Abstract
Axial-flow ventricular assist devices (VADs) can be implanted either through a left thoracotomy with outflow-graft anastomosis to the descending thoracic aorta or through a midline sternotomy with anastomosis to the ascending aorta. Each method has advantages and disadvantages. Because these VADs produce nonpulsatile flow, their hemodynamic characteristics differ from those of pulsatile devices. These differences may have important clinical consequences, particularly in relation to the outflow-graft configuration. We describe a computer-generated flow model that we created to illustrate the flow dynamics and possible clinical consequences of each method.
The simulations indicate that the location of the anastomosis has important qualitative effects on flow in the ascending aorta and aortic arch. At high VAD outputs (≥75%), native cardiac output cannot supply the carotid and subclavian arteries. With a descending aortic anastomosis, net backward flow occurs in the descending aorta to supply these branches. Consequently, the aortic arch has a region with almost no net flow, where fluid particles stagnate over many cardiac cycles, possibly causing thrombogenesis. With an ascending aortic anastomosis, the arch has no stagnant region, although flow turbulence still occurs.
When the aortic valve remains closed, so that the total output occurs through the VAD, the aortic root has a region of nearly stagnant flow. With an ascending aortic anastomosis, a small degree of recirculatory flow may prevent complete stagnation at the aortic root. With the descending aortic anastomosis, however, no recirculation occurs.
These results help delineate the complex flow dynamics and the advantages and drawbacks of each technique.
Key words: Aorta, anastomosis, axial flow, computer simulation, heart-assist devices, heart failure, hemodynamic processes/physiology, nonpulsatile flow, outflow graft, regional blood flow
The new generation of axial-flow ventricular assist devices (VADs) is designed to support patients with severe heart failure. These devices, such as the Jarvik 2000 FlowMaker® (Jarvik Heart, Inc.; New York, NY), the MicroMed DeBakey VAD® (MicroMed Technology, Inc.; Houston, Tex), and the HeartMate® II (Thoratec Corporation; Pleasanton, Calif) can be implanted either through a left thoracotomy with the outflow-graft anastomosis to the descending thoracic aorta or through a midline sternotomy with the anastomosis to the ascending aorta. Each technique has advantages and disadvantages. Because these VADs may produce less pulsatile flow than that of a normal heart, their hemodynamic characteristics are different from those of pulsatile devices. These differences may have important clinical consequences, particularly in relation to the outflow-graft configuration.
Herein, we describe the surgical technique for each approach, and then we discuss a computer-generated flow model that we created to show the differing flow dynamics of each technique and to illustrate their possible clinical consequences. This investigation was prompted by observations of flow stasis at the aortic root in patients with axial-flow devices and by clinical events that may have resulted from the altered blood flow. To fully weigh the risks and potential benefits of the 2 approaches, physicians must understand the surgical technique, advantages and disadvantages, and complex flow dynamics associated with each approach.
Surgical Techniques
Descending Aortic Outflow-Graft Anastomosis
To begin the process of descending aortic outflow-graft anastomosis, anesthesia is induced and the patient is placed in the semilateral left decubitus position for optimal access to the left side of the chest and the left femoral vessels. The left femoral artery and vein are exposed for cannulation, and a left thoracotomy is performed. The left inferior pulmonary ligament is then divided, and the lungs are retracted, preferably without the need for deflation. The descending aorta is exposed below the hilum of the left lung and above the diaphragm. If the patient has atherosclerotic plaques or calcification of the aorta, the supraceliac portion is used. After heparinization has been accomplished, side-biting aortic clamps are applied to the exposed aortic segment, and a 2-cm vertical aortotomy is made. A 16-mm graft is then trimmed appropriately and anastomosed obliquely to the aorta with 4-0 polypropylene sutures. The graft is clamped, and the aortic clamp is released. Once hemostasis has been attained, the driveline is tunneled subcutaneously through the left costophrenic angle and across the midline, exiting 3 to 4 cm below the right costal margin in the midclavicular line. Until ready for use, the FlowMaker LVAD rests on the patient's chest.
The pericardium is incised anterior and posterior to the phrenic nerve, with a 1-cm pedicle remaining on each side. The apex of the left ventricle is inspected, and a sewing ring is sutured to the ventricular apex with intramyocardial 2-0 pledgeted, polyester, braided fiber (Ticron) sutures. Heparin (3 mg/kg) is then given, and femorofemoral cannulation is performed. Cardiopulmonary bypass (CPB) is instituted under normothermic conditions. Then fibrillation is induced, and the left ventricular apex is incised and cored. Before VAD implantation, the ventriculotomy must be inspected to ensure that no thrombus is present.
The Jarvik 2000 FlowMaker implantation proceeds in this fashion: the outflow graft is aimed laterally and posteriorly, curving gently from the ventricular apex around the costophrenic angle to the descending aorta. The pump is secured to the sewing ring with umbilical tapes (Fig. 1). Once the pump and graft are securely in place, the heart is defibrillated, and any intracardi-ac air is evacuated with the guidance of transesophageal echocardiography (TEE). The pump is started at 8,000 rpm, and a 19-gauge needle is introduced into the graft for complete de-airing. The clamp is then removed from the graft. The lungs are expanded, the patient weaned from CPB, and the chest drained and closed routinely.
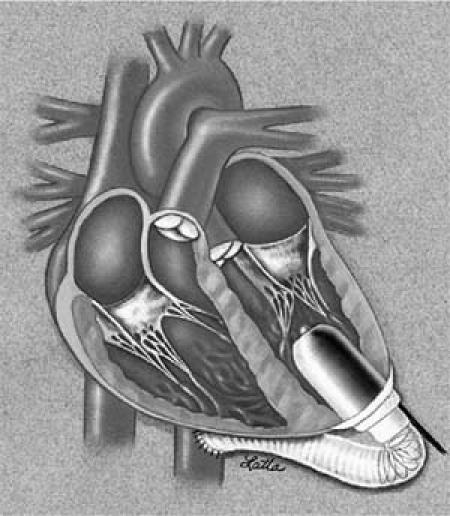
Fig. 1 The Jarvik 2000 FlowMaker® configuration with the outflow-graft anastomosis to the descending aorta.
Recently, we have used an alternative, left-sided subcostal approach, implanting the device on the inferior surface of the heart. This may further decrease the morbidity and risk in patients who have undergone a previous sternotomy.
Ascending Aortic Outflow-Graft Anastomosis
For the ascending aortic outflow-graft anastomosis, the patient is placed in the supine position, and a median sternotomy is performed. Once the pericardium has been opened, the aorta and the right atrium are cannulated. The ascending aorta is inspected for atherosclerosis or calcification. If the aorta is free of disease, the outflow graft is anastomosed by the standard method. The driveline is tunneled from the pericardium to the right subcostal margin. If the patient is in hemodynamically stable condition, the left ventricular apex is inspected, and the sewing ring is attached to the apex with the same type of 2-0 pledgeted polyester Ticron sutures. Cardiopulmonary bypass is initiated, the patient is placed in the Trendelenburg position, and fibrillation is induced. The left ventricular apex is then incised and cored. The outflow graft is positioned posteromedially. The graft is cross-clamped, a 19-gauge needle is inserted into the graft for de-airing, and the pump is started. After complete de-airing has been confirmed by means of TEE, the clamp is removed.
Computer Simulations
To show the effects of outflow-graft anastomosis location on flow in the thoracic aorta, we performed 2-dimensional numerical simulations of flow in an aor-tic model. The model consisted of a curved channel that represented the ascending aorta, aortic arch, and descending aorta, with rigid walls. To represent the outflow graft of the VAD, a short channel was added to either the ascending or descending aortic portion of the model. Inflow from the left ventricle was specified as a periodic function of time, using a period of 1 second, which is consistent with flow rates measured with magnetic resonance imaging by Olufsen and co-authors.1 Inflow through the VAD graft was independent of time. The overall average flow rate was 4 L/min, with a specified fraction being delivered by the VAD. Outflow also occurred at several sites along the “aorta.” These sites represented flow into the coronary, brachiocephalic, left carotid, and left subclavian arteries. Figure 2 shows the geometry of the simulation domain. The Appendix describes the simulations in more detail.
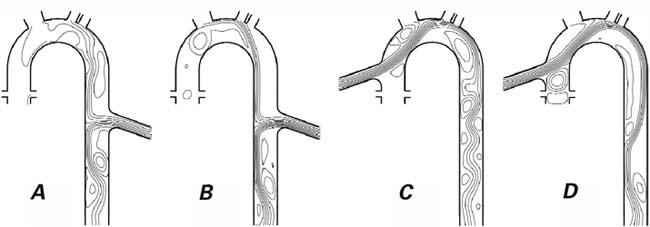
Fig. 2 Streamlines of the cycle-averaged flow in the thoracic aorta and the ventricular assist device (VAD) graft tube with outflow-graft anastomosis to the descending aorta (A, B) versus the ascending aorta (C, D) in a 2-dimensional computer model. The total output (cardiac plus VAD) is 4 L/min. In A and C, 75% of the output occurs through the VAD; in B and D, 100% of the output is through the VAD (the aortic valve remains closed).
Figure 2 also shows representative results of the simulations—specifically, streamlines of the flow averaged over a cardiac cycle for cases in which 75% and 100% of the flow occurred through the VAD. The streamlines are the average flow paths, and the flow veloc-ity is greater where the streamlines are more closely spaced. In this figure, closed loops represent regions where there is average recirculation; because the flow is pulsatile, however, such mean recirculation does not result in the entrapment of fluid particles. In these simulations, the primary concern is the large region in the ascending aorta (Figs. 2A and 2B) in which the average fluid velocity is approximately zero, as indicated by the absence of streamlines.
Our simulations suggest that the location of the anastomosis may have important qualitative effects on flow in the ascending aorta and the aortic arch. At high VAD outputs (75% or greater), the native cardiac output is insufficient to supply the carotid and subclavian arteries. Therefore, when a descending aortic anastomosis is used, a net backward flow in the descending aorta occurs to supply these branch arteries, although there is no evidence of reverse flow in the branch arteries themselves. Because of the reverse flow in the aorta, the flow velocity is approximately zero in a large region of the ascending aorta. In this region, particles stagnate over many cardiac cycles. If a similar flow situation occurred in a natural aorta, it could be conducive to thrombogenesis. In contrast, with use of an ascending aortic outflow-graft anastomosis (Fig. 2C and 2D), the ascending aorta has no such stagnant region.
When the aortic valve does not open (so that all of the output occurs through the VAD), the only flow in the aortic root is that which supplies the coronary arteries. Because this flow is minimal, the aortic root has a region of flow that is nearly stagnant. With an ascending aortic anastomosis, a small degree of recirculatory flow in this region may prevent complete stagnation at the aortic root. With the descending aortic anastomosis, however, no such recirculation areas occur; instead, flow is static on average.
Our simulations are instructive, but the calculations are idealized in several respects. These limitations should be taken into account by anyone who wishes to make quantitative predictions on the basis of these simulations. The most serious limitations are the 2-dimensional nature of the model and the rigid walls of the aorta, which eliminate the effects of 3-dimensional secondary flows and aortic dilation during systole. With 3-dimensional flow, the streamline details —especially the patterns of recirculation—are different. Even in 3 dimensions, however, regions of recirculation are probably present. A 3rd limitation of our model is the simple treatment of the inflow and outflow boundaries (see the Appendix). Moreover, the volumetric flow rate of the VAD is not constant in time, as modeled here, but fluctuates with changing pressures in the aorta. All of these limitations will be overcome in future simulations. Meanwhile, our current simple model of aortic flow can show qualitative hemodynamic differences between descending and ascending aortic outflow-graft anastomoses.
Discussion
The introduction of axial-flow VADs has significantly advanced the therapeutic options for patients with severe heart failure. These continuous-flow devices have minimal size, weight, and energy demands and are easy to implant and control.2 Current research is directed at optimizing their use to prevent known complications such as aortic valve cusp fusion3 and thrombosis in the aorta and branch vessels. Because axial-flow nonpulsatile devices produce nonphysiologic flow, they alter vascular hemodynamics. The classic implantation configuration for VADs, in which the device draws blood from the apex of the left ventricle and pumps it into the descending thoracic aorta, alters the natural hemodynamic pattern in the aorta. This change may affect both the microvasculature and the macrovasculature. Vlachopoulos and coworkers4 used tonometry to examine how VADs affect the vascular system. After device implantation, radial and aortic waveforms were substantially altered, proving that the implantation configuration changes the hemodynamic patterns.4
Optimizing the implantation configuration offers an outstanding opportunity to minimize these changes. The locations of the proximal and distal anastomoses are important in this regard. In previous studies, the ventricle was found to surpass the atrium for the proximal anastomosis.5 Therefore, all current devices exist in parallel to the heart, collecting blood from the left ventricular apex and ejecting it into the ascend-ing or descending aorta. Using a computer model to study the hemodynamic impact of VADs, Platt and associates5 concluded that ascending and descending aortic outflow cannulation yield equal improvements in the cardiac output and myocardial oxygen balance. However, few researchers have examined the impact of outflow-graft location. It is likely that other hemodynamic variables are substantially affected by this variable. For example, altered flow dynamics occur close to the inflow and outflow sites, which may affect not only the potential for thrombosis but also the perfusion of nearby branch vessels.
To predict what alterations will occur, one must view the heart and great vessels as a complex engineering system and, therefore, apply the principles of engineering. For our simulations, an engineering fluid dynamic model, based on the solution of the fluid dynamic equations in a simplified geometric configuration, was used to perform the analysis. The model was driven by measured cardiac output profiles used previously by Olufsen and colleagues.1 Boundary conditions were formulated by using an impedance representation of the rest of the arterial tree. The model should be considered a computational analog to the human aorta that is useful for investigating aortic flow characteristics.
With use of the axial-flow VAD, the flow characteristics of stagnant regions are of particular concern, because of the potential for thrombogenesis. Stasis and mild wall shear stress have been correlated with thrombotic events. According to Piccione,6 the incidence of such events varies from 2.7% to 35%. He cited patient variability, pump design, differing anticoagulant regimens, and observer variability as possible reasons for this wide range. In our patients with the Jarvik 2000 LVAD and a descending aortic anastomosis, we have observed areas of stagnation on TEE while the aortic valve was not opening. This finding was consistent with computer models of a descend-ing aortic anastomosis, which showed a net flow of approximately zero in the aortic arch and root. Therefore, the alteration in flow is a likely source of thrombosis when the outflow graft is anastomosed to the descending aorta.
We could not definitively ascribe adverse effects such as strokes to the aortic root stasis. Nonetheless, we theorized that if the outflow graft were anastomosed to the ascending aorta, the flow dynamics would improve—especially at high pump speeds. This was confirmed by TEE in Jarvik 2000 patients, who had little or no stagnation (“smoke”) even at relatively high VAD speeds. Our computer models reconfirmed these results, showing that recirculatory flow prevented stagnation at the aortic root. The ascending aortic anastomosis minimized the extent of the aorta that was upstream from the outflow, thus minimizing areas of stagnation.
The surgical approach for the Jarvik 2000 is dictated by the location of the outflow graft, so the potential drawbacks of the descending aortic anastomosis (such as valve fusion, stasis, and substantially altered flow) must be weighed against the advantages of the lateral thoracotomy approach. Often, patients who need a VAD have undergone previous sternotomies, making repeat surgical entry into the thoracic cavity difficult and hazardous. Patients who have had multiple sternotomies may have a better surgical outcome or a decreased complication rate with the lateral thoracotomy approach. Certain physical restrictions, such as the presence of vein grafts in coronary artery bypass patients, may dictate the position of the outflow cannula in the clinical setting.7
Through clinical observations, echocardiography, and computer modeling, we have begun to understand the complex physiology imparted by axial-flow VADs. In the future, as we achieve greater understanding of implantation options and subsequent alterations of physiologic flow, it should be possible to tailor the best approach to each patient. Considerations would include preoperative right-sided heart function, the risk posed by a sternotomy, the need for adjunctive procedures such as coronary artery bypass and aneurysmectomy, and the cause of the heart failure.
Appendix
Figure 2 shows the geometry of the 2-dimensional aortic model used for our flow simulations. The channel was 3 cm wide, the inner radius of curvature of the arch was 3.5 cm, and the ascending aortic segment was 4.5 cm long. To represent the VAD graft, a channel measuring 1.6 cm wide and 6.4 cm long was extended at a 20° angle from either the ascending or descending aorta. The outflow graft was centered 1 cm from the curved portion on the ascending segment or 7 cm on the descending segment. The coronary, brachiocephalic, left carotid, and left subclavian branches were represented by patches where flow through the domain boundary depended on the instantaneous pressure at that location and on the individual artery's effective flow resistance, as determined by Olufsen.8
At the aortic root and the inlet of the VAD graft channel, inflow velocities were rendered consistent with specified volumetric flow rates in a circular tube with the same diameter and channel width. At the aortic root, the volumetric flow rate was a periodic function of time, with a period of 1 second, and of time dependence, taken from the magnetic resonance imaging data of Olufsen and associates.1 The magnitude of the flow rate was scaled to obtain the required average flow rate. At the VAD graft inlet, the flow rate was independent of time, being approximately consistent with the rate produced by an axial-flow VAD such as the Jarvik 2000 FlowMaker. In the simulations, the total average output (heart and VAD) was 4 L/min, with a specified percentage of flow occurring through the VAD.
The computational domain was truncated in the descending aorta, where a simple convective outflow boundary condition was used. The overall system pressure was calculated from the outlet flow rate by use of the frequency-dependent complex impedance determined by Olufsen.8 Similarly, the flow rates into the branch arteries were established from the wall pressure, using the average flow resistance of each artery, again as determined by the Olufsen method.8 Simulations were performed with the Fluent computational flow dynamics package (Fluent Inc.; Lebanon, NH) on a grid of approximately 7,000 points, including 30 points across the channel width. The Fluent package solves the governing equations (the Navier-Stokes equations) that describe the conservation of mass and momentum of the fluid. Resolved solutions are reliable descriptions of the behavior of real fluids in the simulated geometry. As described above, the applicability of these simulations to human physiology is limited by the idealized geometry and boundary conditions (for example, a 2-dimensional model and rigid vessel walls). The adequacy of the grid resolution was confirmed by doubling the number of grid points and finding that the result did not change quantitatively. The simulations were run for several cardiac cycles to eliminate initial transients (3 cycles were generally sufficient to reach asymptotic behavior), and only the last cardiac cycle was analyzed.
Footnotes
Address for reprints: O. H. Frazier, MD, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345
E-mail: knowlin@heart.thi.tmc.edu
References
- 1.Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann Biomed Eng 2000;28:1281–99. [DOI] [PubMed]
- 2.Hetzer R, Jurmann MJ, Potapov EV, Hennig E, Stiller B, Muller JH, Weng Y. Heart assist systems—current status [in German]. Herz 2002;27:407–17. [DOI] [PubMed]
- 3.Baradarian S, Dembitsky WP, Jaski B, Abolhoda A, Adamson R, Chillcot S, Daily PO. Left ventricular outflow tract obstruction associated with chronic ventricular assist device support. ASAIO J 2002;48:665–7. [DOI] [PubMed]
- 4.Vlachopoulos C, Mcdonald P, Spratt P, O'Rourke M. Pulse wave analysis in the assessment of patients with left ventricular assist device. J Heart Lung Transplant 2001;20:98–102. [DOI] [PubMed]
- 5.Platt KL, Moore TW, Barnea O, Dubin SE, Jaron D. Performance optimization of left ventricular assistance. A computer model study. ASAIO J 1993;39:29–38. [PubMed]
- 6.Piccione W Jr. Left ventricular assist device implantation: short and long-term surgical complications. J Heart Lung Transplant 2000;19(8 Suppl):S89–94. [DOI] [PubMed]
- 7.Ott RA, Mills TC, Eugene J, Gazzaniga AB. Clinical choices for circulatory assist devices. ASAIO Trans 1990;36:792–8. [DOI] [PubMed]
- 8.Olufsen MS. Structured tree outflow condition for blood flow in larger systemic arteries. Am J Physiol 1999;276(1 Pt 2):H257–68. [DOI] [PubMed]


