Cryptogenic stroke is a diagnosis of exclusion. These are strokes that occur in people who are usually less than 55 years old, without an identifiable cause. Our sensitivity to these events has been heightened because of the new definitions of a transient ischemic attack. Transient ischemic attack (TIA) is a clinical diagnosis of a neurologic deficit without MRI abnormalities: if there is an MRI abnormality, whether or not that person is symptomatic, it is now defined as a stroke. With these new definitions, and the sensitivity of MRI, we are seeing more cryptogenic strokes.
It has been hypothesized that many cryptogenic strokes are caused by small emboli that travel from the legs to the right atrium; during straining (such as a Valsalva maneuver) these emboli can go across a PFO into the left atrium and then travel to the brain, producing a stroke. The problem is that these are very small emboli, approximately 1 to 3 mm, and we can't actually show these small emboli crossing from right to left. However, large emboli have been observed by echocardiography to be trapped in the PFO. So the diagnosis of cryptogenic stroke is a diagnosis of exclusion that is impossible to verify.
What is the scope of the problem? Of the 700,000 strokes per year in the United States, 80% of them are ischemic, and 20% of those are defined as cryptogenic. The prevalence of PFO among this cryptogenic stroke population is about 40% to 50%; in the general population, it's only about 20%. Current estimates are that somewhere between 30,000 and 60,000 strokes per year in the U.S. are caused by paradoxical embolism through a PFO.
There are some other fascinating associations: scuba divers with PFOs are more susceptible to decompression illness. Platypnea-orthodeoxia is a condition of desaturation that occurs when you're standing up but not when you're lying down; these patients are quite symptomatic, with arterial saturations in the low 80s. They also frequently have PFOs; if you close the PFO, the arterial desaturation is alleviated.
Fat emboli during orthopedic surgery or air emboli during neurosurgery may also travel through the venous system. If you don't have a PFO, the fat or the air is trapped in the lungs and doesn't cause much of a problem unless it's massive; but if you have a PFO, then the embolus can go from right to left atrium up to the brain, with devastating neurologic consequences.
Treatment Options for Cryptogenic Stroke and PFO
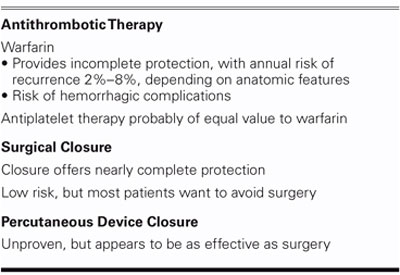
There are several treatment alternatives for cryptogenic stroke (Table I). Warfarin has been used for years, but it is uncertain that this reduces the risk of events, which is still 2% to 8% per year. Antiplatelet therapy appears to be of equivalent value to warfarin. The WARS trial looked at over 2,000 patients with stroke of uncertain origin (atrial fibrillation and high-grade carotid stenosis were excluded); the recurrence rate was quite high—8% per year—but not all of these patients had PFOs. There was a substudy of 630 patients in WARS who were evaluated by transesophageal echocardiography; PFO was significantly more common in patients with cryptogenic stroke. In a subset analysis, they separated patients into those who had a PFO from those patients who did not have a PFO, and then segregated them according to age. Remember, cryptogenic stroke is usually defined as occurring in patients less than 55 years of age; above age 55, strokes are generally felt to be more likely due to atherosclerosis. Interestingly, though, in the lowest age group (<55), the incidence of recurrent stroke or death tended to be lower in patients with a PFO, and it was about equal in the 55 to 65 age range. But above age 65 into the 80s, patients were 3 times more likely to have a stroke or death if a PFO was present, even accounting for other risk factors usually associated with atherosclerosis. As you get older, you probably have more venous emboli and your right atrial pressure goes up, raising the risk of PFO-mediated embolic events to the point where, in the elderly, that risk may be just as important as underlying atherosclerotic disease. These are all observational data at present, but the concept is fascinating and needs to be tested. Perhaps all elderly patients with stroke should be getting their PFOs closed in addition to getting their carotid arteries cleaned out.
TABLE I. Treatment Alternatives
Percutaneous ASD and PFO Closure
We now have options for percutaneous closure of interatrial communications, such as the CardioSEAL device and the Amplatzer device. Placing one of these involves advancing a catheter across the interatrial septum and deploying the device, which springs out and then is detached. The procedure takes about 30 to 45 minutes, does not require general anesthesia, and can even be done without transesophageal echo, using intracardiac echo. It's an outpatient procedure, and patients go home within a few hours. The CardioSEAL device itself is made of Dacron with embedded metal springs. The true “seal” is actually a biologic seal, with scar tissue that forms over the device. The mechanical force of the springs just approximates the tissues; the body's healing process does the rest, to achieve complete closure.
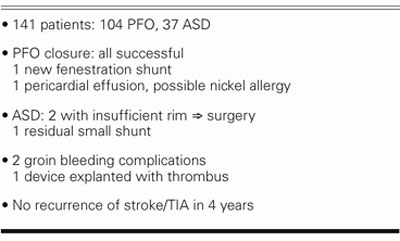
In the last 4 years, we've done 141 procedures (Table II), including atrial septal defect (ASD) closures and about 100 PFOs. All 141 devices were successfully implanted. One had a shunt around it, probably from a tear; and there was 1 case of pericardial effusion. There was 1 residual shunt among the ASDs, and there were some minor bleeding complications. We did have one CardioSEAL device that had significant thrombus, and we subsequently explanted that device. Last year, in the Journal of the American College of Cardiology, we reported a higher incidence of thrombus on the CardioSEAL, and we've stopped using that device and use the Amplatzer now exclusively. Importantly, in our series of 100 patients with PFO closure, we've had no recurrence of stroke or transient ischemic attack over the 4 years that we've been following these cases. Currently, there are 2 ongoing randomized trials that will attempt to test whether device closure of patent foramen ovale is preferable to continuous medical therapy to prevent recurrent stroke from paradoxical embolism.
TABLE II. Results at UCLA: 2001–2004
Migraine
Approximately 12% of the population, or 27 million people in the United States alone, have migraine headaches (Table III). Migraine is 3 times as common in women as in men. The incidence of PFO in patients with migraine is about 50% if migraine is accompanied by visual aura, versus 20% in the general population. Migraine with aura seems to be different from migraine without aura. Migraine patients without aura are no different from the general population in regard to the incidence of PFO.
TABLE III. Migraine Facts
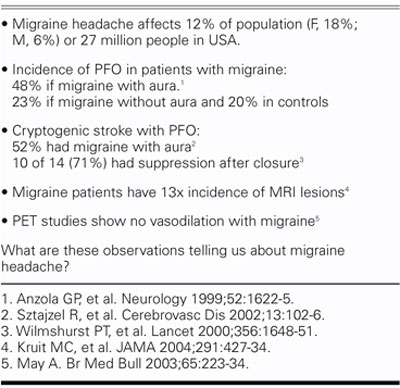
If we look at it another way, the incidence of migraine with aura is about 50% in cryptogenic stroke patients who have a PFO, instead of the 12% found in the general population. Small observational studies have showed that if the PFO is closed, the migraines are suppressed in about 70% of those patients. We also know that migraine sufferers have an increased risk of abnormal MRIs; in 1 study from Denmark, there was a 13-fold higher incidence of MRI lesions in patients with migraine. We also know that the previous view of the pathophysiology of migraine headaches—intense vasospasm followed by vasodilation and headache (Table IV)—is incorrect and that our concept needs to be adjusted (Table V). A recent article in the Journal of the American Medical Association described migraine as a progressive brain disease, with a 5 times higher incidence of posterior circulation infarcts.
TABLE IV. The Presumed Etiology of Migraine Headache: Older Version
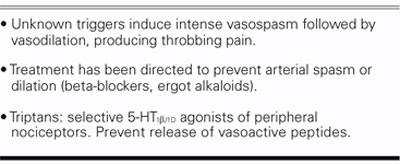
TABLE V. The Presumed Etiology of Migraine Headache: Newer Version
There are 2 recent articles in the Journal of the American College of Cardiology, from our group at UCLA and Mark Reisman's group in Seattle, both showing that in the patients who had an ASD or PFO closed because of cryptogenic stroke, the incidence of migraine headaches was significantly reduced. In our data, migraine was present in 37 out of 89 responders; 24 had aura. Of those patients with aura, 18 of 24 had no more recurrent headache after PFO or ASD closure. Zero. In the patients who did not have complete resolution of their migraine episodes, there was still a significant improvement in the symptoms.
A New Migraine Hypothesis
One hypothesis to explain these observations is that migraine is due to paradoxical embolism through a PFO. Our migraine hypothesis is a little different from that (Table VI). We believe that there are certain individuals who are neurophysiologically susceptible; but migraine symptoms are initiated by chemical substances, and not primarily by embolism. People eat chocolate or nuts, or drink red wine, and get migraine headaches; and there is perimenstrual migraine in women. I believe that there is some chemical that usually is cleared by the lungs in a 1st pass; however, if you have a PFO, you can shunt this chemical directly to the brain, which triggers the migraine headache symptoms in susceptible individuals. Closure of the PFO will reduce or eliminate migraine headaches, I believe, in 75% of patients, especially those migraines associated with aura. The presence of a PFO also increases the risk of embolic TIA in that patient population, and so you may have an abnor-mal MRI due to small emboli going to the brain. A migraine-associated stroke is not due to intense vasospasm, but is actually a paradoxical embolism through the PFO. Closing the PFO will also reduce the risk of associated stroke. These concepts need to be tested in a multicenter randomized clinical trial, which is currently being proposed to the FDA.
TABLE VI. Our Migraine Hypothesis

Ultimately, PFO closure, performed percutaneously in the catheterization laboratory, may come to play an important role in reducing migraine symptoms in certain individuals (particularly those with aura), and in reducing some of the associated risk of stroke. Ongoing randomized studies will be testing this prospectively.
Footnotes
Address for reprints: Jonathan M. Tobis, MD, Adult Cardiac Catheterization Laboratory, UCLA, Center for Health Sciences, BL 394, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1717
E-mail: jtobis@mednet.ucla.edu
Presented at the Texas Heart Institute's symposium “Current Issues in Cardiology;” held at the Sheraton World Resort; 5 March 2005; Orlando


