Abstract
HA95, a nuclear protein homologous to AKAP95, has been identified in immune precipitates of the Epstein-Barr virus (EBV) coactivating nuclear protein EBNA-LP from EBV-transformed lymphoblastoid cells (LCLs). We now find that HA95 and EBNA-LP are highly associated in LCLs and in B-lymphoma cells where EBNA-LP is expressed by gene transfer. Binding was also evident in yeast two-hybrid assays. HA95 binds to the EBNA-LP repeat domain that is the principal coactivator of transcription. EBNA-LP localizes with HA95 and causes HA95 to partially relocalize with EBNA-LP in promyelocytic leukemia nuclear bodies. Protein kinase A catalytic subunit α (PKAcsα) is significantly associated with HA95 in the presence or absence of EBNA-LP. Although EBNA-LP is not a PKA substrate, HA95 or PKAcsα expression in B lymphoblasts specifically down-regulates the strong coactivating effects of EBNA-LP. The inhibitory effects of PKAcsα are reversed by coexpression of protein kinase inhibitor. PKAcsα also inhibits EBNA-LP coactivation with the EBNA-2 acidic domain fused to the Gal4 DNA binding domain. Furthermore, EBNA-LP- and EBNA-2-induced expression of the EBV oncogene, LMP1, is down-regulated by PKAcsα or HA95 expression in EBV-infected lymphoblasts. These experiments indicate that HA95 and EBNA-LP localize PKAcsα at nuclear sites where it can affect transcription from specific promoters. The role of HA95 as a scaffold for transcriptional regulation is discussed.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that latently infects B lymphocytes and causes their efficient proliferation into continuous lymphoblastoid cell lines (LCLs) (28, 51, 52). EBV-infected B lymphoblasts can cause lymphoproliferative diseases in immune-compromised people (53). EBV is also causally associated with Burkitt's lymphoma (BL), nasopharyngeal carcinoma, and Hodgkin's disease (11, 16, 27, 29). Five EBV-encoded nuclear proteins, EBNA-1, -2, -3A, -3C, and -LP, and a latent-infection-associated membrane protein, LMP1, are critical for the conversion of resting B lymphocytes to LCLs (7, 22, 33, 42, 54, 64). EBNA-LP and EBNA-2 are the first two EBV proteins expressed in latent infection of human B lymphocytes (2). EBNA-2 activates transcription of cellular genes, including CD21, CD23, and c-myc and of viral genes, including the EBNAs, LMP1, and LMP2 (1, 2, 8, 17, 32, 60, 69-71). EBNA-2 has the intrinsic ability to self associate and recognizes promoters by binding to RBP-Jκ and PU.1/Spi1, cellular transcription factors that recognize specific DNA sequences (20, 25, 26, 31, 58). Once associated with a promoter, the EBNA-2 acidic domain can recruit basal and activated transcription factors p300, CBP, and PCAF histone acetylases and a p100 transcriptional coactivator (25, 65-67, 73). EBNA-LP strongly coactivates transcription mediated by EBNA-2 or by the EBNA-2 acidic domain (24, 47). In coactivating transcription with EBNA-2, EBNA-LP has a central role in the EBV effects on cell growth and survival.
The experiments reported here focus on the role of a recently described EBNA-LP-associated cell protein, HA95, in regulating gene expression (23). EBNA-LP is comprised of 22- and 44-amino-acid repeats translated from W1W2 exons encoded by the EBV BamHI W 3-kbp DNA repeat and C-terminal 11- and 34-amino-acid sequences encoded by exons derived from the EBV BamY DNA (Fig. 1A) (72). In transient transfection assays, the EBNA-LP repeat domains are similar to wild-type EBNA-LP in strongly coactivating transcription mediated by EBNA-2 or the EBNA-2 acidic domain (24). EBNA-LP is highly phosphorylated during G2/M (36, 50) and can be phosphorylated by p34cdc2 or casein kinase II (36). Phosphorylation on serine 35 is particularly important for coactivation (49, 78).
FIG. 1.
EBNA-LP associates with HA95 through the W4 domain. (A) A schematic diagram of the EBNA-LP open reading frame indicating the four nearly perfect 22 (w1)- and 44 (w2)-amino-acid repeats and the C-terminal 11 (y1) and 34 (y2) amino acids. (B) BJAB, non-EBV-infected, BL cells were converted to expression of wild-type Flag-tagged EBNA-LP (BFLP), EBNA-LP deleted for the last 10 amino acids (BFLPd10), or EBNA-LP deleted for the last 45 amino acids (BFLPW4). IB4 cells were converted to expression of Flag-tagged EBNA-LP (IB4FLP). The Flag-tagged EBNA-LP proteins were immune precipitated (IP). FLP and HA95 in the lysates (5% of starting lysate) and in the immune precipitates were electrophoretically separated in polyacrylamide gels, immune blotted, and detected with HA95 or JF186, an EBNA-LP-specific antibody. Lysates (5% of starting lysate) and anti-myc immune precipitates of BJAB (B), BFLP (BLP), or IB4 parental or myc-tagged HA95 (MHA95)-converted BJAB (BMHA95), BFLP (BLPHA95), or IB4 (IB4HA95) cells were electrophoretically separated in polyacrylamide gels and immune blotted with 9E10 anti-myc or JF186 anti-EBNA-LP antibody.
HA95 is the product of a gene duplication with AKAP95, a nuclear anchor for protein kinase A (PKA) (14, 43, 48). HA95 is similar to AKAP95 in localizing to the nucleus, in localizing to metaphase chromosomes, and in self associating. HA95 does not associate with AKAP95 and differs from AKAP95 in not binding to the PKA RII regulatory subunit (43, 48). Instead, HA95 associates with the lamin B receptor, lamina-associated polypeptide, and emerin on the inner aspect of the nuclear membrane and has a role in mitosis-associated nuclear membrane breakdown and chromatin condensation (43). Since HA95 is also highly associated with EBNA-LP, a coactivator of EBNA-2-mediated transcription, and PKA affects EBNA-2-mediated gene regulation (18, 59), we have investigated whether HA95 and PKA interact with EBNA-LP and affect EBNA-LP- and EBNA-2-mediated transcriptional coactivation.
MATERIALS AND METHODS
Plasmids.
PSG5-Flag-EBNA-LP is nucleotides 11 to 646 of the EBNA-LP T65 cDNA (55) in frame with an N-terminal Flag epitope with a modified pSG5-Flag expression vector (12, 56). EBNA-LP mutants pSG-FLPd10 and pFSGW4 were made by inserting a nonsense codon before the C-terminal 10 or 45 codons (24). pSG-EBNA-2 is the 1.8-kbp BstUI-DraI fragment encompassing the entire EBNA-2 coding region of EBV strain W91 in pSG5 (6). Gal4E2AD is EBV strain B95 EBNA-2 codons 426 to 462 fused to the carboxyl terminus of the Gal4 DNA binding domain (5). p512/+72 LMP1Luc contains the LMP1 promoter sequences cloned into the pGL2-Luc vector (Promega). Plasmid pBamCp8CAT is eight copies of the EBNA-2-responsive −330 to −430 sequence from the EBV BamHI C fragment EBNA promoter (gift of Paul Ling, Baylor College of Medicine) placed upstream of minimal adenovirus E1B promoter in the chloramphenicol acetyltransferase (CAT) reporter plasmid pCAT-3 M (40, 68). Gal4tkLuc is the luciferase reporter plasmid containing five copies of the Gal4 binding site upstream of the thymidine kinase (tk) promoter.
A plasmid, pFC-PKA, that expresses the catalytic subunit of PKA (PKAcs) under control of the cytomegalovirus immediate-early promoter was purchased from Stratagene Corp. (San Diego, Calif.). The PKA inhibitor (cPKI) expression plasmid was the gift of Marc Montiminy (Salk Institute, La Jolla, Calif.). The expression plasmid RSV-CHO-PKA-Calpha V2 for the Chinese hamster ovary (CHO)-derived wild type and the catalytically inactive mutant PKA, abbreviated cPKAcs and cPKAm, were the gift of Richard Maurer (Oregon Health Sciences Center, Portland, Oreg.) (30, 44). The EcoRI/NotI fragment of full-length HA95 cDNA was cloned in frame into a triple-myc epitope containing pcDNA3 vector (Invitrogen) after removing the HA95 stop codon by PCR. The cDNA and surrounding sequences were sequence verified.
Cell lines and cell culture.
BJAB is an EBV-negative BL cell line that has an amplified c-myc gene. The Akata BL cell line is latently infected with a type 1 EBV (63). IB4 is an EBV-transformed B-lymphoblastoid cell line (55). All cells were maintained at 37°C in a 5% CO2-containing humidified atmosphere in RPMI 1640 medium (GIBCO BRL) supplemented with 8 μg of gentamicin per ml and 10% fetal calf serum (HyClone).
Transfection and clonal selection.
Fifteen million BJAB, IB4, or Akata cells in log-phase growth were transfected with 10 μg of reporter plasmid and various protein expression plasmids by an electroporator (Gene Pulser; Bio-Rad Laboratories) with a pulse of 0.2 V at 960 mF. In all cases, the total amount of transfected DNA was held constant by adding empty vector. As an internal control for transfection efficiency, 1 μg of pGK-βGal was cotransfected. Cells were harvested 48 h after transfection, and aliquots of cell lysate were assayed for CAT or luciferase activity (68). Percent acetylation was calculated with a PhosphorImager and ImageQuant software (Molecular Dynamics). Luciferase and β-galactosidase (β-gal) activities were measured with an Opticomp I luminometer (MGM Instrument; Waltham, Mass.).
To derive stable clones of Flag or myc epitope-tagged EBNA-LP or HA95, 1.5 × 107 BJAB or IB4 cells in log-phase growth were cotransfected with 10 μg of wild-type or mutant forms of pSG5-Flag-EBNA-LP, 10 μg of pcDNA-myc-HA95, and 0.5 μg of pSG5-Hyg, an expression plasmid for hygromycin phosphotransferase, with a 0.2-V and 960-mF pulse from a Gene Pulser (Bio-Rad Laboratories). Transfected clones were selected by plating 2,000 cells/well in 98-well culture dishes in medium containing 400 U of hygromycin B (GIBCO BRL) per ml. Stable clones were first identified by immunofluorescence staining after being subcultured for 5 to 6 passages in the presence of hygromycin.
Immune precipitation and immunoblotting.
For immune precipitation, 2 × 107 cells per ml were lysed in NP-40 lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.5% NP-40, 10 mg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride) for 30 min on ice, and the lysate was cleared by centrifugation at 14,000 × g for 15 min. Aliquots corresponding to 107 cells were immune precipitated with 10 μg of anti-Flag (M2) antibody (Sigma). Immune complexes were collected with 30 μl of protein G-Sepharose beads, washed extensively with NP-40 lysis buffer, and eluted from the protein G-Sepharose by being boiled for 3 min in electrophoresis sample buffer. Denatured immune complexes were separated by electrophoresis and were transferred to nitrocellulose membrane (Schleicher & Schuell). The membranes were incubated with polyclonal anti-PKAcsα (Santa Cruz Biotechnology Inc.), monoclonal anti-PKARIIα antibody generated against purified human recombinant GST-RIIα, rabbit polyclonal anti-HA95 (48), monoclonal anti-AKAP95 (clone 47) generated against purified human recombinant GST-AKAP95, monoclonal anti-myc (9E10), monoclonal anti-EBNA-LP (JF186 [19]), or monoclonal anti-LMP1 antibody (S12 [41]) in 5% nonfat dry milk. After repeated washing in phosphate-buffered saline-Tween 20 (PBST) (180 mM NaCl, 3.6 mM KCl, 14 mM Na2HPO4, 2 mM KH2PO4, 0.5% Tween 20), blots were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse (Amersham Life Science) antibodies, washed in PBST, and developed by enhanced chemiluminescence (Amersham Life Science).
Immunofluorescence staining and confocal microscopy.
IB4- or Flag-EBNA-LP-converted IB4 cells (23) were centrifuged at 1,000 × g for 5 min, and about 500 cells in 1 μl were spread on a microwell glass slide at 37°C. Cells were fixed with cold methanol-acetone (1:1). The slides were rehydrated in PBS for 30 min and made permeable with 0.5% Triton X-100 (Sigma) in PBS for 30 min at room temperature. After being washed in PBS, the slides were incubated with blocking buffer (5% bovine serum albumin plus 0.02% NaN3 in PBS) for 1 h and then with antibodies diluted in a blocking buffer for 1 h at room temperature. Primary antibodies included the anti-EBNA-LP mouse monoclonal antibody JF186, the anti-PML monoclonal antibody PG-M3 (Santa Cruz Biotechnology), and anti-HA95 rabbit polyclonal serum. Anti-HA95 antibodies for immunofluorescence staining were affinity purified with the immunized peptide. Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated F(ab)2 fragment of goat anti-mouse immunoglobulin (Ig) (Jackson Laboratories) and Texas red-conjugated goat anti-rabbit Ig (Jackson Laboratories). Cells were sequentially exposed to anti-EBNA-LP monoclonal antibody (1:500), anti-HA95 antibody (1:100), FITC-conjugated anti-mouse Ig (1:40), and Texas red-conjugated anti-rabbit Ig (1:30). The images were recorded on a Zeiss microscope with a Nikon confocal attachment (PCM 2,000) and analyzed with C-Imaging (Compix Inc.) and Adobe Photoshop programs.
In vitro PKA assay.
PKA activity was assayed (PKA assay kit; Upstate Biotechnology, Lake Placid, N.Y.) based on phosphorylation of a specific substrate (Kemptide) by [γ-5′-32P]triphosphate ([γ-32P]ATP) in the presence of PKC and CaM kinase inhibitors. Wild-type or mutant forms of Flag-EBNA-LP (FLP) fusion proteins were purified with anti-Flag antibody conjugated to Sepharose-G beads (M2 beads; Sigma Chemical). After extensive washings with NP-40 lysis buffer, FLP-bound M2 beads (10 μl) were assayed for PKA activity by incubation in 10 μl of substrate cocktail (500 μM Kemptide, LRRASLG, and 10 μM cyclic AMP [cAMP]), 10 μl of inhibitor cocktail (20 μM PKC inhibitor peptide, RFARKGALRRKNV, and 20 μM CaM kinase inhibitor, R24571), and 10 μl of assay dilution buffer (20 mM morpholinepropanesulfonic acid [pH 7.2], 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol). For control, assays were done in the presence of 10 μl of 20 μM protein kinase inhibitor (PKI) peptide (TYADFIASGRTGRRNAI). Reactions were started by adding 10 μl of 0.5 mM ATP in 75 mM MgCl2 and 10 μCi of [γ-32P]ATP (3,000 Ci/mmol) (Amersham), gentle mixing, and incubation at 30°C for 10 min. Reactions were stopped by spotting 25 μl of the reaction mixture onto the center of a P81 phosphocellulose paper and washing 10 times with 0.75% phosphoric acid and once with acetone. Dried papers were exposed to X-ray film and were counted in scintillation fluid.
RESULTS
EBNA-LP 66-amino-acid repeat domain associates with HA95.
Since the EBNA-LP 66-amino-acid repeat domain mediates nearly wild-type transcriptional coactivation, initial experiments investigated the role of this domain in the association of HA95 with EBNA-LP. Cell lines were derived that stably express wild-type or deletion mutant EBNA-LPs, including EBNA-LPW4, which is C-terminally deleted for all 45 unique amino acids and is composed entirely of 66-amino-acid repeats (Fig. 1A). The cells express Flag-tagged EBNA-LP wild-type (FLP) or deletion mutants (FLPW4 or FLPd10) at about threefold higher levels than endogenous EBNA-LP in LCLs (data not shown). The expression of wild-type or mutant FLPs in BJAB or IB4 cells did not significantly affect the level of HA95, which was barely detectable in 5% of the lysate from BJAB or IB4 cells (Fig. 1A and data not shown). Immune precipitation with Flag-specific monoclonal antibody coupled to beads precipitated about 10% of wild-type or mutant FLPs and the coprecipitated about 10% of the HA95 (compare the immune precipitates with 5% of lysate in Fig. 1A). HA95 was as highly associated with FLPW4 or FLPd10 as it was with wild-type FLP. These data indicate that most of the endogenous HA95 stably associates with wild-type EBNA-LP or with the transcriptional coactivating EBNA-LP 66-amino-acid repeat domain.
The reciprocal association of EBNA-LP with HA95 was further investigated with BJAB and IB4 cell lines that stably express a c-myc epitope-tagged HA95 (MHA95). MHA95 was stably overexpressed at higher levels in BJAB cells than in FLP-converted BJAB BL cells and was barely detectable in IB4 LCL cells (Fig. 1B). MHA95 immune precipitation from BJAB or IB4 cells recovered at least 2% of the MHA95. The efficiency of immune precipitation from IB4 cells was more difficult to assess because of the low level of HA95 expression (Fig. 1B). About 1% of the EBNA-LP coimmune precipitated with HA95 from BJAB and at least 2% from IB4 cells (Fig. 1B). These data indicate that a substantial fraction of EBNA-LP stably associates with HA95 in BJAB BL or IB4 LCL cells.
To evaluate the extent to which EBNA-LP interacts directly with HA95, EBNA-LP or EBNA-LPW4 was cloned as a fusion protein C terminal to the GAL4 activating domain in pACT2 and was tested for its interaction in yeast strain Y190 with HA95 fused C terminal to the Gal4 DNA binding domain in pBridge (Clonetech Corp) (13). Multiple colonies of doubly transformed yeast were selected on double drop-out media, and the colonies were assayed for LacZ on nitrocellulose filters soaked with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Six independent yeast clones that were doubly transformed with pACT2-LP and pBridge-HA95 turned light blue after overnight incubation, whereas six independent yeast clones that were doubly transformed with pACT2-LPW4 and pBridge-HA95 turned dark blue after overnight incubation. In contrast, six control Y190 clones that were doubly transformed with pACT2 and pBridge-HA95 and six Y190 clones that were transformed with pACT2-LP or pACT2-LPW4 and pBridge remained colorless after overnight incubation. In a control test for strong interaction, six independent clones of Y190 doubly transformed with pASCY.Jk that expresses RBP-Jκ fused to the GAL4 DNA binding domain and pACT2.3C that expresses EBNA-3C fused to the GAL4 acidic domain turned dark blue by 2 h. Similar results were obtained in a repeat experiment. These data indicate that the transcriptional coactivating 66-amino-acid repeat domains of EBNA-LP interact directly with HA95 in yeast; this interaction is somewhat down-modulated in the context of wild-type EBNA-LP.
HA95 colocalizes with EBNA-LP in IB4 cells.
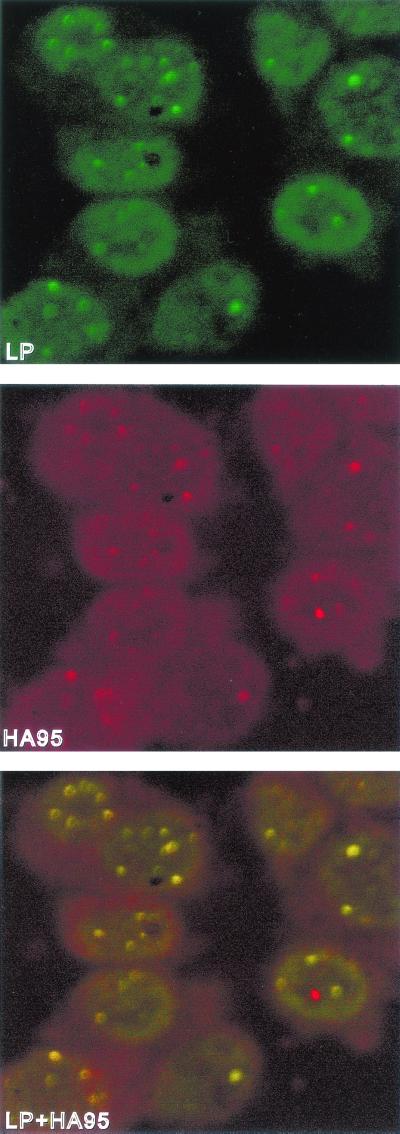
In B lymphoblasts not infected with EBV, HA95 is diffusely distributed through interphase nuclei, is absent from nucleoli, and is concentrated near the nuclear membrane (43, 48), whereas in EBV-infected or uninfected B lymphocytes EBNA-LP is concentrated in PML bodies with some diffuse nuclear distribution (61, 72). To determine if HA95 is partially relocalized with EBNA-LP in PML bodies in LCLs, HA95-specific immune rabbit antibody, an EBNA-LP specific monoclonal antibody, and a PML-specific monoclonal antibody were used to detect endogenous HA95, EBNA-LP, and PML in the EBV-transformed IB4 LCL cells using confocal microscopy. EBNA-LP, identified with a fluorescein-tagged antibody (Fig. 2), was highly concentrated in PML bodies but was also diffusely distributed within nuclei with slight accentuation at the nuclear rim. HA95, identified with a Texas red-labeled antibody (Fig. 2), was more concentrated at the nuclear rim but also partially localized to PML bodies (Fig. 2 and data not shown). Indeed, EBNA-LP and HA95 extensively overlapped in IB4 LCLs; the overall color of PML bodies and the nuclear periphery was yellow rather than green or red when viewed as a merged image (Fig. 2, bottom panel). These data indicate that EBNA-LP and HA95 extensively colocalize and that EBNA-LP causes HA95 to partially relocalize in PML bodies.
FIG. 2.
EBNA-LP and HA95 colocalize in IB4 LCL cells. IB4 cells were immune stained with anti-EBNA-LP-specific JF186 monoclonal antibody and goat anti-mouse antibody coupled to FITC (green) and with anti-HA95-specific affinity-purified rabbit antibody and Texas red (red)-conjugated goat anti-rabbit antibody. Slides were visualized with a Zeiss axioscope microscope fitted with a Nikon PC2000 confocal attachment. The merged green and red images are shown in the bottom panel.
EBNA-LP and HA95 associate with PKAcsα but not with AKAP95.
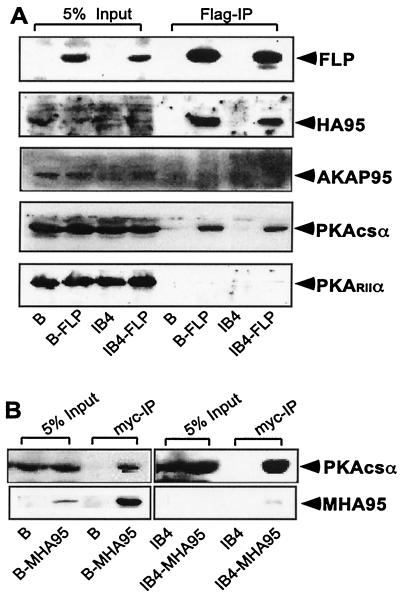
Although HA95 lacks homology to the AKAP95 sequences that bind to the PKA RII subunit and does not coimmune precipitate with AKAP95 or bind to the PKA RII subunit in in-gel assays (36, 40), we considered the possibility that HA95 or EBNA-LP may have conserved or evolved another mechanism for interacting or associating with PKAcs or PKA RII. EBNA-LP was immune precipitated from FLP-converted BJAB or IB4 cells, and immune blots were done to detect FLP, HA95, AKAP95, the PKA RIIα subunit, PKA RIα, or PKAcsα (Fig. 3A and data not shown). As expected, FLP immune precipitation resulted in very similar levels of HA95 coimmune precipitation; HA95 did not coimmune precipitate from cells lacking FLP. These data confirm that HA95 is extensively and specifically associated with EBNA-LP. AKAP 95, PKA RIIα, and PKA RIα were not associated with FLP (Fig. 3A). However, about 3% of the PKAcsα coimmune precipitated with EBNA-LP; PKA did not coimmune precipitate from cells lacking FLP (Fig. 3A). Based on the efficiency of FLP immune precipitation, which we estimate to be less than 30%, these data indicate that at least 10% of the PKAcsα is stably associated with EBNA-LP in EBNA-LP overexpressing BJAB or IB4 cells.
FIG. 3.
EBNA-LP and HA95 associate with the catalytic subunit of PKA. (A) Cell lysates from BJAB (B), BJAB-LP (BFLP), IB4, and IB4-LP (IB4FLP) cells were immune precipitated (IP) with anti-Flag antibody. Lysates (5% of starting lysate) and immune precipitates were analyzed by polyacrylamide gel electrophoresis and immune blotted with JF186, HA95, AKAP95, PKA RIIα, and PKAcsα antibodies. (B) BJAB or IB4 cells converted to myc-HA95 expression (BMHA95) or IB4 (IB4MHA) were lysed and immune precipitated with 9E10 anti-myc antibody. Lysates (5% of starting lysate) and immune precipitates were analyzed by polyacrylamide gel electrophoresis and immune blot with PKAcs-specific antibody.
To determine whether HA95 or EBNA-LP or both were required for PKAcsα coimmune precipitation, myc epitope-tagged MHA95 was immune precipitated from BJAB cells that lack EBNA-LP or from IB4 LCL cells that have endogenous EBNA-LP. myc antibody immune precipitated 15 to 25% of the MHA95 from BJAB or IB4 cells, and 3 to 5% of PKAcsα coimmune precipitated (Fig. 3B and data not shown). No MHA95 or PKAcsα immune precipitated from cells that lacked MHA95. Thus, at least 12% of PKAcsα is specifically associated with HA95 in the presence or absence of EBNA-LP; PKAcsα association with HA95 in the absence of EBNA-LP accounts for most or all of the PKAcsα in the EBNA-LP immune precipitates. In associating with HA95, EBNA-LP does not negatively effect PKAcsα association with HA95.
A direct interaction of HA95 with PKAcsα could not be detected in Y190 yeast. Six independent clones of yeast doubly transformed with pBridge-HA95 and pACT.PKAcsα failed to give blue coloration after overnight growth on X-Gal plates. Six independent Y190 clones doubly transformed in parallel with pBridge-HA95 and EBNA-LP were light blue after overnight incubation. Further, immune blots of lysates of the pBridge-HA95 and pACT.PKAcsα doubly transformed Y190 clones confirmed stable expression of HA95 and PKAcsα cross-reactive proteins of the expected size with antibody to HA95 and PKAcsα. The simplest interpretation of this experiment is that PKAcsα association with HA95 is mediated by a third protein. However, Saccharomyces cerevisiae has endogenous PKA and we cannot exclude the possibility that yeast PKA or a PKA interacting protein may block the interaction of human PKAcsα with HA95.
EBNA-LP and EBNA-LPW4 immune precipitates contain PKA catalytic activity.
PKA catalytic activity was also readily detected in Flag epitope immune precipitates from FLP-, FLPd10-, or FLPW4-converted BJAB cells using Kemptide (LRRASLG) as the PKA substrate and buffer with inhibitors of PKC and CaM kinase, which might otherwise contribute to Kemptide phosphorylation. Flag immune precipitates from FLP, FLPd10, and FLPW4 expressing BJAB cells had five- to sixfold more PKA activity than immune precipitates from control BJAB cells (Fig. 4). PKA specificity was confirmed with the PKA inhibitory peptide TYADFIASGRTGRRNAI, which resulted in nearly complete inhibition of Kemptide labeling (Fig. 4). These data indicate that catalytically active PKA is associated with EBNA-LP in cell lysates and that the association requires only the EBNA-LP W4 domain. EBNA-LP is also phosphorylated in immune precipitate in vitro kinase assays, but EBNA-LP phosphorylation is inhibited by Wortmanin, consistent with the observation that a small amount of DNA-PK is associated with EBNA-LP (23).
FIG. 4.
EBNA-LP is associated with active PKA. Immune precipitates from BJAB (B), BFLP, BFLPd10, or BFLPW4 cells were immune precipitated with M2 beads. The immune precipitates were split and assayed for PKA based on phosphorylation of a specific substrate (Kemptide; Upstate Biotechnology) using [γ-32P]ATP in the presence (dashed lines) or absence (solid lines) of PKA inhibitor (PKA INH). The labeled Kemptide was blotted on P81 phosphocellulose paper (boxed figure). Radioactivity was measured with a PhosphorImager (Molecular Dynamics), and relative counts are indicated in the graph as kinase activity.
PKA or HA95 down-regulates and PKI up-regulates EBNA-LP coactivation with EBNA-2 of the EBV LMP1 or Cp promoters or of an EBNA-2 acidic domain-dependent promoter.
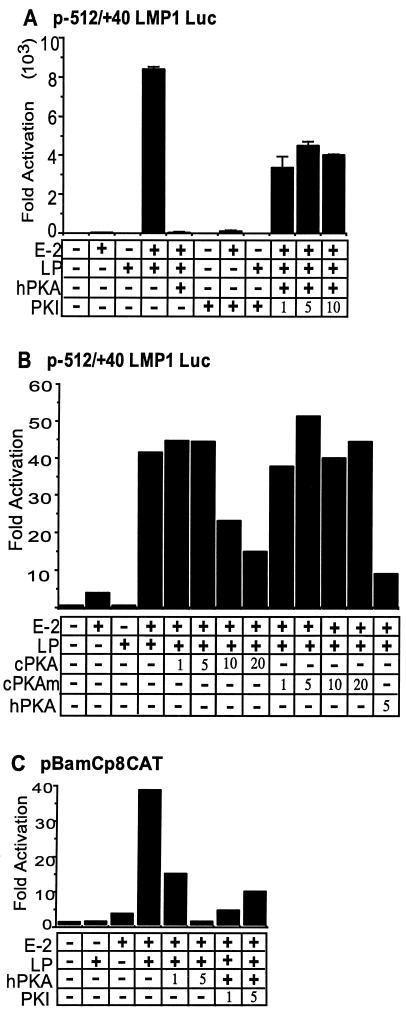
The role of PKA in EBNA-LP and EBNA-2 coactivation of the EBV LMP1 and Cp promoters was evaluated in BJAB cells by cotransfection with GK promoter and β-gal control plasmids, luciferase or CAT reporter, and EBNA-2, EBNA-LP, PKA, HA95, or control expression vector (Fig. 5). As expected, EBNA-LP alone had minimal or no effect on EBV LMP1- or Cp-promoted luciferase or CAT reporter expression, and EBNA-2 had only a small effect, whereas EBNA-2 and EBNA-LP strongly coactivated both promoters (Fig. 5). Coexpression of PKAcsα strongly repressed EBNA-2 and EBNA-LP coactivation of the LMP1 or Cp promoters (Fig. 5). The effect was likely due to PKA catalytic activity, since PKAcsα is readily detected in Western blots (Fig. 2) and total cellular PKAcsα levels did not increase in the transfected cells, despite the 20 to 50% transfection efficiency and the robust effect (Fig. 5A to C and data not shown). To further test the dependence of the repressive effect on PKA catalytic activity, the effect of wild-type and kinase-negative CHO-derived PKAcsα (30) were compared. In multiple experiments, CHO PKAcsα repressed EBNA-LP and EBNA-2 coactivation in a dose-dependent fashion, whereas a kinase-negative point mutant of CHO PKAcsα had no effect (Fig. 5B). Thus, PKAcsα specifically represses EBNA-LP and EBNA-2 coactivation and the effect is dependent on PKA catalytic activity.
FIG. 5.
PKA down-regulates EBNA-2- and EBNA-LP-mediated transactivation of LMP1- and BamC-dependent reporters; PKI reverses this effect. (A) BJAB cells were cotransfected with 10 μg of a −512/+40 LMP1 promoter-luciferase reporter plasmid, pSG-EBNA-2, pSG-EBNA-LP, pPKAcsα, and PKI expression plasmid, un-less indicated by a minus sign or a number. Total amounts of input DNA were balanced by addition of vector plasmid DNA. Luciferase activity was measured 48 h later and was normalized to reporter and empty expression vector control experiment results. (B) BJAB cells were cotransfected with a −512/+40 LMP1 promoter-luciferase reporter plasmid, pSG-EBNA-2, pSG-EBNA-LP, and an expression vector for human PKAcsα, CHO PKAcsα, or catalytic inactive CHO PKAcsα. In these experiments, 5 μg of each plasmid was used, unless indicated by a minus sign or a number. Luciferase activity was measured 18 h later instead of 48 h and was normalized to reporter and empty expression vector control. This result is representative of three independent experiments. (C) Similar experiments are presented with 10 μg of a minimal adenovirus E1b promoter-CAT reporter plasmid that has eight upstream copies of the EBNA-2 responsive, −330 to −430 Cp enhancer (39). CAT activity was measured by [14C]chloramphenicol acetylation. These results are representative of three independent experiments. In all experiments, total input DNA was balanced by addition of vector plasmid DNA.
Expression of PKI had little effect on the EBV promoters alone and only slightly increased EBNA-2 activation (Fig. 5). However, PKI substantially reversed the inhibitory effects of PKA (Fig. 5). The effect of PKI was partially dependent on the amount of cotransfected PKI expression plasmid, although saturation was achieved with 1 to 5 μg of PKI expression plasmid in some experiments, including that shown in Fig. 5A. PKAand PKI had no consistent effects on a cotransfected GK β-gal expression plasmid. Thus, PKI partially reverses the PKA effect, further implicating nuclear PKAcsα in repression of EBNA-LP and EBNA-2 coactivation of the EBV LMP1 and Cp promoters.
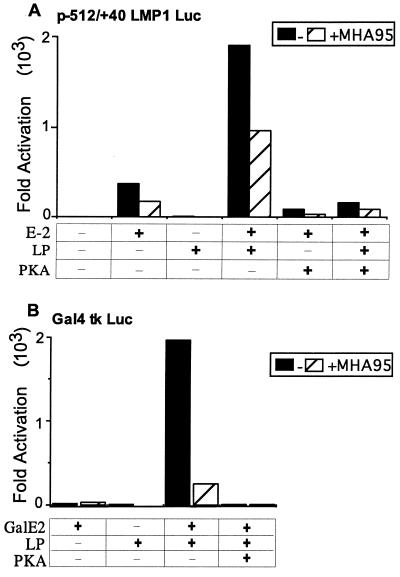
HA95 overexpression also down-regulated EBNA-LP coactivation with EBNA-2 of the LMP1 promoter (Fig. 6A). This probably accounts for the inability to derive LCLs with high-level HA95 expression (Fig. 1B). Furthermore, HA95 expression also down-regulated EBNA-LP and EBNA-2 acidic domain-mediated coactivation of a tk minimal promoter with up-stream Gal4 DNA binding sites, using an EBNA-2 acidic domain fusion to the GAL4 DNA binding domain (Fig. 6B). The HA95 inhibitory effects were consistent and significant, although not as robust as those of PKAcsα, which nearly completely repressed EBNA-LP and EBNA-2 coactivation of the LMP-1 promoter as well as EBNA-LP coactivation of GAL4-EBNA-2 acidic domain activation of a GAL4 enhancer element (Fig. 6A and B). The effects did not substantially increase by transfection with more or less HA95 expression vector. These data indicate that PKA and HA95 can repress EBNA-LP coactivation with the EBNA-2 acidic domain and that components of EBNA-2 other than the acidic domain are not required for this effect. The smaller effect of HA95 relative to that of PKAcsα may be due to a larger pool of free endogenous HA95 or to the dependence on PKAcsα catalytic activity.
FIG. 6.
HA95 suppresses EBNA-LP coactivation with EBNA-2 of the LMP1 promoter or EBNA-LP coactivation with the EBNA-2 acidic domain fused to the Gal4 DNA binding domain of a promoter with multiple Gal4 DNA binding sites. (A) BJAB cells were cotransfected with the −512/+40 LMP1-luciferase reporter plasmid, pSG-EBNA-2, pSG-EBNA-LP, a control expression vector or a PKAcsα expression vector, and pcDNA3 or pcDNA3myc-HA95 (10 μg of each plasmid). Luciferase activity was measured 48 h later. (B) BJAB cells were cotransfected with pG4tkLuc containing five copies of the Gal4 binding site upstream of a tk promoter-driven luciferase reporter, pSG5-Gal4-EBNA-2AcD, pSG5-EBNA-LP, a control expression vector or a PKAcsα expression vector, and pcDNA3 or pcDNA3myc-HA95 (10 μg of each plasmid). Luciferase activity was measured 48 h later. These results are representative of four independent experiments.
PKA and HA95 down-regulate EBNA-LP coactivation with EBNA-2 of LMP1 expression in EBV-infected Akata-BL cells.
The Akata-BL cell line is EBV infected, has a latency I phenotype, and expresses EBNA-1 but not EBNA-2, EBNA-LP, or LMP1 (62). Cotransfection of EBNA-2 and EBNA-LP expression vectors into Akata cells induces LMP1 expression, while EBNA-2 alone has little if any effect (47). To examine the role of PKA and HA95 in regulating the LMP1 promoter in the context of the full EBV genome in an EBV-infected lymphoblast, Akata cells were transfected with EBNA-2 and EBNA-LP expression vectors and with PKA, HA95, or a control empty expression vector (Fig. 7). As expected, EBNA-2 had minimal effects, whereas EBNA-LP and EBNA-2 significantly up-regulated LMP1 expression (Fig. 7). Cotransfection with increasing amounts of PKI expression vector did not further increase LMP1 levels beyond those of EBNA-LP and EBNA-2 (Fig. 7). Cotransfection with increasing amounts of PKAcsα expression vector progressively inhibited EBNA-2 and EBNA-LP induction of LMP1 expression (Fig. 7). Cotransfection with increasing amounts of HA95 expression vector also progressively inhibited LMP1 expression, but the effects were less than those of PKAcsα (Fig. 7). These results implicate PKA and HA95 as negative regulators of EBNA-LP and EBNA-2 coactivation of LMP1 expression from the EBV genome in lymphoblasts.
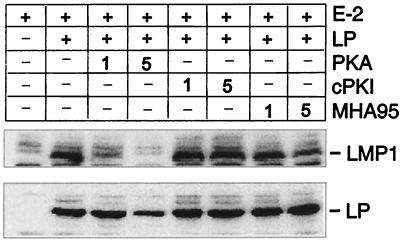
FIG. 7.
EBNA-LP coactivation of LMP1 protein expression from the EBV genome in Akata cells is down-regulated by the coexpression of PKAcs or HA95. Akata cells were transfected with pSG5-EBNA-2, pSG5-EBNA-LP, pPKAcs, cPKI, pcDNAmycHA95, or control expression plasmids to balance the transfections (10 μg of each unless otherwise indicated). LMP1 (upper panel) and EBNA-LP (lower panel) expression were assayed by immune blot with anti-LMP1 (S12) and anti-EBNA-LP (JF186) antibody.
DISCUSSION
EBNA-LP and EBNA-2 are key activators of viral and cellular gene transcription and are essential for initiating and maintaining EBV-infected B-lymphocyte activation, proliferation, and survival (2, 5-7, 24, 31, 32, 42, 47, 57, 65-67, 73, 76). Present knowledge of mechanisms by which EBNA-LP and EBNA-2 coactivate the EBV LMP1 promoter are summarized in Fig. 8. HA95 is likely to have a central role in EBNA-LP and EBNA-2 transcriptional effects. EBNA-LP is extensively associated with HA95, and HA95 is highly associated with EBNA-LP. HA95 binds directly to the EBNA-LP repeat domain that mediates transcriptional activation and associates with this domain in lymphoblasts. EBNA-LP localizes in part to sites of HA95 at the nuclear periphery, and EBNA-LP relocalizes a component of HA95 to PML bodies. Consistent with HA95 being a key mediator of EBNA-LP's key role in transcriptional regulation, stable HA95 expression was at a low level in LCLs, whereas high-level overexpression was apparent in BL lymphoblasts that are not dependent on EBNA-LP and EBNA-2 coactivation for their growth. PKAcsα associates with HA95 and with EBNA-LP HA95 complexes. In lymphoblasts, PKAcsα expression has a catalytic effect in down-modulating EBNA-LP coactivation; the effect is reversed by PKI. HA95 overexpression has a similar, although less robust, effect in down-modulating transcription, consistent with the notion that HA95 is a scaffold for recruitment of PKA for the down-regulation of transcription at sites of EBNA-LP coactivation.
FIG. 8.
Schematic model for the role of HA95 and PKA in EBNA-LP coactivation with EBNA-2 of the EBV LMP1 latency promoter. EBNA-2 can multimerize through two self-associating N-terminal domains (25). EBNA-2 then binds to specific viral and cell promoters through interaction with RBP-Jκ and PU.1 (for a review see reference 34). The EBNA-2 acidic domain mediates transcriptional activation through interactions with p100 (66), TFIIH (65), TFIIB (67), p300/CBP (74), and EBNA-LP (24). The p100 protein is a scaffold for c-Myb (9) and Pim-1 (37). The data herein indicate that the EBNA-LP repeat domain is complexed with HA95. EBNA-LP and HA95 can each self associate (43, 48). HA95 was previously known to be associated with RHA (75, 77). RHA is part of a Pol II holoenzyme complex that includes CBP/p300, BRCA1, and Pol II (3, 38, 46, 77). In recruiting RHA and its associated CBP/p300, BRCA1, and Pol II proteins, HA95 should have positive transcriptional effects. In recruiting PKAcsα, HA95 has negative direct effects on EBNA-LP-mediated coactivation with EBNA-2. PKAcsα may also have positive effects through HA95 by an effect on CREB mediated by CBP that is associated with HA95 through RHA.
HA95 now appears to have several roles. HA95 has 61% overall colinear sequence similarity to AKAP95, including a typical nuclear localization signal, a bipartite nuclear localization signal, and two adjacent zinc finger domains (43, 48, 75, 77). Both proteins associate with interphase nuclei and with mitotic condensed chromosomes. However, HA95 associates with itself but not with AKAP95, lacks the critical amphipathic helical component of the sequence in AKAP95 that interacts with PKA RIIα, and is not bound by PKA RIIα under conditions in which PKA RIIα binds to AKAP95 (48). HA95 further differs from AKAP95 in having N-terminal YG repeats, three nucleoporin-like FG repeats, and a potential SH3 binding domain (75). Moreover, HA95 binds directly to and associates with RNA helicase A (RHA), can shuttle between the nucleus and cytoplasm, and is implicated in RNA transport mediated by constitutive transport elements (75, 77). HA95 also associates with the lamin B receptor and lamina-associated polypeptide 2, integral proteins of the inner nuclear membrane. Antibodies to HA95 inhibit nuclear membrane breakdown and chromatin condensation (43).
The interaction and association of HA95 with RHA (75, 77) are particularly likely to be relevant to the role of HA95 in transcription and to EBNA-LP effects in coactivating transcription. RHA associates with p300/CBP, BRCA1, and Pol II and mediates the association of p300/CBP and BRCA1 with the Pol II complex (3, 15, 46). Thus, HA95 could have a key role in the positive transcriptional effects of the EBNA-LP repeat domain by being a scaffold for recruitment of RHA and its associated proteins to EBNA-LP (see Fig. 8). Furthermore, RHA and HA95 can facilitate posttranscriptional RNA transport, raising the possibility that EBNA-LP may have both transcriptional and posttranscriptional effects on gene expression.
Our data indicate that HA95 is a scaffold for recruitment of PKAcsα to EBNA-LP and that by recruiting PKAcsα HA95 can have a negative effect on EBNA-LP coactivation of transcription with EBNA-2 or with the EBNA-2 acidic domain. EBNA-LP and HA95 are associated with a significant fraction of the cellular PKAcsα, and the EBNA-LP-associated PKAcsα is active in specific phosphorylation assays. PKAcsα overexpression down-regulated EBNA-LP and EBNA-2 coactivation of the EBV latency LMP1 and Cp promoter/reporter constructs in B lymphoblasts not infected with EBV and in EBV-infected B lymphoblasts. PKAcsα had similar effects on EBNA-LP coactivation with an EBNA-2 acidic domain Gal4 DNA binding domain fusion. Moreover, PKI, which both inhibits and increases the PKAcsα export from the nucleus, reversed the inhibitory effects of PKAcsα expression, confirming the specificity of the effect for nuclear PKA. These are significant new findings indicative of a direct role of nuclear PKA in the regulation of transcription in latent EBV infection.
Several aspects of the effect of nuclear PKA on transcriptional regulation require comment. First, PKAcsα down-regulation of EBNA-LP coactivation is mediated by HA95, which associates with PKAcsα even in the absence of EBNA-LP. EBNA-LP is therefore likely to have evolved to mimic the interaction of cellular transcription factors with HA95, and HA95 is likely to also mediate PKAcsα down-regulation of cellular promoters. Second, HA95 can shuttle between the nucleus and cytoplasm and may thereby mediate PKAcsα translocations into or out of the nucleus. The interaction of HA95 with PKA may be direct or indirect, possibly through PKI. Third, our data indicate that the PKA effect requires PKA enzymatic activity. Overall PKA protein levels do not significantly increase following PKA expression vector transfection, and expression of catalytically active PKA was required. Fourth, although PKAcsα coimmune precipitated with EBNA-LP, EBNA-LP is not phosphorylated by PKAcsα. EBNA-LP is phosphorylated in immune precipitates by DNA-PK (23) and is also phosphorylated by casein kinase and p34cdc2 (36, 78). Fifth, the physiological target of PKAcsα in negatively regulating transcription is likely to be a component of a cellular HA95 complex. Potential candidates include HA95, RHA, and the RHA-associated proteins p300/CBP, BRCA1, or Pol II (3, 15, 46). Sixth, PKAcsα association with HA95 is not mediated by PKA RIIα or RIα, since RIIα and RIα were not detected in HA95 immune precipitates, whereas PKAcsα was substantially enriched in HA95 immune precipitates. The failure to detect RI and RIIα is consistent with previous findings that a substantial fraction of nuclear PKAcsα is not associated with regulatory subunits (for reviews see references 10 and 45). HA95 did not directly bind to PKAcsα in yeast, leaving open the possibility that the interaction may be mediated by an as-yet unknown PKA regulatory subunit or PKI.
PKA has a complex role in transcription and in regulation of the LMP1 promoter (Fig. 8) and may have similar effects on cellular promoters. PKAcsα agonists have been reported to have an overall small positive effect on LMP1 expression in LCLs and on EBV Bam W EBNA promoter activity (4, 18, 35). The LMP1 and Bam W promoters are also positively affected by nearby CREB binding sites in some cell lines (18, 35, 59). The positive effects of PKA through these sites appear to be mediated by activation of an ATF-1/CREB-1 heterodimer (59). EBNA-2 can also activate the LMP1 promoter CREB site through an interaction with an ATF-2/c-Jun heterodimer (59). While EBNA-LP recruitment of activated PKAcsα would now be expected to have a negative effect on EBNA-2 and EBNA-LP coactivation in cells that have high levels of free PKAcsα, the effects may differ among cell types. EBNA-LP, CBP, or other putative transcription factors that interact with HA95 or RHA may recruit PKAcsα to promoters that have CRE sites and thereby facilitate CREB activation. Indeed, Forskolin or dibuteryl-cAMP stimulation of PKA had only a slight overall negative effect on EBNA-LP and EBNA-2 coactivation of the LMP1 promoter in EBV negative BJAB BL cells or in EBV-infected Akata BL cells (data not shown).
PKA is required for cAMP stimulation of transcription (21) and is now shown to also be capable of down-regulatory effects through an association with HA95 in the nucleus. These complex transcriptional effects of PKA are likely to be important in the responses of cells to G-protein-coupled receptor ligands. In latent EBV infection, ligands for G-protein-coupled receptors are likely to activate PKA and thereby modulate EBNA-LP and EBNA-2 effects on transcription.
Acknowledgments
This research was supported by grant numbers CA47008, CA85180, and CA87661 from the National Cancer Institute of the U.S. Public Health Service.
Marc Montminy, Richard Maurer, and Jeffrey Lin contributed reagents and advice.
I. Han and Y. Xue contributed equally to this work.
REFERENCES
- 1.Abbot, S. D., M. Rowe, K. Cadwallader, A. Ricksten, J. Gordon, F. Wang, L. Rymo, and A. B. Rickinson. 1990. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J. Virol. 64:2126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, S. F., B. P. Schlegel, T. Nakajima, E. S. Wolpin, and J. D. Parvin. 1998. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat. Genet. 19:254-256. [DOI] [PubMed] [Google Scholar]
- 4.Bell, A., J. Skinner, H. Kirby, and A. Rickinson. 1998. Characterisation of regulatory sequences at the Epstein-Barr virus BamHI W promoter. Virology 252:149-161. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J. I., and E. Kieff. 1991. An Epstein-Barr virus nuclear protein 2 domain essential for transformation is a direct transcriptional activator. J. Virol. 65:5880-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. I., F. Wang, and E. Kieff. 1991. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J. Virol. 65:2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., F. Wang, J. Mannick, and E. Kieff. 1989. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA 86:9558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordier, M., A. Calender, M. Billaud, U. Zimber, G. Rousselet, O. Pavlish, J. Banchereau, T. Tursz, G. Bornkamm, and G. M. Lenoir. 1990. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J. Virol. 64:1002-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dash, A. B., F. C. Orrico, and S. A. Ness. 1996. The EVES motif mediates both intermolecular and intramolecular regulation of c-Myc. Genes Dev. 10:1858-1869. [DOI] [PubMed] [Google Scholar]
- 10.Dell'Acqua, M. L., and J. D. Scott. 1997. Protein kinase A anchoring. J. Biol. Chem. 272:12881-12884. [DOI] [PubMed] [Google Scholar]
- 11.de-The, G., A. Geser, N. E. Day, P. M. Tukei, E. H. Williams, D. P. Beri, P. G. Smith, A. G. Dean, G. W. Bronkamm, P. Feorino, and W. Henle. 1978. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 274:756-761. [DOI] [PubMed] [Google Scholar]
- 12.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16:7098-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durfee, T., K. Becherer, P. L. Chen, S. H. Yeh, Y. Yang, A. E. Kilburn, W. H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. [DOI] [PubMed] [Google Scholar]
- 14.Eide, T., V. Coghlan, S. Orstavik, C. Holsve, R. Solberg, B. S. Skalhegg, N. J. Lamb, L. Langeberg, A. Fernandez, J. D. Scott, T. Jahnsen, and K. Tasken. 1998. Molecular cloning, chromosomal localization, and cell cycle-dependent subcellular distribution of the A-kinase anchoring protein, AKAP95. Exp. Cell Res. 238:305-316. [DOI] [PubMed] [Google Scholar]
- 15.Endoh, H., K. Maruyama, Y. Masuhiro, Y. Kobayashi, M. Goto, H. Tai, J. Yanagisawa, D. Metzger, S. Hashimoto, and S. Kato. 1999. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol. Cell. Biol. 19:5363-5372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Epstein, M., B. Achong, and Y. Barr. 1964. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet i:702-703. [DOI] [PubMed]
- 17.Fahraeus, R., A. Jansson, A. Ricksten, A. Sjoblom, and L. Rymo. 1990. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc. Natl. Acad. Sci. USA 87:7390-7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahraeus, R., L. Palmqvist, A. Nerdstedt, S. Farzad, L. Rymo, and S. Lain. 1994. Response to cAMP levels of the Epstein-Barr virus EBNA2-inducible LMP1 oncogene and EBNA2 inhibition of a PP1-like activity. EMBO J. 13:6041-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finke, J., M. Rowe, B. Kallin, I. Ernberg, A. Rosen, J. Dillner, and G. Klein. 1987. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J. Virol. 61:3870-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman, S. R., E. Johannsen, X. Tong, R. Yalamanchili, and E. Kieff. 1994. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J kappa recombination signal binding protein. Proc. Natl. Acad. Sci. USA 91:7568-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grove, J. R., D. J. Price, H. M. Goodman, and J. Avruch. 1987. Recombinant fragment of protein kinase inhibitor blocks cyclic AMP-dependent gene transcription. Science 238:530-533. [DOI] [PubMed] [Google Scholar]
- 22.Hammerschmidt, W., and B. Sugden. 1989. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature 340:393-397. [DOI] [PubMed] [Google Scholar]
- 23.Han, I., S. Harada, D. Weaver, Y. Xue, W. Lane, S. Orstavik, B. Skalhegg, and E. Kieff. 2001. EBNA-LP associates with cellular proteins including DNA-PK and HA95. J. Virol. 75:2475-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada, S., R. Yalamanchili, and E. Kieff. 2001. Epstein-Barr virus nuclear protein 2 has at least two N-terminal domains that mediate self-association. J. Virol. 75:2482-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel, T., P. D. Ling, S. D. Hayward, and M. G. Peterson. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science 265:92-95. [DOI] [PubMed] [Google Scholar]
- 27.Henle, G., and W. Henle. 1976. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int. J. Cancer 17:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Henle, W., V. Diehl, G. Kohn, H. zur Hausen, and G. Henle. 1967. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science 157:1064-1065. [DOI] [PubMed] [Google Scholar]
- 29.Herbst, H., F. Dallenbach, M. Hummel, G. Niedobitek, S. Pileri, N. Muller-Lantzsch, and H. Stein. 1991. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc. Natl. Acad. Sci. USA 88:4766-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard, P., K. H. Day, K. E. Kim, J. Richardson, J. Thomas, I. Abraham, R. D. Fleischmann, M. M. Gottesman, and R. A. Maurer. 1991. Decreased catalytic subunit mRNA levels and altered catalytic subunit mRNA structure in a cAMP-resistant Chinese hamster ovary cell line. J. Biol. Chem. 266:10189-10195. [PubMed] [Google Scholar]
- 31.Johannsen, E., E. Koh, G. Mosialos, X. Tong, E. Kieff, and S. R. Grossman. 1995. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by J kappa and PU.1. J. Virol. 69:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott, Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 35.Kirby, H., A. Rickinson, and A. Bell. 2000. The activity of the Epstein-Barr virus BamHI W promoter in B cells is dependent on the binding of CREB/ATF factors. J. Gen. Virol. 81:1057-1066. [DOI] [PubMed] [Google Scholar]
- 36.Kitay, M. K., and D. T. Rowe. 1996. Cell cycle stage-specific phosphorylation of the Epstein-Barr virus immortalization protein EBNA-LP. J. Virol. 70:7885-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leverson, J. D., P. J. Koskinen, F. C. Orrico, E. M. Rainio, K. J. Jalkanen, A. B. Dash, R. N. Eisenman, and S. A. Ness. 1998. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol. Cell 2:417-425. [DOI] [PubMed] [Google Scholar]
- 38.Li, J., H. Tang, T. M. Mullen, C. Westberg, T. R. Reddy, D. W. Rose, and F. Wong-Staal. 1999. A role for RNA helicase A in post-transcriptional regulation of HIV type 1. Proc. Natl. Acad. Sci. USA 96:709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling, P. D., J. J. Hsieh, I. K. Ruf, D. R. Rawlins, and S. D. Hayward. 1994. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J. Virol. 68:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling, P. D., J. J. Ryon, and S. D. Hayward. 1993. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J. Virol. 67:2990-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann, K. P., and D. Thorley-Lawson. 1987. Posttranslational processing of the Epstein-Barr virus-encoded p63/LMP protein. J. Virol. 61:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannick, J. B., J. I. Cohen, M. Birkenbach, A. Marchini, and E. Kieff. 1991. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J. Virol. 65:6826-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins, S. B., T. Eide, R. L. Steen, T. Jahnsen, B. S. Skalhegg, and P. Collas. 2000. HA95 is a protein of the chromatin and nuclear matrix regulating nuclear envelope dynamics. J. Cell Sci. 113:3703-3713. [DOI] [PubMed] [Google Scholar]
- 44.Maurer, R. A. 1989. Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J. Biol. Chem. 264:6870-6873. [PubMed] [Google Scholar]
- 45.Montminy, M. 1997. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66:807-822. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima, T., C. Uchida, S. F. Anderson, C. G. Lee, J. Hurwitz, J. D. Parvin, and M. Montminy. 1997. RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90:1107-1112. [DOI] [PubMed] [Google Scholar]
- 47.Nitsche, F., A. Bell, and A. Rickinson. 1997. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J. Virol. 71:6619-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orstavik, S., T. Eide, P. Collas, I. O. Han, K. Tasken, E. Kieff, T. Jahnsen, and B. S. Skalhegg. 2000. Identification, cloning and characterization of a novel nuclear protein, HA95, homologous to A-kinase anchoring protein 95. Biol. Cell 92:27-37. [DOI] [PubMed] [Google Scholar]
- 49.Peng, R., J. Tan, and P. D. Ling. 2000. Conserved regions in the Epstein-Barr virus leader protein define distinct domains required for nuclear localization and transcriptional cooperation with EBNA2. J. Virol. 74:9953-9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petti, L., C. Sample, and E. Kieff. 1990. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology 176:563-574. [DOI] [PubMed] [Google Scholar]
- 51.Pope, J. 1967. Establishment of cell lines from peripheral leukocytes in infectious mononucleosis. Nature 216:810-811. [DOI] [PubMed] [Google Scholar]
- 52.Pope, J. H. M., and W. Scott. 1968. Transformation of huiman foetal leukocytes in vitro by filtrates of a human leukaemic line containing Herpes-like virus. Int. J. Cancer 3:857-866. [DOI] [PubMed] [Google Scholar]
- 53.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, 4th ed., vol. 2. Lippincott, Williams and Wilkins, Philadelphia, Pa.
- 54.Robertson, E., and E. Kieff. 1995. Reducing the complexity of the transforming Epstein-Barr virus genome to 64 kilobase pairs. J. Virol. 69:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sample, J., M. Hummel, D. Braun, M. Birkenbach, and E. Kieff. 1986. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc. Natl. Acad. Sci. USA 83:5096-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seed, B. 1995. Developments in expression cloning. Curr. Opin. Biotechnol. 6:567-573. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair, A. J., I. Palmero, G. Peters, and P. J. Farrell. 1994. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 13:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sjoblom, A., A. Jansson, W. Yang, S. Lain, T. Nilsson, and L. Rymo. 1995. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J. Gen. Virol. 76:2679-2692. [DOI] [PubMed] [Google Scholar]
- 59.Sjoblom, A., W. Yang, L. Palmqvist, A. Jansson, and L. Rymo. 1998. An ATF/CRE element mediates both EBNA2-dependent and EBNA2-independent activation of the Epstein-Barr virus LMP1 gene promoter. J. Virol. 72:1365-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung, N. S., S. Kenney, D. Gutsch, and J. S. Pagano. 1991. EBNA-2 transactivates a lymphoid-specific enhancer in the BamHI C promoter of Epstein-Barr virus. J. Virol. 65:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szekely, L., K. Pokrovskaja, W. Q. Jiang, H. de The, N. Ringertz, and G. Klein. 1996. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J. Virol. 70:2562-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takada, K., K. Horinouchi, Y. Ono, T. Aya, T. Osato, M. Takahashi, and S. Hayasaka. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147-156. [DOI] [PubMed] [Google Scholar]
- 63.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomkinson, B., E. Robertson, and E. Kieff. 1993. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 67:2014-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong, X., R. Drapkin, D. Reinberg, and E. Kieff. 1995. The 62- and 80-kDa subunits of transcription factor IIH mediate the interaction with Epstein-Barr virus nuclear protein 2. Proc. Natl. Acad. Sci. USA 92:3259-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong, X., R. Drapkin, R. Yalamanchili, G. Mosialos, and E. Kieff. 1995. The Epstein-Barr virus nuclear protein 2 acidic domain forms a complex with a novel cellular coactivator that can interact with TFIIE. Mol. Cell. Biol. 15:4735-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong, X., F. Wang, C. J. Thut, and E. Kieff. 1995. The Epstein-Barr virus nuclear protein 2 acidic domain can interact with TFIIB, TAF40, and RPA70 but not with TATA-binding protein. J. Virol. 69:585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsang, S. F., F. Wang, K. M. Izumi, and E. Kieff. 1991. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J. Virol. 65:6765-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, F., C. D. Gregory, M. Rowe, A. B. Rickinson, D. Wang, M. Birkenbach, H. Kikutani, T. Kishimoto, and E. Kieff. 1987. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc. Natl. Acad. Sci. USA 84:3452-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang, F., H. Kikutani, S. F. Tsang, T. Kishimoto, and E. Kieff. 1991. Epstein-Barr virus nuclear protein 2 transactivates a cis-acting CD23 DNA element. J. Virol. 65:4101-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, F., L. Petti, D. Braun, S. Seung, and E. Kieff. 1987. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J. Virol. 61:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang, L., S. R. Grossman, and E. Kieff. 2000. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc. Natl. Acad. Sci. USA 97:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang, S., M. Guo, H. Ouyang, X. Li, C. Cordon-Cardo, A. Kurimasa, D. J. Chen, Z. Fuks, C. C. Ling, and G. C. Li. 2000. The catalytic subunit of DNA-dependent protein kinase selectively regulates p53-dependent apoptosis but not cell-cycle arrest. Proc. Natl. Acad. Sci. USA 97:1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westberg, C., J. P. Yang, H. Tang, T. R. Reddy, and F. Wong-Staal. 2000. A novel shuttle protein binds to RNA helicase A and activates the retroviral constitutive transport element. J. Biol. Chem. 275:21396-21401. [DOI] [PubMed] [Google Scholar]
- 76.Yalamanchili, R., X. Tong, S. Grossman, E. Johannsen, G. Mosialos, and E. Kieff. 1994. Genetic and biochemical evidence that EBNA 2 interaction with a 63-kDa cellular GTG-binding protein is essential for B lymphocyte growth transformation by EBV. Virology 204:634-641. [DOI] [PubMed] [Google Scholar]
- 77.Yang, J. P., H. Tang, T. R. Reddy, and F. Wong-Staal. 2001. Mapping the functional domains of HAP95, a protein that binds RNA helicase A and activates the constitutive transport element of type D retroviruses. J. Biol. Chem. 276:30694-30700. [DOI] [PubMed] [Google Scholar]
- 78.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]