Abstract
As the prevalence and incidence of ischemic heart disease continue to increase, so does interest in ischemic heart failure management. Limitations of current therapies have led to research aimed at regenerating and repairing ischemically damaged myocardium through stem-cell therapy. Cell types being evaluated include embryonic stem cells, fetal and neonatal cardiomyocytes, skeletal myoblasts, bone marrow stem cells, peripheral blood CD34+ cells, endothelial progenitor cells, cardiac progenitor cells, and fibroblasts. Preclinical animal studies and promising early results of clinical trials now under way suggest that stem-cell therapy may soon become an important new tool in heart failure management.
Key words: Bone marrow stem cells/cytology; cardiac myocytes; cell differentiation; cell transplantation; cells, cultured; embryo/cytology; heart failure; hematopoietic stem cell transplantation; hybrid cells/cytology; myoblasts, skeletal; myocardial infarction/pathology/therapy; myocardium/cytology; peripheral blood stem cell transplantation; stem cells
Ischemic heart disease is a major health problem. It is widely prevalent in the United States (5 million individuals) and is increasing in incidence (approximately 400,000 to 600,000 new patients each year). Heart failure due to ischemic heart disease is expected to become an even greater problem as the aging of the population and improved treatment of acute myocardial infarction lead to longer post-myocardial infarction survival.1 Therefore, the management of heart failure is receiving greater attention.
Ischemic heart failure is characterized by the loss of critical amounts of cardiomyocytes after a sudden decrease in myocardial perfusion. For most of the early 20th century, the infarcted heart was thought to compensate endogenously for this loss through hypertrophy of surrounding viable myocardium, in keeping with the long-held belief that the heart is a terminally differentiated or postmitotic organ (that is, unable to regenerate itself at the cellular level). This belief was first challenged by Linzbach in the late 1940s2,3 and was ultimately proved wrong by numerous animal trials demonstrating that cardiomyocytes could and did proliferate under conditions of severe hemodynamic stress and increased mitotic index found in the healthy myocardium of an infarcted heart.2,3 As a result, other endogenous mechanisms for repairing damage caused by myocardial ischemia were proposed, including hyperplasia and migration of bone-marrow–derived stem cells to damaged myocardium resulting in angiogenesis and the generation of viable new myocardium.4 None of these endogenous repair mechanisms, however, is sufficient to restore lost myocardium or cardiac function.4 Instead, the ischemic loss of myocardium initiates a detrimental cascade of events, including formation of a noncontractile scar, ventricular wall thinning, hemodynamic overload, ventricular remodeling, heart failure, and eventually death.
Despite advances, current therapeutic approaches to ischemic heart failure remain very limited. Heart failure, regardless of the cause, can be treated pharmacologically or surgically. Pharmacologic options consist mainly of β-blockers, diuretics, and angiotensin-converting enzyme inhibitors. Surgical options include left ventricular reshaping by means of endocardial patches or passive constraints, implantation of mechanical left ventricular assist devices, and heart transplantation. Transplantation is severely limited by the unpredictable and limited availability of organs, immunosuppressive complications, and long-term graft failure.1,5 Therefore, new and more efficient heart failure therapies are needed, including those with the ultimate goal of restoring function by regenerating and repairing damaged myocardium.
Stem-cell therapy offers the most promise in this regard, especially in light of recent advances in the understanding of stem-cell biology and transplantation. Herein, we provide a general overview of the current state of stem-cell therapy for ischemic heart failure, including the types of stem cells used, results of preclinical animal studies, and early results of clinical trials in human beings.
Stem-Cell Types
Stem cells are defined as cells that are clonogenic (capable of producing exact duplicates), self-renewing (capable of dividing indefinitely), and potent (capable of differentiating into multiple cell lineages).6 Several generations of stem cells can be distinguished, according to their potential for differentiation, as being totipotent, pluripotent, multipotent, or unipotent.7
Stem cells in the very early stages of embryonic development are often referred to as totipotent, or omnipotent. Totipotent cells can give rise to any type of cell: cells of the trophoblast and cells of the 3 germ layers (endoderm, mesoderm, and ectoderm), all of which are necessary for complete embryonic development. This means that from 1 totipotent cell, a living organism can develop. Only fertilized oocytes and blastomeres of embryos at stages 2 through 8 are capable of generating a fully viable organism.8 However, totipotent stem cells are not considered true stem cells, because they can divide only a limited number of times before undergoing further differentiation and specialization.9
Stem cells that can give rise to cells of all 3 embryonic germ layers but not to trophoblasts are considered pluripotent. Three types of pluripotent stem cells can be derived from the mammalian embryo: embryonic stem (ES) cells, embryonic germ (EG) cells, and embryonic carcinoma (EC) cells. The ES cells are derived from the inner cell mass of the blastocyst at day 5 post-fertilization. The EG cells are derived from embryonic genital ridges at 9.5 to 12.5 days post-coitus. Both ES and EG cells can develop into cells of the 3 germ layers. The EC cells emerge from undifferentiated parts of germ cell tumors; they are also less able to differentiate, are usually aneuploid (that is, having varying amounts of DNA), and are malignant in laboratory animals.8,10
Stem cells that give rise to cells of different lineages within a single germ layer are considered multipotent. Multipotent (that is, adult) stem cells emerge in fetal and early postnatal life when specific gene expression patterns are imprinted.11 Adult stem cells are responsible for maintaining cellular homeostasis of tissues by replenishing cells that are lost through maturation and senescence or through injury (Table I). It is still debatable whether multipotent stem cells survive in all self-renewing organs for a lifetime or are continuously supplied from a central pool of stem cells, which is currently believed to be bone marrow.6,12 It is possible that every self-renewing organ contains a small pool of stem cells that can adjust to the normal turnover of tissue-specific cells. However, when injury occurs, this small pool of stem cells can no longer produce sufficient amounts of tissue-specific cells. This has led to the hypothesis that bone marrow stem cells (BMSCs) are mobilized from the bone marrow (central pool) and directed to the injured tissue or organ.
TABLE I. Adult Stem Cells and Their Primary Routes of Differentiation
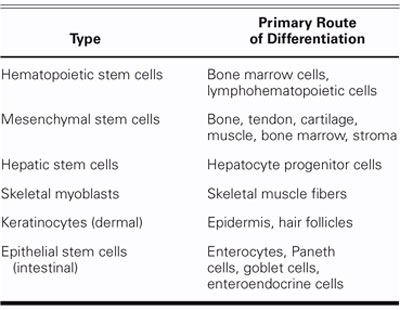
Tissue-specific progenitor (unipotent) stem cells arise from multipotent cells. After a limited number of divisions, unipotent stem cells begin to differentiate and give rise to cells of specific tissue types. These tissue-specific cells then impart function and structure to tissues and organs by becoming their integral units.11
Stem-Cell Differentiation
At present, there are 4 accepted theories to explain the differentiation of adult stem cells into tissue-specific cells. None of them are mutually exclusive. The 1st theory posits a putative primordial equivalent of the embryonic stem cell (the so-called “stem cell of all stem cells”); the antigenic signature of this cell has recently been defined as CD34−, CD44−, CD45−, c-kit−, and major-histocompatibility-complex (MHC) classes I and II.6 This primordial equivalent can be found in bone marrow and peripheral blood and can give rise to various lineage-restricted stem cells.6,11 The 2nd theory posits several types of circulating lineage-restricted adult stem cells. The 3rd theory is grounded on the assumption that already differentiated tissue-specific cells regain the properties of a stem cell through a process of dedifferentiation and subsequent redifferentiation into new cells of another tissue.6 This theory goes against the long-held belief that adult stem cells are tissue-specific cells that can differentiate only into cells of the tissues in which they reside.6,11
The 4th theory, which is supported by most experimental and clinical data, suggests that circulating hematopoietic stem cells (HSCs) predestined to become primarily hematopoietic cells can, under certain circumstances, deviate from that predestined pathway in a process called transdifferentiation.6,10,11 The molecular mechanisms involved in this process remain very obscure and are the focus of intensive research efforts, but further discussion of these mechanisms lies beyond the scope of this review.
Some have questioned the validity of the 4th hypothesis in light of recent studies of mice with metabolic liver disease. These studies have provided evidence 1) that bone marrow cells can fuse with healthy hepatocytes to produce hybrid cells (tetraploid or even hexaploid hybrids) that display phenotypes of both cell types13–15 and 2) that cell fusion is the major source of bone-marrow–derived hepatocytes.13–15 These findings have been offset, however, by many other animal studies showing that stem-cell–derived tissue-specific cells have a normal, diploid phenotype (polyploidy is not uncommon in liver tissue).10,11
Although it is theoretically possible for hybrid tetraploid cells to somehow regain the tetraploid karyotype in a diploid state (thus masking the occurrence of cell fusion), the fact remains that cell fusion has a very low estimated frequency of 1:10,000 to 1: 100,000. Therefore, it is highly unlikely that cell fusion would account for the extensive tissue regeneration that has been achieved with stem-cell therapy.10
Therapeutic stem-cell transplantation is founded on 2 principal assumptions: first, as already mentioned, that heart failure is due to the irreversible loss of a critical number of cardiomyocytes; and second, that the function of the infarcted heart can be improved by repopulating areas of dead myocardium with new contractile cells.1 Unfortunately, spontaneous multiplication of cardiomyocytes in areas surrounding an infarct cannot completely compensate for lost cells,2 and the conversion of in-scar fibroblasts to contractile cells requires a complicated, time-consuming process of genetic manipulation that, to date, has not been shown to result in the generation of functional myocytes that can integrate themselves into the surrounding viable myocardium. Therefore, obtaining contractile cells from an exogenous pool and engrafting them into postinfarction myocardial scar tissue seems to be the best solution.1,16
Potential Sources of Cardiac Stem Cells
To restore function to nonviable infarcted myocardium, optimal stem-cell transplantation (also called cell cardiomyoplasty) should generate contractile cells that integrate both functionally and structurally into the surrounding viable muscle. This integration occurs via the formation of cell-to-cell contacts such as cardiac-specific intercalated discs consisting of desmosomes and gap junctions. The desmosomes give myocardial tissue the necessary physical strength; the gap junctions, which consist of the cardiac muscle-specific protein connexin 43, enable communication between cardiomyocytes that results in coordinated and synchronous contraction of myocardial tissue. The contractility of engrafted cells can occur naturally (for example, in the case of fetal cardiomyocytes or skeletal myoblasts) or be induced by transdifferentiation (for example, in the case of adult stem cells). Endothelial cells and fibroblasts have also been used to restore function to nonviable myocardium, but the results have been substantially inferior to those achieved with contractile cells.1,2,8,10
Embryonic Stem Cells
Embryonic stem cells are very conducive to stem-cell transplantation therapy, mainly because they are pluripotent. These cells are also weakly immunogenic, since they express only moderate amounts of MHC class I and no MHC class II proteins, and they can readily differentiate into nearly any cell in the body.10,17 In vitro differentiation of ES cells into cardiomyocytes occurs in embryoid bodies. This differentiation can be divided into 3 stages: early, intermediate, and terminal. In the early stage, pacemaker-like cells are produced, and in the intermediate stage, atrial and ventricular cells and cells of the heart conduction system develop. During the terminal stage, well-organized bundles of myofibrils can be observed with clearly distinguishable A, I, and Z bands, and intercalated discs that contain desmosomes and gap junctions. Cardiomyocytes derived from terminally differentiated ES cells respond to β-adrenergic stimulation in vitro.8
In rat studies,17 it was found that ES cells transplanted into ischemically injured myocardium became normal myocardial cells. These cells remained viable for as long as 4 months and brought about both a reduction in infarct area and improvements in regional and global myocardial function and contractility. Similarly encouraging results have come from studies of human ES (hES) cells.5 Theoretically, ES cells are an unlimited source of cells for transplantation since, under certain conditions (for example, incubation with leukemia inhibiting factor), they can survive indefinitely in culture.5 The hES cells can also differentiate in vitro into cardiomyocytes having electrophysiologic, structural, and contractile properties characteristic of neonatal cardiomyocytes, and even displaying atrial and ventricular subtypes. Furthermore, hES cells can be genetically engineered to gain an improved resistance to ischemia.8,10 However, hES cells have drawbacks that might limit their therapeutic feasibility and effectiveness; drawbacks include potential immune rejection, difficult-to-obtain pure cell cultures, and limited proliferative capacity.
Fetal and Neonatal Cardiomyocytes
Fetal and neonatal cardiomyocytes are not true stem cells, since they are already differentiated and do not, in practical terms, divide. Nevertheless, they can be used for cell cardiomyoplasty in ischemic myocardium. These cells are obtained from fetal and neonatal hearts, respectively, and then grown in tissue cultures. In animal models of myocardial infarction (MI), the cells have been successfully engrafted through epicardial injection into ischemic myocardium. These cells have also been shown to connect with host cardiomyocytes through intercalated discs containing desmosomes and gap junctions—a sign of their structural and functional integration into the host myocardium.
In rat studies, engraftment of fetal or neonatal cardiomyocytes into ischemic myocardium was shown to result in left ventricular wall thickening, reduced dyskinesis, and increased ejection fraction.18–20 The engrafted fetal or neonatal cardiomyocytes also survived for as long as 6 months after implantation. These promising results are offset, however, by the strong immunogenicity and small-scale production of engrafted cells and, above all, by the significant ethical issues raised by their use.1
Skeletal Myoblasts
Histologically, skeletal myoblasts (SMs) are committed progenitors of skeletal muscle cells. Normally, they reside under the basement membrane of skeletal muscle fibers, where they remain quiescent until recruited for repair or regeneration of damaged muscle tissue. Skeletal myoblasts were the 1st potential cardiac stem cells to be explored extensively because of their autologous origin, high proliferative potential, commitment to a myogenic lineage, and resistance to ischemia. The last property is an especially important one for cells intended for implantation in the hypoxic environment of a postinfarction scar.1,21,22
Studies in rats and human beings confirmed that implanted SMs could repopulate scar tissue, resulting in ventricular wall thickening, elevated ejection fraction, and improved contractility.5,21–23 However, as shown by fluorescent studies, the engrafted cells also developed a peculiar phenotype of hyperexcitable myotubes with contractile activity that was completely unaffected by neighboring cardiomyocytes.24 Other investigators found that SM-derived cardiomyocytes did not necessarily integrate into the surrounding host myocardium, as evidenced by the normal expression in vitro versus downregulated expression in vivo of 2 key proteins involved in electromechanical cell integration—N-cadherin and connexin 43.25 Therefore, the contribution of SM-derived cardiomyocytes to improved cardiac function cannot be explained in terms of electromechanical integration.24
One hypothesis proposes that the elastic properties of implanted SMs limit the expansion of the postinfarction scar. This concept has merit, provided that the cells are injected soon after an ischemic episode, since it seems unlikely that complete postischemia ventricular remodeling could be reversed by injection of SMs (or any other stem cells, for that matter).1,21 A 2nd hypothesis is that SM-derived cardiomyocytes contribute directly to improved systolic function. This has merit because several groups have shown that SMs injected into diseased myocardium substantially improve contractile function.21–23 One group has even demonstrated that SMs and fetal cardiomyocytes equally improve postinfarction cardiac function,26 which is interesting, considering that fetal cardiomyocytes express connexin 43 and become functionally integrated, whereas myocytes do not. This suggests that normal heart function may not necessarily require connexin 43 and that there are different ways to induce grafted SM-derived cells to contract, such as by stretching or by direct transmembrane channeling of electric currents.1,24 It has also been hypothesized that SM-derived cells exert a paracrine effect on surrounding myocardial cells, perhaps through hepatocyte growth factor and insulin growth factor I, which SM-derived cells are known to express. These factors may act as antifibrotic agents that protect peri-infarction tissues and possibly recruit stem cells for myocardial repair, indirectly increasing the contractility of the damaged heart. However, these are only speculations and warrant additional research.
Bone Marrow Stem Cells
Bone marrow is composed of various types of cells of specific phenotypes and function. Bone marrow cells can be transplanted either as total, unfractionated bone marrow or as a well-defined subpopulation of BMSCs.1,27 Bone marrow has recently gained attention as a potential source of multipotent stem cells for cell cardiomyoplasty, particularly because of its easy accessibility, autologous origin, and ability to transdifferentiate into either myocardial or vascular cells.
Total unfractionated bone marrow is usually aspirated from the iliac crest and immediately injected into damaged myocardium. Although this approach seems very simple, feasible, and straightforward, it is not effective. Studies in sheep showed no improvement in regional or global cardiac function after injection of unfractionated bone marrow into scar tissue1 and no hemodynamic improvement after injection into infarcted myocardium.28 Histologic analysis showed that grafted cells did not transdifferentiate into either cardiomyocytes or vascular cells.28 These results may be explained by the fact that total bone marrow contains very few multipotent cells capable of transdifferentiating into cardiomyocytes, per unit of volume. Questions therefore remain with regard to the efficiency of using total unfractionated bone marrow.
Another, slightly more complicated method is to inject a well-defined subpopulation of multipotent BMSCs into damaged myocardium. Preclinical results suggest that this approach is effective, although hematopoietic subpopulations appear to be more effective than nonhematopoietic subpopulations29,30: in a mouse model of MI, a nonhematopoietic subpopulation of BMSCs (CD34−, c-kit+, Sca-1+) known to re-generate the hematopoietic system was shown histologically to regenerate and revascularize cardiac tissue. However, these histologic findings were not accompanied by functional data, leaving in question the functional efficacy of this treatment.29,30
In other mouse studies,31 a hematopoietic subpopulation of BMSCs (CD34+, lin−, c-kit+), containing both short-term repopulating progenitor cells and long-term repopulating HSCs, was shown to regenerate ischemic myocardium. It is currently believed that long-term repopulating HSCs can transdifferentiate into cardiomyocytes and endothelial cells, making them capable of mediating the regeneration of damaged myocardium.31 In 1 study,32 fluorescently labeled CD34+, lin−, c-kit+ HSCs were injected into healthy myocardium adjacent to an infarct that had been induced several hours earlier by left coronary artery occlusion. Nine days later, a new band of myocardium occupying 68% of the infarcted area and extending across the entire infarct had appeared in 40% of the experimental cases. Further histologic analysis of the new myocardium revealed small fetal-like cardiomyocytes that were positive for A-actinin (a protein specific for cardiac and skeletal muscle); cardiac-specific myosin; several transcription factors known to promote stem-cell differentiation and activate cardiac gene programs (GATA-4, MEF2, and Csx/Nkx 2.5); and connexin 43.31,32 Thirty-six percent of the fluorescently stained cardiomyocytes were also positive for BrdU, a sign that the HSC-derived cardiomyocytes were at 1 point mitotically active. Nineteen percent of fluorescent cells were also positive for Ki-67, a marker of active cell cycling.32 The investigators also identified fluorescent endothelial and smooth muscle cells in the new myocardial tissue, indicating that revascularization accompanied myocardial regeneration. Improved cardiac function was confirmed by hemodynamic studies that showed a 30% to 40% improvement in left ventricular systolic and diastolic pressures after HSC injection. As further proof that HSCs were responsible for the therapeutic effects, it was shown that the lin−, c-kit+ subpopulation could not reconstitute bone marrow in irradiated animals and therefore does not contain HSCs.12,29,31,32
The mobilization of HSCs from bone marrow can apparently be enhanced by cytokine factors, as shown by studies in a rat model of MI.28,31,33 Treatment with cytokine stem-cell factor (SCF)—which binds to the c-kit tyrosine kinase receptor, and granulocyte colony-stimulating factor (G-CSF) led to a 250-fold increase in circulating HSCs. This in turn resulted in a band of new myocardium that occupied 76% of the infarct, a 61% reduction in 30-day mortality (from 78% to 17%), and substantially improved cardiac function. The phenotypes of engrafted cells included developing cardiomyocytes, endothelial cells, and smooth muscle cells. These results, together with those discussed above, suggest that lin−, c-kit+ cells can improve both short- and long-term outcomes of ischemic cardiomyopathy by transdifferentiating into physically and functionally integrated cardiomyocytes and vascular cells.
Recently, multipotent mesenchymal stem cells (MSCs) have gained attention for their therapeutic potential. Also known as bone marrow stromal cells, MSCs are self-renewing clonal precursors of nonhematopoietic tissues derived from mesodermal germ layer. They are relatively easy to obtain from autologous bone marrow, to expand in vitro without sacrificing multipotency, and to cryopreserve for future use. Normally, MSCs can differentiate into osteocytes, chondrocytes, and adipocytes. Under certain conditions, however, they can differentiate into other cell types. When cultured with vascular endothelial growth factor (VEGF), MSCs can differentiate in-to endothelial cells.34 When treated with the DNA-demethylating agent 5-azacytidine (5-aza), they can differentiate into cardiomyogenic (CMG) cells, which can subsequently form myotubes connected by intercalated discs that beat spontaneously and synchronously.5,35 Like cardiomyocytes, CMG myotubes have well-organized sarcomeres and centrally positioned nuclei. They also express cardiac-specific transcription factors in a pattern that is seen in the early embryonic heart and display functional adrenergic and muscarinic receptors on their surfaces.34
The exact mechanisms by which MSCs may improve cardiac function remain controversial.36–39 One candidate mechanism is de novo angiogenesis, as shown recently in rats whose ischemic hearts were injected with 5-aza–treated MSCs.36–38 The MSCs appeared to improve left ventricular function by becoming engrafted, undergoing site-specific differentiation, and contributing to de novo angiogenesis in hibernating myocardium despite not being functionally integrated (as indicated by their segregation from the surrounding viable myocardium by a band of scar tissue). Another possible mechanism is cell fusion, which would theoretically allow damaged myocardial and endothelial cells to preserve their structural and functional integrity by commandeering additional cytoplas-mic material and new genetic material from the fused cells.37–39
Unfortunately, 5-aza can also induce uncontrollable expression of a variety of genes, and this has raised concerns about its clinical safety. Several studies suggest that 5-aza may not be required in order to induce MSCs to differentiate into functionally integrated cardiomyocytes and that physiologic components of the cardiac microenvironment may suffice.38,40 Conversely, some groups have reported very low levels of MSC engraftment in vivo, despite the injection of large numbers of MSC cells.4,39,41
At the Texas Heart Institute, allogeneic MSC injections were performed in a canine model of chronic ischemia induced by ameroid constriction. The MSCs differentiated into smooth muscle cells and endothelial cells,42 which resulted in increased vascularity and improved cardiac function both at rest and under stress. These results suggest that the ischemic burden of the left ventricle decreased after cell therapy.42
One group4 found that MSCs genetically engineered to over-express the serine threonine kinase Akt, which transmits a powerful survival signal, became 17 times more resistant than normal MSCs to the hypoxic environment of the ischemic myocardium. Moreover, when transplanted into areas surrounding ischemic myocardium, these genetically altered cells inhibited myocardial remodeling and collagen deposition and almost completely normalized systolic and diastolic function. These cells could not, however, induce new blood vessel growth.
Clearly, MSC therapy can restore some function to the ischemic heart. In addition, this ability is apparently enhanced when other stem cells are used as well, as recently shown in an ischemic swine model in which human MSCs and fetal cardiomyocytes were transplanted together.43
Other Stem Cells
In addition to the stem cells already discussed, other sources of cardiac stem cells are currently under investigation. These include peripheral blood CD34+ cells, endothelial progenitor cells, fibroblasts, and cardiac progenitor cells.
Adult peripheral blood CD34+ cells are of interest as potential cardiac stem cells, because they are known to be able to transdifferentiate (albeit into hepatocytes)44 and they can be obtained from autologous sources without the need for painful bone marrow aspiration. In vivo studies in a mouse model of MI have shown that these cells can transdifferentiate into cardiomyocytes, endothelial cells, and smooth muscle cells and that such transdifferentiation is significantly increased in the presence of local tissue injury.45
Endothelial progenitor cells have the ability to promote therapeutic neovascularization. These CD34+ mononuclear cells normally reside in bone marrow and are recruited into the peripheral blood in response to tissue ischemia. Once mobilized, endothelial progenitor cells specifically home in on ischemic areas, where they differentiate into endothelial cells to form new vessels. Studies in a swine model have shown that autologous transplantation of endothelial progenitor cells and their subsequent differentiation into mature endothelial cells can attenuate chronic myocardial ischemia and enhance neovascularization, and can consequently preserve or improve left ventricular function.46 The evidence also suggests that transplantation of endothelial progenitor cells may even inhibit myocardial fibrosis.
Fibroblasts of autologous origin have the potential to inhibit host matrix degradation, to improve diastolic function by replacing scar tissue with more elastic muscle-like tissue, and to enhance regional hypertrophy and neovascularization. In addition, they may prevent infarct expansion and ventricular remodeling by increasing scar thickness, which reduces wall stress. In rat studies, fibroblasts that were harvested from ischemic myocardial scar tissue and pericardium and then genetically manipulated to express the gene for the muscle-specific transcription factor MyoD were shown to differentiate into multinucleated, typically cross-striated myotubes that were indistinguishable from myotubes derived from primary skeletal myoblasts.16 One month after implantation into postinfarction scars, these genetically engineered myogenic cells had still not been functionally integrated but did occupy a substantial portion of the scars. The effects on ventricular remodeling and function were not evaluated. Nevertheless, the reported findings suggest that genetically engineered fibroblasts may be especially useful in the elderly, a population whose supplies of autologous primary skeletal myoblasts or BMSCs are often limited.16
Cardiac progenitor cells isolated from adult myocardium have a phenotype (Sca-1+, lin−, CD45−, CD31+, CD38+) very similar to that of skeletal myoblasts and express most cardiogenic transcription factors but no cardiac structural genes.47 Like MSCs, they can be induced to differentiate into cardiomyocytes in vitro by treatment with 5-aza.47 However, very little is known about cardiac progenitor cells, and further research to characterize them is warranted.
Delivery Methods
Stem cells can be delivered into the ischemic myocardium either by invasive or by noninvasive means. Invasive epicardial injection is done on a surgically exposed heart. Then, depending on the protocol used, cardiac stem cells are injected into the infarction itself or into the viable myocardial tissue surrounding it. However, in light of the trend toward less invasive diagnostic and therapeutic procedures, percutaneous approaches are under investigation. Catheter-based transendocardial injection, which is technically feasible and functionally efficient, has been widely confirmed to be accurate for delineating and identifying scarred and viable myocardium and for differentiating degrees of infarct transmurality. The electromechanical mapping platform offers a benefit over surgical and intracoronary approaches, because the viability of the site can be determined before each injection. Injections can then be limited to targeted, viable areas of hibernating myocardium. Many treated sites in patients with ischemic heart failure are in areas of totally occluded epicardial vascular beds, making intracoronary delivery impossible. Furthermore, potential ischemia provoked by coronary manipulation is avoided with use of this method. This approved procedure seems safer for these chronically ill, high-risk patients because it avoids associated surgical morbidity and mortality.
Catheter-based infusion through the coronary sinus is under study but is currently limited in its clinical application. There have been sporadic reports of successful intracoronary injections of stem cells during open-heart revascularization procedures, but larger trials are needed.
Also under consideration are noninvasive methods of targeting the ischemic myocardium with stem cells that take advantage of endogenous mechanisms. Recent studies in a rat model showed that endogenous signaling via cytokines can enhance mobilization, homing, and transdifferentiation of stem cells.11 Two possible explanations for this have been proposed. In the 1st mechanism, cytokines are released into the blood in response to cellular necrosis in the injured myocardium; from there, they mobilize BMSCs to enter the peripheral circulation. The mobilized stem cells then home in on adhesive receptors presented by the injured tissue and, once there, begin to differentiate into tissue-specific cells (that is, cardiomyocytes). In the 2nd mechanism, stem cells from the bone marrow continually circulate throughout the body, constantly traveling through all kinds of tissues. When myocardial injury occurs, the stem cells exit the circulation at the injury site and infiltrate. Both mechanisms assume that stem cells originate in a common pool (that is, the bone marrow), as discussed above.11
Little is known about the signals involved in the mobilization and homing of stem cells to the injured myocardium. It is currently believed that the cytokines SCF, G-CSF, and stromal-cell–derived factor-1 (SDF-1) and their receptors play a major role.1
Despite their relatively high efficiency, all of the above-mentioned delivery methods are hampered by a major limitation of cell cardiomyoplasty, namely, cell death. As many as 90% of transplanted cells die shortly after implantation as a result of physical stress from the implantation procedure itself, myocardial inflammation, or myocardial hypoxia. This does, however, suggest ways to improve the timing of cell cardiomyoplasty. Therapy too soon after an ischemic episode, when the damaged tissue is still inflamed, may increase the risk of cell death; therapy too late may decrease the chances of reverse myocardial remodeling.1
Clinical Trials
Early results from phase I human trials of stem-cell therapy for ischemic heart disease are beginning to emerge. In 1 trial,48 10 patients who had experienced MI were treated by bypass surgery and were also injected with skeletal myoblasts derived from the musculus vastus lateralis into the postinfarction scar. The combined therapy resulted in ventricular wall thickening across 60% of the treated ischemic myocardium and improved systolic function. In general, this combined therapy was also safe and effective, although 4 patients did have some ventricular tachycardia that was apparently due to the stem-cell transplantation. No definite conclusions can be drawn about the benefits of the stem-cell therapy itself until larger, multicenter, randomized, controlled studies that do not involve concomitant revascularization can be done.
In another trial,31 6 patients who had experienced MIs underwent implantation of autologous BMSCs into peri-infarction regions during a coronary artery bypass grafting procedure. After 9 to 16 months of follow-up, the patient-reported exercise capacity and the mean ejection fraction had noticeably improved in all cases, and no ventricular arrhythmias had occurred. In 5 patients, myocardial perfusion scans showed significant improvement in cardiac function (mean ejection fraction increased from 0.37 to 0.48).
In a controlled trial,49 10 patients who had suffered an acute MI were treated with BMSCs that were delivered to infarcted areas by a high-pressure balloon catheter 5 to 9 days after the ischemic event. The delay between MI and treatment was due to the time required to aspirate, separate, harvest, and culture the stem cells. When compared with untreated control patients at 3-month follow-up, the treated patients showed no change in ejection fraction. However, they did have a significant reduction in infarct size and a significant increase in stroke volume. No adverse effects were reported.
In the largest clinical trial of its kind so far,50 21 high-risk patients with end-stage ischemic heart disease were enrolled in a prospective, nonrandomized trial of autologous mononuclear BMSCs. After thorough baseline evaluation, 14 patients received stem-cell treatment and 7 patients (controls) did not. In brief, autologous mononuclear BMSCs were injected transendocardially into viable myocardium identified by electromechanical mapping. At the 2-month follow-up visit, treated patients showed a significant reduction in total reversible defect and a significant increase in global left ventricular function. At 4 months, treated patients showed improved left ventricular ejection fraction (from 0.20 at baseline to 0.29) and reduced end-systolic volume. This technique appears to be feasible, safe, and effective.
Several other clinical trials of stem-cell therapy for ischemic heart failure are under way in the United States. Their therapeutic results have not yet been published.
Conclusions
Stem-cell therapy for ischemic heart failure is very promising. Initial clinical trials have provided good results. However, major issues still need to be resolved before stem-cell therapy can be introduced fully into clinical practice. These issues include defining the strategies that can be used to drive stem cells to differentiate into specific cardiac cells in vitro, optimizing the protocols for enriching and purifying specific cell type populations in vitro, developing large-scale culture techniques, determining the efficacy of various transplantation techniques, and characterizing the properties of stem-cell grafts in vivo.12 The greatest obstacle of all to full acceptance of stem-cell therapy in the clinic appears to be the ethical issues raised by the use of hES cells.5,9
Acknowledgments
We thank Emerson C. Perin, MD, PhD, for his helpful comments and review of this manuscript. We also thank Guilherme V. Silva, MD, for his helpful discussion of the manuscript, and Mr. Jude Richard for editing the manuscript.
Footnotes
Address for reprints: Doreen Rosenstrauch, MD, PhD, Texas Heart Institute, MC 2-255, P.O. Box 20345, Houston, TX 77225-0345
E-mail: Doreen.Rosenstrauch@uth.tmc.edu.
This work was partially supported by a grant from the Roderick Duncan MacDonald Research Fund at St. Luke's Episcopal Hospital, Houston, Texas (Dr. Rosenstrauch).
References
- 1.Menasche P. Cell transplantation in myocardium. Ann Thorac Surg 2003;75(6 Suppl):S20–8. [DOI] [PubMed]
- 2.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res 1998;83:1–14. [DOI] [PubMed]
- 3.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001;344: 1750–7. [DOI] [PubMed]
- 4.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195–201. [DOI] [PubMed]
- 5.Hughes S. Cardiac stem cells. J Pathol 2002;197:468–78. [DOI] [PubMed]
- 6.Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med 2003;349:570–82. [DOI] [PubMed]
- 7.Rosenstrauch D, Kadipasaoglu K, Shelath H, Zoldhelyi P, Frazier OH. Auricular cartilage tissue engineering. In: Yaszemski MJ, Trantolo DF, Lewandrowski K, Hasirci V, Altobelli DE, Wise DL, editors. Tissue engineering and novel delivery systems. New York: Marcel Dekker; 2003. p. 253–64.
- 8.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002;91:189–201. [DOI] [PubMed]
- 9.Council for Responsible Genetics. Genewatch. Available at: www.gene-watch.org. Accessed March 12, 2004.
- 10.Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res 2002;91:866–76. [DOI] [PubMed]
- 11.Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res 2002;91:1092–102. [DOI] [PubMed]
- 12.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann N Y Acad Sci 2003;996: 152–7. [DOI] [PubMed]
- 13.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature 2002;416:545–8. [DOI] [PubMed]
- 14.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897–901. [DOI] [PubMed]
- 15.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422: 901–4. [DOI] [PubMed]
- 16.Etzion S, Barbash IM, Feinberg MS, Zarin P, Miller L, Guetta E, et al. Cellular cardiomyoplasty of cardiac fibroblasts by adenoviral delivery of MyoD ex vivo: an unlimited source of cells for myocardial repair. Circulation 2002;106 (12 Suppl 1):I125–30. [PubMed]
- 17.Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 2002; 92:288–96. [DOI] [PubMed]
- 18.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation 1996;94(9 Suppl):II332–6. [PubMed]
- 19.Scorsin M, Hagege AA, Marotte F, Mirochnik N, Copin H, Barnoux M, et al. Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation 1997;96(9 Suppl):II-188–93. [PubMed]
- 20.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, et al. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg 1996;62:654–61. [DOI] [PubMed]
- 21.Tambara K, Sakakibara Y, Sakaguchi G, Lu F, Premaratne GU, Lin X, et al. Transplanted skeletal myoblasts can fully replace the infarcted myocardium when they survive in the host in large numbers. Circulation 2003;108(Suppl 1):II259–63. [DOI] [PubMed]
- 22.Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, et al. Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci 2001;938:208–20. [DOI] [PubMed]
- 23.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 2003;41:879–88. [DOI] [PubMed]
- 24.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A 2003;100:7808–11. [DOI] [PMC free article] [PubMed]
- 25.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol 2000; 149:731–40. [DOI] [PMC free article] [PubMed]
- 26.Scorsin M, Hagege A, Vilquin JT, Fiszman M, Marotte F, Samuel JL, et al. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg 2000;119:1169–75. [DOI] [PubMed]
- 27.Horwitz EM. Bone marrow transplantation: it's not just about blood anymore! Pediatr Transplant 2003;7 Suppl 3:56–8. [DOI] [PubMed]
- 28.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, et al. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation 2003;108 Suppl 1:II247–52. [DOI] [PubMed]
- 29.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361: 45–6. [DOI] [PubMed]
- 30.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395–402. [DOI] [PMC free article] [PubMed]
- 31.Orlic D, Kajstura J, Chimenti S, Bodine SM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant 2003;7 Suppl 3:86–8. [DOI] [PubMed]
- 32.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci 2001;938:221–30. [DOI] [PubMed]
- 33.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 2001;98:10344–9. [DOI] [PMC free article] [PubMed]
- 34.MacKenzie TC, Flake AW. Human mesenchymal stem cells: insights from a surrogate in vivo assay system. Cells Tissues Organs 2002;171:90–5. [DOI] [PubMed]
- 35.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 2003;108 Suppl 1:II253–8. [DOI] [PubMed]
- 36.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002;416: 542–5. [DOI] [PubMed]
- 37.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature 2002;416:545–8. [DOI] [PubMed]
- 38.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999;100 (19 Suppl):II247–56. [DOI] [PubMed]
- 39.Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg 2000;120:999–1005. [DOI] [PubMed]
- 40.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 2002;73:1919–26. [DOI] [PubMed]
- 41.Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs 2001;25:187–93. [DOI] [PubMed]
- 42.Silva GV, Litovsky S, Assad JAR, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005;111:150–6. [DOI] [PubMed]
- 43.Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP, Xiao YF. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg 2002;74:1568–75. [DOI] [PubMed]
- 44.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002;346:738–46. [DOI] [PubMed]
- 45.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 2003;108:2070–3. [DOI] [PubMed]
- 46.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–8. [DOI] [PubMed]
- 47.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 2003;100:12313–8. [DOI] [PMC free article] [PubMed]
- 48.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003;41:1078–83. [DOI] [PubMed]
- 49.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913–8. [DOI] [PubMed]
- 50.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107:2294–302. [DOI] [PubMed]


