google Scholar]
- McGeown M. G., Douglas J. F., Brown W. A., Donaldson R. A., Kennedy J. A., Loughridge W. G., Mehta S., Nelson S. D., Doherty C. C., Johnstone R. Advantages of low dose steroid from the day after renal transplantation. Transplantation. 1980 Apr;29(4):287–289. doi: 10.1097/00007890-198004000-00005. [DOI] [PubMed] [Google Scholar]
- Morris P. J., Chan L., French M. E., Ting A. Low dose oral prednisolone in renal transplantation. Lancet. 1982 Mar 6;1(8271):525–527. doi: 10.1016/s0140-6736(82)92042-6. [DOI] [PubMed] [Google Scholar]
- Reisman L., Lieberman K. V., Burrows L., Schanzer H. Follow-up of cyclosporine-treated pediatric renal allograft recipients after cessation of prednisone. Transplantation. 1990 Jan;49(1):76–80. doi: 10.1097/00007890-199001000-00017. [DOI] [PubMed] [Google Scholar]
- Rolin H. A., 3rd, Hall P. M., Wei R. Inaccuracy of estimated creatinine clearance for prediction of iothalamate glomerular filtration rate. Am J Kidney Dis. 1984 Jul;4(1):48–54. doi: 10.1016/s0272-6386(84)80026-8. [DOI] [PubMed] [Google Scholar]
- Salaman J. Cyclosporine mono-drug therapy. Transplant Proc. 1988 Jun;20(3 Suppl 3):117–120. [PubMed] [Google Scholar]
- Schulak J. A., Mayes J. T., Moritz C. E., Hricik D. E. A prospective randomized trial of prednisone versus no prednisone maintenance therapy in cyclosporine-treated and azathioprine-treated renal transplant patients. Transplantation. 1990 Feb;49(2):327–332. doi: 10.1097/00007890-199002000-00020. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Weil R., 3rd, Iwatsuki S., Klintmalm G., Schröter G. P., Koep L. J., Iwaki Y., Terasaki P. I., Porter K. A. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980 Jul;151(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- Stiller C. R., Opelz G. Should cyclosporine be continued indefinitely? Transplant Proc. 1991 Feb;23(1 Pt 1):36–40. [PubMed] [Google Scholar]
- Thiel G., Harder F., Loertscher R., Brünisholz M., Landmann J., Brunner F., Follat F., Wenk M., Mihatsch M. Cyclosporine alone or in combination with prednisone in cadaveric renal transplantation. Transplant Proc. 1984 Oct;16(5):1187–1190. [PubMed] [Google Scholar]
- Vathsala A., Weinberg R. B., Schoenberg L., Grevel J., Goldstein R. A., Van Buren C. T., Lewis R. M., Kahan B. D. Lipid abnormalities in cyclosporine-prednisone-treated renal transplant recipients. Transplantation. 1989 Jul;48(1):37–43. doi: 10.1097/00007890-198907000-00009. [DOI] [PubMed] [Google Scholar]




