Abstract
Cyclin E/Cdk2 is a critical regulator of cell cycle progression from G1 to S in mammalian cells and has an established role in oncogenesis. Here we examined the role of deregulated cyclin E expression in apoptosis. The levels of p50-cyclin E initially increased, and this was followed by a decrease starting at 8 h after treatment with genotoxic stress agents, such as ionizing radiation. This pattern was mirrored by the cyclin E-Cdk2-associated kinase activity and a time-dependent expression of a novel p18-cyclin E. p18-cyclin E was induced during apoptosis triggered by multiple genotoxic stress agents in all hematopoietic tumor cell lines we have examined. The p18-cyclin E expression was prevented by Bcl-2 overexpression and by the general caspase and specific caspase 3 pharmacologic inhibitors zVAD-fluoromethyl ketone (zVAD-fmk) and N-acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO), indicating that it was linked to apoptosis. A p18-cyclin E276-395 (where cyclin E276-395 is the cyclin E fragment containing residues 276 to 395) was reconstituted in vitro, with mutagenesis experiments, indicating that the caspase-dependent cleavage was at amino acid residues 272 to 275. Immunoprecipitation analyses of the ectopically expressed cyclin E1-275, cyclin E276-395 deletion mutants, and native p50-cyclin E demonstrated that caspase-mediated cyclin E cleavage eliminated interaction with Cdk2 and therefore inactivated the associated kinase activity. Overexpression of cyclin E276-395, but not of several other cyclin E mutants, specifically induced phosphatidylserine exposure and caspase activation in a dose-dependent manner, which were inhibited in Bcl-2-overexpressing cells or in the presence of zVAD-fmk. Apoptosis and generation of p18-cyclin E were significantly inhibited by overexpressing the cleavage-resistant cyclin E mutant, indicating a functional role for caspase-dependent proteolysis of cyclin E for apoptosis of hematopoietic tumor cells.
The cyclins and their catalytic subunits, the cyclin-dependent kinases (CDKs), control cell cycle progression by regulating events that drive the transitions between cell cycle phases (13, 14). The activity of these CDKs is regulated positively by cyclins, their associated catalytic partners, and negatively by binding of CDK inhibitors (CKIs). Activation of cyclin/CDK complexes results in a cascade of protein phosphorylations that ultimately induces cell cycle progression. Cyclins were first identified in clam and sea urchin embryos, where they were observed to accumulate during interphase and to be degraded during mitosis (16). The human G1 cyclins, the D- and E-type cyclins, were identified functionally by screening of human cDNA libraries for sequences that could complement G1 cyclin mutations in Saccharomyces cerevisiae (31, 35, 65). The cyclin E mRNA levels show a periodic pattern of expression, being synthesized during the G1 phase of the cell cycle, with levels increasing sharply in late G1, followed by accumulation of cyclin E protein and then down regulation in S phase (11, 32, 35; reviewed in reference 51).
Cyclin E binds Cdk2 with a resulting peak of associated kinase activity at the G1/S boundary (11, 32). Cyclin E/Cdk2 complexes have been shown to play an essential and rate-limiting role in the transition between G1 and S phase (46, 52, 61, 62), as well as for the initiation of DNA replication (26, 33, 46). How cyclin E/Cdk2 promotes S-phase entry is poorly defined, since few downstream effectors of cyclin E/Cdk2 are known. While cyclin E complexes phosphorylate the protein product of the retinoblastoma tumor suppressor gene (pRb), which is also phosphorylated by the D-type cyclin-Cdk4/6 complexes (10, 17, 28), it is likely that other substrates exist during late G1. Unlike cyclin D, cyclin E remains essential in the absence of pRb, since (i) its inducible expression in fibroblasts accelerates G1/S progression without affecting the kinetics of pRb phosphorylation (53); (ii) unlike the D-type cyclins, cyclin E is essential for cell cycle progression in pRb-deficient cells (46); (iii) ectopic expression of cyclin E bypasses pRb-mediated cell cycle arrest (1, 40); and (iv) cyclin E is required for S-phase entry in Drosophila melanogaster (12). These findings highlight a fundamental difference between the cyclin D and cyclin E complexes, strongly suggesting that other key rate-limiting substrates exist for cyclin E/Cdk2 (1, 40). Moreover, all functions of cyclin D1 can be replaced by cyclin E, suggesting that cyclin E is the downstream target of cyclin D1 and the master regulator of S-phase entry (18).
Apoptosis, a universal genetic program of cell death in higher eukaryotes, is a basic process involved in cellular development and differentiation (50). Apoptosis may be essential for the prevention of tumor formation, and its deregulation is widely believed to be involved in pathogenesis of many human diseases, including cancer (reviewed in reference 60). In almost all instances, deregulated cell proliferation and suppressed cell death together provide the underlying platform for neoplastic progression (15). In cancer, called a disease of deregulated cell proliferation, one principal target is the late G1 cell cycle regulated by pRb (23). Defects in this pathway, which may be universal in human cancer, include deletions of the Rb gene itself and deregulation of the CDKs that phosphorylate and functionally inactivate pRb, either through direct overactivation of CDKs or through genetic loss of their inhibitors (56). We have shown that the absence of Rb is capable of activating an apoptotic response associated with p53 stabilization and increased expression of genes dependent on E2F transcription (2), such as cyclin E. Cyclin E has been shown to be deregulated and overexpressed in several solid tumors, including breast, colon, and prostate carcinomas (29, 56). We have recently shown that cyclin E levels are also regulated by genotoxic stress and that cyclin E activation plays a functional role in apoptosis of hematopoietic cells (43).
The activation of a cascade of the ICE/CED-3 family of cysteine proteases (termed caspases) is a common and critical regulator of the execution phase of apoptosis, triggered by many factors, including genotoxic agents (e.g., γ-irradiation or treatment with anticancer agents [7, 19, 20]). Once the cells are committed to cell death, apoptogenic factors, the best known of which is cytochrome c, are released from mitochondria to initiate the caspase cascade (7, 38). Cytochrome c acts as a cofactor to stimulate the complexing of Apaf-1 with caspase 9 (36), which then initiates activation of the caspase cascade. Caspases are synthesized as inactive precursors, which are activated by proteolytic cleavage to generate active enzymes which then may further proteolytically cleave proteins crucial for the maintenance of cellular cytoskeleton, DNA repair, signal transduction, and cell cycle control (25). There are many in vivo caspase substrates, including transcription factors, kinases, enzymes involved in DNA repair, and cytoskeletal proteins (reviewed in reference 25). In addition, several proteins essential for cell cycle regulation, such as pRb (27, 59), MDM2 (6), PITSLRE (3), p21WAF1/CIP1, and p27Kip1 (34) are also caspase targets.
Apoptotic targets of CDKs are likely to exist, given reports of an interplay between the cell cycle control processes and apoptosis (44, 45), with recent reports indicating that the apoptosis-regulatory proteins themselves can directly impinge on the cell cycle machinery (4, 37, 42, 47). Clearly, induction of apoptosis by various stimuli has been shown to require activation of Cdk2 (54, 66), whereas forced expression of CKIs in cultured cells (43, 66), in neurons (48), or during myocyte differentiation (63) prevents apoptosis. Importantly, similar observations were made in noncycling developing thymocytes, in which Cdk2 was activated; conversely, Cdk2 inhibition eliminates apoptosis (21). Moreover, conditional cyclin A expression was reported to be involved in apoptosis of fibroblasts (58), whereas ectopic expression of cyclins D1 in rat fibroblasts under conditions of serum starvation led to apoptotic cell death (22). We found that cyclin E was upregulated rapidly during apoptosis induced by genotoxic stress in hematopoietic cells, resulting in caspase activation and exposure of the phosphatidylserine on the plasma membrane. Consistent with a role of cyclin E in apoptosis, its overexpression greatly sensitizes these cells to radiation, while its inhibition by a dominant-negative Cdk2 blocks cell death (43).
We have been interested in elucidating the role of cyclin E in apoptosis of hematopoietic cells. In this study, we show that the p50-cyclin E is proteolytically cleaved and thus converted to a p18 C-terminal cyclin E fragment. We examined the temporal expression of this cyclin E derivative, its genesis, and its potential role in apoptosis. p18-cyclin E becomes the most abundant form of cyclin E during the course of apoptosis induced by multiple genotoxic agents in all hematopoietic tumor cell lines we have examined. Cyclin E cleavage results in elimination of its binding to Cdk2 and, therefore, inactivation of its associated kinase activity and cell cycle function. Overexpression of p18-cyclin E276-395 (where cyclin E276-395 is the cyclin E fragment containing residues 276 to 395) triggers apoptosis, while expression of a cleavage-resistant mutant prevents it. Our data indicate that cyclin E has a dual role in apoptosis of tumor cells of hematopoietic origin, with a distinct role for cyclin E276-395 in the amplification of the apoptotic process.
MATERIALS AND METHODS
Cells and treatments.
The IM-9 and U266 multiple myeloma and the Molt-4 lymphoma cell lines were obtained from the American Type Culture Collection and maintained in RPMI medium with 10% fetal bovine serum (BioWhittaker), l-glutamine, and penicillin-streptomycin (100 U/ml). 293 T human kidney cells were maintained in Dulbecco modified Eagle medium (Gibco-BRL) containing 10% fetal bovine serum and antibiotics.
Cells (2 × 105/ml) were irradiated with 4 to 20 Gy (137Cs source; fixed dose rate of 2.8 Gy/min [20]), or treated with etoposide (VP16; 10 μM [20]). The cell-permeable caspase inhibitors (50 μM) were added to cells 0.5 h prior to irradiation (unless otherwise stated) and remained in the medium until the time of cell lysis for protein extraction. The following pharmacologic inhibitors (BIOMOL) were used: N-acetyl-Tyr-Val-Ala-Asp-fluoromethyl ketone (YVAD-fmk), N-acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO), N-acetyl-Val-Glu-Ile-Asp-fluoromethyl ketone (VEID-fmk), and N-acetyl-Ile-Glu-Thr-Asp-cho (IETD-CHO), specific for caspases 1, 3, 6, and 8, respectively. For caspase activity assays the DEVD-p-nitroanilide (DEVD-pNA) substrate was used, and the release of pNA was determined at 405 nm in a 96-well microtiter plate reader, as described (43).
Immunoblot and immunoprecipitation analyses.
Following irradiation or treatment with VP16, 2 × 105 cells/ml were lysed at 4°C for 30 min in buffer A (25 mM HEPES [pH 7.5], 100 mM NaCl, 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol [DTT], 1% NP-40, aprotinin and leupeptin [each at 2 μg/ml], and 1 mM phenylmethylsulfonyl fluoride). Protein (20 μg/lane) was resolved by sodium dodecyl sulfate-11% polyacrylamide gel electrophoresis (SDS-12% PAGE), followed by transfer to nitrocellulose membrane and blocking in 5% nonfat dry milk for 1 h at room temperature. The blots were then incubated with the appropriate primary antibody in phosphate-buffered saline with 0.05% Tween 20 (PBST) containing 5% nonfat dry milk for 16 h at 4°C followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Kirkegaard & Perry Laboratories). The following primary antibodies were used: rabbit anti-cyclin E (C-19; Santa Cruz), mouse anti-cyclin E (HE-12; Santa Cruz), cyclin A (Santa Cruz), Cdk2 (M2; Santa Cruz), and actin (Sigma Chemical Co.).
Immunoprecipitation analyses were carried out with 150 μg of protein from cell lysates using the same buffer as above except 0.1% NP-40 was used instead of 1% NP-40. Immunoblot analyses were then carried out with mouse antihemagglutinin (anti-HA; HA.11, BAbCo), Cdk2, cyclin E, and cyclin A monoclonal antibodies, followed by incubation with the appropriate secondary antibody conjugated to HRP, after which the blots were developed using enhanced chemiluminescence (ECL kit; Amersham) and exposed to X-ray film.
Site-directed mutagenesis and generation of recombinant proteins and expression vectors.
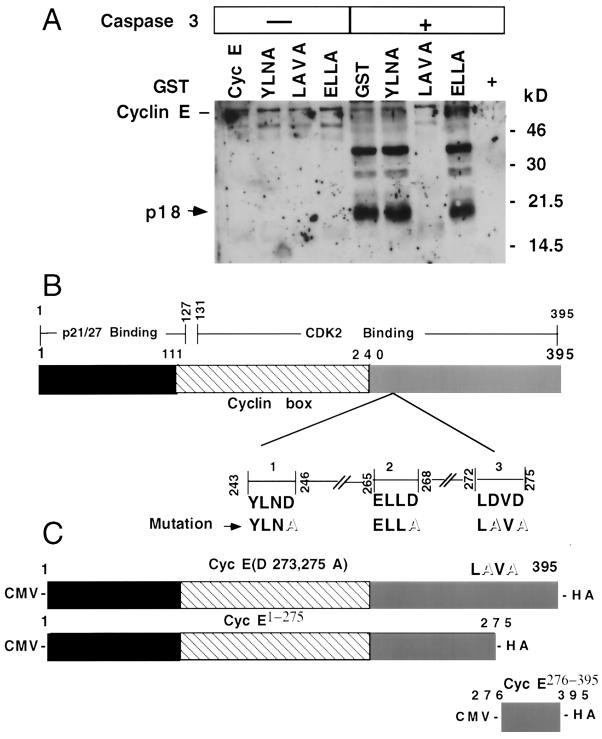
Based on the potential caspase cleavage sites in the C terminus of the cyclin E, Asp residues were changed to Ala at the following positions: 243YLND246 to YLNA, 265ELLD268 to ELLA, and 272LDVD275 to LAVA. The primers 5′-AGC TGT TGG TTC TCT GTG TCC TGG-3′, 5′-GTT GCA TAT CTA AAT GAG TTA CAT GAA G-3′, 5′-CTC TGT GTC CTG GAA GTT GAA TGC CTT GAA TTT-3′, and 5′-TTC TCG CGC AAC TAG TCA GTG G-3′ were used as selection primers with the Clontech site-directed mutagenesis method. Glutathione S-transferase (GST)-tagged recombinant proteins were grown in Escherichia coli and then purified and further used for in vitro cleavage assay. The cyclin E cleavage-resistant mutant (cyclin E Clr; LD273VD275 to LA273VA275), C-terminal fragment (cyclin E276-395), and N-terminal fragment (cyclin E1-275) were cloned into the BamHI-XhoI sites of the pCDNA1.1 vector containing an HA tag at the C terminus. The primer pairs used for the PCRs were 5′-GCG GAT CCA TGA AGG AGG ACG GCG GC-3′ and 5′-CTT GCC TCG AGC GCC ATT TCC GGC CCG CT-3′ (using as a template GST-cyclin E Clr), 5′-GCG GAT CCA TGG ACT GCC TTG AAT TTC CT-3′ and 5′-CTT GCC TCG AGC GCC ATT TCC GGC CCG CT-3′ (using as a template GST-cyclin E), 5′-GCG GAT CCA TGA AGG AGG ACG GCG GC-3′ and 5′-CTT GCC TCG AGA ACA TCC AGG ACA CAG AG-3′ (using as a template GST-cyclin E) to synthesize the cyclin E Clr, cyclin E276-395, and cyclin E1-275 expression vectors, respectively. All primers were synthesized by Integrated DNA Technologies, and the final constructs were verified by DNA sequencing.
Transfections and flow cytometry studies.
Cells were transiently transfected with wild-type cyclin E and various derivative mutant constructs containing an HA tag at the C terminus (2 μg of DNA), cloned into the mammalian expression vector pCDNA1.1 under the control of the cytomegalovirus promoter. Transfections were performed with IM-9 cells using DMRIE-C (Gibco-BRL) or FuGENE 6 (Roche Molecular Biochemicals) and with 293 T cells using Lipofectamine (Gibco-BRL) as described (7, 43) and according to the manufacturer's specifications.
To allow sorting of the transfected cells, IM-9 cells were cotransfected with the pEGFP vector (Clontech) and the cyclin E expression constructs, at a 1:1 ratio. Out of 107 cells, about 105 cells were collected at 16 h posttransfection with a FACS Vantage cell sorter based on their green fluorescent protein (GFP) expression. The GFP-expressing cells were then either subjected to annexin V-fluorescein isothiocyanate (annexin V-FITC) staining, using a FACScan device followed by analysis with the CellQuest software (Becton Dickinson), or to a caspase activity assay, as described (43). Alternatively, sorted cells were maintained in culture for further analysis following radiation treatment. IM-9 cells were also cotransfected with cyclin E Clr and pCDNA3, as described (43), followed by selection in the presence of G418 (0.8 to 1 mg/ml) to generate stable transfectants. Transfection of Bcl-2 and characterization of the Bcl-2-overexpressing IM-9/Bcl-2 stable clone have been previously described (7).
Histone H1 kinase assay.
For immunocomplex kinase assays, the lysis buffer was buffer A, except the concentration of NP-40 was 0.1% instead of 1% and it was supplemented with 1 mM Na3VO4 and 50 mM NaF as phosphatase inhibitors. Cell lysates (250 μg) were incubated with 0.5 μg of antibody for 16 h at 4°C, followed by incubation with protein A-Sepharose beads for 2 h at 4°C. Beads were washed three times with lysis buffer (1 ml each time) and one time with kinase buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM DTT). Beads were suspended in 30 μl of kinase buffer containing 1 μg of histone H1 as the substrate (Calbiochem), 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM Na3VO4, 1 mM NaF, 20 μM ATP, and 10 μCi of [γ-32P]ATP. After incubation for 30 min at 30°C with occasional mixing, the reactions were stopped with 10 μl of 4× Laemmli buffer, boiled for 10 min, and resolved by SDS-PAGE. Phosphorylated proteins were visualized by autoradiography.
In vitro cleavage assay.
The GST-cyclin E fusion proteins were expressed in the E. coli BL21 cells grown in Luria-Bertani medium to exponential phase and induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. Cells were pelleted and resuspended in lysis buffer (0.5% NP-40, 20 mM Tris HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, and protease inhibitor cocktails) and sonicated. Following centrifugation, the supernatants were incubated with glutathione-Sepharose beads (Sigma Chemical Co.) for 1 h at 4°C. The beads were washed four times with the same lysis buffer containing protease inhibitors. Proteins were eluted with a solution containing 100 mM Tris HCl [pH 8.0], 120 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, and 20 mM glutathione. Protein concentrations were determined by the Bio-Rad assay. The recombinant activated caspase 3 was expressed in E. coli and prepared as described (19, 41). GST-cyclin E (100 ng) was incubated with recombinant caspase 3 in reaction buffer (100 mM HEPES [pH 7.5], 20% glycerol, 5 mM DTT, 0.5 mM EDTA) for 16 h at 30°C and subjected to immunoblot analyses using the anti-cyclin E (C-19; Santa Cruz) antibody.
RESULTS
Expression of cyclin E during genotoxic stress-induced apoptosis.
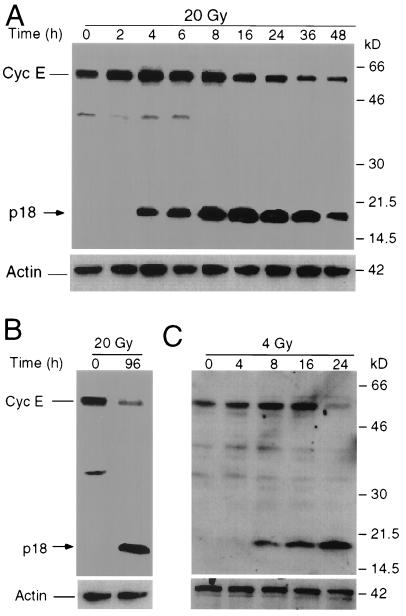
Genotoxic stress agents, such as ionizing radiation or etoposide, kill multiple-myeloma as well as T-cell-lymphoma cells (7, 19, 20). However, the possible involvement of cell cycle regulatory proteins in apoptosis triggered by genotoxic stress agents is unclear. To determine the possible role of cell cycle regulators in apoptosis, we examined the possible role played by cyclin E in the apoptotic process of multiple-myeloma IM-9 cells. Cells were collected and extracted at various times between 0 and 48 h following treatment with ionizing radiation, and cellular lysates were then subjected to Western blot analysis with antibodies to several cell cycle proteins, including cyclin E. This analysis revealed that cyclin E was upregulated early in a time- and dose-dependent manner, with its levels reaching a threefold increase 4 to 8 h following γ-irradiation (Fig. 1) (43). Interestingly, a novel p18-cyclin E protein species was induced at 4 h following irradiation of IM-9 cells (Fig. 1A). The levels of this cyclin E species increased in a time-dependent manner, becoming more abundant at 8 to 16 h following irradiation. p18-cyclin E became the major form of cellular cyclin E starting at 16 h postirradiation and remained such afterwards. This p18 species could be detected with an antibody specific to the C terminus of the protein (C-19) but not with antibodies specific to the N terminus (data not shown). In contrast, the levels of the full-length p50-cyclin E declined from 8 to 48 h following irradiation. This pattern of cyclin E expression was not limited to IM-9 cells, since similar observations were made for U-266 multiple myeloma and Molt-4 lymphoma (Fig. 1B and C), as well as Jurkat and RPMI 8226 cells (data not shown). All irradiated tumor hematopoietic cell lines generated a similar p18-cyclin E, which became the most abundant cyclin E species during apoptosis. These data indicate that p18-cyclin E was produced during radiation-induced apoptosis in all human hematopoietic tumor cell lines examined.
FIG. 1.
Expression of p50- and p18-cyclin E following radiation. Multiple-myeloma IM-9 (A), U-266 (B), and lymphoma Molt-4 (C) cells were irradiated (20, 20, and 4 Gy, respectively) and lysed at the indicated times, and 20 μg of protein was subjected to SDS-12% PAGE as described in Materials and Methods. Western blot analyses were performed using rabbit anti-cyclin E (C-19) and mouse anti-β-actin primary antibodies, the corresponding HRP-conjugated secondary antibodies, and ECL reagent used as a chemiluminescent agent. The full-length cyclin E, which is 50 kDa and is found predominantly in both normal and tumor cells, is the EL form (i.e., the 15-amino-acid elongated variant of cyclin E [46, 49]). Abbreviations: Cyc E, cyclin E; p18, novel cyclin E-derivative.
Dose-dependent expression of p18-cyclin E is prevented by Bcl-2.
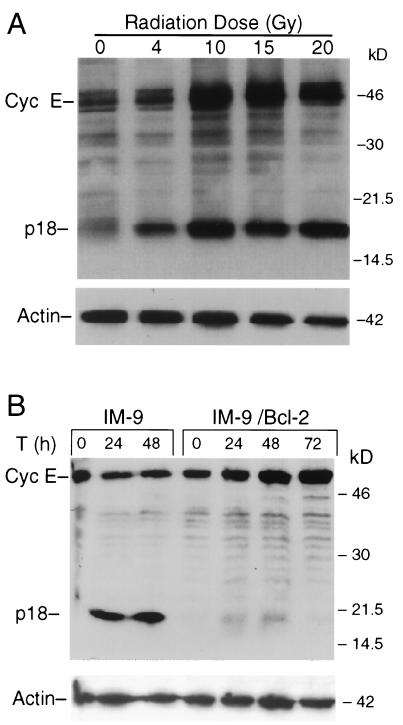
To further examine the expression of p18-cyclin E, IM-9 cells were subjected to different doses of ionizing radiation. The abundance of the p18-cyclin E was increased in a dose-dependent manner, with radiation doses as low as 2 to 4 Gy inducing expression of the p18-cyclin E (Fig. 2A). Induction of the p18-cyclin E was less efficient and appeared at later times at the lower doses (data not shown). For further experiments we have used the 10-Gy dose of radiation, in which case the p18-cyclin E species appeared at 6 h.
FIG. 2.
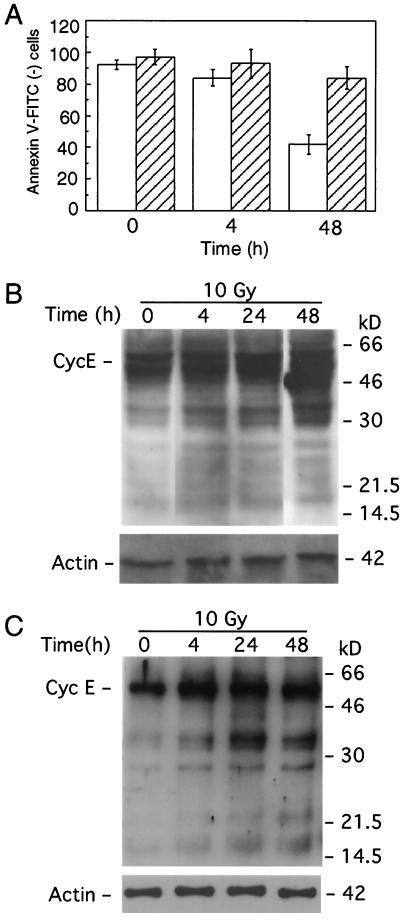
Dose-dependent expression of p18-cyclin E is prevented by Bcl-2. (A) IM-9 cells were irradiated at the indicated radiation doses and lysed 16 h later. Western analyses were performed, as described in the legend to Fig. 1, using rabbit anti-cyclin E (C-19) and mouse antiactin antibodies. (B) To determine the effect of Bcl-2 overexpression on cyclin E cleavage, parental as well as Bcl-2-overexpressing IM-9 cells were irradiated (10 Gy) and lysed at the indicated times (T). Western blot analyses were performed using anti-cyclin E (C-19) and β-actin antibodies as described for Fig. 1. Cyc E, cyclin E.
To further determine whether the induction of p18-cyclin E was associated with apoptosis, the levels of the p50- and p18-cyclin E were examined in cells stably overexpressing the antiapoptotic protein Bcl-2. Ectopic expression of Bcl-2 in IM-9 cells prevents radiation-induced apoptosis, as measured by annexin V-FITC staining and caspase activation (7). In irradiated IM-9/Bcl-2 cells, the p18-cyclin E was undetectable during the 72-h postirradiation period examined. Moreover, there was no decrease in the levels of full-length p50-cyclin E (Fig. 2B). Instead, there was a continuous accumulation of cyclin E during the 72-h postirradiation period examined. Taking together all of the above findings, it can be concluded that the time- and dose-dependent induction of p18-cyclin E is associated with radiation-induced apoptosis.
Induction of the p18- but not the p50-cyclin E is caspase dependent.
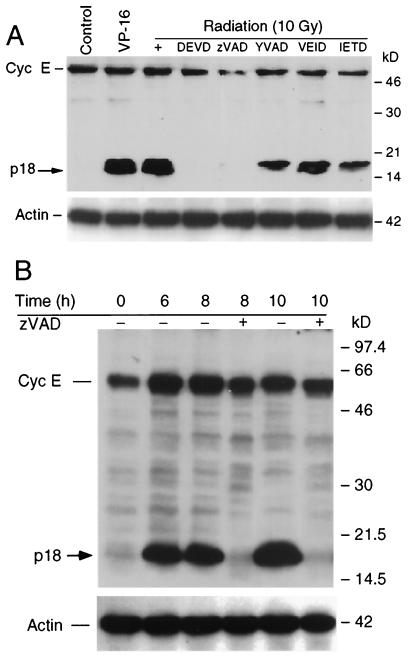
The p18-cyclin E species could be the result of alternative splicing or posttranscriptional modification. Although alternative splicing, as well as generation of smaller truncated fragments of cyclin E, has been described (29, 30, 46, 55), none of these cyclin E-derived products are as small as p18. The appearance of a smaller C-terminal cyclin E species is reminiscent of similar truncations of many cellular proteins during apoptosis as a result of the proteolytic cleavage by caspases (25). To address whether the p18-cyclin E derivative fragment was caspase mediated, we examined the effect of various pharmacologic caspase inhibitors in vivo. IM-9 cells were irradiated following preincubation with multiple caspase inhibitors (50 μM) for 30 min, and Western blot analyses were used to examine the expression of cyclin E in cells isolated 16 h postirradiation. The p18-cyclin E species was generated following irradiation, as well as treatment with 10 μM etoposide (VP16; an inhibitor of topoisomerase I), indicating that its presence was not restricted to irradiation but was rather a more general phenomenon associated with genotoxic stress-induced apoptosis of hematopoietic cells. Importantly, the general pancaspase inhibitor zVAD-fmk and the caspase 3 inhibitor DEVD-CHO efficiently prevented the appearance of the cyclin E-derived fragment. In contrast, YVAD, IETD, and VEID, inhibitors of caspases 1, 6, and 8, did not prevent the appearance of the p18 fragment. These results indicate that cleavage of cyclin E is mediated by caspase 3 or a caspase 3-like activity (Fig. 3A).
FIG. 3.
The expression of p18, but not of p50 cyclin E, is caspase 3 dependent. (A) IM-9 cells were irradiated (10 Gy) or treated with VP-16 (10 μM) for 16 h. To examine the effect of caspase inhibitors on cyclin E (Cyc E) cleavage, cells were preincubated for 0.5 h prior to irradiation with the general pancaspase inhibitor zVAD-fmk and the inhibitors of caspases 3, 1, 6, and 8 (50 μM), DEVD-CHO, YVAD, IETD, and VEID, respectively. Cells were collected at 16 h following radiation, lysed, and subjected to Western blot analyses using anti-cyclin E (C-19) and actin antibodies. (B) To examine the levels of p18- and p50-cyclin E in the presence of caspase inhibitors, IM-9 cells were pretreated with zVAD-fmk (50 μM) and irradiated (10 Gy). Cells were collected at 6, 8, and 10 h following radiation and lysed, and Western blot analyses were performed as described in the legend to Fig. 1.
We next wanted to determine whether the regulation of the expression levels of the p50- and p18-cyclin E species could be dissociated. For this purpose, we examined their expression levels following treatment with caspase inhibitors at different time points following irradiation. IM-9 cells were irradiated following their preincubation with the pancaspase inhibitor zVAD-fmk. While zVAD-fmk blocked the genesis of p18-cyclin E, it had no effect on the radiation-dependent induction of p50-cyclin E. These findings indicate that increased expression of p50-cyclin E is an event upstream of caspase activation, while induction of p18-cyclin E is a later event (Fig. 3B).
Decreased levels of p50- and induction of p18-cyclin E correlate with loss of cyclin E-associated kinase activity.
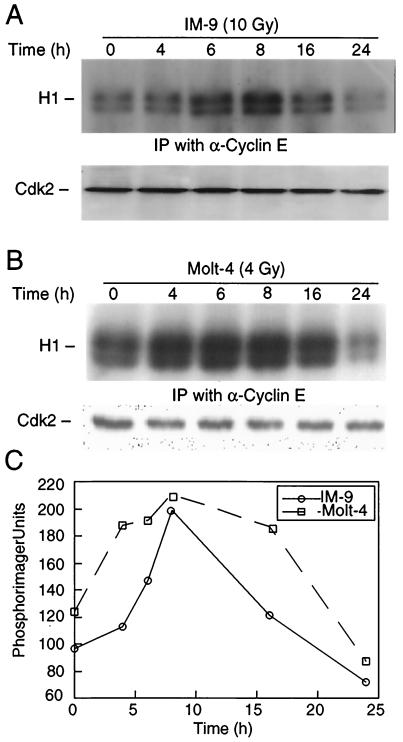
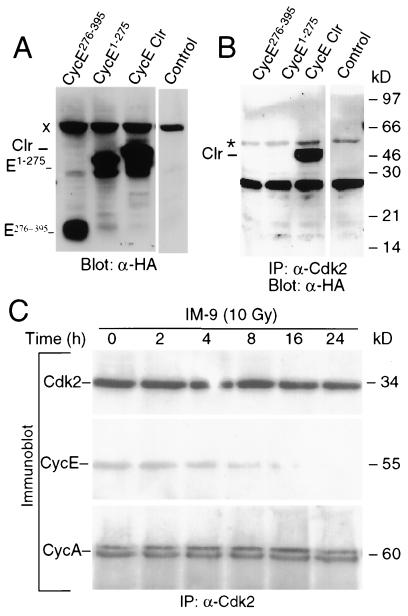
Next we sought to examine the functional significance of cyclin E cleavage by determining the cyclin E/Cdk2-associated kinase activity, the best-characterized cellular function of cyclin E. Cyclin E-associated kinase activity was measured by the phosphorylation of histone H1 in immunoprecipitates prepared from control and irradiated IM-9 and Molt-4 cells. We have previously shown that cyclin E/Cdk2 kinase activity was increased in IM-9 cells, with a kinetics reflecting the changes in the levels of cyclin E (43). Similarly, we found that there was an initial increase, at 4 to 8 h following irradiation, in phosphorylation of the histone H1 substrate (Fig. 4A). H1 phosphorylation reached a more-than-twofold increase during this time, thus reflecting the increased levels of the p50-cyclin E (Fig. 1A). However, the cyclin E/Cdk2 kinase activity started to decrease at 16 h postirradiation, becoming almost completely absent within 24 h. In contrast, there was no change in the levels of Cdk2 as determined by immunoblotting. This indicates that cyclin E/Cdk2 kinase activity reflects the expression levels of p50-cyclin E, and its decrease parallels the timing of p50-cyclin E proteolytic cleavage in IM-9, as well as in Molt-4 cells (Fig. 4B and C).
FIG. 4.
Cyclin E cleavage correlates with loss of associated kinase activity. IM-9 (A) and Molt-4 (B) cells were irradiated (10 and 4 Gy, respectively) and lysed at the indicated times. Cellular lysates containing 250 μg of protein were subjected to immunoprecipitation (IP) with 0.5 μg of anti-cyclin E antibody (C-19). Immunocomplex kinases were assayed using histone H1 (H1) as a substrate. From the same cell lysates, 10 μg of protein was subjected to SDS-10% PAGE and immunoblotting with anti-Cdk2 antibody (M2). (C) Phosphorimager values for the kinase activity, as determined above, were plotted with respect to time after irradiation.
Mapping the caspase-dependent proteolytic cleavage site in cyclin E.
If induction of p18-cyclin E was the result of caspase-mediated cleavage of p50-cyclin E, then caspase recognition sequences must exist in cyclin E. Examining the sequence of cyclin E for putative caspase recognition and cleavage sequences, we identified three such sites at positions 243 to 246, 265 to 268, and 272 to 275, representing the amino acid sequences Tyr-Leu-Asn-Asp (YLND), Glu-Leu-Leu-Asp (ELLD), and Leu-Asp-Val-Asp (LDVD), respectively. The Asp residues at positions 246, 268, 273, and 275 were mutated to Ala by site-directed mutagenesis. The GST fusion proteins generated from these different mutants of cyclin E were then used to examine whether they served as substrates for caspase 3-dependent cleavage. Recombinant caspase 3 was incubated with these fusion proteins in an in vitro cleavage assay, with proteins being subsequently separated by SDS-PAGE and analyzed by immunoblotting with an anti-cyclin E (C-19) antibody. The full-length p50-cyclin E, as well as the cyclin E mutants at positions 243 to 246 and 265 to 268 (YLND to YLNA and ELLD to ELLA, respectively), were efficiently cleaved by caspase 3 in vitro, generating a p18-cyclin E. In contrast, the cyclin E mutant at positions 272 to 275 (LDVD to LAVA) was resistant to caspase 3-mediated cleavage (Fig. 5A). These results indicate that the caspase 3 recognition and cleavage sequence of cyclin E is at position 272 to 275. This cleavage site is located at the C terminus of cyclin E in the Cdk2-binding domain (Fig. 5B). The appearance of the p18-cyclin E fragment could be effectively prevented by preincubating GST-cyclin E with 100 nM zVAD-fmk prior to performing the in vitro cleavage assay with recombinant caspase 3 (data not shown), further confirming the caspase dependence for the genesis of p18-cyclin E.
FIG. 5.
Caspase-dependent cleavage of cyclin E. (A) To map the cleavage site in cyclin E, GST-tagged wild-type and GST-tagged mutant cyclin E (lanes YLNA, LAVA, and ELLA) proteins were isolated from E. coli, examined for in vitro cleavage using recombinant caspase 3 (16 h at 30°C), and subjected to immunoblot analyses using anti-cyclin E (C-19) antibody. (B) A model for cyclin E interacting domains is shown, with the domains of cyclin E interaction with p21, p27, and Cdk2 depicted as previously suggested (5). Cdk2 interacts with most of the cyclin box and the C terminus of cyclin E (amino acid residues 131 to 395); the entire N terminus and partial cyclin box of cyclin E (amino acid residues 1 to 127) are responsible for p21 and p27 binding (5). The putative caspase recognition and cleavage sequences in cyclin E are also shown, with the replacement of the aspartate with alanine residues depicted in boldface type and outlined letters, respectively. (C) Expression vectors of cleavage-resistant cyclin E, cyclin E Clr (D273, 275A), cyclin E1-275, and cyclin E276-395 were constructed by subcloning the respective fragments generated by PCR into the pcDNA1.1 vector. The resulting expression constructs are driven by a cytomegalovirus (CMV) promoter and contain an HA tag fusion at the C terminus.
Interaction of Cdk2 is eliminated following cyclin E cleavage during apoptosis.
The proteolytic cleavage of cyclin E could have an impact on its associated kinase activity. To examine this possibility, we constructed expression vectors containing the cyclin E cleavage-resistant mutant (cyclin E Clr; LD273VD275 to LA273VA275), the C-terminal cyclin E fragment (cyclin E276-395), and the N-terminal fragment (cyclin E1-275),containing an HA tag at the C terminus (Fig. 5C). To overcome the relatively low transfection efficiency of IM-9 cells, cyclin E, cyclin E Clr, cyclin E276-395, and cyclin E1-275 were expressed in 293 T cells (Fig. 6A). Biochemical assays were then performed to investigate the interaction of cyclin E and its derivative fragments with the conventional catalytic partner Cdk2. As expected, immunoprecipitation analyses with anti-Cdk2 antibodies, followed by immunoblotting with anti-HA antibodies to detect cyclin E-HA in these transiently transfected cells, indicated that cyclin E Clr efficiently interacts with Cdk2 (Fig. 6B). In contrast, similar analyses have shown that neither cyclin E1-275 nor cyclin E276-395 interacted with Cdk2. Since binding is essential for cyclin E/Cdk2 kinase activity, these data indicate that cleavage of cyclin E eliminates the interaction of Cdk2 with cyclin E and inactivates its associated kinase activity. To determine whether such interactions were also observed in vivo, immunoprecipitation analyses were performed in control and irradiated IM-9 cells using an anti-cyclin E antibody. Immunoblot analyses of these immunoprecipitates revealed that the interaction of Cdk2 with cyclin E was eliminated in a time-dependent manner following irradiation (Fig. 6C). In contrast, the interaction of Cdk2 with cyclin A, another catalytic partner of Cdk2, was not affected. Similarly, the expression of Cdk2 also remained unchanged during the entire period following irradiation.
FIG. 6.
Cyclin E cleavage eliminates cyclin E's interaction with Cdk2. (A) 293 T cells were transiently transfected with the HA epitope-tagged cyclin E276-395, cyclin E1-274, and cyclin E Clr using Lipofectamine. At 13 h posttransfection, cells were lysed in 0.1% NP-40 lysis buffer and analyzed by Western blotting (20 μg of protein) with anti-HA antibody. (B) The interactions of Cdk2 with cyclin E276-395, cyclin E1-275, and cyclin E Clr were examined using the above lysates (150 μg of protein) immunoprecipitated with 1 μg of polyclonal anti-Cdk2 antibody. Western blot analyses were then performed with the monoclonal anti-HA antibody, as described above. Control lanes contain anti-rabbit antiserum. (C) IM-9 cells were irradiated (10 Gy) and lysed at the indicated time points, which was followed by immunoprecipitation with anti-Cdk2 and immunoblotting with anti-Cdk2, anti-cyclin E, and anti-cyclin A monoclonal antibodies. “Clr” represents cyclin E Clr. “x” and “∗” designate a nonspecific band and the immunoglobulin G band, respectively, which provide suitable controls.
Cyclin E276-395 expression triggers cell death.
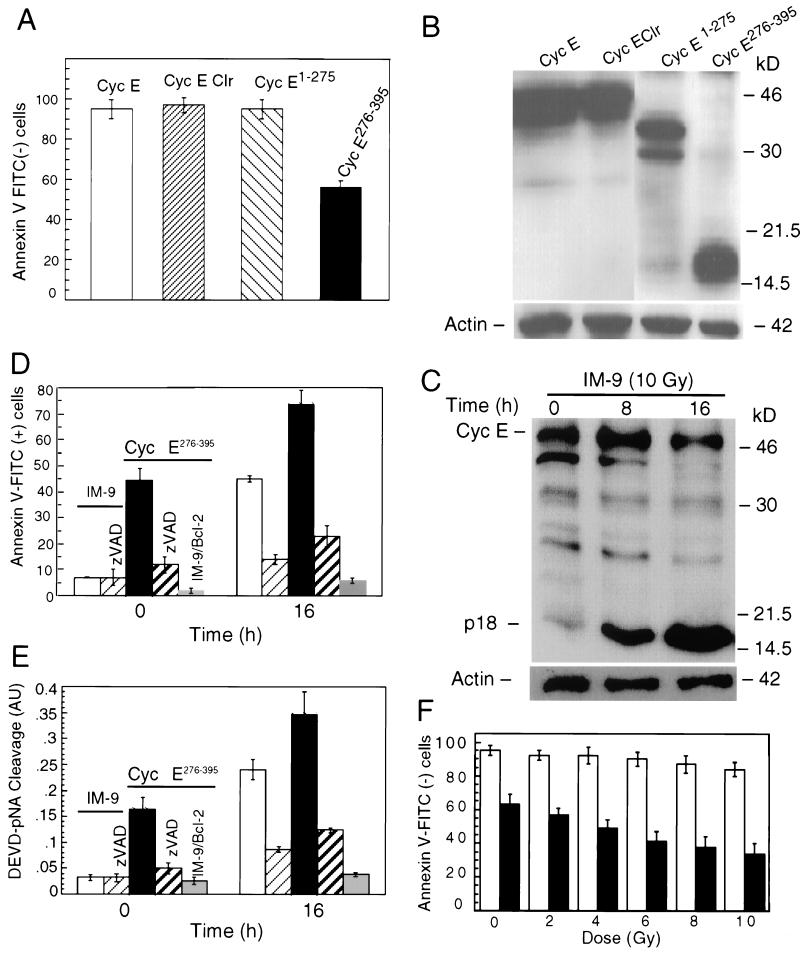
To address whether the p18-cyclin E, generated following proteolytic cleavage of p50-cyclin E during apoptosis, had any role in the cell death process in individual cells, IM-9 cells were transiently transfected with the expression plasmids for cyclin E, cyclin E Clr, cyclin E1-275, and cyclin E276-395, all containing an HA epitope at the C terminus. At 16 h posttransfection, these cells were collected to determine phosphatidylserine exposure by annexin V-FITC staining, an early marker of apoptosis. Expression of cyclin E1-275, cyclin E Clr, or cyclin E had no significant effect on viability of IM-9 cells. In contrast, expression of cyclin E276-395 induced annexin V-FITC staining in about 40% of these cells (Fig. 7A). Expression levels of all transfected cyclin E constructs, as determined by immunoblotting with antibodies to HA and β-actin as a control, were quite comparable (Fig. 7B). Most importantly, the expression levels of HA-cyclin E276-395 were comparable to the levels of p18-cyclin E generated at 16 h following irradiation of IM-9 cells, as determined by their relative expression and that of β-actin, used as a protein loading control (Fig. 7C).
FIG. 7.
Expression of cyclin E276-395 induces apoptosis. IM-9 cells were transiently transfected with the HA epitope-tagged cyclin E276-395, cyclin E1-275, cyclin E Clr, and cyclin E mutants. In addition, cells were cotransfected (1:1 ratio) with enhanced GFP and sorted 16 h later for enhanced GFP expression by fluorescence-activated cell sorting. (A) Cells were analyzed for annexin V-FITC staining as a marker of early apoptosis. (B) To determine expression of cyclin E and derivative mutants in transfected cells, Western blot analyses were performed in these cells with anti-HA monoclonal antibody. (C) In addition, irradiated IM-9 cells (10 Gy) were collected at the indicated times and subjected to immunoblotting with anti-cyclin E. β-Actin levels were also determined as a positive loading control. Immunoblotting, antibody incubation, and further blot processing in these two experiments were performed at the same time. In addition, cyclin E276-395 was expressed in IM-9 or IM-9/Bcl2 cells, which was followed by irradiation in the absence or presence of zVAD-fmk. The total number of apoptotic cells was determined by annexin V-FITC staining and flow cytometry (D) or caspase activation, as measured by DEVD-pNA cleavage activity (E). IM-9 and IM-9/Bcl-2 cells are represented by empty and gray bars, respectively. Caspase activity is shown in arbitrary units (AU). (F) The radiation dose response was determined at 8 h in cells transfected with cyclin E276-395 compared to parental IM-9 cells. Empty and black bars represent IM-9 cells and those transiently expressing cyclin E276-395, respectively; gray and striped bars represent cells treated with zVAD or those stably expressing Bcl-2. Data shown in panels A, D, and E are the mean values of two independent duplicate experiments.
To further examine the potential apoptotic role of cyclin E276-395 expression, control and transfected cells were incubated in the presence of zVAD-fmk, a broad-spectrum caspase inhibitor. A similar experiment was done in cells stably overexpressing Bcl-2 (IM-9/Bcl-2). Phosphatidylserine exposure and caspase activation, two key steps in the commitment and execution phases of apoptosis, were then examined. Expression of cyclin E276-395-induced annexin V-FITC staining, as a measure of phosphatidylserine exposure, was detected in about 40% of these cyclin E276-395-transfected cells, compared to only 5% in IM-9 cells (Fig. 7D). Following 16 h after radiation treatment, there was increased annexin V-FITC staining, with about 75% of the transfected cells staining positively, compared to only 40% in parental IM-9 cells. Moreover, about threefold more caspase activity was observed in cells expressing cyclin E276-395 compared to IM-9 cells, as measured by the DEVD-pNA cleavage assay (Fig. 7E). A radiation dose-response curve clearly indicates that cells expressing cyclin E276-395 were more susceptible to radiation-induced apoptosis compared to parental IM-9 cells (Fig. 7F). Both annexin V-FITC staining and caspase activation were prevented by zVAD-fmk treatment or stable expression of Bcl-2. These data demonstrate that cyclin E276-395 expression induces apoptosis in a caspase-dependent and Bcl-2-inhibitable manner.
If cyclin E cleavage plays a direct role in apoptosis, then preventing this cleavage should affect the apoptotic process. To examine this possibility, we stably expressed cyclin E Clr in IM-9 cells. As predicted, apoptosis following irradiation was eliminated in the presence of cyclin E Clr, under conditions in which only about 40% of IM-9 cells remained annexin V negative (Fig. 8A), thus indicating the physiological role of cyclin E cleavage in apoptosis. The levels of expression of cyclin E Clr were comparable to that of cyclin E276-395, which caused apoptosis (Fig. 7B). Using anti-HA antibodies, it was determined that there was no proteolytic cleavage of cyclin E Clr following irradiation up to 48 h following irradiation (Fig. 8B). Interestingly, the appearance of the endogenous p18-cyclin E, detected following irradiation of IM-9 cells (Fig. 7B), was prevented in cyclin E Clr-expressing cells, as determined by immunoblotting with the C-terminal anti-cyclin E antibodies (Fig. 8C).
FIG. 8.
Expression of cyclin E Clr prevents apoptosis. (A) IM-9 parental cells and those stably expressing cyclin E Clr were subjected to annexin V-FITC staining and flow cytometry at the indicated times following irradiation. Western blot analysis of cyclin E Clr-expressing cells following irradiation was performed to examine the cleavage of ectopic cyclin E, using anti-HA antibodies (B), and endogenous cleavage of cyclin E, using anti-cyclin E (C-19) antibodies (C). Empty and striped bars represent parental IM-9 cells and those stably expressing cyclin E Clr, respectively.
DISCUSSION
In this study we have identified and characterized a novel p18 cyclin E-derived fragment. Its time- and dose-dependent expression during apoptosis was inversely correlated with that of p50-cyclin E, the predominant cyclin E in most human cells (49). The conversion of cyclin E from a p50 to a p18 fragment may be a general process, as it is produced in all hematopoietic tumor cell lines we have examined, and following treatment with multiple genotoxic stress agents which trigger apoptosis. A similar cyclin E derivative has not been previously reported, even though both splice variants and cyclin E truncations have been found in some tumors (49). In addition, some low-molecular-weight forms of cyclin E have been previously reported in solid tumors and shown not to be the result of genomic rearrangements of the cyclin E gene (29, 30). Both normal and tumor cells contain these variants (24, 29). The truncated cyclin E fragments and splicing variants previously described are all considerably larger than p18-cyclin E276-395 and efficiently bind to Cdk2, and the bound Cdk2 complexes have active kinase activity. In fact there was an increased level of kinase activity in those cells, which led to faster cell cycle progression and proliferation (24, 49). In contrast to all previous reports, the p18-cyclin E generated during apoptosis through a more severe truncation of cyclin E can no longer bind Cdk2 and thus is lacking any associated active kinase activity.
The degradation of cyclin E in late S phase is thought to take place largely through a ubiquitin- and proteasome-dependent pathway. The SCF pathway, which targets cyclins for ubiquitination on the basis of site-specific auto-phosphorylation of Thr 380 of cyclin E (8, 57, 64), and a pathway involving Cul-3, an E3-type ubiquitin-protein ligase which selects cyclin E for ubiquitination on the basis of its assembly into Cdk complexes, may be complementary ways to control cyclin abundance. Here we show that caspase-dependent proteolytic cleavage is an additional mechanism used by hematopoietic tumor cells to regulate the cellular functions of cyclin E during apoptosis. The apoptosis dependence, sensitivity to caspase inhibitors, and in vitro cleavage of cyclin E indicate that cyclin E276-395 is generated during apoptosis following proteolytic attack by a caspase. p18-cyclin E genesis is prevented by Bcl-2 under conditions in which Bcl-2 blocks apoptosis up to at least 96 h following irradiation. In addition, Bcl-2 expression maintains the induced level of full-length p50-cyclin E and is most likely to do so by preventing its cleavage. Our data clearly indicate that caspase 3, or a caspase 3-like enzyme, directly cleaves cyclin E, since (i) generation of p18-cyclin E during apoptosis is prevented when cells are treated with general (zVAD) and specific caspase 3 (DEVD) pharmacologic inhibitors, but not when inhibitors of caspase 1, 6, or 8 are used; (ii) p18-cyclin E can be reconstituted in vitro in a reaction containing p50-cyclin E and purified caspase 3; and (iii) cyclin E cleavage is prevented when the aspartates 273 and 275 are mutated. In contrast to our findings in hematopoietic cells, it was reported previously that Cdk2 is activated during apoptosis of endothelial cells through cleavage of p21 and p27 (34). However, we found that not to be the case in the hematopoietic cells we have examined, with p21 being slightly upregulated but not cleaved (data not shown).
Interestingly, even though the cyclin E cleavage site has an aspartate residue in the P4 position, the cleavage site of cyclin E does not represent a prototypical DEVD caspase 3 consensus sequence. It is likely that during apoptosis the caspase tetrapeptide recognition sequence LDVD is exposed on the surface of cyclin E and perhaps facilitated by a favorable protein conformation, it becomes accessible to caspase 3. Its cleavage results in inactivation of the associated kinase and shutdown of the cell cycle machinery associated with the execution phase of apoptosis. In addition, even though cyclin E1-275 still retains the full cyclin box and part of the C terminus of cyclin E (Fig. 5B and 6B), it no longer interacts with Cdk2, as determined in cells transfected with various cyclin E mutants or by examining these interactions in IM-9 cells where p18-cyclin E is generated during radiation-induced apoptosis. In contrast, the binding of Cdk2 to cyclin A remained unaffected (Fig. 6C). These data reinforce the notion that the precise structural conformation of cyclin E is likely to be critical for retention of its ability to bind Cdk2 and thus sustain a functional kinase activity.
What is the consequence of cyclin E proteolytic cleavage, and how is cyclin E276-395 mechanistically linked to the apoptotic events? Clearly, the cleavage of cyclin E inactivates the associated kinase activity since its proteolytic products, cyclin E276-395 and cyclin E1-275, can no longer interact with Cdk2, thus abolishing cyclin E/Cdk2-associated kinase activity. Therefore, caspase 3-mediated cleavage of cyclin E might be a mechanism to modulate its apoptotic function. One possible consequence of cyclin E cleavage is that it represents a mechanism to inactivate cyclin E function and shut down cell proliferation during the execution phase of apoptosis. A second possibility is that the p18-cyclin E may acquire a new function which contributes to apoptosis. A third possibility could be a combination of the previous two. In support of the second interpretation, expression of cyclin E276-395 but not cyclin E, cyclin E Clr, or cyclin E1-275 triggers apoptosis. In addition, exposure of IM-9 cells expressing cyclin E276-395 to genotoxic agents, such as ionizing radiation, makes them much more susceptible to apoptosis compared to parental cells. However, this is strikingly inhibited when cyclin E276-395 is expressed in IM-9 cells stably expressing Bcl-2 or treated with zVAD-fmk. This indicates that cyclin E276-395 induces apoptosis in a caspase-dependent manner which could be prevented by Bcl-2 expression. In addition, overexpression of cyclin E Clr prevents apoptosis as well as the generation of the endogenous p18 fragment of cyclin E following irradiation. This indicates a dominant effect of this cleavage-resistant mutant which abolishes the proteolytic cleavage of cyclin E, further indicating that cleavage of cyclin E has a significant contribution to apoptosis of these hematopoietic cells.
Mitochondria play an essential role in apoptosis through redistribution of intermembrane mitochondrial proteins, such as cytochrome c (9). We have recently shown distinct initial and amplification stages of cytochrome c release following irradiation of IM-9 cells (7). The early phase consisted in the release of low levels of cytochrome c into the cytosol preceding caspase activation with no effects on mitochondrial function. The second phase was dependent on a feedback amplification loop linking caspase 3 activation to mitochondrial dysfunction, consisting in a late-stage massive cytochrome c release (7). Based on our present and previous studies (43), we propose a dual role for cyclin E in apoptosis of hematopoietic cells. We suggest that the increased cyclin E levels might be implicated in the initiation phase, while p18-cyclin E might participate, directly or indirectly, in the amplification of the mitochondrial phase of the apoptotic response initiated by cytochrome c release and caspase activation. Similar functions have been attributed to Bcl-2 and Bad (7). The two distinct functions exhibited by cyclin E during apoptosis are supported by the fact that, while caspase 3 inhibitors could block cyclin E cleavage, they are unable to prevent the induction of cyclin E, indicating that cleavage does not affect the early upregulation of p50-cyclin E which we have previously reported (43). Therefore, the increased expression of cyclin E is an event upstream of caspase activation, while its cleavage is the result of the activated caspase(s). When cells receive a death stimulus, during the commitment phase, cyclin E is induced and caspases are activated. The activation of caspases may be mediated, directly or indirectly, by the induction of cyclin E, most likely through the phosphorylation of a cellular substrate(s) by cyclin E/Cdk2. Moreover, during the execution phase of apoptosis, cleavage of cyclin E by caspase(s) converts it to p18-cyclin E. The resulting p18-cyclin E molecule may contribute to the amplification of the caspase cascade and eventually accelerate the death process and thus directly contribute to the execution phase of apoptosis. One possible mechanism by which p18-cyclin E might participate in apoptosis is through a feedback mechanism (7) by which it could interfere with the function of Bax and/or other proapoptotic proteins or contribute to the release of proapoptogenic factors from mitochondria. Alternatively, it might interact with another molecule, possibly affecting a novel apoptotic pathway.
Among the different substrates cleaved by caspase 3, DFF45, PAK2, and PKC-δ have been shown to be directly related to different characteristics of apoptosis, such as chromatin condensation, DNA fragmentation, membrane blebbing, and morphological changes (25, 39). However, the majority of cellular caspase substrates, of which at least 100 have been reported (25), were discovered serendipitously and have not been examined in terms of their mechanistic link to apoptosis. Therefore, the exact role of the cleavage products of most caspase substrates during apoptosis is not clear. Here we suggest that cyclin E is another caspase 3 substrate, with the resulting p18-cyclin E acquiring a new role in the amplification of the apoptotic process. The substrates for p18-cyclin E276-395 may include caspases, proteins that will induce such types of proteases, or even proapoptotic Bcl-2 family members. Perhaps p18-cyclin E interacts with a proapoptotic molecule or participates in a protein complex containing a number of death-inducing molecules and thus modulates their function. For example, it has been reported that Bax transactivation may be dependent on Cdk2 (21). Additional studies will be required to clarify the precise role of cyclin E cleavage in apoptosis of hematopoietic cells.
In summary, our data indicate that cyclin E has a dual role in apoptosis induced by genotoxic stress. Cyclin E activation as an early response is likely to play a critical role in the initiation of cell death, while its proteolytic cleavage is likely to further contribute to the execution phase of apoptosis. Therefore, cyclin E plays an important role in apoptosis of hematopoietic cells, in addition to its reported key regulatory role in the control of the G1-to-S-phase transition and the initiation of DNA replication. Cyclin E is therefore unique among most proteins in its essential role in three fundamental biological processes. Taken together with the earlier finding of a caspase 3-dependent inactivation of the CKIs p21 and p27 in endothelial cells, these findings suggest that targeting cell cycle regulators is a general event in apoptosis. Further investigations into the role of cyclin E in cell death are likely to shed new light on how these events might also be coupled to cell cycle control and proliferation.
Acknowledgments
We thank E. S. Alnemri and S. M. Srinivasula (Thomas Jefferson University), A. Koff (Memorial Sloan-Kettering Cancer Center), and B. Amati and K. Alevizopoulos (Swiss Institute for Experimental Cancer Research [ISREC]) for various constructs. We also thank C. Stanko (Cleveland Clinic Flow Cytometry Core) for help with flow cytometry and Erica Dupree for critical reading of the manuscript.
This work was supported by U.S. Public Service research grant CA81504, research grant CA82858 from the National Cancer Institute to A.A., and a research grant from the Radiological Society of North America (RSNA) to J.C.B.
REFERENCES
- 1.Alevizopoulos, K., J. Vlach, S. Hennecke, and B. Amati. 1997. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 16:5322-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almasan, A., Y. Yin, R. E. Kelly, E. Y. Lee, A. Bradley, W.-W. Li, J. R. Bertino, and G. M. Wahl. 1995. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. USA 92:5436-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyaert, R., V. J. Kidd, S. Cornelis, M. Van de Craen, G. Denecker, J. M. Lahti, R. Gururajan, P. Vandenabeele, and W. Fiers. 1997. Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J. Biol. Chem. 272:11694-11697. [DOI] [PubMed] [Google Scholar]
- 4.Brady, H. J., G. Gil-Gomez, J. Kirberg, and A. J. Berns. 1996. Bax alpha perturbs T cell development and affects cell cycle entry of T cells. EMBO J. 15:6991-7001. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., P. Saha, S. Kornbluth, B. D. Dynlacht, and A. Dutta. 1996. Cyclin-binding motifs are essential for the function of p21CIP1. Mol. Cell. Biol. 16:4673-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, L., V. Marechal, J. Moreau, A. J. Levine, and J. Chen. 1997. Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J. Biol. Chem. 272:22966-22973. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Q., B. Gong, and A. Almasan. 2000. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clurman, B. E., R. J. Sheaff, K. Thress, M. Groudine, and J. M. Roberts. 1996. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 10:1979-1990. [DOI] [PubMed] [Google Scholar]
- 9.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy, S. F., P. W. Hinds, K. Louie, S. I. Reed, A. Arnold, and R. A. Weinberg. 1993. Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73:499-511. [DOI] [PubMed] [Google Scholar]
- 11.Dulic, V., E. Lees, and S. I. Reed. 1992. Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257:1958-1961. [DOI] [PubMed] [Google Scholar]
- 12.Duronio, R. J., A. Brook, N. Dyson, and P. H. O'Farrell. 1996. E2F-induced S phase requires cyclin E. Genes Dev. 10:2505-2513. [DOI] [PubMed] [Google Scholar]
- 13.Ekholm, S. V., P. Zickert, S. I. Reed, and A. Zetterberg. 2001. Accumulation of cyclin E is not a prerequisite for passage through the restriction point. Mol. Cell. Biol. 21:3256-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664-1672. [DOI] [PubMed] [Google Scholar]
- 15.Evan, G. I., and K. H. Vousden. 2001. Proliferation, cell cycle and apoptosis in cancer. Nature 411:342-348. [DOI] [PubMed] [Google Scholar]
- 16.Evans, T., E. T. Rosenthal, J. Youngblom, D. Distel, and T. Hunt. 1983. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33:389-396. [DOI] [PubMed] [Google Scholar]
- 17.Ewen, M. E., H. K. Sluss, C. J. Sherr, H. Matsushime, J. Kato, and D. M. Livingston. 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73:487-497. [DOI] [PubMed] [Google Scholar]
- 18.Geng, Y., W. Whoriskey, M. Y. Park, R. T. Bronson, R. H. Medema, T. Li, R. A. Weinberg, and P. Sicinski. 1999. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 97:767-777. [DOI] [PubMed] [Google Scholar]
- 19.Gong, B., and A. Almasan. 2000. Apo2 ligand/TNF-related apoptosis-inducing ligand and death receptor 5 mediate the apoptotic signaling induced by ionizing radiation in leukemic cells. Cancer Res. 60:5754-5760. [PubMed] [Google Scholar]
- 20.Gong, B., Q. Chen, B. Endlich, S. Mazumder, and A. Almasan. 1999. Ionizing radiation-induced, Bax-mediated cell death is dependent on activation of serine and cysteine proteases. Cell Growth Differ. 10:491-502. [PubMed] [Google Scholar]
- 21.Hakem, A., T. Sasaki, I. Kozieradzki, and J. M. Penninger. 1999. The cyclin-dependent kinase Cdk2 regulates thymocyte apoptosis. J. Exp. Med. 189:957-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, E. K., M. Begemann, A. Sgambato, J. W. Soh, Y. Doki, W. Q. Xing, W. Liu, and I. B. Weinstein. 1996. Increased expression of cyclin D1 in a murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ. 7:699-710. [PubMed] [Google Scholar]
- 23.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 24.Harwell, R. M., D. C. Porter, C. Danes, and K. Keyomarsi. 2000. Processing of cyclin E differs between normal and tumor breast cells. Cancer Res. 60:481-489. [PubMed] [Google Scholar]
- 25.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, P. K., S. Chevalier, M. Philippe, and M. W. Kirschner. 1995. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol. 130:755-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janicke, R. U., P. A. Walker, X. Y. Lin, and A. G. Porter. 1996. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 15:6969-6978. [PMC free article] [PubMed] [Google Scholar]
- 28.Kato, J., H. Matsushime, S. W. Hiebert, M. E. Ewen, and C. J. Sherr. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7:331-342. [DOI] [PubMed] [Google Scholar]
- 29.Keyomarsi, K., D. Conte, Jr., W. Toyofuku, and M. P. Fox. 1995. Deregulation of cyclin E in breast cancer. Oncogene 11:941-950. [PubMed] [Google Scholar]
- 30.Keyomarsi, K., and A. B. Pardee. 1993. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc. Natl. Acad. Sci. USA 90:1112-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koff, A., F. Cross, A. Fisher, J. Schumacher, K. Leguellec, M. Philippe, and J. M. Roberts. 1991. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66:1217-1228. [DOI] [PubMed] [Google Scholar]
- 32.Koff, A., A. Giordano, D. Desai, K. Yamashita, J. W. Harper, S. Elledge, T. Nishimoto, D. O. Morgan, B. R. Franza, and J. M. Roberts. 1992. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 257:1689-1694. [DOI] [PubMed] [Google Scholar]
- 33.Krude, T., M. Jackman, J. Pines, and R. A. Laskey. 1997. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell 88:109-119. [DOI] [PubMed] [Google Scholar]
- 34.Levkau, B., H. Koyoma, E. W. Raines, B. E. Clurman, B. Herren, K. Orth, and J. M. Roberts. 1997. Cleavage of p21/cip1/waf1 and p27kip1 mediates apoptosis of endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell 1:553-563. [DOI] [PubMed] [Google Scholar]
- 35.Lew, D. J., V. Dulic, and S. I. Reed. 1991. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66:1197-1206. [DOI] [PubMed] [Google Scholar]
- 36.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 37.Linette, G. P., Y. Li, K. Roth, and S. J. Korsmeyer. 1996. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc. Natl. Acad. Sci. USA 93:9545-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 39.Liu, X., H. Zou, C. Slaughter, and X. Wang. 1997. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89:175-184. [DOI] [PubMed] [Google Scholar]
- 40.Lukas, J., T. Herzinger, K. Hansen, M. C. Moroni, D. Resnitzky, K. Helin, S. I. Reed, and J. Bartek. 1997. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 11:1479-1492. [DOI] [PubMed] [Google Scholar]
- 41.MacFarlane, M., M. Ahmad, S. M. Srinivasula, T. Fernandes-Alnemri, G. M. Cohen, and E. S. Alnemri. 1997. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 272:25417-25420. [DOI] [PubMed] [Google Scholar]
- 42.Mazel, S., D. Burtrum, and H. T. Petrie. 1996. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J. Exp. Med. 183:2219-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazumder, S., B. Gong, and A. Almasan. 2000. Cyclin E induction by genotoxic stress leads to apoptosis of hematopoietic cells. Oncogene 19:2828-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meikrantz, W., and R. Schlegel. 1995. Apoptosis and the cell cycle. J. Cell. Biochem. 58:160-174. [DOI] [PubMed] [Google Scholar]
- 45.Meikrantz, W., and R. Schlegel. 1996. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J. Biol. Chem. 271:10205-10209. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsubo, M., A. M. Theodoras, J. Schumacher, J. M. Roberts, and M. Pagano. 1995. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol. 15:2612-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Reilly, L. A., D. C. Huang, and A. Strasser. 1996. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 15:6979-6990. [PMC free article] [PubMed] [Google Scholar]
- 48.Park, D. S., E. J. Morris, J. Padmanabhan, M. L. Shelanski, H. M. Geller, and L. A. Greene. 1998. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J. Cell Biol. 143:457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter, D. C., and K. Keyomarsi. 2000. Novel splice variants of cyclin E with altered substrate specificity. Nucleic Acids Res. 28:E101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raff, M. C. 1992. Social controls on cell survival and cell death. Nature 356:397-400. [DOI] [PubMed] [Google Scholar]
- 51.Reed, S. I. 1997. Control of the G1/S transition. Cancer Surv. 29:7-23. [PubMed] [Google Scholar]
- 52.Resnitzky, D., M. Gossen, H. Bujard, and S. I. Reed. 1994. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol. Cell. Biol. 14:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resnitzky, D., and S. I. Reed. 1995. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol. Cell. Biol. 15:3463-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlegel, J., I. Peters, S. Orrenius, D. K. Miller, N. A. Thornberry, T. T. Yamin, and D. W. Nicholson. 1996. CPP32/apopain is a key interleukin 1 beta converting enzyme-like protease involved in Fas-mediated apoptosis. J. Biol. Chem. 271:1841-1844. [DOI] [PubMed] [Google Scholar]
- 55.Sewing, A., V. Ronicke, C. Burger, M. Funk, and R. Muller. 1994. Alternative splicing of human cyclin E. J. Cell Sci. 107:581-588. [DOI] [PubMed] [Google Scholar]
- 56.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 57.Singer, J. D., M. Gurian-West, B. Clurman, and J. M. Roberts. 1999. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 13:2375-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sofer-Levi, Y., and D. Resnitzky. 1996. Apoptosis induced by ectopic expression of cyclin D1 but not cyclin E. Oncogene 13:2431-2437. [PubMed] [Google Scholar]
- 59.Tan, X., S. J. Martin, D. R. Green, and J. Y. J. Wang. 1997. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J. Biol. Chem. 272:9613-9616. [DOI] [PubMed] [Google Scholar]
- 60.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 61.Tsai, L. H., E. Lees, B. Faha, E. Harlow, and K. Riabowol. 1993. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene 8:1593-1602. [PubMed] [Google Scholar]
- 62.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 63.Wang, J., and K. Walsh. 1996. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 273:359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Won, K. A., and S. I. Reed. 1996. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 15:4182-4193. [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong, Y., T. Connolly, B. Futcher, and D. Beach. 1991. Human D-type cyclin. Cell 65:691-699. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, B. B., H. Li, J. Yuan, and M. W. Kirschner. 1998. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 95:6785-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]