Abstract
Ventricular septal defects complicate approximately 1% to 2% of cases of acute myocardial infarction. Such postinfarction defects require urgent surgical treatment because, on medical treatment alone, 60% to 70% of patients die within the first 2 weeks. Despite the development of various surgical techniques for repair of postinfarction ventricular septal defect, the condition carries a high risk of recurrence and subsequent death.
We describe a modification of the infarct exclusion technique in which the septal portion of the patch is reinforced by the right ventricular free wall. This modification appears to prevent leaks to the right ventricle through the ventricular septal defect, from anywhere around the patch. We applied this modified technique to 4 patients with anteroapical postinfarction ventricular septal defect. There was 1 early death, due to mesenteric artery occlusion secondary to embolus. No residual shunt was found during the postoperative period. We believe that our modification to the infarct exclusion technique might reduce both operative mortality and recurrence, by supporting friable endocardial tissue with right ventricular wall. We suggest that it be considered for use in patients with anteroapical ventricular septal defect and no severe right ventricular dysfunction.
Key words: Cardiac surgical procedures/methods; heart rupture, post-infarction/surgery; heart septal defects, ventricular/surgery; myocardial infarction/complications; recurrence; ventricular septal rupture/surgery
Postinfarction ventricular septal defects (VSDs) complicate approximately 1% to 2% of cases of acute myocardial infarction. Postinfarction VSDs are most commonly located in the anteroapical septum (60%) as a result of a full-thickness anterior infarction secondary to occlusion of the left anterior descending coronary artery. Postinfarction VSDs require urgent surgical treatment because the results of medical treatment are disappointing, with 60% to 70% of patients dying within the first 2 weeks.1,2
Postinfarction VSD was first successfully corrected by Cooley in 1957.3 Subsequently, different surgical techniques have been devised to treat postinfarction VSDs.4–8 In the early 1990s, Cooley6 modified his endoaneurysmorrhaphy technique (used in the repair of left ventricular [LV] aneurysm) for application to postinfarct VSD (Figs. 1A and 1B). In the present study, we describe a slight modification of this technique in which the septal portion of the patch is reinforced by the right ventricular wall.

Fig. 1 A, B) Standard infarct exclusion technique: endoventricular circular patch plasty.
LV = left ventricle; RV = right ventricle; VSD = ventricular septal defect
Surgical Technique
Cardiopulmonary bypass was established via the ascending aorta with bicaval cannulation. The combination of roller pump and membrane oxygenator was used in all patients. The patient's body temperature was lowered to 28 °C. The left side of the heart was decompressed via the right superior pulmonary vein. Myocardial protection was achieved with antegrade blood cardioplegic solutions and topical cold saline.
In performing the apical left ventriculotomy, we preserved the left anterior descending coronary artery. A demarcation zone between necrotic tissue and intact tissue was determined macroscopically. As in endoaneurysmorrhaphy, a Dacron patch was prepared in accordance with the size of the defect and the left ventricular cavity. The patch was sutured to the noninfarcted free wall of the left ventricle with continuous 3-0 polypropylene suture. Interrupted mattress sutures were then passed sequentially through the septal portion of the patch, the left ventricular septum, the free wall of the right ventricle, and a buttressing pledget of Teflon, as shown in Figures 2A and 2B. The right ventricular free wall was then pulled securely against the interventricular septum, reinforcing the septum and excluding a small part of the right ventricle. The ventriculotomy was closed with a 3-0 continuous polypropylene suture.
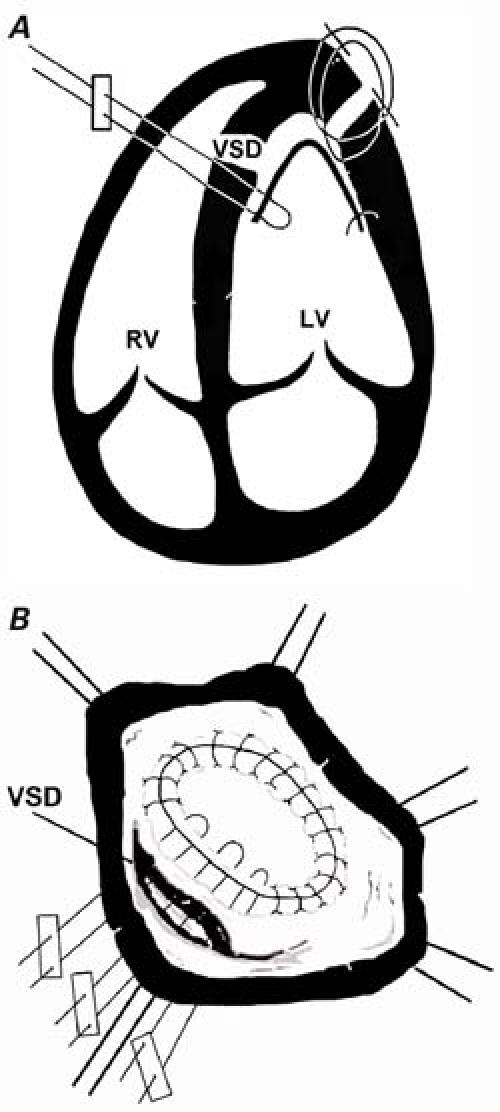
Fig. 2 A, B) Modified infarct exclusion technique: after the left ventricular defect has been excluded, multiple interrupted mattress sutures are passed sequentially through the septal portion of the patch, the left ventricular septum, the free wall of the right ventricle, and a buttressing pledget of Teflon. The right ventricular free wall is then drawn against the septum, to reinforce both the patch and the friable tissue that surrounds it. A small part of the right ventricle is necessarily excluded.
LV = left ventricle; RV = right ventricle; VSD = ventricular septal defect
Patients and Results
In 1999, we began using this technique in patients with anteroapical postinfarction VSD. Four men (mean age, 57 years; range, 47–64) underwent surgery with this modified technique between the years 1999 and 2004. All patients presented with anterior apical VSD. We assigned patients to surgery in accordance with the Killip classification system: patients in classes III (pulmonary edema) and IV (cardiogenic shock) underwent operation. Two of our patients were in class III, and the other 2 were in class IV. Three patients had double-vessel disease, and 1 had single-vessel disease.
Coronary angiography and echocardiography were performed preoperatively in all patients. The mean left ventricular ejection fraction was 30% ± 7%. The left-to-right shunt, measured by oximetry, ranged from 2 to 4.5 (average, 3.1). One patient underwent surgery while in cardiogenic shock. The mean preoperative pulmonary artery pressure was 45 ± 7 mmHg, and right atrial pressure was 13 ± 4 mmHg. All patients were supported perioperatively by intraaortic balloon counterpulsation. The time between myocardial infarction and VSD development was 14 days (range, 7–19 days), and repair was performed within an average of 1 to 2 days after diagnosis. In 3 patients, coronary artery bypass grafting was performed at the time of VSD repair. The average cross-clamp time was 95 min (range, 80–120 min), and the average cardiopulmonary bypass time was 154 min (range, 110–170 min). All patients demonstrated satisfactory postoperative hemodynamics. The mean pulmonary artery pressure was 32 ± 5 mmHg and right atrial pressure was 7 ± 5 mmHg, as measured on the 2nd postoperative day.
One early postoperative death occurred 35 days after repair of the VSD, due to mesenteric artery embolism.
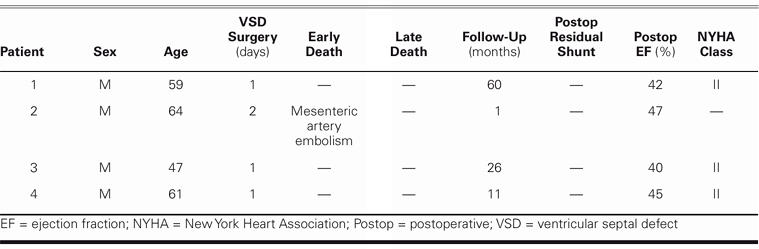
Follow-up varied in length from 11 to 60 months (mean, 22 mo). There was no residual shunt on postoperative echocardiography. There was mild mitral regurgitation in 2 patients. At the final follow-up visit, all 3 patients were in New York Heart Association functional class II (Table I).
Table I. Patient Data
Discussion
Postinfarction VSD occurs in approximately 2% of patients hospitalized with an acute infarction. Congestive heart failure and cardiogenic shock develop due to extensive infarction of the LV and left-to-right shunt.1,3 Postinfarction VSD has essentially a 100% mortality rate within 1 year, unless surgically treated.9
The 1st successful repair of postinfarction VSD was accomplished by Cooley and colleagues in 1957.3 Since that time, modifications to the surgical technique have improved operative results and reduced recurrence of the VSD.4–8 In 1994, Cooley6 modified his technique of left ventricular endoaneurysmorrhaphy for application to postinfarction VSD. This procedure, also known as infarct exclusion, has several advantages: it does not require resection of myocardium, it maintains ventricular geometry (which can enhance ventricular function), and it avoids tension on friable muscle (which can diminish postoperative bleeding).6–8 Overall, the technique of infarct exclusion has improved the results of surgery in patients with postinfarction VSD.6–8
In the present study, we have modified the infarct exclusion technique slightly by reinforcing the septal portion of the patch with the right ventricular free wall. We think that this modification not only reinforces the circular patch but prevents leaks to the right ventricle through the VSD, from anywhere around the patch.
According to recently published results of surgery for postinfarct VSD,4,9 the surgical mortality rate ranges from 13% to 42%, with the mortality rate for patients in shock ranging from 44% to 58%. Recurrence of VSD after infarct exclusion occurs in approximately 10% to 25% of patients, due to rupture of the necrotic and friable endocardial tissue.4–7 We hypothesize that support of the friable endocardial tissue with right ventricular wall prevented recurrence in our patients.
Preoperatively, right ventricular dysfunction is an important risk factor in surgery of postinfarct VSD. Right ventricular dysfunction is related to several factors: left ventricular dysfunction, right ventricular infarction or ischemia, and right ventricular volume overload.10 Anterior infarcts involve significantly more of the LV than do inferior infarcts. Conversely, inferior infarcts involve a much larger amount of right ventricular myocardium.11,12 All patients in our study had anterior myocardial infarction and no right ventricular dysfunction. It might be supposed that this modified technique would cause a reduction in right ventricular cavity and hemodynamic problems as a consequence. However, we observed no impairment in the hemodynamic parameters measured in our patients, which could indicate that this approach does not cause important hemodynamic problems.
We believe that our modification to the infarct exclusion technique might reduce both operative mortality and the recurrence of VSD. We suggest that this modified technique be considered for use in patients with anteroapical VSD and no severe right ventricular dysfunction.
Acknowledgment
This study was supported by the Akdeniz University Research Projects Unit.
Footnotes
Address for reprints: Dr. Ilhan Golbasi, Akdeniz Universitesi Tip Fak., Kalp Damar Cerrahisi Anabilim Dali, 07070 Antalya, Turkey
Email: golbasi@akdeniz.edu.tr
References
- 1.Bouchart F, Bessou JP, Tabley A, Redonnet M, Mouton-Schleifer D, Haas-Hubscher C, Soyer R. Urgent surgical repair of postinfarction ventricular septal rupture: early and late outcome. J Card Surg 1998;13:104–12. [DOI] [PubMed]
- 2.Killen DA, Piehler JM, Borkon AM, Gorton ME, Reed WA. Early repair of postinfarction ventricular septal rupture. Ann Thorac Surg 1997;63:138–42. [DOI] [PubMed]
- 3.Cooley DA, Belmonte BA, Zeis LB, Schnur S. Surgical repair of ruptured interventricular septum following acute myocardial infarction. Surgery 1957;41:930–7. [PubMed]
- 4.Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, Sosnowski AW. Post infarction ventricular septal defect - can we do better? Eur J Cardiothorac Surg 2000;18: 194–201. [DOI] [PubMed]
- 5.Daggett WM. Postinfarction ventricular septal defect repair: retrospective thoughts and historical perspectives. Ann Thorac Surg 1990;50:1006–9. [DOI] [PubMed]
- 6.Cooley DA. Repair of postinfarction ventricular septal defect. J Card Surg 1994;9:427–9. [DOI] [PubMed]
- 7.David TE, Armstrong S. Surgical repair of postinfarction ventricular septal defect by infarct exclusion. Semin Thorac Cardiovasc Surg 1998;10:105–10. [DOI] [PubMed]
- 8.Cooley DA. Postinfarction ventricular septal rupture. Semin Thorac Cardiovasc Surg 1998;10:100–4. [DOI] [PubMed]
- 9.Labrousse L, Choukroun E, Chevalier JM, Madonna F, Robertie F, Merlico F, et al. Surgery for post infarction ventricular septal defect (VSD): risk factors for hospital death and long term results. Eur J Cardiothorac Surg 2002;21: 725–32. [DOI] [PubMed]
- 10.Frassani R, Gelsomino S. A right atrial approach in redo postinfarction ventricular septal defect. Cardiovasc Surg 1999; 7:656–8. [DOI] [PubMed]
- 11.Lowe JE, Gall SA Jr. As originally published in 1989: Correlates of survival in patients with postinfarction ventricular septal defect. Updated in 1997. Ann Thorac Surg 1997;63: 1508–9. [DOI] [PubMed]
- 12.Cummings RG, Califf R, Jones RN, Reimer KA, Kong YH, Lowe JE. Correlates of survival in patients with postinfarction ventricular septal defect. Ann Thorac Surg 1989;47: 824–30. [DOI] [PubMed]


