Abstract
Lysophosphatidic acid (LPA) is a natural phospholipid with multiple biological functions. We show here that LPA induces phosphorylation and inactivation of glycogen synthase kinase 3 (GSK-3), a multifunctional serine/threonine kinase. The effect of LPA can be reconstituted by expression of Edg-4 or Edg-7 in cells lacking LPA responses. Compared to insulin, LPA stimulates only modest phosphatidylinositol 3-kinase (PI3K)-dependent activation of protein kinase B (PKB/Akt) that does not correlate with the magnitude of GSK-3 phosphorylation induced by LPA. PI3K inhibitors block insulin- but not LPA-induced GSK-3 phosphorylation. In contrast, the effect of LPA, but not that of insulin or platelet-derived growth factor (PDGF), is sensitive to protein kinase C (PKC) inhibitors. Downregulation of endogenous PKC activity selectively reduces LPA-mediated GSK-3 phosphorylation. Furthermore, several PKC isotypes phosphorylate GSK-3 in vitro and in vivo. To confirm a specific role for PKC in regulation of GSK-3, we further studied signaling properties of PDGF receptor β subunit (PDGFRβ) in HEK293 cells lacking endogenous PDGF receptors. In clones expressing a PDGFRβ mutant wherein the residues that couple to PI3K and other signaling functions are mutated with the link to phospholipase Cγ (PLCγ) left intact, PDGF is fully capable of stimulating GSK-3 phosphorylation. The process is sensitive to PKC inhibitors in contrast to the response through the wild-type PDGFRβ. Therefore, growth factors, such as PDGF, which control GSK-3 mainly through the PI3K-PKB/Akt module, possess the ability to regulate GSK-3 through an alternative, redundant PLCγ-PKC pathway. LPA and potentially other natural ligands primarily utilize a PKC-dependent pathway to modulate GSK-3.
Lysophosphatidic acid (LPA; 1-acyl-2-lyso-sn-glycero-3-phosphate) is a natural phospholipid that exhibits pleiotrophic biological functions, ranging from rapid morphologic changes to long-term stimulation of cell proliferation and survival (28, 42, 46, 62). Although not present at significant concentrations in plasma or freshly isolated blood, LPA is generated and released during blood clotting and can therefore contribute to wound healing and tissue regeneration and repair (18, 25, 27). Recent studies on the pathophysiological roles of LPA have revealed abnormalities in LPA production and function in cardiovascular and neoplastic diseases (19, 27, 46, 66, 67). The most striking findings are in ovarian cancer, where LPA is present in elevated levels in ascites and probably in the plasma of patients (66, 67). LPA may thus contribute to the progression of human cancer, offering a diagnostic marker and/or therapeutic target for ovarian malignancies.
LPA interacts with G-protein-coupled receptors (46). Several members (Edg-2/Vzg-1, Edg-4, and Edg-7, also known as LPA1, LPA2, and LPA3, respectively) of the endothelial-cell differentiation gene family have been identified to be high-affinity receptors for LPA (24, 26). The other members of the family, including Edg-1, Edg-3, Edg-5, Edg-6, and Edg-8, are receptors for the structurally related phospholipid sphingosine-1-phosphate (34). In contrast to the three highly related LPA receptors, a dissimilar receptor cloned from the genus Xenopus, PSP24, was reported to be a high-affinity LPA receptor (30, 59), although its physiological role in mammals remains controversial (40). Recent data have suggested the existence of additional, non-Edg receptors for LPA (35). Most cell types in culture express one or more LPA receptors and respond to LPA, making it difficult to characterize the receptor dependence of a particular response to LPA. Activation of LPA receptors stimulates a number of well-characterized signaling pathways via the heterotrimeric G proteins G(i), G(q), and G(12/13), including inhibition of adenylate cyclase (62), activation of the Ras-Raf-Erk pathway (36), stimulation of phospholipase C (PLC) and PLD (62), tyrosine phosphorylation of focal-adhesion proteins (53), and stress fiber formation (51).
In the present study, we demonstrate that LPA induces phosphorylation and inhibition of glycogen synthase kinase 3 (GSK-3) through specific Edg receptors. GSK-3 was originally identified as a serine/threonine kinase that regulates glycogen synthesis (37). Later studies implicated GSK-3 in multiple biological processes. GSK-3 phosphorylates a broad range of substrates, including several transcription factors (50) and the translation factor eIF2B (65). GSK-3 has also been implicated in the regulation of cell fate in Dictyostelium spp. (31) and is a component of the Wnt signaling pathway required for Drosophila and Xenopus development (17, 32, 49, 55). More recent studies indicate a role for GSK-3β in the control of cell proliferation and survival in mammalian cells (12, 48).
Upon stimulation with insulin or other factors, GSK-3 is rapidly phosphorylated at serine 21 (GSK-3α) and serine 9 (GSK-3β), resulting in inhibition of its protein kinase activity (11, 56, 57). Protein kinase B (PKB/Akt), a serine/threonine kinase located downstream of phosphatidylinositol 3-kinase (PI3K), has been demonstrated to phosphorylate both of these sites in vitro and in vivo (10), suggesting that certain growth factors repress GSK-3 activity through the PI3K-PKB/Akt signaling cascade. We and others have recently identified cyclic AMP (cAMP)-dependent protein kinase A (PKA) as a novel GSK-3 kinase that phosphorylates and inactivates both isoforms of GSK-3 (21, 44), suggesting that GSK-3 represents an important convergence point integrating signals from multiple signaling cascades. Here we report that LPA stimulates GSK-3 phosphorylation and inactivation via an intracellular signaling pathway involving PKC, independent of the previously identified PI3K-PKB/Akt and cAMP-PKA pathways. The results indicate that, depending on the stimulatory context, the activity of GSK-3 can be regulated through multiple different signaling mechanisms.
MATERIALS AND METHODS
Reagents.
LPA (oleoyl; C18:1) was purchased from Sigma or Avanti. Before use, LPA solution was freshly made in phosphate-buffered saline containing 1% fatty acid-free bovine serum albumin (Roche Molecular Biochemicals). Platelet-derived growth factor (PDGF), the phorbol ester TPA (12-O-tetradecanoylphorbol-13-acetate) and wortmannin were obtained from Sigma. H89, LY294002, GF109203X, the GF109203 inactive analog bisindolylmaleimide V, and Ro31-8220 were from Calbiochem. Insulin-like growth factor 1 (IGF-1) was from Upstate Biotechnology (Lake Placid, N.Y.). Insulin was from Gibco-BRL. Microcystin LR was from Biomol (Plymouth Meeting, Pa.). The plasmid pcDNA3/GS-PKCɛ was purchased from Invitrogen. The activated form of PKCɛ was generated by deleting amino acids 156 to 162 as described previously (52).
Cell lines.
Swiss 3T3, NIH 3T3, HEK293, Cos-7, and OVCAR-3 were obtained from the American Type Culture Collection. The ovarian cancer cell line A2780CP was kindly provided by T. C. Hamilton (Fox Chase Cancer Center, Philadelphia, Pa.). These cell lines were frozen at early passages and used for less than 10 weeks in continuous culture. Swiss 3T3, NIH 3T3, and HEK293 were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (Sigma). Cos-7 and A2780CP cells were maintained in RPMI 1640 plus 10% fetal bovine serum. Before stimulation with LPA or other growth factors, cells were grown to subconfluence in 60-mm dishes and starved in serum-free medium for 8 to 24 h unless otherwise indicated.
Transfection.
HEK293 cells were plated in 60-mm dishes. The following day, at ca. 60% confluence, the cells were transfected (1.5 μg of DNA/dish for stable transfection and 6 μg of DNA/dish for transient transfection) by using Fugene 6 according to the protocol of the supplier (Roche Molecular Biochemicals). In cotransfection experiments, the molar ratio of hemagglutinin (HA)-GSK-3β or HA-Erk1 expression vector and Edg receptor expression vectors or constitutively active PKC constructs was adjusted to 1:5 to achieve coexpression of an Edg receptor or a mutant PKC with HA-GSK-3β or HA-Erk1.
Establishment of stable clones expressing PDGFRβ.
To establish stable clones expressing the wild-type (WT) and mutant PDGF receptor β subunit (PDGFRβ), HEK293 cells were subcultured at a 1:30 dilution and grown in the presence of 1.0 mg of G418/ml 48 h after transfection with pLXSN-based PDGFRβ vectors (60, 61). Discrete G418-resistant colonies were isolated by ring cloning or by picking colonies directly by using pipettes. Cloned cultures were expanded sequentially in 24-well plates, six-well plates, and 60-mm dishes. Positive clones were determined by immunoblotting with antibody against the human PDGFRβ (Santa Cruz Biotechnology).
RT-PCR.
The expression of Edg-2, Edg-4, and Edg-7 was examined by reverse transcription-PCR (RT-PCR). Total RNA was isolated from cultured cell lines by using TriPure isolation reagent (Roche Molecular Biochemicals) according to the manufacturer's instructions. Before RT-PCR, RNA samples were treated with DNase (DNA-free DNase treatment and removal kit; Ambion, Austin, Tex.). The oligonucleotide primer pairs and expected sizes of PCR products were as follows: Edg-2, 5′-CAAAATGAGGCCTTACGACGCCA and 5′-TCCCATTCTGAAGTGCTGCGTTC, 621 bp; Edg-4, 5′-GCGCGCGGATCCACCATGGTCATCATGGGCCAGTGCT and 5′-GCGCGGTCGACTCAGTCCTGTTGGTTGGGTTGA, 1,236 bp; Edg-7, 5′-CTGATGTTTAACACAGGCCC and 5′-GACGTTGGTTTTCCTCTTGA, 402 bp; and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-CCCATGGCAAATTCCATGGCACCG and 5′-GTCATGGATGACCTTGGCCAGGGG, 344 bp.
The RT-PCR was performed by using a Titan one-tube RT-PCR system (Roche Molecular Biochemicals). The 1× RT-PCR buffer contains 1 μg of RNA, 0.4 μM sense and antisense primers, 0.2 mM concentrations of each deoxynucleoside triphosphate, 5 mM dithiothreitol (DTT), 1.5 mM MgCl2, 5 U of RNasin, 0.5 U of reverse transcriptase, and 0.5 U of Taq DNA polymerase in a final volume of 50 μl. The mixes were first incubated at 60°C for 30 min to allow RT, followed by PCR for 35 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min. PCR products were visualized by staining with ethidium bromide after electrophoresis (2% agarose).
Western blotting.
Cells were lysed in sodium dodecyl sulfate (SDS) sample buffer or ice-cold Triton X-100 lysis buffer (1% Triton X-100, 50 mM HEPES [pH 7.4], 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 100 mM NaF, 10 mM sodium pyrophosphate, protein inhibitor mixture [Roche Molecular Biochemicals]). Total cellular protein was separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon (polyvinylidene difluoride), and immunoblotted with antibodies according to the protocols of the manufacturers. Anti-phospho-GSK-3 (New England Biolabs) is a rabbit polyclonal antibody that recognizes both GSK-3α phosphorylated at serine 21 and GSK-3β phosphorylated at serine 9. Anti-phospho-GSK-3β (New England Biolabs) is a rabbit polyclonal antibody reactive with only GSK-3β phosphorylated at serine 9. Anti-GSK-3 (Upstate Biotechnology or Santa Cruz Biotechnology) is a phosphorylation-independent monoclonal antibody reactive with both GSK-3α and β. Anti-phospho-PKB/Akt (New England Biolabs) is a rabbit polyclonal antibody that reacts with PKB/Akt phosphorylated at threonine 308. Anti-PKB/Akt (New England Biolabs) is a phosphorylation-independent antibody against total cellular PKB/Akt. Anti-phospho-Erk antibody (Promega) is a rabbit polyclonal antibody reactive with phosphorylated Erk1 and Erk2. Anti-PKC isotype-specific antibodies were from Santa Cruz. Anti-HA monoclonal antibody was from BAbCO. Immunocomplexes were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia) by using horseradish peroxidase-conjugated secondary antibodies (Bio-Rad).
In vitro kinase assays.
After stimulation, cells were lysed for 30 min on ice in freshly made lysis buffer (20 mM Tris-HCl [pH 7.4], 137 mM NaCl, 1 mM EDTA, 20 mM NaF, 1% Triton X-100, 10% glycerol, 1 mM DTT, 1 mM sodium vanadate, 5 μg of aprotinin/ml, 5 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride, 1 μM microcystin LR). The lysates were clarified by centrifugation for 10 min at 14,000 rpm in a microcentrifuge. For measuring GSK-3β activity in vitro, ca. 100 μg of total cellular protein was diluted in freshly made lysis buffer and immunoprecipitated with 1 μg of anti-GSK-3β antibody (mouse monoclonal anti-rat GSK-3β, Transduction Laboratories, Lexington, Ky.). After 2 h of rotation at 4°C, protein G-Sepharose was added for another 1 h of incubation. Immunoprecipitates were washed twice with lysis buffer, twice with wash buffer (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA, 0.2 mM sodium vanadate, 1 μM microcystin LR), and twice with kinase buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 0.2 mM EGTA). Activity of the immunoprecipitated GSK-3 was assayed in a total volume of 40 μl of kinase buffer containing 3.75 μg of phosphoglycogen synthase peptide 2, 15 μM cold ATP, and 10 μCi of [γ-32P]ATP. After 20 min of incubation at 30°C, reaction mixtures were centrifuged, and 15 μl of the supernatant was spotted onto Whatman P81 phosphocellulose paper. Filters were washed in three changes of 0.75% phosphoric acid, rinsed in acetone, dried, and counted in a liquid scintillation counter. The data were normalized on GSK-3β protein levels determined by immunoblotting analysis of the remaining reaction mixtures.
The assay for Akt protein kinase activity was carried out as previously described (21).
PKC phosphorylation of GSK-3 and other PKC substrates in vitro.
Recombinant human PKC isoforms, purified rabbit GSK-3, histone H1, PKCɛ substrate peptide, and PKC lipid activator were purchased from Upstate Biotechnology. The phosphorylation reaction for the classical isoforms of PKC (α, β, and γ) was performed in 50 μl of kinase buffer (20 mM morpholinepropanesulfonic acid [pH 7.4], 25 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM DTT, 1 mM CaCl2, 15 mM MgCl2, 100 μM ATP, 100 μg of phosphatidylserine/ml, 10 μg of diglycerides/ml, and 0.05 U of purified GSK-3/reaction) in the absence or presence of each of PKC enzyme (40 ng). Reaction with novel and atypical isotypes of PKC (δ, ɛ, ζ, and η) was conducted in the above buffer with CaCl2 omitted. After 15 min at 30°C, the reaction was stopped by adding an equal volume of 2× SDS sample buffer. Phosphorylation of GSK-3 was analyzed by immunoblotting with the GSK-3 phospho-specific antibody. PKC phosphorylation of standard substrates (histone H1 and PKCɛ substrate peptide, 5 μg/reaction) was performed under the same conditions except that 10 μCi of [γ-32P]ATP was included in the reaction. The incorporation of 32P into substrates was quantified as described for the in vitro kinase assay for GSK-3.
RESULTS
LPA induces phosphorylation and inhibition of both isoforms of GSK-3.
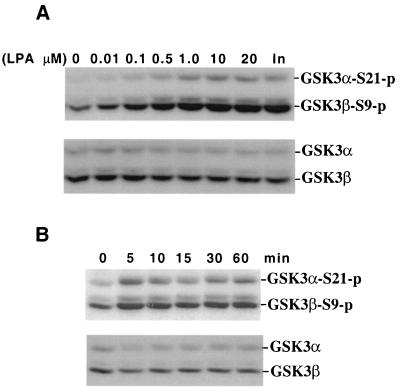
GSK-3 not only functions as a regulator of metabolism but also plays a critical role in signal transduction of many growth factors such as IGF-1, insulin, and neuronal growth factor (10, 21, 48). To assess whether GSK-3 is also regulated by LPA, a prototypic ligand working through G protein-coupled receptors (46), we incubated Swiss 3T3 cells with different concentrations of oleoyl LPA. Phosphorylation of GSK-3 was determined by immunoblotting with a GSK-3 phospho-specific antibody that recognizes GSK-3α phosphorylated at serine 21 and GSK-3β phosphorylated at serine 9. LPA treatment induced dosage- and time-dependent phosphorylation of GSK-3α and GSK-3β at the respective serine residues (Fig. 1). The effect of LPA on GSK-3 was seen at submicromolar concentrations and reached a maximum at 1 to 10 μM LPA. The level of GSK-3 phosphorylation induced by ≥1 μM LPA was comparable to that of 0.1 μg of insulin/ml, a known potent inducer of GSK-3 phosphorylation (10). The induction of GSK-3 phosphorylation by LPA was immediate and sustained. A maximal effect was observed 5 min after addition of LPA. By 15 min, phosphorylation declined but returned to almost peak levels by 1 h. The phosphorylation then gradually decreased (data not shown). Consistent with the inhibitory effect of phosphorylation of the serine residues on GSK-3 activity (10, 56), LPA treatment inhibited GSK-3β kinase activity as determined by in vitro kinase assays (see Fig. 3).
FIG. 1.
LPA induces phosphorylation of GSK-3α at serine 21 (S21) and GSK-3β at serine 9 (S9) in a dosage- and time-dependent manner. Serum-starved Swiss 3T3 cells were stimulated with LPA. After stimulation, cell lysates were prepared and analyzed for GSK-3 phosphorylation by Western blotting with a GSK-3 phospho-specific antibody as detailed in Materials and Methods. Reprobing with a phosphorylation-independent antibody against total GSK-3α and GSK-3β was included to show equal loading among samples. (A) Cells were stimulated for 5 min with indicated concentrations of LPA or with insulin (In, 0.1 μg/ml). (B) Cells were incubated with 10 μM LPA for different periods of time (minutes) as indicated. Experiments were performed at least three times with consistent results.
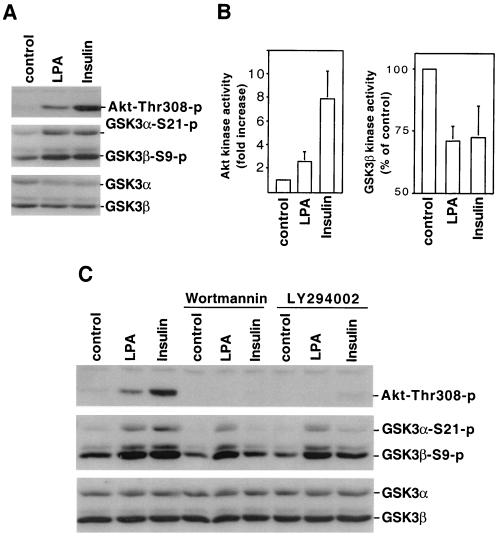
FIG. 3.
LPA-induced GSK-3 phosphorylation does not correlate with activation of the PI3K-PKB/Akt cascade. (A) Serum-starved Swiss 3T3 cells were stimulated with LPA (10 μM, 5 min) or insulin (0.1 μg/ml, 5 min). Cell lysates were prepared and analyzed for phosphorylation of PKB/Akt (Thr-308) and GSK-3 by immunoblotting with PKB/Akt or GSK-3 phospho-specific antibodies. Reprobing with an antibody against total GSK-3α and GSK-3β was performed to show equal loading among samples. Shown is a representative experiment of four independent assays. (B) PKB/Akt and GSK-3β kinase activity in LPA- and insulin-stimulated cells was measured by in vitro kinase assays as described in Materials and Methods. PKB/Akt activity was quantified by densitometry as reported previously (21) and presented as fold increases over unstimulated control cells. GSK-3β activity was presented as percentages of the activity of unstimulated control cells. The results for both PKB/Akt and GSK-3β are means ± the standard deviation of three independent experiments. (C) Swiss 3T3 cells were stimulated with LPA (10 μM) or insulin (0.1 μg/ml) for 5 min in the presence of the PI3K inhibitors, wortmannin (0.1 μM), or LY294002 (10 μM) or vehicle. Wortmannin, LY294002, or vehicle was added to culture 45 to 60 min before stimulation. Cell lysates were analyzed for PKB/Akt and GSK-3 phosphorylation as in panel A.
The effect of LPA on GSK-3 phosphorylation is mediated by specific Edg receptors.
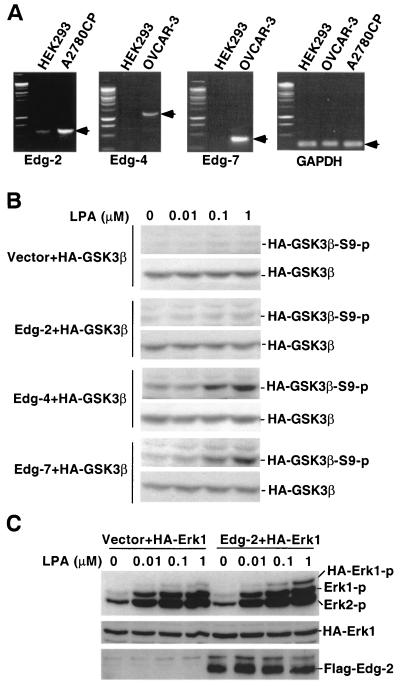
Because the optimum dose and the rapid response suggest that LPA induced GSK-3 phosphorylation through a receptor-mediated process, we evaluated the role of the known LPA receptors, Edg-2, Edg-4, and Edg-7 (24, 26, 59), in the response to LPA. A number of cell lines were screened for the ability of LPA to stimulate GSK-3 phosphorylation. In addition to Swiss 3T3, LPA increased GSK-3 phosphorylation in NIH 3T3, 10T1/2, and Cos-7 cells (data not shown). However, LPA treatment led to only minimal GSK-3 phosphorylation in HEK293 cells (Fig. 2B). In previous studies, HEK293 cells have been shown to exhibit low to absent responses to LPA and have therefore been used to study receptor dependency of certain LPA-induced biological processes (43, 59). To determine the expression profile of LPA receptors in HEK293 cells, we performed RT-PCR analysis of total cellular RNA from HEK293 cells by using cell lines known to express Edg receptors as positive controls. As assessed by RT-PCR, low levels of Edg-2 were present in HEK293 cells. A faint band indicative of a very low level of Edg-7 mRNA was seen in the cell line whereas Edg-4 was not detectable (Fig. 2A).
FIG. 2.
LPA-induced GSK-3 phosphorylation is mediated by Edg-4 or Edg-7 but not Edg-2. (A) Total cellular RNA was isolated from HEK293, A2780CP, and OVCAR-3 cells. Expression of Edg-2, Edg-4, Edg-7, and GAPDH mRNA was analyzed by RT-PCR as described in Materials and Methods. PCR products consistent with expected sizes are indicated by arrows. (B) HEK293 cells were cotransfected with HA-GSK-3β, along with an empty vector or an Edg expression vector. Two days after transfection, cells were starved for 8 h and stimulated with LPA at indicated concentrations for 5 min. Cell lysates were prepared and analyzed for GSK-3β phosphorylation by immunoblotting with a GSK-3β phospho-specific antibody that reacts only with GSK-3β phosphorylated at serine 9. Expression of transfected HA-GSK-3β was verified by immunoblotting with an anti-HA monoclonal antibody. (C) HEK293 cells were cotransfected with HA-Erk1, along with an empty vector or Edg-2 expression vector. Cells were treated as in panel B, and lysates were analyzed for Erk phosphorylation by immunoblotting with an Erk phospho-specific antibody. Expression of Flag-Edg-2 in transfected cells was confirmed by immunoblotting with anti-Flag-M2 antibody. Similar results were obtained from three independent experiments.
We cotransfected HEK293 cells with HA-tagged GSK-3β (HA-GSK-3β), along with a control vector or an Edg-2, Edg-4, or Edg-7 expression vector. After starvation, transfected cells were stimulated with a range of concentrations of LPA (0.01 to 1.0 μM). Phosphorylation of HA-GSK-3β was examined by immunoblotting with an anti-phospho-GSK-3β antibody that is reactive only with GSK-3β phosphorylated at serine 9. As demonstrated in Fig. 2B, LPA did not induce HA-GSK-3β phosphorylation in control vector-transfected cells. Nor did it increase HA-GSK-3β phosphorylation in Edg-2-transfected HEK293 cells. In contrast, transfection with Edg-4 or Edg-7 reconstituted HA-GSK-3β phosphorylation in response to LPA. As shown in Fig. 2C, Edg-2 protein of a predicted molecular mass (∼42 kDa) was expressed in Edg-2-transfected cells. To confirm that Edg-2 was indeed functional in HEK293 cells, we cotransfected the cells with Edg-2 expression vector and an HA-tagged Erk1 (HA-Erk1) expression construct. Phosphorylation of Erk, which is associated with activation of the kinase was analyzed by immunoblotting with an Erk phosphorylation-specific antibody. Expression of Edg-2 dramatically potentiated LPA-induced phosphorylation of cotransfected HA-Erk1 (Fig. 2C). Transfection of Edg-2 sensitized LPA-induced phosphorylation of the endogenous Erk1 and Erk2 to a lesser extent, a finding consistent with expression of Edg-2 only in a fraction of the whole-cell population analyzed. The observation establishes that expression of Edg-4 or Edg-7, but not of Edg-2, in HEK293 cells is sufficient to mediate LPA-induced GSK-3 phosphorylation. The data, however, do not exclude the possibility that HEK293 cells may lack a specific G protein that is required to link Edg-2 to GSK-3 phosphorylation and that Edg-2 could signal to GSK-3 in other cell types.
LPA-induced GSK-3 phosphorylation does not correlate with activation of the PI3K-PKB/Akt cascade.
PI3K-dependent activation of PKB/Akt is an intermediary in the phosphorylation and inactivation of GSK-3 induced by insulin and other growth factors (10, 21, 48). In Swiss 3T3 cells, insulin is a potent activator of PI3K, as reflected by the strong phosphorylation of its downstream target PKB/Akt at threonine 308 (Fig. 3A). Phosphorylation of threonine 308 and serine 473 is associated with PKB/Akt activation, and therefore measuring PKB/Akt phosphorylation status offers a surrogate index for PKB/Akt activation (20, 21). Indeed, insulin stimulated a prominent increase in PKB/Akt activity, as determined by in vitro kinase assays (Fig. 3B). Compared to insulin, LPA is a weaker activator of PKB/Akt, inducing only modest increases in PKB/Akt phosphorylation and activity (Fig. 3A and B). However, the effects of LPA on GSK-3 phosphorylation and activity were comparable to those of insulin (Fig. 3B), suggesting that PKB/Akt may not be entirely responsible for LPA-triggered GSK-3 phosphorylation and inhibition.
To assess the role of the PI3K-PKB/Akt pathway in LPA-induced GSK-3 phosphorylation, Swiss 3T3 cells were treated with the PI3K specific inhibitors, LY294002 or wortmannin prior to stimulation with LPA or insulin. GSK-3 phosphorylation induced by insulin was essentially eliminated by pretreatment with LY294002 or wortmannin, demonstrating the efficacy of the inhibitors. The effect of LPA on GSK-3 phosphorylation was, however, only minimally reduced by these PI3K inhibitors (Fig. 3C). As expected, wortmannin and LY294002 blocked both insulin- and LPA-induced PKB/Akt activation, as reflected by loss of PKB/Akt phosphorylation at threonine 308 in response to either agent (Fig. 3C). The differential effects of PI3K inhibitors on insulin and LPA signaling suggest that LPA induces GSK-3 phosphorylation primarily through a PI3K-PKB/Akt-independent route.
LPA-induced GSK-3 phosphorylation is sensitive to PKC inhibitors.
In addition to the PI3K-PKB/Akt module, we and others have demonstrated that cAMP-dependent PKA can directly mediate GSK-3 phosphorylation and inactivation (21, 44). LPA, however, has been previously shown to downregulate intracellular cAMP levels in multiple cell lines, including 3T3 cells (62, 63). Consistent with this, the PKA inhibitor H89 (22) did not alter LPA-induced GSK-3 phosphorylation despite its ability to block GSK-3 phosphorylation induced by the cAMP analog (8-bromo-cAMP) (data not shown).
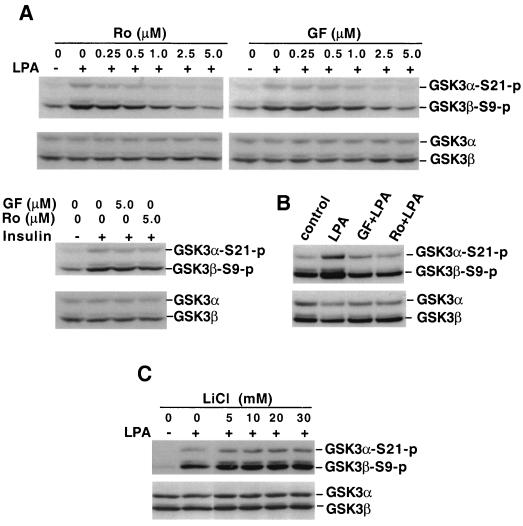
Since PKA, PKB/Akt, and PKC are structurally related protein kinases, exhibiting a high homology especially within their catalytic domains (2, 7, 39), it is conceivable that some isotypes of PKC may also function as GSK-3 kinases or may function as a component of a signaling network leading to GSK-3 phosphorylation. Indeed, it has been reported that Wnt proteins induce inactivation of GSK-3 through a PKC-dependent mechanism (6, 9). Thus, we assessed the possibility that one or more PKC isoforms might be involved in LPA signaling to GSK-3 by using the specific PKC inhibitors Ro31-8220 and GF109203X, which are effective against both phorbol ester-sensitive and -insensitive PKC isoforms (14, 15). Pretreatment of Swiss 3T3 cells with either of these inhibitors decreased LPA-induced GSK-3 phosphorylation in a dosage-dependent manner (Fig. 4A). At 2.5 μM, both inhibitors achieved nearly complete blockade of LPA-induced GSK-3 phosphorylation, with Ro31-8220 demonstrating a higher efficiency, a finding consistent with their relative potency against PKC (14, 15). Even 5 μM Ro31-8220 or GF109203X, however, had a limited effect on insulin-stimulated GSK-3 phosphorylation (Fig. 4A), indicating that these compounds do not directly affect GSK-3 phosphorylation or cause nonspecific inhibition of cellular signaling. The delayed peak of GSK-3 phosphorylation 1 h after LPA treatment (Fig. 1) was also sensitive to Ro31-8220 and GF109203X (Fig. 4B). Since Erk has been suggested to participate in GSK-3 inactivation in response to epidermal growth factor (EGF) (54), we sought to determine whether Ro31-8220 or GF109203X would inhibit LPA-induced GSK-3 phosphorylation by compromising LPA-stimulated Erk activation. By an undefined mechanism, however, treatment of Swiss 3T3 cells with Ro31-8220 or GF109203X potentiated LPA-stimulated Erk activation. The MEK-1 inhibitor PD98059 had little effect on LPA-induced GSK-3 phosphorylation (X. Fang et al., unpublished data), excluding the possibility of involvement of Erk in LPA-mediated GSK-3 phosphorylation. Hers et al. recently reported that Ro31-8220 and GF109203X directly inhibit GSK-3 activity (33). It is thus possible that in intact cells, these drugs may act to reduce auto- or transphosphorylation of GSK-3 rather than achieving their effects via targeting PKC enzymes. To address this possibility, we tested the effect of the GSK-3 inhibitor lithium (33) on LPA-mediated GSK-3 phosphorylation. As demonstrated in Fig. 4C, lithium (up to 30 mM) did not alter LPA signaling to GSK-3 phosphorylation, suggesting that inhibition of GSK-3 activity itself does not block LPA-induced GSK-3 phosphorylation.
FIG. 4.
LPA-induced GSK-3 phosphorylation is sensitive to PKC inhibitors. (A) Serum-starved Swiss 3T3 cells were stimulated with LPA (10 μM, 5 min) in the absence or presence of indicated concentrations of the PKC inhibitor Ro31-8220 (Ro) or GF109203X (GF). In the bottom panel, Swiss 3T3 cells were stimulated with insulin (0.1 μg/ml, 5 min) without or with Ro31-8220 (Ro, 5 μM) or GF109203X (GF, 5 μM). Ro31-8220 or GF109203X was added to culture where indicated 45 to 60 min before stimulation. Cell lysates were prepared and analyzed for GSK-3α and GSK-3β phosphorylation by immunoblotting with a GSK-3 phospho-specific antibody as described in Fig. 1. Shown are representative experiments of three independent assays. (B) Swiss 3T3 cells were stimulated with LPA (10 μM) for 1 h with or without GF109203 (GF, 2.5 μM) or Ro31-8220 (2.0 μM). Cell lysates were analyzed for GSK-3 phosphorylation as in panel A. (C) Swiss 3T3 cells were stimulated with LPA (10 μM, 5 min) in the presence of lithium chloride (LiCl) at the indicated concentrations. LiCl was added to medium 75 min before LPA stimulation. Cell lysates were prepared and analyzed as in panel A.
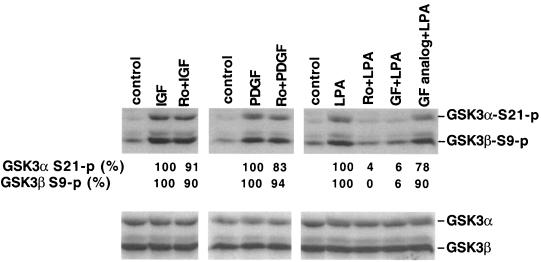
To further assess this distinct mechanism of GSK-3 regulation by LPA, we compared the effects of PKC inhibitors on GSK-3 phosphorylation induced by growth factors which have been shown to link to GSK-3 through the PI3K-PKB/Akt cascade. Similar to insulin, IGF-1 and PDGF induction of GSK-3 phosphorylation was largely resistant to Ro31-8220, with consistently less than 20% inhibition of ligand-induced GSK-3 phosphorylation in Swiss 3T3 cells (Fig. 5). EGF-induced GSK-3 phosphorylation was also resistant to PKC inhibitors (data not shown). Similar results were obtained in HEK293 cells stimulated with IGF-1, insulin or EGF, or when another PKC inhibitor, GF109203X, was used. Compared to insulin, IGF-1, PDGF, or EGF, LPA is unique since its induction of GSK-3 phosphorylation is highly sensitive (>80% inhibition) to Ro31-8220 or GF109203X (Fig. 5), suggesting a predominant role for a PKC-dependent pathway in LPA-mediated GSK-3 regulation. In an effort to determine whether GSK-3 phosphorylation induced by peptide growth factors is in part due to the PKC pathway, we tested the effect of PKC inhibitors when Swiss 3T3 cells were stimulated with lower, more physiologic concentrations of PDGF. At the PDGF concentrations examined (from 50 to 0.4 ng/ml), we did not observe an apparent reduction in GSK-3 phosphorylation by Ro31-8220 or GF109203X (data not shown). However, as we reported recently, inhibition of PKC is accompanied by enhanced activation of the PI3K-PKB/Akt pathway (45), which may compensate for the loss of the potential contribution of PKC enzymes in the regulation of GSK-3.
FIG. 5.
PKC inhibitors do not block GSK-3 phosphorylation induced by peptide growth factors. Serum-starved Swiss 3T3 cells were stimulated for 10 min with IGF-1 (50 ng/ml), PDGF (50 ng/ml), or LPA (10 μM) in the presence of Ro31-8220 (Ro, 2.25 μM), GF109203X (GF, 2.0 μM), the GF109203X inactive analog bisindolylmaleimide V (GF analog, 2.0 μM), or vehicle. These compounds were added to culture where indicated 45 to 60 min before stimulation with IGF-1, PDGF, or LPA. Cell lysates were prepared and analyzed for GSK-3α and GSK-3β phosphorylation by immunoblotting as described in Fig. 1. The bands representing phosphorylated α or GSK-3β were quantified by densitometry. The numbers represent the percent relative intensities, with the values of unstimulated cells (control) defined as background and the values of cells stimulated in the absence of PKC inhibitors minus background defined as 100%. Similar results were obtained from three independent experiments.
TPA stimulates GSK-3 phosphorylation and downregulation of intracellular PKC activity inhibits the effect of LPA on GSK-3.
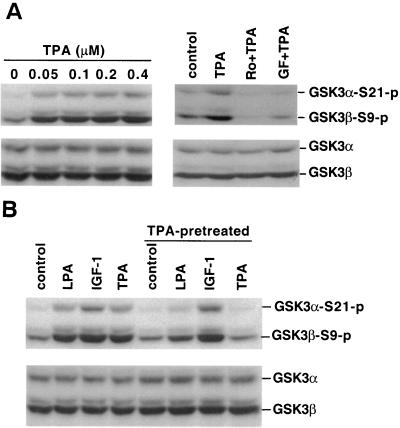
Acute treatment with the PKC agonist TPA stimulated phosphorylation of both isoforms of GSK-3 in Swiss 3T3 (Fig. 6A) and other cell lines, including HEK293 (data not shown), providing further support for the involvement of PKC in the regulation of GSK-3. This effect of TPA was completely abolished by the PKC inhibitors Ro31-8220 and GF109203X, suggesting that mammalian cells contain a signaling pathway in which activation of TPA-sensitive isoform(s) of PKC can be found upstream of GSK-3.
FIG. 6.
TPA induces GSK-3 phosphorylation, and chronic treatment with TPA compromises LPA-mediated GSK-3 phosphorylation. (A) Serum-starved Swiss 3T3 cells were stimulated with TPA for 10 min at the indicated concentrations. In the right panel, cells were stimulated with TPA (0.1 μM, 10 min) in the presence of Ro31-8220 (Ro, 4.5 μM), GF109203X (GF, 2 μM), or vehicle. Ro31-8220, GF109203X, or vehicle was added to culture 45 to 60 min before stimulation with TPA. (B) Swiss 3T3 cells were preincubated with 0.2 μM TPA or vehicle in serum-free medium for more than 24 h before stimulation with LPA (10 μM, 5 min), IGF-1 (50 ng/ml, 5 min), or TPA (0.1 μM, 10 min). For both panels A and B, cell lysates were prepared and analyzed for GSK-3α and GSK-3β phosphorylation by immunoblotting as in Fig. 1. Similar results were obtained in three independent experiments.
Prolonged treatment of Swiss 3T3 cells with TPA for more than 24 h to deplete endogenous PKC activity (1) severely compromised induction of GSK-3 phosphorylation by LPA. In contrast, the response to IGF-1 or insulin (data not shown) was unaffected by chronic TPA treatment (Fig. 6B). Although TPA-sensitive PKCs clearly contribute to LPA-induced GSK-3 phosphorylation, the incomplete inhibition of LPA's effect by prolonged treatment with TPA suggests the involvement of TPA-insensitive PKCs or other kinases.
PKCs phosphorylate GSK-3 at regulatory serine residues in vitro and in vivo.
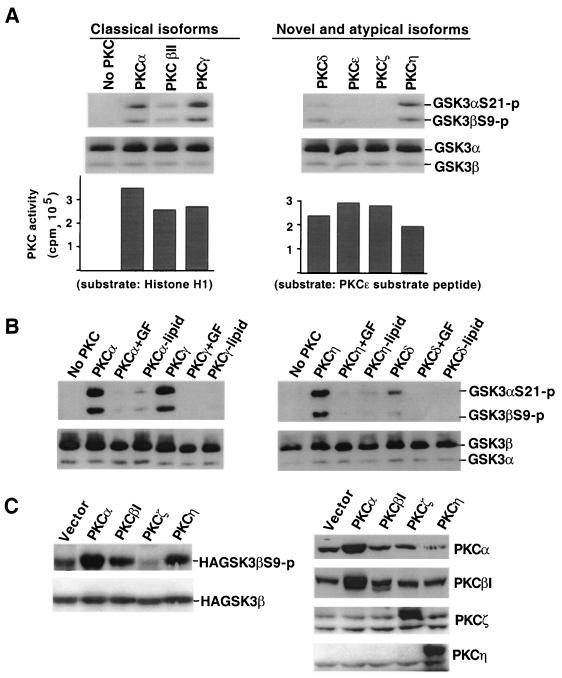
To determine whether PKC itself could directly phosphorylate GSK-3, an in vitro kinase assay was performed by using recombinant human PKC isoforms and purified GSK-3. Phosphorylation of GSK-3 was detected by Western blotting with the GSK-3 phospho-specific antibody (Fig. 7). In the presence of ATP, several isoforms (α, βΙΙ, γ, η, and δ) of PKC phosphorylated both GSK-3α at Ser 21 and GSK-3β at Ser 9 (Fig. 7A). Among these isotypes, PKCβII and PKCδ were relatively weak compared to their activities toward other standard PKC substrates (Fig. 7A). PKCɛ and PKCζ did not phosphorylate GSK-3. There was no detectable phosphorylation of GSK-3 in the absence of PKC enzymes. The ability of PKCs to phosphorylate GSK-3 was further confirmed by experiments showing that in vitro GSK-3 phosphorylation by PKC enzymes (α, γ, and η) was sensitive to the PKC inhibitor GF109203X and was dependent on the presence of PKC lipid activators (Fig. 7B). These data suggest that some isoforms of PKC can phosphorylate the RTSSF sequence in GSK-3α and the RTTSF motif in GSK-3β (10). These sites, originally identified as the PKB/Akt consensus sequence (10), can also be phosphorylated by cAMP-dependent PKA in vivo and in vitro (21, 44), indicating plasticity for phosphorylation of these sites in GSK-3 by structurally related protein kinases.
FIG. 7.
Specific PKC isotypes can phosphorylate GSK-3α at serine 21 and GSK-3β at serine 9 in vitro and in vivo. (A) Different isoforms of PKC were incubated with purified GSK-3 in an in vitro kinase assay as described in Materials and Methods. Reaction mixes were diluted in SDS sample buffer and analyzed for phosphorylation of GSK-3α at serine 21 and of GSK-3β at serine 9 by immunoblotting with a GSK-3 phospho-specific antibody as described in Fig. 1. Reprobing with a phosphorylation-independent antibody against total GSK-3α and GSK-3β (substrates) was included to show equal loading among samples. The bottom panel shows activity of the PKC isotypes toward standard PKC substrates (histone H1 for classical forms and PKCɛ substrate peptide for novel and atypical forms). (B) PKCα, PKCγ, PKCη, and PKCδ were incubated with GSK-3 in the presence of the PKC inhibitor GF109203X (+GF, 0.15 μM for PKCα and PKCγ, 0.6 μM for PKCη and PKCδ) or without lipid activators (−lipid) as indicated. The reaction mixes were analyzed as in panel A. (C) HEK293 cells were cotransfected with HA-GSK-3β, along with an empty vector or a constitutively active PKC construct as indicated. Two days after transfection, cells were starved for 12 h, and lysates were prepared and analyzed for HA-GSK-3β phosphorylation levels with a GSK-3β phospho-specific antibody. Expression of transfected HA-GSK-3β was detected by immunoblotting with an anti-HA monoclonal antibody. Expression of PKC proteins was confirmed by immunoblotting with PKC isotype-specific antibodies. The anti-PKCβ1 antibody cross-reacts with PKCα, whereas the other anti-PKC antibodies did not exhibit cross-reactivity. Each of these experiments was performed at least twice with consistent results.
We next assessed the effect of expression of constitutively active forms of PKC isotypes on GSK-3 phosphorylation in vivo. HEK293 cells were cotransfected with HA-GSK-3β, along with an empty vector or a dominant active PKC isoform (α, β1, ζ, η, and ɛ). As shown in Fig. 7C, expression of constitutively active PKCα, PKCβ1, and PKCη enhanced phosphorylation of cotransfected GSK-3β, with the strongest activity seen with PKCα transfection. Consistent with the results in the in vitro kinase assay (Fig. 7A), transfection with dominant active PKCζ did not lead to an increase in GSK-3β phosphorylation (Fig. 7C). Nor did constitutively active PKCɛ induce GSK-3β phosphorylation (data not shown). Expression of the PKC isoforms in transfected HEK293 cells was verified by immunoblotting with PKC isotype-specific antibodies (Fig. 7C). These in vitro and in vivo experiments provide strong evidence that PKCs may function in vivo as GSK-3 kinases or upstream mediators of GSK-3 regulation,
The PLCγ-PKC pathway is sufficient for PDGF-induced GSK-3 phosphorylation.
GSK-3 phosphorylation induced by the peptide growth factors examined was largely resistant to PKC inhibitors in contrast to the effect of LPA, suggesting that PKC has no or limited input to GSK-3 regulation by insulin, IGF-1, PDGF, or EGF. These factors, however, are all activators of PKC (58), and we demonstrated that activation of PKC with TPA is sufficient to induce GSK-3 phosphorylation (Fig. 6). One possibility for this discrepancy is that the PKC-mediated pathway leading to GSK-3 phosphorylation is dormant or compromised when the PI3K-PKB/Akt pathway is most strongly activated by stimuli such as insulin, IGF-1, and PDGF.
The human PDGF receptor, particularly PDGFRβ, has been well characterized with respect to its associating proteins (60, 61). For instance, tyrosines 740 (Y-740) and 751 (Y-751) are necessary for stable association and activation of PI3K, Y-771 allows binding of GAP, Y-1021 is essential for the binding and activation of PLCγ, and Y-1009 is required for interacting with the phosphotyrosine phosphatase Syp (41, 60, 61). Therefore, mutations at these tyrosine residues can be used to investigate the role of these signaling molecules in PDGF signal transduction (60, 61). We thus assessed whether PDGF is capable of inducing GSK-3 phosphorylation through a mutant receptor (Y-1021) that retains normal association with PLCγ but lacks signaling interactions with PI3K and other known functional pathways (Y-740/Y-751, Y-771, and Y-1009 were all converted to phenylalanine) (61).
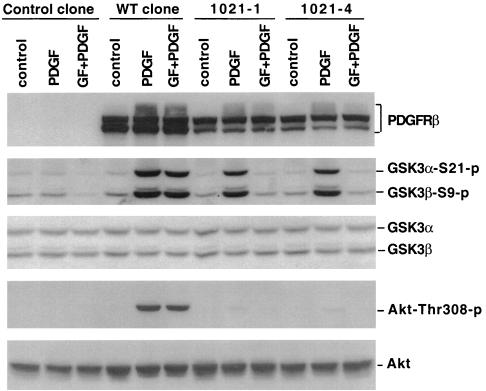
HEK293 cells do not express PDGF receptors and lack responses to PDGF as described previously (20). We therefore established stable HEK293 clones that expressed WT and the mutant PDGFRβ (Y-1021). As expected, expression of the WT PDGFRβ restored the response to PDGF in HEK293. As demonstrated in Fig. 8, PDGF induced a strong phosphorylation of GSK-3 in a WT clone but not in a clone derived from control-vector DNA-transfected cells. Similar to the observation in Swiss 3T3 cells, the response to PDGF in the WT clone is largely resistant to the PKC inhibitor GF109203X (Fig. 8) or Ro31-8220 (data not shown).
FIG. 8.
The PLCγ pathway is sufficient for PDGF-induced GSK-3 phosphorylation. HEK293 cells were transfected with an empty backbone vector, WT PDGFRβ, or mutant PDGFRβ (Y-1021). Stable clones carrying empty vector (control clone) or expressing WT PDGFRβ (WT clone) or mutant PDGFRβ (Y-1021-1 and Y-1021-4) were established. After starvation in serum-free medium for more than 12 h, these clones were stimulated with PDGF B/B (75 ng/ml, 10 min) in the presence of GF109203X (GF, 2.5 μM) or vehicle. Cells were preincubated with GF109203X or vehicle for 45 to 60 min before stimulation with PDGF B/B. Cell lysates were analyzed for phosphorylation of GSK-3 and PKB/Akt (threonine 308) by immunoblotting as described in Materials and Methods. Reprobing with phosphorylation-independent antibodies against total GSK-3α and GSK-3β or total PKB/Akt was included to show equal loading among samples. The expression of PDGFRβ in WT and Y-1021 clones, but not in the control clone, was verified by immunoblotting with an anti-PDGFRβ antibody. Similar results were obtained in three independent experiments
A number of Y-1021 clones were established and confirmed by Western blotting to express the mutant PDGFRβ that retains the ability to couple to PLCγ with the ability to couple to other signaling molecules including PI3K abolished (61). Strikingly, PDGF induced GSK-3 phosphorylation in these Y-1021 clones (Y-1021-1 and Y-1021-4) at levels similar to that seen in the WT clone (Fig. 8). Consistent with an essential role for Y-740/Y-751 in PI3K-PKB/Akt activation (23, 61), PDGF failed to stimulate PKB/Akt phosphorylation in Y-1021-1 and Y-1021-4 clones. Furthermore, in contrast to the observation in the WT clone, PDGF-induced GSK-3 phosphorylation in Y-1021-1 and Y-1021-4 clones was sensitive to the PKC inhibitor GF109203X (Fig. 8) or Ro31-8220 (data not shown), indicating a selective requirement of PKC activity for PDGF signal to GSK-3 in these mutant Y-1021 clones.
DISCUSSION
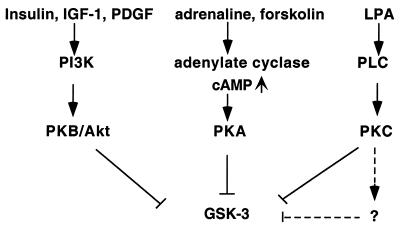
The results in the present study demonstrate that the lysophospholipid LPA induces phosphorylation and inactivation of the multifunctional serine/threonine kinase GSK-3 through specific Edg receptors and an intracellular signaling pathway involving PKC. This mode of GSK-3 regulation is unique to LPA compared to insulin, IGF-1, PGDF, and EGF, which do not require PKC activity for induction of GSK-3 phosphorylation in Swiss 3T3 cells. Consistent with previous studies of insulin, IGF-1, and PDGF (10, 11, 21), our data confirm that these peptide growth factors primarily regulate GSK-3 via the PI3K pathway. In addition, changes in intracellular cAMP levels also regulate GSK-3 activity as GSK-3 can be phosphorylated and inhibited by cAMP-dependent PKA (21, 44). Therefore, as indicated in Fig. 9, GSK-3 represents a point of convergence integrating information from multiple signaling pathways and cell surface receptors. Analogous to GSK-3, many other functionally important proteins, such as Raf and BAD, also lie downstream of more than one signaling cascade (3, 4, 13, 23, 52). The multiple levels of cross talk among signaling pathways may provide plasticity in the modulation of functions of biologically critical molecules.
FIG. 9.
Convergence of multiple intracellular signaling pathways at GSK-3.
LPA signals through the Edg subfamily members (Edg-2, Edg-4, and Edg-7) that can couple to multiple G proteins to evoke a great variety of responses (46). Although it is currently unknown why multiple LPA receptors exist, it has been suggested that each receptor might be functionally linked to only a subset of cellular responses to LPA (8, 19, 38). For instance, Edg-2 is much more effective than Edg-4 and Edg-7 in inhibiting adenylate cyclase (8, 38). In addition, while Edg-2 and Edg-4 mediate acute neurite retraction, Edg-7 does not (8, 38). In order to elucidate the receptor specificity for LPA-induced GSK-3 phosphorylation, we compared the effects of Edg-2, Edg-4, and Edg-7 receptors in transient-transfection experiments. Our results clearly establish that Edg-4 and Edg-7, but not Edg-2, can mediate LPA-induced GSK-3 phosphorylation. This conclusion, however, does not exclude the possibility for participation of other untested LPA receptors, such as PSP24, in the process. Because of the essential role of PKC activity in the regulation of GSK-3 by LPA, it appears that LPA binding to Edg-4 or Edg-7 results in activation of PLCβ and the subsequent generation of diacylglycerol and inositol trisphosphate. These second messengers will, in turn, activate classical and novel PKC isoforms, contributing to GSK-3 phosphorylation.
Our in vitro and in vivo phosphorylation experiments indicate that several types of PKC, namely, PKCα, PKCβ, PKCγ, PKCη, and PKCδ, are able to phosphorylate both isoforms of GSK-3 at regulatory serine residues. In contrast, PKCɛ and PKCζ fail to phosphorylate these sites of GSK-3. Our results were largely consistent with a previous report by Goode et al. (29), who assessed the effects of individual PKC isoforms on GSK-3 kinase activity in vitro. However, differential effects on GSK-3α and GSK-3β isoforms of PKC enzymes were noted in that report (29). Instead of assessing site-specific phosphorylation of GSK-3, Goode et al. examined the general phosphorylation of GSK-3 by PKC with [γ-32P]ATP. Auto- or transphosphorylation of GSK-3α at other sites could cause heavy background, obscuring the effect of PKC enzymes on site-specific phosphorylation. Most recent studies, including the present one, have shown coordinate or similar regulation of GSK-3α and GSK-3β in both in vivo and in vitro circumstances, although their biological functions might be quite diverse (10, 21). In spite of the ability of certain PKC enzymes to phosphorylate GSK-3 in vitro and in vivo, we have been unable to detect a direct association between endogenous GSK-3 and PKC isotypes in Swiss 3T3 cells by coimmunoprecipitation (data not shown). This implies that the associations are not strong enough to detect by coimmunoprecipitation or that another kinase acting downstream of PKC directly phosphorylates GSK-3, as illustrated in Fig. 9.
To determine whether activation of PKC is sufficient to induce GSK-3 phosphorylation, we expressed WT and mutant PGDFRβ in HEK293 cells that are naturally refractive to PDGF. In these transfectants, PDGF not only induces GSK-3 phosphorylation through the WT PDGFRβ receptor but also does so through a mutant receptor that retains normal association with PLCγ but lacks the ability to couple to other signaling molecules, including PI3K (61). Induction of GSK-3 phosphorylation through such mutant receptors requires PKC activity, differing from the mechanism through which the WT receptor influences GSK-3. These data indicate that growth factors, such as PDGF, which regulate GSK-3 activity mainly through the PI3K-PKB/Akt module (5, 10, 11, 21, 57) can utilize a PKC-mediated pathway for GSK-3 phosphorylation. This potential route to regulate GSK-3 is redundant and suppressed under normal conditions where interactions with other pathways, including that of PI3K, are present. This prioritization of signaling pathways is analogous to the regulation of growth factor-induced Erk activation (4, 52). Although the Grb2/SOS/Ras/Raf pathway and the PLCγ-PKC-Raf cascade can interact to regulate Erk, the former pathway is fully capable of stimulating the Erk response to a growth factor, such as EGF, when endogenous PKC activity is blocked by prolonged treatment with TPA or by specific PKC inhibitors (4, 52; Fang, unpublished). However, activation of PKC alone, for example, by TPA or other PKC lipid activators or by expression of dominant active PKC isoforms, is sufficient to evoke Erk activation, a phenomenon compatible with PKC isotypes serving as redundant activators of Raf (4, 52).
In our experiments, PDGF-stimulated GSK-3 phosphorylation becomes PKC dependent only when activation of the PI3K and other signaling pathways is impaired. This switch to a PKC-mediated pathway was not reproduced when PI3K activity was pharmacologically inhibited by wortmannin or LY294002 (data not shown). This suggests that the uncoupling of the PKC-mediated pathway to GSK-3 phosphorylation in cells expressing the WT PDGFRβ is not due to PI3K or PKB/Akt enzyme activity. The selection therefore appears to occur at the receptor level or is conferred by activation of GAP and/or Syp, which are also disrupted in the Y-1021 mutant (61). Alternatively, in the absence of interactions with PI3K, GAP, and/or Syp, the PDGF receptor may induce a greater magnitude and duration of PLCγ activation that is transmitted through PKC into GSK-3 phosphorylation. Whatever the mechanism, our results are consistent with the concept that activation of PKC can induce GSK-3 phosphorylation. LPA and potentially other natural ligands, which are not potent activators of PI3K, may primarily use a PKC pathway to modulate GSK-3 activity.
The involvement of PKC activity in LPA-mediated GSK-3 phosphorylation is reminiscent of Wnt-regulated GSK-3β inactivation (6, 9). GSK-3β is a critical component of the Wnt signaling pathway. Wnt proteins induce downregulation of GSK-3β activity via a PKC-dependent mechanism (6, 9). However, a recent study indicates that Wnt causes GSK-3β inhibition independently of serine-9 phosphorylation (16). There are other regulatory mechanisms, such as phosphorylation of tyrosine residues, for the control of GSK-3β activity (47, 64). Wnt signaling couples to the cytosolic and nuclear accumulation of β-catenin and the subsequent transactivation of β-catenin target genes associated with GSK-3β inhibition (6). These effects, however, are not observed when GSK-3β activity is inhibited through N-terminal phosphorylation of serine 9, for example, by insulin (16). Similar to insulin, LPA-induction of GSK-3β inactivation is not accompanied by an apparent translocation of β-catenin from the membrane to cytosol (data not shown). Thus, inhibition of GSK-3β alone is insufficient to induce β-catenin accumulation and translocation. The fact that inhibition of GSK-3 can be mediated by several distinct pathways, leading to at least two distinct consequences, underscores the importance of this enzyme in coordinating responses of cells to their environment.
Acknowledgments
The work was supported by the National Institutes of Health grants CA82716 and CA64602 to G.B.M. and by Atairgin Technologies Research grant LS99-225RG and Lynne Cohen Foundation Ovarian Cancer Research grant 80095031 to X.F.
We are grateful to A. Kazlauskas (Harvard Medical School) for providing PDGFRβ expression vectors; W. H. Moolenaar and G. C. Zondag (The Netherlands Cancer Institute) for the Flag-Edg-2 expression vector; E. J. Goetzl and S. An (UCSF) for the Edg-4 expression vector; J. Aoki (The University of Tokyo) for the Flag-Edg-7 expression vector; P. Parker ((Imperial Cancer Research Fund) for constitutively active PKCα, PKCβ1, PKCζ, and PKCη constructs; and M. Cobb (University of Texas Southwestern Medical Center) for the HA-Erk1 expression vector.
REFERENCES
- 1.Adams, J. C., and W. J. Gullick. 1989. Differences in phorbol-ester-induced downregulation of protein kinase C between cell lines. Biochem. J. 257:905-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:271-274. [DOI] [PubMed] [Google Scholar]
- 3.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and independent mechanisms. Science 286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 4.Cai, H., U. Smola, V. Wixler, I. Eisenmann-Tappe, M. T. Diaz-Meco, J. Moscat, U. Rapp, and G. M. Cooper. 1997. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol. Cell. Biol. 17:732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, E. Y., N. M. Mazure, J. A. Cooper, and A. J. Giaccia. 2001. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 61:2429-2433. [PubMed] [Google Scholar]
- 6.Chen, R. H., W. V. Ding, and F. McCormick. 2000. Wnt signaling to β-catenin involves two interactive components. J. Biol. Chem. 275:17894-17899. [DOI] [PubMed] [Google Scholar]
- 7.Coffer, P. J., and J. R. Woodgett. 1991. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201:475-481. [DOI] [PubMed] [Google Scholar]
- 8.Contos, J. J., I. Ishii, and J. Chun. 2000. Lysophosphatidic acid receptors. Mol. Pharmacol. 58:1188-1196. [DOI] [PubMed] [Google Scholar]
- 9.Cook, D., M. J. Fry, K. Hughes, R. Sumathipala, J. R. Woodgett, and T. C. Dale. 1996. Wingless inactivates glycogen synthase kinase-3 via an intracellular signaling pathway which involves a protein kinase C. EMBO J. 15:4526-4536. [PMC free article] [PubMed] [Google Scholar]
- 10.Cross, D. A. E., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-788. [DOI] [PubMed] [Google Scholar]
- 11.Cross, D. A. E., D. R. Alessi, J. R. Vandenheede, H. E. McDowell, H. S. Hundal, and P. Cohen. 1994. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem. J. 303:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, H., Y. Meng, and R. F. Bulleit. 1998. Inhibition of glycogen synthase kinase 3beta activity regulates proliferation of cultured cerebellar granule cells. Brain Res. Dev. Brain Res. 111:177-188. [DOI] [PubMed] [Google Scholar]
- 13.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 14.Davis, P. D., L. H. Elliott, W. Harris, C. H. Hill, S. A. Hurst, E. Keech, M. K. Kuma, G. Lawton, J. S. Nixon, and S. E. Wilkinson. 1992. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity J. Med. Chem. 35:994-1001. [DOI] [PubMed] [Google Scholar]
- 15.Davis, P. D., C. H. Hill, E. Keech, G. Lawton, J. S. Nixon, A. D. Sedgwick, J. Wadsworth, D. Westmacott, and S. E. Wilkinson. 1989. Potent selective inhibitors of protein kinase C. FEBS Lett. 259:61-63. [DOI] [PubMed] [Google Scholar]
- 16.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez, I., K. Itoh, and S. Y. Sokol. 1995. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc. Natl. Acad. Sci. USA 92:8498-8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichholtz, T., K. Jalink, I. Fahrenfort, and W. H. Moolenaar. 1993. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem. J. 291:677-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang, X., D. Gaudette, T. Furui, M. Mao, V. Estrella, A. Eder, T. Pustilnik, T. Sasagawa, R. Lapushin, S. Yu, R. B. Jaffe, J. R. Wiener, J. R. Erickson, and G. B. Mills. 2000. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann. N. Y. Acad. Sci. 905:188-208. [DOI] [PubMed] [Google Scholar]
- 20.Fang, X., S. Yu, A. Eder, M. Mao, R. C. Bast, Jr., D. Boyd, and G. B. Mills. 1999. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18:6635-6640. [DOI] [PubMed] [Google Scholar]
- 21.Fang, X., S. X. Yu, Y. Lu, R. C. Bast, Jr., J. R. Woodgett, and G. B. Mills. 2000. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. USA 97:11960-11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Findik, D., Q. Song, H. Hidaka, and M. Lavin. 1995. Protein kinase A inhibitors enhance radiation-induced apoptosis. J. Cell. Biochem. 57:12-21. [DOI] [PubMed] [Google Scholar]
- 23.Franke, T. F., S. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima, N., and J. Chun. 2001. The LPA receptors. Prostaglandins 64:21-32. [DOI] [PubMed] [Google Scholar]
- 25.Gaits, F., O. Fourcade, F. Le Balle, G. Gueguen, B. Gaige, A. Gassama-Diagne, J. Fauvel, J. P. Salles, G. Mauco, M. F. Siman, and H. Chap. 1997. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 410:54-58. [DOI] [PubMed] [Google Scholar]
- 26.Goetzl, E. J., and S. An. 1999. A subfamily of G protein-coupled cellular receptors for lysophospholipids and lysosphingolipids. Adv. Exp. Med. Biol. 469:259-264. [DOI] [PubMed] [Google Scholar]
- 27.Goetzl, E. J., and K. Lynch. 2000. Preface: the omnific lysophospholipid growth factors. Ann. N. Y. Acad. Sci. 905:xi-xiv. [DOI] [PubMed]
- 28.Goetzl, E. J., Y. Kong, and B. Mei. 1999. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J. Immunol. 162:2049-2056. [PubMed] [Google Scholar]
- 29.Goode, N., K. Hughes, J. R. Woodgett, and P. J. Parker. 1992. Differential regulation of glycogen synthase kinase 3β by protein kinase C isotypes. J. Biol. Chem. 267:16878-16882. [PubMed] [Google Scholar]
- 30.Guo, Z., K. Liliom, K., D. J. Fischer, I. C. Bathurst, L. D. Tomei, M. C. Kiefer, and G. Tigyi. 1996. Molecular cloning of a high-affinity receptor for the growth factor-like lipid mediator lysophosphatidic acid from Xenopus oocytes. Proc. Natl. Acad. Sci. USA 93:14367-14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harwood, A. J., S. E. Plyte, J. R. Woodgett, H. Strutt, and R. R. Kay. 1995. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell 80:139-148. [DOI] [PubMed] [Google Scholar]
- 32.He, X., J. P. Saint-Jeannet, J. R. Woodgett, H. E. Varmus, and I. B. Dawid. 1995. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374:617-622. [DOI] [PubMed] [Google Scholar]
- 33.Hers, I., J. M. Tavare, and R. M. Denton. 1999. The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro31-8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett. 460:433-436. [DOI] [PubMed] [Google Scholar]
- 34.Hla, T. 2001. Sphingosine 1-phosphate receptors. Prostaglandins 64:135-142. [DOI] [PubMed] [Google Scholar]
- 35.Hooks, S. B., W. L. Santos, D. S. Im, C. E. Heise, T. L. Macdonald, and K. R. Lynch. 2000. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 276:4611-4621. [DOI] [PubMed] [Google Scholar]
- 36.Howe, L. R., and C. J. Marshall. 1993. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J. Biol. Chem. 268:20717-20720. [PubMed] [Google Scholar]
- 37.Hughes, K., E. Nikolakaki, S. E. Plyte, N. F. Totty, and J. R. Woodgett. 1993. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 12:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii, I., J. J. Contos, N. Fukushima, and J. Chun. 2000. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 58:895-902. [DOI] [PubMed] [Google Scholar]
- 39.Jones, P. F., T. Jakubowicz, F. J. Pitossi, F. Maurer, and B. A. Hemmings. 1991. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasawa, Y., K. Kume, T. Izumi, and T. Shimizu. 2000. Mammalian PSP24s (alpha and beta isoforms) are not responsive to lysophosphatidic acid in mammalian expression systems. Biochem. Biophys. Res. Commun. 276:957-964. [DOI] [PubMed] [Google Scholar]
- 41.Kazlauskas, A., G. S. Feng, T. Pawson, M. Valius. 1993. The 64-kDa protein that associates with the platelet-derived growth factor receptor beta subunit via Tyr-1009 is the SH2-containing phosphotyrosine phosphatase Syp. Proc. Natl. Acad. Sci. USA 90:6939-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh, J. S., W. Lieberthal, S. Heydrick, and J. S. Levine. 1998. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J. Clin. Investig. 102:716-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, M. J., S. Thangada, C. H. Liu, B. D. Thompson, and T. Hla. 1998. Lysophosphatidic acid stimulates the G-protein-coupled receptor Edg-1 as a low affinity agonist. J. Biol. Chem. 273:22105-22112. [DOI] [PubMed] [Google Scholar]
- 44.Li, M., X. Wang, M. K. Meintzer, T. Laessig. M. J. Birnbaum, and K. A. Heidenreich. 2000. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol. Cell. Biol. 20:9356-9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao, M., X. Fang, Y. Lu, R. LaPushin, R. C. Bast, Jr., and G. B. Mills. 2000. Inhibition of growth factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 352:475-482. [PMC free article] [PubMed] [Google Scholar]
- 46.Moolenaar, W. H. 1999. Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 253:230-238. [DOI] [PubMed] [Google Scholar]
- 47.Murai, H., M. Okazaki, and A. Kikuchi. 1996. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 392:153-160. [DOI] [PubMed]
- 48.Pap, M., and G. M. Cooper. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929-19932. [DOI] [PubMed] [Google Scholar]
- 49.Pierce, S. B., and D. Kimelman. 1995. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development 121:755-765. [DOI] [PubMed] [Google Scholar]
- 50.Plyte, S. E., K. Hughes, E. Nikolakaki, B. J. Pulverer, and J. R. Woodgett. 1992. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim. Biophys. Acta 1114:147-162. [DOI] [PubMed] [Google Scholar]
- 51.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389-399. [DOI] [PubMed] [Google Scholar]
- 52.Schonwasser, D. C., R. M. Marais, C. J. Marshall, and P. J. Parker. 1998. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18:790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seufferlein, T., and E. Rozengurt. 1994. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. Signaling pathways and cross-talk with platelet-derived growth factor. J. Biol. Chem. 269:9345-9351. [PubMed] [Google Scholar]
- 54.Shaw, M., and P. Cohen. 1999. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 461:120-124. [DOI] [PubMed] [Google Scholar]
- 55.Siegfried, E., T. B. Chou, and N. Perrimon. 1992. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71:1167-1179. [DOI] [PubMed] [Google Scholar]
- 56.Stambolic, V., and J. R. Woodgett. 1994. Mitogen inactivation of glycogen synthase kinase-3β in intact cells via serine 9 phosphorylation. Biochem. J. 303:701-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland, C., I. A. Leighton, and P. Cohen. 1993. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 296:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamm, I., and T. Kikuchi. 1991. Activation of signal transduction pathways protects quiescent BALB/c-3T3 fibroblasts against death due to serum deprivation. J. Cell Physiol. 148:85-95. [DOI] [PubMed] [Google Scholar]
- 59.Tigyi, G., D. J. Fischer, D. Baker, D. A. Wang, J. Yue, N. Nusser, T. Virag, V. Zsiros, K. Liliom, D. Miller, and A. Parrill. 2000. Pharmacological characterization of phospholipid growth-factor receptors. Ann. N. Y. Acad. Sci. 905:34-53. [DOI] [PubMed] [Google Scholar]
- 60.Valius, M., C. Bazenet, and A. Kazlauskas. 1993. Tyrosine 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor β subunit and are required for binding of phospholipase Cγ and a 64-kilodalton protein, respectively. Mol. Cell. Biol. 13:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valius, M., and A. Kazlauskas. 1993. Phospholipase Cγ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell 73:321-334. [DOI] [PubMed] [Google Scholar]
- 62.van Corven, E. J., A. Groenink, K. Jalink, T. Eichholtz, and W. H. Moolenaar. 1989. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 59:45-54. [DOI] [PubMed] [Google Scholar]
- 63.Wang, D. J., N. N. Huang, E. J. Heller, and L. A. Heppel. 1994. A novel synergistic stimulation of Swiss 3T3 cells by extracellular ATP and mitogens with opposite effects on cAMP levels. J. Biol. Chem. 269:16648-16655. [PubMed] [Google Scholar]
- 64.Wang, Q. M., C. J. Fiol, A. A. DePaoli-Roach, and P. J. Roach. 1994. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation J. Biol. Chem. 269:14566-14574. [PubMed] [Google Scholar]
- 65.Welsh, G. L., and C. G. Proud. 1993. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem. J. 294:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu, Y., D. C. Gaudette, J. D. Boynton, A. Frankel, X. J. Fang, A. Sharma, J. Hurteau, G. Casey, A. Goodbody, A. Mellors, B. J. Holub, and G. B. Mills. 1995. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Cancer Res. 1:1223-1232. [PubMed] [Google Scholar]
- 67.Xu, Y., Z. Shen, D. W. Wiper, M. Wu, R. E. Morton, P. Elson, A. W. Kennedy, J. Belinson, M. Markman, and G. Casey. 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 280:719-723. [DOI] [PubMed] [Google Scholar]