Affecting over half a million people per year, stroke is the third leading cause of death in the United States. Approximately 30% of strokes are caused by carotid occlusive disease.1 The traditional methods of treating carotid stenosis have included medical or surgical therapy. Patients with symptomatic moderate or severe cervical carotid artery stenosis have been treated with carotid endarterectomy (CEA). Medical therapy with aspirin and more recently with clopidogrel (Plavix) has been reserved for mild-to-moderate (asymptomatic) carotid stenosis or noncervical carotid stenosis.
First performed nearly 26 years ago, carotid artery stenting (CAS) has, until recently, been only a footnote in the treatment of carotid artery disease, because it was performed at only a few centers for a few select indications2 (Fig. 1).
Fig. 1 The RX AccuLink Carotid Stent (Top: 8 mm × 4 cm; Bottom: 8 mm × 6 mm × 4 cm). The advantage of nitinol is its continued expansion when in association with the higher temperature of blood. (Courtesy of Guidant, Inc.; Santa Clara, Calif)
Coupled with the recent development and improvement of distal embolic protection devices (EPD), CAS has become a viable option in the treatment of carotid artery occlusive disease (Fig. 2). Carotid endarterectomy, until 1997, was the only therapy for carotid revascularization. However, in high-risk patients, CAS may actually offer similar or better results when all of the data, trials, and results are reviewed. When evaluating CAS versus CEA and “the controversy,” it is important to look at the history of CEA and CAS, the landmark trials, and future directions.

Fig. 2 RX Accunet Embolic Protection System (Guidant, Inc.; Santa Clara, Calif). This membrane has pores of only 150 microns and thus filters any débris that is liberated during the stenting procedure.
Surgical Therapy
Doctors Cooley, Debakey, and Eastcott are credited with the first surgical treatment of carotid artery occlusive disease in the 1950s.3 Carotid endarterectomy gradually gained acceptance, and approximately 100,000 procedures were performed annually in the 1980s.
Lack of clinical trial data, however, led to confusion concerning the role of CEA in the treatment of carotid artery disease. Forty years after the first carotid artery endarterectomy was successfully performed, randomized trials were designed to evaluate the indications and efficacy of CEA.
The first large-scale trials to evaluate CEA in symptomatic patients with carotid artery stenosis were the North American Symptomatic Carotid Endarterectomy Trial (NASCET),4 the European Symptomatic Carotid Trial (ESCT),5 and the Veterans Administration Symptomatic Trial.
The NASCET trial began enrolling patients in 1988, and results were first published in 1991.4 The initial trial, which included 659 symptomatic patients with severe carotid artery disease (70%–99% stenosis), compared surgical therapy to the best medical therapy at that time (aspirin and coumadin). Clearly, in this group, surgical therapy was superior to medical therapy in ipsilateral stroke rates (9% vs 26%) and death rates (2.5% vs 13.1%) over 2 years. This has accounted for a relative risk reduction with surgical therapy of 65%.
The second part of the trial enrolled 2,226 patients divided into 2 groups: moderate stenosis (50%–69%) and mild stenosis (30%–49%). In 5 years, the moderate stenosis group also showed a moderate decrease in the risk of ipsilateral stroke. Importantly, CEA did not demonstrate any benefit in symptomatic patients with mild stenosis.
The ESCT5 enrolled 3,024 symptomatic patients in 12 European countries and obtained results similar to those of NASCET. The Veterans Administration trial was terminated prematurely after the results of NASCET and ESCT were released, because of the overwhelming benefit of surgery over medical treatment in these groups.
It is interesting to note that, in certain subsets of patients—for example, women—CEA was not found to be beneficial in the moderate stenosis group. This is difficult to explain by data analysis. Also, it should be noted that these patients were a relatively low-risk population: less than a fourth had ischemic heart disease and 12% had diabetes mellitus.
In the Asymptomatic Carotid Artery Study (ACAS), 1,662 patients were randomized to either CEA or aspirin.6 Despite the fact that only 9% of patients were available for follow-up at 5 years, the authors concluded that the risk of stroke or death was decreased by treatment with CEA (5.1%) compared with aspirin (11%). Although a benefit from surgical therapy was identified, this should not have prevented adequate data collection in order to clearly elucidate the long-term clinical benefit of one therapy over another. Patients over the age of 79 years, those with a life expectancy of less than 5 years, or those with a disorder that could “seriously complicate surgery” were excluded from the surgery. As noted by many reports of clinical trials to date, the exclusion of certain subsets can distort the eventual perceived outcomes of those trials.
In the numerous trials reported to date, the patient's perioperative risk of any stroke or death was 6% in the symptomatic group, whereas it was only 2.3% in the asymptomatic group. It is possible that several factors could explain these numbers. First, these trials evaluated low-risk patients, excluding those over the age of 80 years and those with major coronary artery disease, severe pulmonary disease, or congestive heart failure. Second, the surgeons in these trials were high-volume operators at large centers. Medicare analysis has shown that lower-volume centers have a higher mortality rate than those of high-volume centers (2.5% vs 1.7%).7 Finally, some difficult carotid artery disease scenarios were not included in the study, such as high anatomical cervical bifurcation, contralateral carotid artery occlusion, restenotic lesions after endarterectomy, severe intracranial stenosis, and individuals who were post-radiation for head or neck cancer (Fig. 3). Therefore, these numbers represent low-risk patients in high-volume centers with experienced surgeons and may not reflect the true state of carotid artery endarterectomy as it existed at that time.
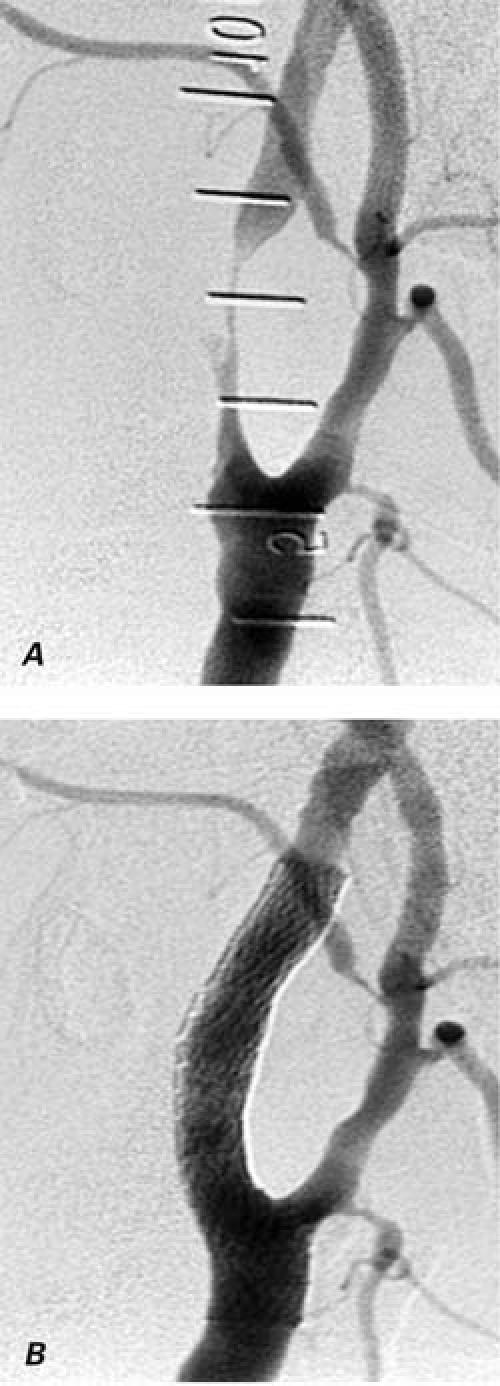
Fig. 3 Angiogram of a section of the cervical internal carotid artery A) with 99% stenosis 5 years after radiation therapy, and B) after the successful deployment of a 10-mm-diameter by 4-cm-length self-expanding nitinol stent.
Endovascular Therapy
Initial Experience: Pre-Distal Protection
The first carotid angioplasty was performed by Mathias in 1979, followed a decade later by the first stent deployment in 1989.2 Until recently, carotid artery stenting was performed in very few centers, in patients who were not surgical candidates—whether for anatomic or medical reasons. This procedure included the use of large 7F–8F iliac angioplasty balloons, 8F–9F balloon-expandable stents, and other equipment unacceptable by today's cardiovascular standards. Therefore, early series reports comprised small cohorts or case reports.
The largest review of carotid artery stenting before distal protection comes from the Global Carotid Artery Registry, collected from 12 countries and 53 centers beginning in 1997.8 This registry includes 12,254 CAS procedures. Approximately 66% of the patients had no cerebral distal protection in their endovascular procedures. The technical success rate was 98.9%.
From this bi-yearly report,8 one can see that CAS performed with cerebral protection yielded a significantly lower event rate than without protection. This can be demonstrated in both the symptomatic and asymptomatic patient populations. Furthermore, as in the surgical cohorts, asymptomatic patients also fared better than those who were actively symptomatic. Restenosis rates were approximately 2% per year over 3 years.
The Carotid Artery and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), published in 2001,9 compared percutaneous angioplasty (not necessarily stenting) to CEA. Randomization of 504 patients in the endovascular arm included mainly angioplasty patients. High-risk surgical patients were excluded from the trial, including those with recent myocardial infarction, uncontrolled hypertension, diabetes mellitus, severe respiratory disease, renal disease, cervical spondylosis, or inaccessible carotid stenosis. The results demonstrated no difference in disabling stroke or death in the endovascular versus the CEA group (6.4% vs 5.9%) and no significant difference in ipsilateral stroke rate at 3 years.
One of the criticisms of the endovascular treatment group was the restenosis rate of 14% versus 4% in the CEA group. However, a closer analysis demonstrates that only 23% of patients received a stent. In addition, the stents that were used were the older-generation, larger iliac-approved devices such as the Palmaz (Cordis Endovascular Corporation, a Johnson & Johnson company; Miami Lakes, Fla), Wallstent (Schneider, Boston Scientific; Natick, Mass), and Strecker (Boston Scientific) stents. The authors concluded that there were no differences in death or stroke rates between endovascular therapy and CEA, albeit with wide confidence intervals.
Advancement: The Age of Distal Protection
In general, the primary complication from CAS was peri-procedural stroke from the liberation of débris during balloon inflation. Therefore, in theory, if distal embolization could be mitigated, CAS could be performed with lower risk. The first distal protection device was actually developed by Theron in 1990;10 however, recent technical refinements have enabled the widespread use of a variety of distal embolic protection devices (EPDs) in conjunction with CAS.
In 2001, Medicare announced that it would reimburse hospitals and physicians involved in CAS, but only under an FDA Investigational Device Exemption (IDE). With this landmark decision came the birth of SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial).11 This was the first FDA-sanctioned trial involving CAS with the simultaneous use of EPDs. The aim of this trial was not to show that stenting is superior to surgery, but that it is not inferior by a difference of 3%. A total of 747 patients were enrolled in this trial. The study protocol randomized high-risk patients defined as:
Age greater than 80 years
Severe pulmonary disease
Severe congestive heart failure or concomitant severe coronary artery disease
Contralateral cervical carotid artery occlusion
Unstable angina
Carotid stenosis inaccessible to vascular surgeons
Previous radiation therapy in the carotid stenosis site
Symptomatic patients with 50% luminal stenosis or asymptomatic patients with 80% luminal stenosis were accepted into this randomized trial. Cardiologists, radiologists, surgeons, and neurologists all participated in the decisions on which patients would be allowed into the trial. Actually, the nonrandomized stent arm (NRSA) of this trial was defined by the surgeons themselves. The EPD used in SAPPHIRE was the Angioguard, by Cordis. The study had 3 arms:
Randomized CEA vs CAS with EPD (334 patients)
Nonrandomized stent arm for patients who were refused surgery (406 patients)
Nonrandomized surgical arm for patients who were refused CAS (7 patients)
At 2 years, patients receiving CAS with EPD fared significantly better than those undergoing CEA in the composite endpoint of death, stroke, and myocardial infarction (12.0% vs 20.1%, P<0.05). However, this result was driven primarily by myocardial infarction, because there was no significant difference in death or stroke (the trend still favored CAS). In symptomatic patients, no statistically significant difference existed between the CAS and the CEA groups (16.8% vs 16.5%) (Fig. 4) Again in asymptomatic patients, no difference existed between the CAS and the CEA groups (5.4 vs 10.2, P = 0.20).
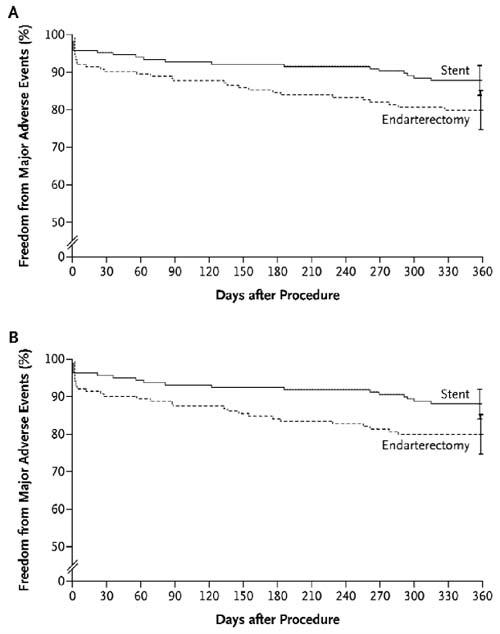
Fig. 4 Freedom from major adverse events at 1 year. In the intention-to-treat analysis (A), the rate of event-free survival at 1 year was 87.9% among patients randomly assigned to carotid stenting, as compared with 79.9% among those randomly assigned to endarterectomy (P = 0.053). In the actual-treatment analysis (B), the rate of event-free survival at 1 year was 88.0% among patients who received a stent, as compared with 79.9% among those who underwent endarterectomy (P = 0.048). I bars represent 1.5 times the SE. (From: Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501. Copyright © 2004 Massachusetts Medical Society. All rights reserved.)
The Acculink for Revascularization of Carotids in High-Risk Patients (ARCHeR)12 was a prospective trial with CAS and EPD using the RX AccuLink Carotid Stent System and the RX Accunet Embolic Protection System in high-risk surgical patients. This study enrolled, in 3 single-arm trials, 581 patients who met the following criteria: 50% symptomatic stenosis or 80% asymptomatic stenosis with one of the following:
Two or more coronary lesions
Severe pulmonary disease
End-stage renal disease
History of neck radiation
Contralateral carotid occlusion
Myocardial infarction in the previous 30 days
Uncontrolled diabetes mellitus12
The primary endpoint of death, myocardial infarction, and stroke at up to 2 years in ARCHeR 1 and 2 were 8.3% and 10.2%, respectively. ARCHeR 1 used the Acculink nitinol stent without cerebral protection, whereas Archer 2 used a cerebral protection device. ARCHeR 3 was similar in the usage of the stent and EPD; however, the monorail delivery system was used exclusively during this phase of the trial. Not enough patients in this trial have reached 1 year; therefore, the ARCHeR 3 results are unavailable.
Currently, a new large study, Carotid Revascularization Endarterctomy vs Stenting Trial (CREST), is under way to compare CAS and CEA in low-risk patients. The results are eagerly awaited, for they might further define the role of CAS. As you are reading this, a second trial (ACT 1, Abbott) in the United States is under way, which randomizes low-risk patients to either CEA or CAS with EPD.
Conclusions
Carotid endarterectomy has been a mainstay in the treatment of carotid occlusive disease. Many critics have viewed carotid artery stenting as a niche procedure, which they feel will not withstand the pressures of multidiscipline physician and government involvement. Unfortunately, in the CEA trials, investigators have looked only at low-risk patients and have extrapolated the results to high-risk patients in clinical practice.
Others complain that there are no long-term data. Then, on the basis of recent trial data, it is fair to state that in high-risk patients, CAS is the procedure of choice. In the future, as endovascular therapy becomes further refined, with better EPDs, better stents (drug-eluting), and more experienced operators, mortality and morbidity rates associated with the procedure will continue to decline.
History is fickle. When surgeons pioneered carotid endarterectomy, they outpaced medical therapy. Surgical therapy was a moving target, and medical therapy could not keep up. Times are again changing. Endovascular therapy—with its innovations—is now the moving target, while surgical therapy has had minor changes. Just as medical therapy has a role in mild-to-moderate lesions, endovascular therapy has a role in this huge population, but its exact nature remains to be seen.
Footnotes
Address for reprints: Neil E. Strickman, MD, FACC, FACP, 6624 Fannin, Suite 2480, Houston, TX 77030
E-mail: nedepper@hgcardio.com
Presented at the 14th International Meeting of the Denton A. Cooley Cardiovascular Surgical Society, 6–10 October 2004, Houston, Texas
References
- 1.American Heart Association. Heart and stroke facts: 1994 statistical supplement. Dallas: American Heart Association; 1993.
- 2.Mathias K, Jager H, Hennigs S, Gissler HM. Endoluminal treatment of internal carotid artery stenosis. World J Surg 2001;25:328–36. [DOI] [PubMed]
- 3.DeBakey ME. Carotid endarterectomy revisited. J Endovasc Surg 1996;3:4. [DOI] [PubMed]
- 4.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53. [DOI] [PubMed]
- 5.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–87. [PubMed]
- 6.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–8. [PubMed]
- 7.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA 1998;279:1278–81. [DOI] [PubMed]
- 8.Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv 2003;60:259–66. [DOI] [PubMed]
- 9.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729–37. [PubMed]
- 10.Theron J, Courtheoux P, Alachkar F, Bouvard G, Maiza D. New triple coaxial catheter system for carotid angioplasty with cerebral protection. AJNR Am J Neuroradiol 1990; 11:869–77. [PMC free article] [PubMed]
- 11.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–501. [DOI] [PubMed]
- 12.Wholey MH. Oral presentation, 2003 American College of Cardiology annual meeting.


