Abstract
In open-heart surgery, sternal wound infection after median sternotomy is a critical complication. The intracutaneous suture is conventionally used in closing sternal incisions. In this prospective, randomized, controlled clinical trial, intracutaneous and transcutaneous suture techniques for closing the sternum were compared with respect to postoperative sternal wound infections and cosmetic results.
In this study, we included 100 patients who had undergone open-heart surgery. Skin wounds were closed with intracutaneous suture in 50 patients and with transcutaneous suture in the remaining 50. Superficial or deep sternal infections that developed within 6 postoperative weeks were evaluated.
Cosmetic results were similar in the 2 groups. Deep wound infections were not observed in either group. Superficial infection of postoperative sternal wounds occurred at rates of 2% (n = 1) and 16% (n = 8) for transcutaneous and intracutaneous techniques, respectively (P = 0.016). One patient in the transcutaneous group and 6 patients in the intracutaneous group who developed superficial sternal infections were diabetic.
Although the use of the transcutaneous suture technique in closing sternal incisions of cardiac surgery patients provided no cosmetic improvement, it decreased the risk of superficial sternal infection and reduced the length of postoperative hospital stay, particularly in diabetic patients.
Key words: Comparative study, diabetes complications, drainage, mediastinitis/etiology, postoperative complications/prevention & control, prospective studies, risk factors, sternum/surgery, suppuration, surgical wound dehiscence, surgical wound infection/prevention & control, suture techniques
Sternal wound infections after median sternotomy in open-heart surgeries are a major complication; they are associated with extended hospital stay, increased hospital costs, and higher mortality and morbidity rates.1–4
The incidence of mediastinal wound infection in patients undergoing median sternotomy for cardiopulmonary bypass has been reported in various studies to be between 0.9% and 20%.5 Sternal dehiscence concomitant with mediastinal or sternal infection is observed in 0.4% to 5% of patients. Mortality rates in these patients are 13% to 39.6%.6,7 A study carried out in 2000 by Puc and colleagues8 on 1,039 patients established that the incidence of morbidity in association with sternal wounds was 2.4%, while 6 out of 19 patients with sternal wound infections experienced dehiscence.
Severity can range from superficial wound infections, which do not reach the sternum, to fulminant mediastinitis that involves the sternum, heart, and major arteries.1,2 While superficial infections are limited to the skin and subcutaneous tissues, deep infections involve sternal osteomyelitis, sternal dehiscence, and mediastinitis.9,10 The rate of deep sternal infection after open-heart surgery is about 0.2% to 2.9%.1–4 Staphylococci are the most important pathogens in sternal infections.1,2,4 The development of sternotomy complications is multifactorial,11 and many studies demonstrate the variables in patients and procedures.11–15
In this study, we compared intracutaneous (IC) and transcutaneous (TC) suture techniques in open-heart surgery with respect to superficial and deep sternal wound infection development, pathologic agents, therapy, cosmetic results, and possible risk factors.
The transcutaneous suture technique can also be described as an interrupted mattress suture technique. The intracutaneous technique is alternatively called a subcuticular suture technique.
Patients and Methods
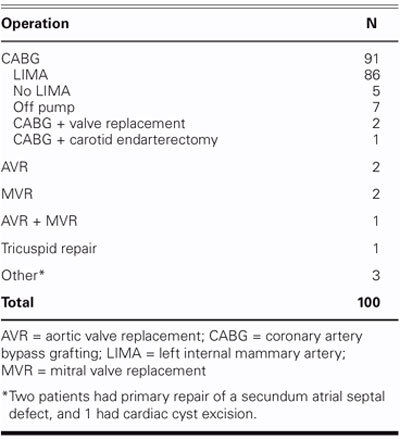
One hundred consecutive patients who were undergoing open-heart surgery at our institution from October 2002 through March 2004 were enrolled in this prospective randomized trial. The approval of the Ethics Committee of Dokuz Eylul University was obtained (Ethics Committee Protocol No. 211), and informed consent forms were signed by all the patients before the study began. Sixty-nine men and 31 women (mean age, 64.52 ± 10.44 years; range, 19–82 years) were enrolled. Patients who were on immunosuppressive or steroid therapy, who were obese (body mass index ≥30), or who had insulin-dependent diabetes mellitus were excluded. There were 31 men (62%) and 19 women (38%) in the TC group (mean age, 64.82 ± 11.32 years; range, 19–76 years), and the IC group had 38 men (76%) and 12 women (24%) (mean age, 61.14 ± 10.08 years; range, 24–82 years). Of the patients, 89 underwent coronary artery bypass grafting (CABG); all surgical procedures are listed in Table I.
TABLE I. Surgical Procedures Undertaken in 100 Sternotomies
Surgical Techniques
Before surgery, each patient took a chlorhexidine hydrochloride (4%) shower for preoperative skin preparation. After the operative site was washed with 4% chlorhexidine in the operating room, it was further treated against cutaneous infection with Poviiodeks Antiseptic (KIM-PA; Istanbul, Turkey), which contains 10% polyvinylpyrrolidone-iodine complex (a solution chemically identical to Betadine); then it was covered with a sterile drape.
Anesthesia was induced by diazepam and fentanyl and was carried out with fentanyl, midazolam, isoflurane, and nitrous oxide. The skin was incised with a sterile lancet, and pericardial and presternal tissues were cut by electrocauterization. Bone wax was used in only 3 patients in each group, for a total of 6 patients who had sternal bleeding after sternotomy. The surgical approach for all of the patients consisted of median sternotomy, cardiopulmonary bypass (CPB), and moderate systematic hypothermia (28–32 °C). While ice slush was used routinely for topical cooling, 72% of the patients were given crystalloid cardioplegic solution, and the remaining 28%, cold blood cardioplegic solution. All CPB patients were heparinized with non-fractionated heparin and were oxygenated by means of a Dideco D-708 membrane oxygenator (Simplex; Mirandola, Italy). An initial heparin dose of 3 mg/kg was given until the activated coagulation time (ACT) reached 480 seconds. After CPB, protamine sulphate was given (10 mg/mL) until preoperative ACT values could be achieved. Heparin and protamine quantities, ACT values, mediastinal blood loss, and transfused blood volumes were recorded during the first 3 postoperative days for all of the patients. All patients underwent standard placement of mediastinal and left pleural chest drains.
In both groups, steel wires were used for closing the sternum, and 2 layers of polyglactin 910 suture (Vicryl 2.0, Ethicon, Inc.; Somerville, NJ) were used to close subcutaneous tissues. We selected at random 50 patients to undergo IC and 50 patients to undergo TC. Intracutaneous closure was performed with polycaprolate absorbable polyfilament (Dexon 4.0, United States Surgical Corporation; Norwalk, Conn). Transcutaneous closure was performed with nonabsorbable monofilament (Prolene 4.0, Ethicon, Inc.; Somerville, NJ).
In the transcutaneous suture technique, the surgeon starts the suture at a point at least 1 cm from the edge of the incision. Then, as deep a bite as possible is performed, in order to hold both the cutaneous and subcutaneous tissues. After the suture comes through the contralateral side, a small bite is taken in proximity to the incision edges, to complete the suture. The nonabsorbable transcutaneous sutures were removed on the 14th day postoperatively.
Antibiotic therapy was as follows: an initial 1-g dose of intravenous (IV) cephazolin, a 1st-generation cephalosporin, was administered 30 minutes before the operation, followed by 1-g IV cephazolin every 8 hours. Those patients who were allergic to penicillin received 1-g IV vancomycin every 12 hours, until their drains were removed.
Postoperative wound inspection was done regularly every day during the first 5 days, at the end of the 2nd week, and at the end of the 6th week. The cosmetic results were evaluated at the end of the 6th postoperative week. Wound infections were evaluated according to the specific wound site evaluation scheme.16 Each wound was assigned a score of 0 to 7, in which 0 was the optimal postoperative wound. The wound site evaluation scheme is as follows: Grade 0: Optimal wound appearance; Grade 1: One infection finding (erythema, edema, increased pain); Grade 2: Two infection findings or hemo-serous discharge; Grade 3: Three infection findings or hemo-serous discharge; Grade 4: Two infection findings and hemo-serous discharge; Grade 5: Three infection findings and hemo-serous discharge; Grade 6: Two infection findings and purulent discharge; and Grade 7: Three infection findings and purulent discharge.1
The following criteria for identifying deep sternal wound infections and mediastinitis, as reported by the Centers for Disease Control and Prevention,17 were considered:
Isolation of an organism from a culture of non-purulent mediastinal tissue or fluid
Observation of mediastinitis during operation
Isolation of a microorganism from blood or from a culture of purulent mediastinal drainage fluid accompanied by chest pain or sternal instability or fever above 38 °C.9
Hematologic and biochemical values were assessed for all the patients in each group, both preoperatively and postoperatively. C-reactive protein and white blood cell values were checked frequently, and wound smear and aspiration cultures were obtained from patients who developed infections. An agar plate that contained hematin, blood, cysteine, lactose, and electrolyte was used for the culture. The proliferation was evaluated after 2 days of incubation in CO2 at 35 °C. Tissue cultures were incubated for 5 days in thioglycolate. Upon detection of bacteria, agar plates were used with antibiotic disks to establish antibiotic resistance. Antibiotic therapy was initiated in accordance with the results of an antibiogram and after consultation with infectious disease specialists.
Follow-up was completed in 100% of the 100 patients. Patients were informed regarding complications that might develop after being discharged. Wound sites were evaluated by the same surgeon who performed the operation. Similarly, follow-up evaluation of all patients was carried out by the same surgeon during hospital appointments, and the study was completed at the end of the 6th week.
Statistical Methods
The χ2 test and Fisher's exact test were used for univariate analysis, and Student's t-test was used for continuous variables. The major findings evaluated were the total numbers of infected patients, of superficial wound infections, and of patients with diabetes mellitus. We used a multivariate logistic model for predicting the efficacy and determining the risk factors associated with both therapeutic approaches.
Results
In each group, 2 early deaths (4%) were observed, and those patients were excluded from the study. Preoperative (Table II), operative (Table III), and postoperative (Table IV) values were recorded and analyzed statistically. Differences between the 2 groups were compared.
TABLE II. Demographic Characteristics of 100 Cardiac Surgery Patients
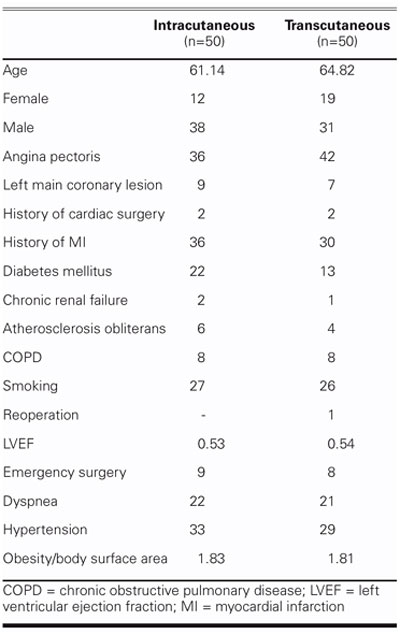
TABLE III. Operative Information on 100 Cardiac Surgery Patients
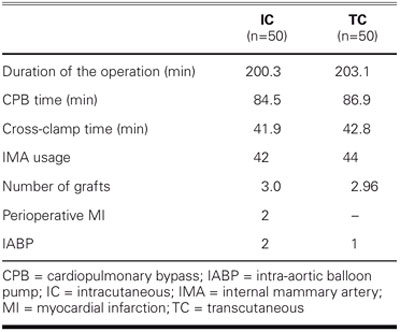
TABLE IV. Postoperative Information on 100 Cardiac Surgery Patients
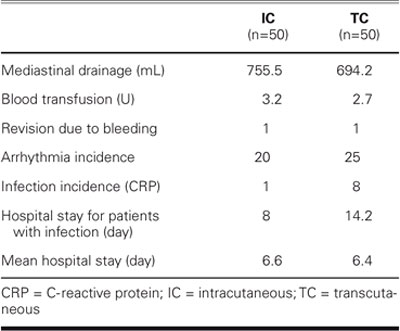
Postoperatively, patients were observed in the cardiovascular intensive care unit until they were hemodynamically stable and extubated. The drains were retained until drainage was less than 100 cc in 12 hours. Wound inspection was performed daily, and infection findings were assessed clinically on the 5th postoperative day. The total incidence of early wound infection for the 6-week follow-up period was 2% for the TC group and 16% for the IC group (P = 0.016). No late wound infections were observed in either group.
Six of the 8 IC group patients who developed superficial wound infections were diabetic. Six (27.3%) of the 22 diabetic patients in the IC group developed superficial wound infections, whereas only one (7.7%) of the 13 diabetic patients in the TC group was observed with a superficial wound infection. This patient, a woman, was also the only patient in the TC group who developed a superficial infection. Three of the 8 patients in the IC group who developed superficial wound infections were women, but sex was not observed to be a significant factor regarding superficial wound infections. On the other hand, the incidence of wound infection was higher in diabetic patients in the IC group than in the TC group (P = 0.007). The mean ages of the patients with superficial sternal infection were comparable for the 2 groups, and all patients with such infection had undergone CABG by means of a left internal mammary artery (LIMA) graft. The infection score for the only patient in TC group who developed a superficial wound infection was 1. In the IC group, the infection scores of 1 (3 patients), 2 (2 patients), and 3 (3 patients) were higher.
Deep wound infection was not observed in either group, and reoperation was not needed for sternal wound stabilization. Three of the 8 patients who developed superficial wound infections in the IC group had microbiologic proliferation in their wound cultures: 1 had Staphylococcus aureus, and 2 had Pseudomonas aeruginosa. The remaining 5 patients in the IC group and 1 patient in the TC group did not have proliferation. Patients who had proliferation of pathogenic microorganisms were placed on an antibiotic regimen set by our infectious diseases clinic. Only 2 patients in the IC group required wound revision, both on the 7th day. Both of these patients were diabetic; their wounds were débrided and resutured by primary suturing. Cosmetic scores for these 2 patients who required revision were 8 and 9 at the end of the 6th week after the revision. At the end of the 6th postoperative week, cosmetic scoring was performed by assigning a number from 1 to 10 (with 10 as the best score). Visual scores by the patients themselves averaged 8.7 in the IC group and 8.5 in the TC group, whereas visual scoring by the surgeons who carried out the monitoring averaged 8.4 and 8.6 for the IC and TC groups, respectively. The cosmetic results assessed by the patients and by the surgeons were not significantly different between the IC and TC groups. The patient with superficial infection in the TC group was discharged on the 8th postoperative day; the mean hospital stay for the 8 patients in the IC group was 14.2 days (range, 9–18 days).
Discussion
Limited information exists in the literature regarding superficial infections in open-heart surgery. The most comprehensive study was carried out by Risnes and coworkers.10 The pathogenesis of postoperative sternal infection is rather complex, involving multiple variables.1–4,12,18,19 In any event, the skin closure technique is a major risk factor.6 The results established in our study of 100 patients (9% sternal infection rate; n = 9) are consistent with the mediastinal wound infection rates of 0.9% to 20% reported in the literature for cardiac surgery patients who undergo median sternotomy.5 Our infection rate in the IC group was rather high, when compared with most reported series, but our overall rate of 9% for all 100 patients (2% for TC group and 16% for IC group) was certainly acceptable.
It has been proposed that serious noncardiac underlying diseases are the determining factors behind deep and superficial sternal infections.1,2,6,19 Various other studies have indicated that the use of strong antimicrobial agents in complex cardiovascular operations may be an important risk factor.20,21 Left ventricular ejection fraction (EF), the duration of preoperative hospital stay, a history of myocardial infarction (MI), angina pectoris, dyspnea, and presence of endocarditis are not critical risk factors in the development of postoperative sternal infections.10 There is no relationship between New York Heart Association (NYHA) functional class and infection rates,10 nor do duration of the operation, CPB time, and cross-clamp time correlate with the incidence of infection.10 Several other variables, such as prolonged ventilation time, tracheotomy, reexploration of the wound because of bleeding, number of blood transfusions, and postoperative MI, also have no impact on the incidence of infection.10 In our series, none of the preoperative, perioperative, and postoperative factors presented in Tables II–IV—other than diabetes mellitus—affected superficial sternal infection in either group.
As might be expected, we found diabetes mellitus to be the major factor for the development of superficial sternal wound infections. A more interesting finding is that 27.3% of the diabetic patients in the IC group developed superficial sternal wound infections, while only 7.7% of the diabetic patients in the TC group developed such infections. The incidence of superficial sternal wound infections was therefore 3.5 times higher among the diabetic patients of our IC group. It is well known that diabetes increases the infection risk through its effects on cellular and humoral immunity.22 At first glance (Table II), a higher ratio of diabetes mellitus patients is seen in our IC group than in our TC group. This, however, can lead a misunderstanding in comparing the groups. Fisher's exact test shows the difference to be statistically insignificant (P = 0.093). Also, we repeated Fisher's exact test after removing the 35 patients with diabetes. The result yielded a P value of 0.362, which was also insignificant. Deep sternal wound infection was found to be higher among men2 in a multicenter randomized trial conducted by the Parisian Mediastinitis Study Group23 and in other studies.2,11 Furthermore, bilateral internal thoracic artery (ITA) grafting increases deep sternal wound infection risk by decreasing sternal blood circulation;7,24,25 in diabetic patients, it can also lead to sternal nutritional problems. Radial, inferior epigastric, or gastroepiploic artery grafts can be used in diabetic patients, as alternatives to the ITA.
Deep sternal infection (mediastinitis) that develops after cardiac surgery is a major postoperative complication,10 which has an occurrence rate of 0.16% to 5%.1–4,9 Mortality rates vary from 14% to 47%.7,24 Sternal dehiscence can lead to deep wound infections by facilitating bacterial translocation. Osteomyelitis is a common sequela of dehiscence. Moreover, the absence of mediastinal drainage can create a suitable culture medium for translocated bacteria.2–4 Sternal infection doubles hospital costs.5 Because we had no incident of deep sternal infection or sternal dehiscence, no surgical intervention was required for this in our 100-patient cohort study.
When Risnes and colleagues10 compared the 2 suture techniques, the IC technique had significantly higher total infection and superficial wound infection than did the TC technique. Blood pooled in the available dead zone at the sutured surgical site often promotes postoperative wound infection.10 It has been reported that TC suturing minimizes the dead zone, thereby decreasing the wound infection incidence.26 Intracutaneous suturing, on the other hand, tends to hinder wound drainage, which can cause subdermal hematoma and superficial infections.10 Bacterial adhesion to surgical suture is also a critical factor in postoperative wound infection development.10 In general, bacteria with low virulence, such as Staphylococcus epidermidis, exhibit high adhesion capacity to biomaterials.27,28
Intracutaneous suturing may promote the passage of superficial bacteria to the suture and tissue under the skin. Adhesions here may facilitate interfilamentous diffusion and protection from phagocytosis, thereby enabling infection to develop easier. Absorbable sutures need oxygen in order to dissolve, and when the oxygen supply is not adequate, that too can promote postoperative wound infections.7,16,26 The relationship between the prevention of postoperative phagocytosis and the easier survival of bacteria is of critical importance in wound healing and in susceptibility to infections.26,27,29 Healing of the surgical wound and connective tissue depend on fast growth of new cells and on synthesis of collagens. After initial vasodilation, blood in damaged vessels coagulates and the larger smooth-muscle vessels contract.21,27,28 Inadequate circulation limits healing. In the TC technique, the tissues can be brought together, and dead spaces generally do not exist. Subsequent serous or purulent fluids can be drained by applying pressure on the neighboring area. The disadvantages of the IC technique can be attributed to the possibility of dead space and to the inability to drain fluids, when they collect within the deeper layers.
Gram-positive bacteria are the most common organisms isolated from postoperative sternal infections. Staphylococcus aureus or S. epidermidis is identified in 70% to 80% of the cases.30,31 The prevalence of mixed infections is about 40%.7,32 Gram-negative bacteria and fungal agents lead to severe mediastinitis. The most common 10 pathogens as reported by Jonkers and associates18 in 2003 were S. aureus (26%), P. aeruginosa (10%), Enterococcus faecalis (9.5%), S. epidermidis (8%), Escherichia coli (7.7%), Proteus mirabilis (6.9%), Enterobacter cloacae (5.4%), Bacteroides fragilis (2.3%), other coagulase-negative staphylococci (2.3%), and Morganella morganii (1.7%). In our study, 3 patients with microbiologic proliferation in their wound cultures developed wound infections: 1 had S. aureus, and the other 2 had P. aeruginosa.
The pathogenesis of postoperative sternal wound infections involves many variables. Our study demonstrated that when IC and TC suture closures were compared, the incidence of superficial sternal wound infection was significantly higher in IC closures. The 2 techniques produced comparable cosmetic results. We conclude that the use of TC suture technique in closing the sternal skin tissue can provide better protection against the risk of superficial infection.
Footnotes
Address for reprints: Ozalp Karabay, MD, Cardiovascular Surgery Department, Dokuz Eylul University Medical School, Inciralti – Ismir 35340, Turkey
E-mail: ozalp.karabay@deu.edu.tr
References
- 1.Vaska PL. Sternal wound infections. AACN Clin Issues Crit Care Nurs 1993;4:475–83. [PubMed]
- 2.Blanchard A, Hurni M, Ruchat P, Stumpe F, Fischer A, Sadeghi H. Incidence of deep and superficial sternal infection after open heart surgery. A ten years retrospective study from 1981 to 1991. Eur J Cardiothorac Surg 1995;9:153–7. [DOI] [PubMed]
- 3.Farinas MC, Gald Peralta F, Bernal JM, Rabasa JM, Revuelta JM, Gonzalez-Macias J. Suppurative mediastinitis after open-heart surgery: a case-control study covering a seven-year period in Santander, Spain. Clin Infect Dis 1995;20:272–9. [DOI] [PubMed]
- 4.Cheung EH, Craver JM, Jones EL, Murphy DA, Hatcher CR Jr, Guyton RA. Mediastinitis after cardiac valve operations. Impact upon survival. J Thorac Cardiovasc Surg 1985; 90:517–22. [PubMed]
- 5.Tegnell A, Aren C, Ohman L. Coagulase-negative staphylococci and sternal infections after cardiac operation. Ann Thorac Surg 2000;69:1104–9. [DOI] [PubMed]
- 6.Orita H, Shimanuki T, Fukasawa M, Inui K, Goto S, Washio M, Horikawa H. A clinical study of postoperative infections following open-heart surgery: occurrence and micro-biological findings in 782 cases. Surg Today 1992;22: 207–12. [DOI] [PubMed]
- 7.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61: 1030–6. [DOI] [PubMed]
- 8.Puc MM, Antinori CH, Villaneuva DT, Tarnoff M, Heim JA. Ten-year experience with Mersilene-reinforced sternal wound closure. Ann Thorac Surg 2000;70:97–9. [DOI] [PubMed]
- 9.Borger MA, Rao V, Weisel RD, Ivanov J, Cohen G, Scully HE, David TE. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998;65:1050–6. [DOI] [PubMed]
- 10.Risnes I, Abdelnoor M, Baksaas ST, Lundblad R, Svennevig JL. Sternal wound infections in patients undergoing open heart surgery: randomized study comparing intracutaneous and transcutaneous suture techniques. Ann Thorac Surg 2001;72:1587–91. [DOI] [PubMed]
- 11.Andenaes K, Lingaas E, Amland PF, Giercksky KE, Abyholm F. Preoperative bacterial colonization and its influence on postoperative wound infections in plastic surgery. J Hosp Infect 1996;34:291–9. [DOI] [PubMed]
- 12.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs superficial infection. Chest 1996;110:1173–8. [DOI] [PubMed]
- 13.Breyer RH, Mills SA, Hudspeth AS, Johnston FR, Cordell AR. A prospective study of sternal wound complications. Ann Thorac Surg 1984;37:412–6. [DOI] [PubMed]
- 14.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles and quantitative methods. New York: Van Nostrand Reinhold Company; 1982.
- 15.Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980;60:27–40. [DOI] [PubMed]
- 16.Andenaes K, Amland PF, Lingaas E, Abyholm F, Samdal F, Giercksky KE. A prospective, randomized surveillance study of postoperative wound infections after plastic surgery: a study of incidence and surveillance methods. Plast Reconstr Surg 1995;96:948–56. [DOI] [PubMed]
- 17.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control 1988;16:128–40. [DOI] [PubMed]
- 18.Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90-days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg 2003;23:97–102. [DOI] [PubMed]
- 19.Stahle E, Tammelin A, Bergstrom R, Hambreus A, Nystrom SO, Hansson HE. Sternal wound complications—incidence, microbiology and risk factors. Eur J Cardiothorac Surg 1997;11:1146–53. [DOI] [PubMed]
- 20.Braxton JH, Marrin CA, McGrath PD, Ross CS, Morton JR, Norotsky M, et al. Mediastinitis and long-term survival after coronary artery bypass graft surgery. Ann Thorac Surg 2000;70:2004–7. [DOI] [PubMed]
- 21.Chu CC, Williams DF. Effects of physical configuration and chemical structure of suture materials on bacterial adhesion. A possible link to wound infection. Am J Surg 1984; 147:197–204. [DOI] [PubMed]
- 22.Pocock SJ. Clinical trials: a practical approach. Chichester (UK): John Wiley & Sons; 1983.
- 23.The Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective multicenter study. J Thorac Cardiovasc Surg 1996;111: 1200–7. [DOI] [PubMed]
- 24.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1–6. [DOI] [PubMed]
- 25.Cameron A, Kemp HG Jr, Green GE. Bypass surgery with the internal mammary artery graft: 15 year follow-up. Circulation 1986;74(5 Pt 2):III30–6. [PubMed]
- 26.Amland PF, Andenaes K, Samdal F, Lingaas E, Sandsmark M, Abyholm F, Giercksky KE. A prospective, double-blind, placebo-controlled trial of a single dose of azithromycin on postoperative wound infections in plastic surgery. Plast Reconstr Surg 1995;96:1378–83. [DOI] [PubMed]
- 27.Heughan C, Zederfeldt B, Grislis G, Hunt TK. Effect of dextran solutions on oxygen transport in wound tissue. An experimental study in rabbits. Acta Chir Scand 1972;138: 639–43. [PubMed]
- 28.Johnson P, Frederiksen JW, Sanders JH, Lewis V, Michaelis LL. Management of chronic sternal osteomyelitis. Ann Thorac Surg 1985;40:69–72. [DOI] [PubMed]
- 29.Gurevitch J, Kramer A, Locker C, Shapira I, Paz Y, Matsa M, Mohr R. Technical aspects of double-skeletonized internal mammary artery grafting. Ann Thorac Surg 2000;69: 841–6. [DOI] [PubMed]
- 30.Grossi EA, Culliford AT, Krieger KH, Kloth D, Press R, Baumann FG, Spencer FC. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg 1985;40: 214–23. [DOI] [PubMed]
- 31.Demmy TL, Park SB, Liebler GA, Burkholder JA, Maher TD, Benckart DH, et al. Recent experience with major sternal wound complications. Ann Thorac Surg 1990;49:458–62. [DOI] [PubMed]
- 32.Sarr MG, Gott VL, Townsend TR. Mediastinal infection after cardiac surgery. Ann Thorac Surg 1984;38:415–23. [DOI] [PubMed]


