There's been a voracious uptake of drug-eluting stents worldwide since their introduction in 2002. In January 2005, Boston Scientific Corporation announced that 1 million TAXUS stents had been implanted.1 This was followed in February 2005 by Cordis Corporation's announcement that 1 million patients had received approximately 1.5 million CYPHER stents.2 So there are now over 2.5 million stents implanted; at about $2,000 each, that equals $5 billion. At the Thoraxcenter, it's been no different: very early in April 2002, we made the decision that this was to be our preferred therapy. We are now approaching 3 years of use, and over 8,000 drug-eluting stents have been implanted in nearly 4,000 patients at our institution.
Early Stent Thrombosis
There is a potential downside to these stents, namely stent thrombosis. There are 2 distinct types: early stent thrombosis, which occurs within the first 30 days, and late stent thrombosis, which occurs after 30 days. For bare-metal stents, the incidence of early stent thrombosis is low. Cutlip and coworkers3 pooled 6 trials with a total of 6,000 patients and documented an incidence of 0.9%. Orford4 looked at 4,500 patients in the Mayo Clinic registry and reported an incidence of 0.51%. The problem is that patients with stent thrombosis have a 70% to 87% risk of death or nonfatal MI.
We recently published a series of 2,512 patients who underwent stenting: 506 with bare-metal stents, 1,017 with sirolimus-eluting stents, and 989 with paclitaxel-eluting stents (Table I).5 Long-term clopidogrel use was different in the 3 groups, but our focus was on the first 30 days, and all 3 had the same dual antiplatelet therapy during that period. The population was pretty typical for a tertiary cath lab. Multivessel disease was present in over half of our patients; unstable angina was the indication in a third, and acute MI in a quarter. In the drug-eluting era, we treated a mean of 1.4 vessels and implanted 2.2 or 2.3 stents per patient, for a total length of 42 to 44 mm. With drug-eluting stents, we became more aggressive and implanted more stents and longer stent lengths. Bifurcations were treated in 18% of patients. The incidence of angiographic stent thrombosis in the 3 groups was basically the same, 1%. However, three quarters of these patients with stent thrombosis presented with an acute MI, and of those, 3 died, giving us a mortality rate of 12% (3 patients out of 26 with stent thrombosis) at 30 days. When a broader clinical definition of “possible” stent thrombosis was applied—including patients with sudden death or out-of-hospital cardiac arrest or myocardial infarction not attributable to another lesion—the 30-day overall incidence was about 1.5%, similar for all 3 groups. Importantly, stent thrombosis, if it occurred, tended to do so in the first 11 days, which suggests that, acutely, a mechanical cause is to blame.
TABLE I. Patient Characteristics in Early Stent Thrombosis Study

Late Stent Thrombosis
Late stent thrombosis may occur if there's impaired re-endothelialization of the vessel and the implanted stent, as was seen with coronary brachytherapy in the early days of its use. The delayed effects of drug-eluting stents in human beings are at present unknown. Virmani6 and colleagues reported on a patient enrolled in the E-SIRIUS trial who received 2 sirolimus-eluting stents: at 8-month angiography and IVUS, he demonstrated vascular enlargement, and at 18 months post-implantation, he died as a consequence of late stent thrombosis. On autopsy, aneurysm formation and a localized hypersensitivity reaction were found. The authors suspected that a reaction to the polymer might have been the cause.
In a research letter in the Lancet last year,7 we reported 4 cases of late stent thrombosis: 2 patients were from our center, and 2 were from the Washington Hospital Center (Table II). All 4 patients were on dual antiplatelet therapy, aspirin, and clopidogrel for 3 to 6 months, followed by aspirin therapy. In all 4 patients, stent thrombosis occurred very late, at least 11 months after stent implantation. It occurred at a time remote from the mandatory 6-month term of antiplatelet therapy that is recommended for aspirin and clopidogrel. These incidents were, however, related to the cessation of aspirin: these patients were on no antiplatelet therapy at the time of the events. In 3 of the 4 patients, the aspirin was stopped for elective surgery; the remaining patient stopped it on his own. All 4 patients received a single large drug-eluting stent, 3 or 3.5 mm in diameter. All presented with an acute myocardial infarction 4 to 14 days after stopping aspirin. These late thromboses occurred more than 11 months after stent implantation, at a time when re-endothelialization or healing following drug-eluting stent implantation should have been complete. Patients 3 and 4 are very interesting because each had a bare-metal stent implanted in another vessel at around the same time as the drug-eluting stent, and the bare-metal stent was patent at the time of the stent thrombosis.
TABLE II. Patient Characteristics of Late Angiographic Stent Thrombosis (LAST)

We took this a bit further and looked at our over-all incidence of late angiographic stent thrombosis (LAST) with drug-eluting stents.8 We took the identical population that we used when we looked at early stent thrombosis: 2,006 patients with drug-eluting stents. We followed each of these patients for at least a year, with a mean follow-up of 1.5 years. There were 3 sirolimus-eluting stent thromboses and 5 paclitaxel-eluting stent thromboses, all of which were angiographically documented, yielding an overall incidence of 0.35%, with an upper limit to the confidence interval of 0.72%. Of the 8 patients, 2 died, for a mortality rate of 25%. Importantly, no cases of stent thrombosis were seen in patients on dual antiplatelet therapy. In 3 of the 8 patients, both aspirin and clopidogrel had been stopped. Five patients were on aspirin. In 2 of these, stent thrombosis occurred within a month of stopping clopidogrel. Three patients were clinically very stable on aspirin monotherapy. In 2 patients, thrombosis occurred very late, 25 and 26 months after stent implantation. Unfortunately, both patients presented in cardiogenic shock and died.
To summarize our perspectives of LAST, late stent thrombosis occurs both in patients who are taking aspirin and in those who are not, and cessation of antiplatelet therapy by cardiologists or other practitioners requires careful consideration. The risks of stopping antiplatelet therapy have to be weighed against the benefits of stopping it, for instance, for surgical procedures. The incidence of LAST is about 0.35% angiographically, but it's probably clinically higher, because some cases present as sudden cardiac death or MI and might never be documented. The cause is unknown and needs to be elucidated. Impaired re-endothelialization, due to the toxic nature of the agent(s), is a plausible and logical explanation; other mechanisms, such as hypersensitivity and expansive remodeling, need to be explored.6 The treatment, unfortunately, is unclear. For the secondary prevention of LAST, in patients who've already had one episode, it seems intuitive to prescribe dual antiplatelet therapy for an indefinite period of time, and that's our current practice. However, for the primary prevention of LAST, remember that long-term dual antiplatelet therapy is associated with an increased risk of major bleeding, up to 2% in the 1st year.
Neointima Formation
Continued neointima formation over time is a phenomenon observed with drug-eluting stents. We know that drug-eluting stents are effective because they reduce neointima or scar formation. However, in contrast with bare-metal stents, in which neointima formation peaks at about 6 months and then regresses, with drug-eluting stents the neointima may continue to grow; the true duration of this process has not been well described. ENDEAVOR I was a study done in Australia and New Zealand with ABT-578, an analog of sirolimus. Both IVUS and angiography have documented continued neointima formation during the 4–12 month period.9 In TAXUS II10 at 6 months, the neointimal area with drug-eluting stents encompassed about 10 to 15 mm2, which was much lower than with bare stents (about 30 mm2). However, at 2 years, neointimal volume continues to increase with drug-eluting stents, while with bare stents it regresses. The FIM study was the pilot study of sirolimus. Serial IVUS studies were done at 4, 12, 24, and 48 months. Between zero and 2 years, there was significant neointimal growth; but between 2 and 4 years, it appeared to plateau, suggesting that neointimal formation does stop with drug-eluting stents, but takes a lot longer compared with bare-metal stents.11
Drug-Eluting Stents versus Surgery
Some very important questions are going to be confronted in the ARTS II, FREEDOM, and SYNTAX studies. The preliminary 6-month results of ARTS II were presented last year at the Transcatheter Cardiovascular Therapeutics symposium. This was a study of 607 patients with multivessel disease that compared patients receiving sirolimus-eluting stents with a historical control group of CABG-treated patients from ARTS I. In comparison with patients in ARTS I, those in ARTS II were older and had more diabetes and more triple-vessel disease; and more stents and longer stents were implanted. Despite being a more complex population, at 6 months, the ARTS II stent group had outcomes that were at least as good, if not better than, the outcomes in the surgical patients in ARTS I (but bear in mind that this is a historical comparison, and not a randomized trial). The 12-month results were presented at the ACC meeting and demonstrate that the use of sirolimus-eluting stents for multivessel disease resulted in a 1-year major adverse cardiac and cerebral event rate of 10.5%.12 FREEDOM is an NIH-sponsored randomized trial looking at diabetic patients with multivessel disease, and comparing drug-eluting stents with surgery. The SYNTAX randomized trial will compare TAXUS stents with surgery in patients with triple-vessel disease and left main disease.
Stent versus Stent Comparisons
Which drug-eluting stent is better, the sirolimus- or paclitaxel-eluting stent? Before we can really talk about “better,” we need to understand what better means. Different companies push different endpoints (Table III). Some will say that one endpoint is more important; others will say the opposite. It's up to you as clinicians to decide for yourselves. There are focused anatomic endpoints, such as ultrasound measurements of the percentage of volume obstruction or angiographic measurements of stenosis severity. There are also clinical criteria, and these come in many definitions. Again, you have to decide what you feel matters most for you or your patients.
TABLE III. End-Point Determinants Used in Various Clinical Trials

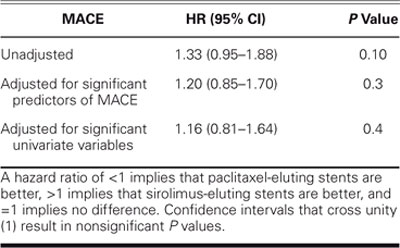
I've already talked a little bit about our own T-SEARCH registry,13 a nonrandomized, real-world, unselected, all-comers experience. This is a pretty com-plex group: unstable angina in about a third, acute MI in about a quarter, cardiogenic shock in 13%, and left main stenting in 3% to 4% of patients. The paclitaxel group was more complicated, receiving more stents and longer stents than did the sirolimus group. And, not surprisingly, this group had a slightly higher incidence of major adverse cardiac events (hazard ratio, 1.33, Table IV). When you adjust for their increased complexity, the hazard ratio is 1.16—slightly, but not significantly higher (P = 0.4). ISAR-DESIRE, a study published in JAMA earlier this year, compared sirolimus- and paclitaxel-eluting stents against balloon angioplasty for people with restenosis after bare-metal stent placement and showed that sirolimus-eluting stents had less late loss and less revascularization, but a similar amount of restenosis compared with paclitaxel-eluting stents.14 Two additional comparative studies, SIRTAX and REALITY, were also presented at the 2005 ACC meeting.
TABLE IV. Adjusted Hazard Ratios by Stent Type of Major Adverse Cardiac Events (MACE), a Composite of Death, Nonfatal Myocardial Infarction, or Target Vessel Revascularization at 1-Year Follow-Up from theT-SEARCH Registry
Future Devices
The -limus family—sirolimus, rapamycin—has been shown to be very effective in reducing restenosis. A number of analogs are also being investigated: everolimus by Guidant,15 tacrolimus by Sorin, biolimus-A9 by BioSensors16 and Terumo, and ABT-578 by Medtronic9 and Abbott (Fig. 1). Pimecrolimus is yet another one in the early stages. Paclitaxel, used in Boston Scientific's TAXUS stent, is now being used by 2 competitors working on new delivery systems. Conor Medsystems embeds paclitaxel in a biodegradable polymer inside novel laser-cut wells within the stent itself, and an Indian company called Sahajanand uses a 3-layer durable polymer. Totally different is a pro-healing approach by Orbus Medical, which uses CD-34 antibodies to capture circulating endothelial progenitor cells, theoretically to hasten re-endothelialization.17 And, finally, while not strictly a drug-eluting stent, biodegradable stents such as the one being investigated by Biotronik hold promise.

Fig. 1 Future devices
Summary
Early stent thrombosis occurs in about 1% to 1.5% of patients with drug-eluting stents, very similar to the rate with bare-metal stents. Late stent thrombosis is more of a concern with drug-eluting stents, with an incidence of at least 0.35%. I would urge caution if you feel you have to stop antiplatelet therapy in patients with drug-eluting stents. While neointima formation peaks at 6 months and then may actually regress with bare-metal stents, it continues to grow with drug-eluting stents—although this process appears to plateau by 4 years with sirolimus. With the others, we have to wait and see. We still don't know the best drug-eluting stent. Trials are under way to compare stents with surgery, and the future brings the arrival of a number of exciting new devices and approaches that are now entering clinical trials.
Footnotes
Address for reprints: Professor Patrick W. Serruys, MD, PhD, Ba 583, Thoraxcenter, Erasmus Medical Center, Dr Molewaterplein 40, 3015GD Rotterdam, The Netherlands
E-mail: p.w.j.c.serruys@erasmusmc.nl
Presented at the Texas Heart Institute's symposium “Current Issues in Cardiology;” held at the Sheraton World Resort; 5 March 2005; Orlando
References
- 1.Boston Scientific announces implantation of millionth TAXUS® Express® coronary stent system (January 19, 2005). Retrieved June 1, 2005 from Boston Scientific website: http://www.bostonscientific.com/common_templates/articleDisplayTemplate.jsp?task=tskPressRelease.jsp§ionId=2&relId=24,25&uniqueId=ABPR5502. Vol. 2005.
- 2.Stories of survival and hope mark the one million patient milestone for the CYPHER(R) sirolimus-eluting coronary stent (February 14, 2005). Retrieved February 16, 2005 from Johnson and Johnson website: http://www.jnj.com/news/jnj_news/20050214_094141.htm.
- 3.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 2001;103:1967–71. [DOI] [PubMed]
- 4.Orford JL, Lennon R, Melby S, Fasseas P, Bell MR, Rihal CS, et al. Frequency and correlates of coronary stent thrombosis in the modern era: analysis of a single center registry. J Am Coll Cardiol 2002;40:1567–72. [DOI] [PubMed]
- 5.Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Sonnenschien K, et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol 2005;45:947–53. [DOI] [PubMed]
- 6.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109:701–5. [DOI] [PubMed]
- 7.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519–21. [DOI] [PubMed]
- 8.Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 2005;45:2088–92. [DOI] [PubMed]
- 9.Meredith IT. ENDEAVOR I final clinical results. Presented at the European Society of Cardiology Congress, Munich, Germany 2004.
- 10.Serruys PW. What is the pattern of neointimal suppression over time? Presented at TCT 2004, Washington D.C., USA 2004.
- 11.Aoki J, Abizaid AC, Serruys PW, Ong AT, Boersma E, Sousa JE, et al. Evaluation of four-year coronary artery response after sirolimus-eluting stent implantation by using serial quantitative IVUS and computer assisted grey-scale value analysis for plaque composition in event-free patients. J Am Coll Cardiol. In press. [DOI] [PubMed]
- 12.Serruys PW. A.R.T.S. II. Arterial Revascularization Therapies Study Part II of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. Presented at the 54th Annual Scientific Session of the American College of Cardiology, Orlando, Florida, USA, 2005. [DOI] [PubMed]
- 13.Ong AT, Serruys PW, Aoki J, Hoye A, van Mieghem CA, Rodriguez-Granillo GA, et al. The unrestricted use of paclitaxel-versus sirolimus-eluting stents for coronary artery disease in an unselected population: one-year results of the Taxus-Stent Evaluated at Rotterdam Cardiology Hospital (T-SEARCH) registry. J Am Coll Cardiol 2005;45:1135–41. [DOI] [PubMed]
- 14.Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA 2005;293:165–71. [DOI] [PubMed]
- 15.Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. Euro Intervention 2005; 1:58–65. [PubMed]
- 16.Grube E, Hauptmann KE, Buellesfeld L, Lim V, Abizaid A. Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. Euro Intervention 2005;1:53–57. [PubMed]
- 17.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol 2005;45:1574–9. [DOI] [PubMed]


