Abstract
From April 2000 through September 2001, we studied 11 patients with the Jarvik 2000 —a left ventricular assist device with an axial-flow pump that provides continuous blood flow—to determine the echocardiographic characteristics. All patients underwent complete echocardiographic examination, including outflow-graft flow evaluation 24 hours after implantation and each month thereafter for the duration of support. Data were obtained at each pump setting (8,000–12,000 rpm in 1,000-rpm increments) and with the pump off.
Left ventricular dimensions and shortening fraction and the duration of aortic valve systolic opening decreased as pump speed increased. Although the aortic valve remained closed at higher pump speeds, pump outflow-graft flow remained pulsatile, because of the systolic thrust of the assisted ventricle. Systolic dominance of phasic flow was more pronounced at lower pump speeds, due to normalization of the diseased heart's Starling response. When the aortic valve was closed continuously, echocardiographic contrast (indicating blood stasis) was noted in the aortic root. Because of the pump outflow graft's proximity to the chest wall, device output could be measured independently of cardiac contributions. Mean peak outflow-graft flow velocities were 0.75 ± 0.30 m/s (systolic) and 0.41 ± 0.13 m/s (diastolic). When the pump was turned off briefly, there was minimal regurgitation through the device into the left ventricle.
This 1st echocardiographic heart function analysis of the Jarvik 2000 confirms that the device unloads the ventricle and increases cardiac output. Cardiac responses to device-speed changes can be evaluated readily with echocardiography in the early and late postoperative period.
Key words: Jarvik 2000, heart-assist devices, echocardiography, evaluation studies, heart failure, hemodynamics, imaging
Axial-flow pumps are a new generation of left ventricular assist devices (LVADs) that offer a number of advantages over conventional pulsatile pumps.1,2 Axial-flow pumps are small enough that they can be implanted as assist devices in a wide size-range of patients. Implantation is relatively simple, and complications are minimal. Axial-flow pumps generate constant unidirectional flow, obviating the need for heart valve prostheses with their attendant cost and risk of infection. Because these pumps are not positive-displacement (pulsatile) devices, they do not require a compliance chamber. They are powered by external wearable batteries and receive energy via small percutaneous drivelines. Axial-flow pumps are now being studied in clinical trials to evaluate durability, safety, and efficacy.
The Jarvik 2000 (Jarvik Heart, Inc.; New York, NY) is an axial-flow pump that is currently under clinical evaluation. The 55-mm-long cylindrical pump is 25 mm in diameter and is implanted in the left ventricular cavity. Implantation can be done via a limited low left lateral thoracotomy, a median sternotomy, or a left subcostal approach below the diaphragm. The outflow graft (Hemashield®, Boston Scientific Corporation; Natick, Mass) is 16 mm in diameter and is anastomosed to the ascending aorta, the descending thoracic aorta (the site used in this group of patients),3 or the supraceliac aorta via a subdiaphragmatic approach (Fig. 1). The Jarvik 2000 is designed to provide up to 7 L/min of flow at a maximum pump speed of 12,000 rpm under usual physiologic conditions. Because no clinically reliable integrated flow sensors are available, state-of-the-art echocardiography is vital in assessing device performance and overall cardiovascular hemodynamic variables. Herein, we summarize our echocardiographic findings in patients enrolled in a bridge-to-transplant protocol at our institution.
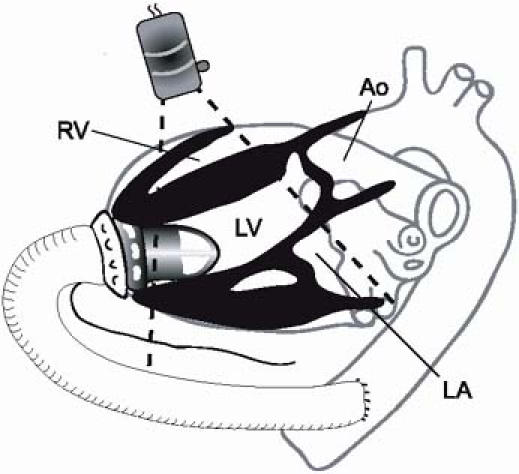
Fig. 1 Schematic representation of the Jarvik 2000. The axial-flow pump is implanted into the left ventricular cavity through a low left lateral thoracotomy. The outflow graft extends from the left ventricular apex to the descending thoracic aorta.
Ao = aorta; LA = left atrium; LV = left ventricle; RV = right ventricle
Methods
From April 2000 through September 2001, we evaluated 11 cardiac transplant candidates who had received a Jarvik 2000 LVAD because of end-stage cardiomyopathy. The protocol was approved by our institutional review board. Before receiving the device, all patients had given their informed consent for the trial. The series included 8 men and 3 women (mean age, 51.2 ± 8.7 [SD] years) who had severe, chronic cardiac failure. The cause was ischemic in 5 patients and nonischemic in 6 patients. Before pump implantation, the mean left ventricular end-diastolic volume was 338 ± 99 mL. In all patients studied, the cardiac index was <2 L/min/m2, and the pulmonary wedge pressure was >20 mmHg by thermodilution measurement using a Swan-Ganz catheter.
Transthoracic echocardiographic studies (Sequoia C265, Acuson, Mountainview, Calif; or HP Sonos 5500, Agilent, Andover, Mass) were performed 24 hours after device implantation and monthly thereafter for the duration of mechanical support (mean, 79 days; range, 13–214 days). Each study (28 total) included 2-dimensional (2D), color-flow (CF) Doppler, pulsed-wave (PW) Doppler, and continuous-wave (CW) Doppler evaluation from the parasternal, apical, subcostal, and suprasternal views. All images were obtained at each of the 5 pump settings (ranging from 8,000 to 12,000 rpm, in 1,000-rpm increments). After stabilization of the patients' clinical status, 24 “pump-off” studies were performed. (All patients had at least 1 study with the device off.) Simultaneous right-sided heart catheterization data were available for 9 patients.
M-Mode and 2-Dimensional Imaging
We used M-mode imaging of the aortic valve to assess the duration of aortic-valve opening.4 Using standard protocols,5 we performed 2D imaging to measure the left ventricular end-systolic and end-diastolic dimensions and fractional shortening, as well as the diameters of the left and right ventricular outflow tracts (LVOT and RVOT). We noted the right ventricular dimensions and function. We also confirmed apical positioning of the pump and visualized the outflow graft–aorta anastomosis in the parasternal long-axis view (Fig. 2A).
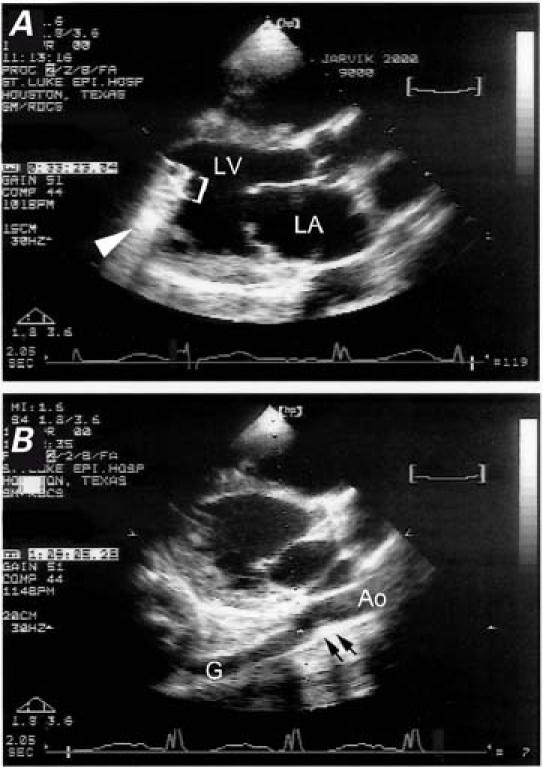
Fig. 2 Two-dimensional echocardiograms in the parasternal long-axis view in a patient with the Jarvik 2000 left ventricular assist device. A) The device (bracket) is visible inside the left ventricular cavity, and the reverberation artifact is indicated by the arrowhead. B) The outflow graft–descending thoracic aorta anastomosis (arrows) is visible behind the left atrium.
Ao = descending thoracic aorta; G = graft; LA = left atrium; LV = left ventricle
Doppler Imaging
With Doppler methods, we obtained the LVOT and RVOT PW-derived velocity-time integrals (VTIs) at all pump settings and used them to calculate the left- and right-sided stroke volume (SV) and cardiac output (CO).6 The severity of valvular regurgitation was evaluated with CW and CF Doppler methods. The systolic pulmonary artery pressure was calculated by using the peak velocity of tricuspid regurgitation.
To measure the flow velocity in the outflow graft, we aligned the PW Doppler sample volume with graft flow at a site 1 cm proximal to the aortic anastomosis (Fig. 2B). We recorded the VTIs of the systolic and diastolic flow through the outflow graft, as well as the peak systolic and diastolic flow velocities. For all measurements, we took care to minimize the angle of incidence between the actual flow and the interrogating Doppler beam.
For indirect estimation of the pump flow, we used the formula LVAD CO = (RVOT CO) − (LVOT CO), wherein RVOT CO represents the total right-sided CO and LVOT CO represents the total output through the aortic valve.
The regurgitant fraction through the deactivated pump was calculated with the following formula: Regurgitant Fraction = (Graft CSA) × (Diast Regurg VTI)/(RVOT CO), wherein Graft CSA is the outflow graft's cross-sectional area and Diast Regurg VTI is the PW Doppler-derived VTI of regurgitant diastolic flow through the outflow graft during a single cardiac cycle.
Results
M-Mode and 2-Dimensional Imaging
Good-to-excellent–quality M-mode and 2D images of the heart were obtained from most views in all examinations, even when performed on the day after device implantation. As the pump speed increased from baseline to 12,000 rpm, the left ventricular end-diastolic diameter decreased from 71 ± 8 to 60 ± 10 mm, and the left ventricular end-systolic diameter decreased from 63 ± 7 to 54 ± 10 mm.
The duration of aortic valve systolic opening decreased as the pump speed increased (Fig. 3), correlating directly with the LVOT SV and inversely with the RVOT SV. In most studies, aortic valve systolic opening ceased at higher speeds (Fig. 4). The degree of pulsatility of flow in the outflow graft did not predict the absence of systolic aortic valve opening.
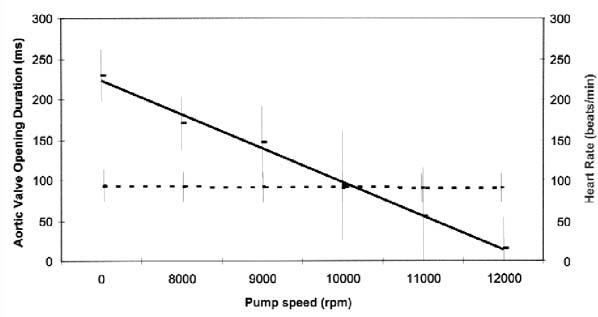
Fig. 3 Graph shows the duration of aortic valve opening at increasing pump speeds in patients with the Jarvik 2000. In all 11 patients (28 studies), the average duration decreased progressively with increasing pump speeds, correlating inversely with the total cardiac index. No significant change in the heart rate was noted.
Continuous line = average aortic valve opening duration; broken line = heart rate
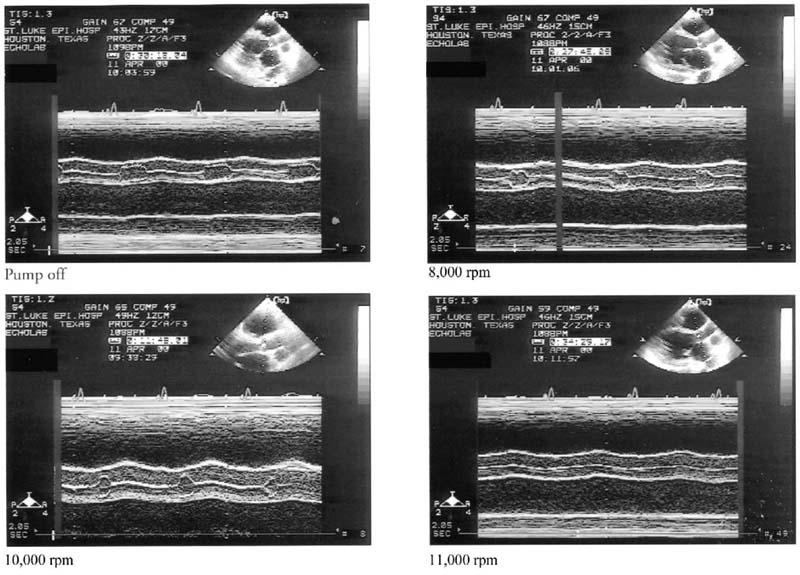
Fig. 4 M-mode evaluation of the aortic valve in a patient with the Jarvik 2000. In pump-off mode, the aortic valve opening was maximal. Between 8,000 and 10,000 rpm, the duration of aortic valve opening decreased progressively. The valve was closed at 11,000 rpm.
When the aortic valve was closed during systole, we noted spontaneous echocardiographic contrast in the sinuses of Valsalva and even in the ascending aorta, which suggested stagnation of blood flow in these areas. Upon reduction of the pump speed, phasic aortic valve opening resumed, and the spontaneous contrast was dispelled within a few cardiac cycles. In 1 patient who had minimal aortic insufficiency, we observed a small jet of spontaneous contrast regurgitating into the left ventricle. The same patient later developed visible thrombus in the noncoronary sinus of Valsalva. This patient had almost a complete loss of left ventricular systolic function, and the aortic valve was closed during systole even at the lowest pump speed.
Apical and parasternal imaging of the pump in the left ventricle was possible in all 11 patients. Despite a significant shadowing artifact related to the mechanical device, we could adequately evaluate all cardiac structures and the regional left ventricular wall motion (with the exception of the apex, which was occupied by the LVAD). In 9 of 10 patients, the parasternal long-axis view revealed the outflow graft-to-descending thoracic aorta anastomosis posterior to the left atrium (Fig. 2B). In 2 patients, the graft was also visible in the apical long-axis view.
Doppler Imaging
During pump operation, the CF, PW, and CW Doppler studies were successful but were variably affected by a device-associated artifact, seen as a dense field of mosaic-like color downfield from the device (Fig. 5). This artifact became worse at higher pump speeds. In 1 patient, it interfered significantly with CF Doppler assessment of the severity of mitral and tricuspid regurgitation in the apical views. Despite the artifact, in 2 other patients who had moderate mitral regurgitation at baseline, we were able to document a progressive and significant reduction in the regurgitation, which almost disappeared at the highest pump setting (12,000 rpm). None of the patients had significant aortic or pulmonary insufficiency. By manipulating the imaging window and the PW sample volume size and position, we could successfully evaluate Doppler flow signals from the LVOT, RVOT, and outflow graft. Flow sampling of the pulmonary vein, however, was not usually possible. We were able to measure the peak tricuspid regurgitation velocity in 19 studies (68%) when the pump was on and in all studies when the pump was off (when the high-frequency Doppler artifact disappeared).
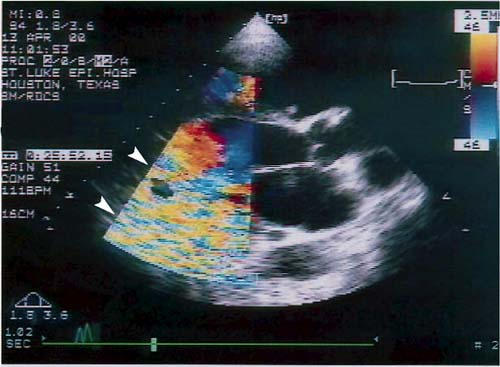
Fig. 5 Two-dimensional echocardiogram with color-Doppler sector over the device shows a device-generated, high-frequency presumed reverberation artifact (arrowheads), which variably affected the quality of Doppler imaging.
The RVOT SV and CO increased at all pump settings compared with baseline and increased significantly upon each increment in speed (Fig. 6). By means of linear regression, we calculated that the total CO increased by 290 mL/min for each 1,000-rpm increase in pump speed over 8,000 rpm (r2 = 0.95). Simultaneously, the LVOT SV decreased progressively and became zero in several studies, correlating with the duration of aortic valve opening. In 1 patient, LVOT output variations suggestive of pulsus alternans appeared at the highest pump setting at which the aortic valve still opened.
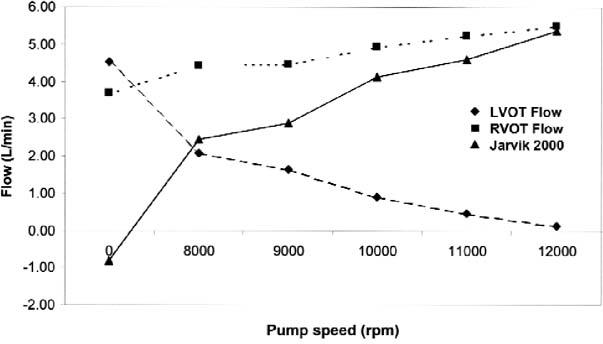
Fig. 6 The effects of the pump speed on the total cardiac output (right ventricular outflow-tract flow), aortic valve output (left ventricular outflow-tract flow), and pump flow in 11 patients with the Jarvik 2000 left ventricular assist device. All values are averages derived from 28 studies in 11 patients and are corrected for body surface area. As the pump speed increased, the pump flow and total cardiac output increased, while flow through the aortic valve decreased. In many cases, the aortic valve remained closed at high pump speeds, and the entire cardiac output occurred through the device.
Device flow, indirectly calculated as the difference between RVOT and LVOT flow, increased progressively with higher pump speeds (Fig. 6). By means of linear regression, we calculated that pump flow increased by 755 mL/min for each 1,000-rpm increase in pump speed over 8,000 rpm (r2 = 0.98).
Flow in the Outflow Graft
During Pump Operation
During pump operation, adequate analysis of graft flow was possible in 9 of the 11 patients. When the pump was on, we demonstrated phasic laminar outflow-graft CF and PW Doppler flow velocities in all 9 assessable patients. We made efforts to ensure that the graft flow and the Doppler sample volume were coaxially aligned. Systolic dominance of phasic flow was more pronounced at lower pump speeds (Fig. 7A). The systolic/diastolic VTI ratios and peak velocities became significantly less as the pump speed increased. Concomitantly, the mean blood pressure progressively increased, and the pulse pressure decreased (Fig. 7B). At the maximum pump speed, the mean peak systolic velocities were 0.75 ± 0.30 m/s, and the mean peak diastolic velocities were 0.41 ± 0.13 m/s.
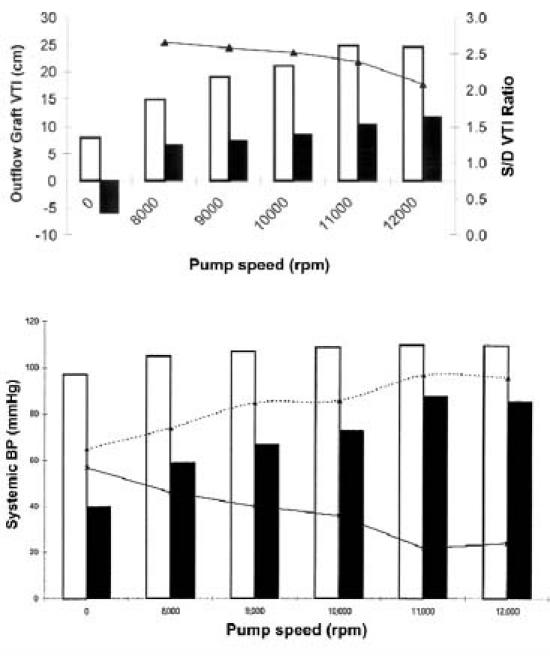
Fig. 7 Effect of the pump speed on the outflow-graft flow and blood pressure in patients with the Jarvik 2000. A) In 9 patients (20 studies), the average velocity–time integrals (VTIs) of diastolic, systolic, and total outflow-graft flow increased progressively as the pump speed increased, while the average S/D VTI ratio decreased. (S/D = systolic/diastolic; continuous line = systolic/diastolic VTI ratio; clear bars = systolic VTI; filled bars = diastolic VTI) B) The average systolic, diastolic, and mean blood pressures (BP) increased progressively and the average pulse pressure decreased as the pump speed increased. (Dotted line = mean blood pressure; continuous line = pulse pressure; clear bars = systolic blood pressure; filled bars = diastolic blood pressure)
The outflow graft's total VTI increased progressively with increments in the pump speed and was directly proportional to the indirect estimate of device flow (LVAD CO = RVOT CO − LVOT CO). In some patients, there was a discrepancy between the direct calculation of graft flow (using the cross-sectional area of the graft and the graft VTI) and the indirect estimate of the LVAD flow. This discrepancy was possibly due to 1) inability to align the CW Doppler interrogation beam parallel to the outflow graft, causing underestimation of the outflow-graft VTI, 2) overlapping of the images of the outflow graft, causing overestimation of the graft's cross-sectional area, and 3) errors in measuring the RVOT diameter.
In 2 of the 11 patients, adequate analysis of graft flow was not possible: in 1 of these patients, the anastomosis was made at the level of the abdominal aorta; and in the other, the echocardiographic image was of poor quality.
During Pump-Off Testing
In 3 of the 9 assessable patients, although an adequate outflow-graft Doppler signal was visible during pump operation, the signal was too weak (probably because of very low flow through the graft) for adequate evaluation when the pump was turned off. In the other 6 patients, we observed complex antegrade and retrograde graft flow (Fig. 8) during pump-off testing. Systolic flow was predominantly antegrade (toward the aorta), and diastolic flow was predominantly retrograde (toward the ventricle).
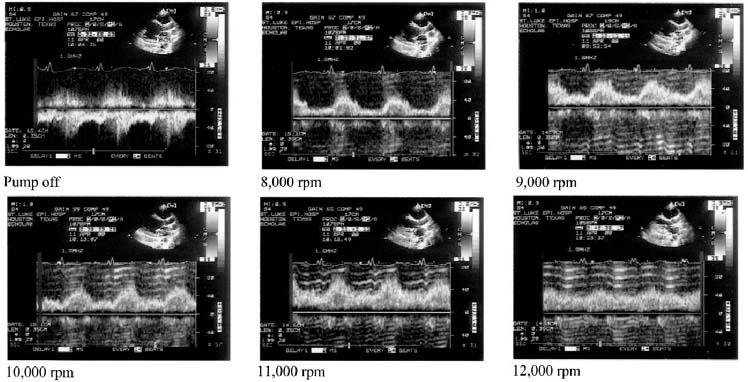
Fig. 8 Pulsed-wave Doppler profile of flow through the outflow graft, while the pump was off and at all pump speeds (8,000–12,000 rpm), in a patient with the Jarvik 2000 left ventricular assist device. When the pump was off, systolic flow was predominantly antegrade, toward the aorta. During diastole, there was low-velocity retrograde flow toward the left ventricle. In this patient, the estimated regurgitant fraction was 30%.
Because the Jarvik pump is valveless, diastolic regurgitation occurs through the outflow graft into the left ventricle when the device is not operating. In the 6 patients that could be assessed, the estimated regurgitant fractions were 6%, 8%, 9%, 12%, 26%, and 30%, respectively. Most of these values would correspond to mild aortic regurgitation.
Discussion
In our experience, echocardiography has been a valuable tool for assessing and managing the Jarvik 2000 LVAD, even during the early postoperative period. Because standard echocardiographic practices were used for these 11 patients, experienced clinical sonographers easily learned the protocol. However, they had to pay careful attention to excluding an unusual device-associated reverberation artifact from basal regions when conducting Doppler interrogations. In this early series, the unique Doppler information acquired from the outflow graft depended on the use of the descending thoracic aorta as the anastomosis site. When the outflow graft-to-ascending aorta approach is used, direct measurement of outflow-graft flow is not technically possible because of the attendant sternotomy wound and the absence of coaxial Doppler measurement windows. In our patients, the left lateral thoracotomy approach permitted coaxial alignment of our Doppler interrogating beam from parasternal windows even during the early postoperative period. This enabled us to characterize outflow-graft flow pulsatility at varying pump speeds and reverse flow characteristics in the pump-off mode. Extending the outflow graft from the apex of the left ventricle allows excellent access for imaging windows. The outflow graft–descending thoracic aorta anastomosis site allows not only access for imaging windows but also accurate PW Doppler measurements in the outflow graft.
Compared with our previous imaging experience using traditional, pusher-plate LVADs, overall cardiac imaging is improved by the small size of the Jarvik 2000. Echocardiographic evaluation of traditional LVADs is further complicated by the difficulty of obtaining adequate Doppler signals in both the inflow and outflow cannulas. Proper alignment with flow in these large, pulsatile devices is frequently a problem. The ungated, forceful, pulsatile flow in traditional LVADs is dissociated from native ventricular function, complicating hemodynamic evaluation. In contrast, the continuous flow of the axial-flow pump produces steady-state hemodynamic conditions, allowing easy evaluation with standard echocardiographic techniques. Although the Jarvik 2000 axial-flow pump operates continuously in the in vitro setting, its placement in the contracting left ventricle results in a continuous relationship between the inflow and outflow pressures. This causes a constant variation in pump flow even when the aortic valve is not open. The higher the pump speed, the lower the impact of left ventricular systole on device flow. This finding correlates with the reduction in systemic pulse pressure observed in our series.
In patients with severe cardiomyopathy and concomitant enlargement of the left ventricle, a reservoir for the device's inflow conduit is usually present. In such patients, the Jarvik 2000 axial-flow pump generates outputs comparable with those seen in animal and in vitro studies of this device—that is, the calculated total cardiac output increases in a linear relationship to the pump speed. If the ventricular reservoir is inadequate, however, this increase will be accompanied by low cardiac output and minimal nonpulsatile arterial pressure. Under these conditions, the increasing pump speeds will only further decrease right ventricular function and left ventricular filling, and the cardiac output will decrease rather than increase.
When the reservoir is adequate and the pump is functioning normally, indirect calculation of the output through the pump (LVAD CO) is equal to the difference between the outputs through the RVOT and the LVOT. Using PW Doppler techniques, we found a constant relationship between indirect and direct flow measurements in all patients. Difficulty in achieving co-axial Doppler interrogating beam alignment with the graft blood flow vector and errors in calculating the graft area probably explain the lack of exact agreement between these 2 methods.
When the device was turned off, there was forward flow into the aorta during systole and regurgitant flow into the left ventricle during diastole, as in mild aortic insufficiency. None of our patients developed clinical signs of decompensation related to acute aortic insufficiency. Because the pump was never turned off for more than 10 minutes, we were unable to evaluate the long-term effects of the regurgitation and increased ventricular volume loading.
The presence of spontaneous echocardiographic contrast in the aortic root at high pump speeds, when the aortic valve is closed throughout systole, can be corrected by lowering the output of the pump and enabling the ventricular contractility to open the aortic valve. In addition to reducing the risk of thrombus formation, the presence of a pulse with the reduced flow of the Jarvik 2000 ensures an adequate reservoir for device function. The main clinical adversity encountered in this new technology has resulted from over-aggressive unloading of the ventricle, which can lead to a downward spiral of low cardiac output and death. At optimal use, this device maintains pulsatility and achieves normal blood flow in the circulatory system.
We observed an unusual device-associated spectral and color-Doppler artifact that likely was attributable to impeller reverberation, since it was observed only in the imaging sector regions immediately downfield from the device. It was prominent in all the Doppler modes and was not significant in the 2D imaging modes. This artifact slightly hindered Doppler evaluation, particularly the CW studies of tricuspid valve flow and the PW signals from the pulmonary veins. Because of the artifact, the systolic pulmonary artery pressure could be estimated in only two thirds of the studies. Evaluation of the severity of mitral regurgitation in the apical views was occasionally difficult. However, decreased functional mitral regurgitation was observed in some patients at higher pump speeds, presumably because of reduced left ventricular chamber size with increased left ventricular assistance. Pulsed-wave Doppler signals were minimally affected and could be adequately interpreted with the exception of signals from pulmonary venous flow.
Summary
The Jarvik 2000 is a promising new device for treating patients with heart failure. Our echocardiography-derived flow data, obtained at varying pump speeds and in the pump-off setting, demonstrated that the axial-flow pump is well suited for use as a true left ventricular assist device. Echocardiography spectral Doppler patterns clearly showed that, although the device operates continuously, a degree of characteristic phasic aortic flow is maintained because of an unimpeded left ventricular systolic contribution. By unloading the failed left ventricle, the pump improves native cardiac function. By working synchronously with the natural heart, this device enabled many of our study patients to resume symptom-free and active lives.
Echocardiography is optimally suited for evaluating the performance of the Jarvik 2000. In our study, standard echocardiographic techniques allowed accurate measurement of overall systemic cardiac output (the RVOT CO calculation). By subtraction of the LVOT CO from the RVOT CO, we were able to measure the device flow indirectly and correlate it with direct device flow measurements obtained from outflow-graft Doppler measurements. Aortic valve-opening observations allowed appropriate pump speed selection for prevailing loading conditions. We also measured systolic pulmonary artery pressure and valve regurgitation lesions. Mild regurgitation through the deactivated device was documented by echocardiography and was tolerated by patients in the setting of a period of left ventricular recovery.
Acknowledgments
The authors thank Kathy Miller, BS, for helping to coordinate the research for this study; and Sue Maisey, RDMS, and Igor Rodenko, RDMS, for their skilled ultrasonography assistance.
Footnotes
Address for reprints: Raymond F. Stainback, MD, FASE, 6624 Fannin, Suite 2480, Houston, TX 77030
Dr. Croitoru is now with Piedmont Cardiology Associates, PA, Green-wood, South Carolina.
References
- 1.Jarvik RK. System considerations favoring rotary artificial hearts with blood-immersed bearings. Artif Organs 1995; 19:565–70. [DOI] [PubMed]
- 2.Nose Y, Tsutsui T, Butler KC, Jarvik R, Takami Y, Nojiri C, et al. Rotary pumps: new developments and future perspectives. ASAIO J 1998;44:234–7. [DOI] [PubMed]
- 3.Parnis SM, Macris MP, Jarvik R, Robinson JL, Kolff JW, Anai H, et al. Five month survival in a calf supported with an intraventricular axial flow blood pump. ASAIO J 1995; 41:M333–6. [DOI] [PubMed]
- 4.Picard MH. M-mode echocardiography: principles and examination techniques. In: Weyman AE, editor. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lea & Febiger; 1994. p. 282–301.
- 5.Weyman AE. Standard plane positions—standard imaging planes. In: Weyman AE, editor. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lea & Febiger; 1994. p. 98–123.
- 6.Marshall SA, Weyman AE. Doppler estimation of volumetric flow. In: Weyman AE, editor. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lea & Febiger; 1994. p. 955–78.


