Abstract
Bleeding and thrombus formation are common problems with life-threatening implications in patients receiving a left ventricular assist device. We describe the anticoagulation protocol for the 1st patient in the United States to undergo successful implantation of the HeartMate® II left ventricular assist system.
Key words: Anticoagulants/administration and dosage; blood coagulation tests; cardiac surgical proce-dures; heart-assist devices; human; male; monitoring, physiologic/instrumentation; activated partial thromboplastin time; platelet function tests; prothrombin time; thromboelastography
Bleeding and thromboembolic events are common complications associated with left ventricular assist device (LVAD) implantation, occurring in 60% and 30% of patients, respectively.1,2 Bleeding during the early postoperative period can lead to poor outcomes and increased demands on blood resources. After LVAD implantation, excessive thrombin generation and platelet activation are of particular concern. Therefore, the delicate manipulation of the coagulation system—from a procoagulant state intraoperatively to an anticoagulated state postoperatively—is an important element of the LVAD implantation procedure. We describe the hematologic findings of and the anticoagulation protocol used for the 1st person in the United States to undergo implantation of a HeartMate® II left ventricular assist system (LVAS) (Thoratec Corporation; Pleasanton, Calif). We also provide data regarding biochemical markers of thrombin generation after LVAD implantation.
Case Report
In November 2003, a 20-year-old man was admitted to our institution in cardiogenic shock. Although the patient had been in relatively good health before hospital admission, he had used cocaine and had smoked several packs of cigarettes a day for several years. He had no history of recent viral illness, and the result of a human immunodeficiency virus (HIV) test was negative. He had no family history of cardiomyopathy. A coronary angiogram showed normal coronary arteries but severe global hypokinesis.
Initially, we achieved partial improvement of the patient's hemodynamic status by inserting an intra-aortic balloon pump and administering inotropic medications; however, the patient remained in a debilitated cardiac state.
Seventeen days after admission, the patient underwent HeartMate II implantation. His preoperative coagulation profile included a slightly elevated international normalized ratio (INR) of 1.3, a normal activated partial thromboplastin time (aPTT) of 31 seconds, and a platelet count of 375 × 109/L (with 9% platelet function), as assessed by the aggregation response to low-dose adenosine diphosphate (ADP). The preoperative D-dimer was 16.77 (normal range, <2.5).
Due to decreased clotting-factor activity, especially factor VII, the patient underwent preoperative plasma exchange to maximize his procoagulant status. The cardiopulmonary bypass circuit was primed with fresh frozen plasma (FFP) to minimize procedure-related hemodilution. Intraoperative blood loss was estimated to be 2 liters. Transfusions included 8 units of red blood cells, 11 units of FFP, and 6 platelet doses. Serial measurement of antithrombin III (ATIII) levels during the early postoperative period showed significantly reduced levels (nadir of 55%). On postoperative day 2, 1500 units of ATIII was administered to supplement ATIII activity. The postoperative anticoagulation protocol included
10% low-molecular-weight dextran at 25 mL/hr until chest tube drainage reached <50 mL/hr.
Intravenous heparin to attain a target aPTT of 45 to 55 seconds.
Aspirin (100 mg) daily and dipyridamole (75 mg) 3 times a day.
Warfarin to achieve a target INR of 2.5 to 3.5 (upon removal of chest tubes and no evidence of bleeding).
Heparin discontinuation after overlap with warfarin and achievement of the target INR for 2 to 3 consecutive days.
We used several tests to assess the bleeding-to-coagulation ratio, including standard assays (prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen, and platelet count). In addition, we measured levels of D-dimer, ATIII, thrombin-antithrombin complex, and prothrombin fragment F-1.2, all of which are markers for clot production and lysis (Fig. 1). In addition to the aforementioned assays, thromboelastographic (TEG) monitoring was performed for global assessment of cellular and enzyme-mediated contributors to coagulation. Daily TEG assays were performed to guide anticoagulant therapy.

Fig. 1 Levels of thrombin–antithrombin (TAT) complex and prothrombin fragment F-1.2, which are markers for clot production and lysis.
Because of the greater risk for bleeding during the very early postoperative period, we elected to use low-molecular-weight dextran as our starting anticoagulant agent. Low-molecular-weight dextran has been shown to affect both protein and platelet-based coagulation through, as yet, unclear mechanisms.3 We began administering low-molecular-weight dextran on postoperative day 3, when we determined that the TEG maximal amplitude was elevated. On day 4, bivalirudin was administered, rather than heparin, because of concerns about positive heparin antibody serology (as detected by PF-4 enzyme-linked immunosorbent assay [ELISA]) and low ATIII levels. The starting dose of bivalirudin was 0.5 mg/kg per hour administered by continuous infusion with titration to a target aPTT of 45 to 55 seconds. The bivalirudin infusion rate was increased by 20% until the aPTT target was achieved (monitoring every 4 hours).
Serial aPTT values showed a prompt increase in the target range after bivalirudin administration (Fig. 2). Warfarin was started on postoperative day 7, and a target INR of 2.5 to 3.5 was achieved on day 13.
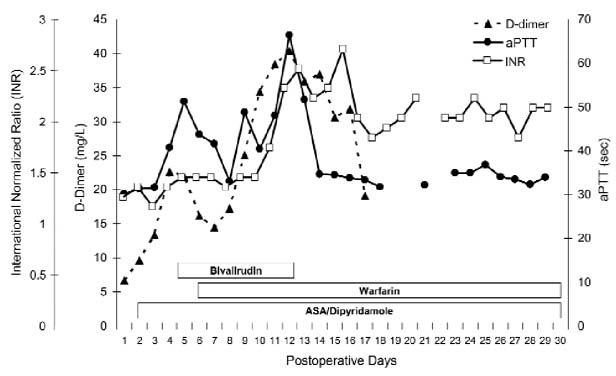
Fig. 2 Serial INR (squares), aPTT (circles) and D-dimer (triangles) values plotted against the anticoagulant course of the first 30 postoperative days. Note the rising D-dimer level, with a decrease late in the course.
ASA = aspirin; INR = international normalized ratio; aPTT = activated partial thromboplastin time
During the 2nd postoperative week, the patient had several episodes of epistaxis related to local trauma, and he required several blood transfusions. The patient was discharged home after 6 months. He had no physical limitations and was able to continue regular physical exercise. The HeartMate II LVAS is functioning well; there has been no mechanical problem reported or any evidence of clot interference. The patient is currently on the heart transplant waiting list (1B status).
Discussion
Our patient was the first in the United States to undergo successful implantation of a HeartMate II LVAS. Epistaxis was the main bleeding complication that we encountered, and no thromboembolic events occurred. The use of TEG monitoring was helpful in determining when anticoagulation therapy should be started, because TEG is uniquely capable of showing the combined interaction of coagulation factors, platelet content, and platelet function in the process of clot production in whole blood. It is also helpful for monitoring both hyper- and hypocoagulopathic situations. We used the values of the reaction time (R), clot kinetics (K), and angle (α) as markers for clotting factor deficiencies, and maximal amplitude (MA) as a marker for platelet dysfunction.4
Fries and colleagues5 reported that, in a patient receiving biventricular support, standard coagulation profile tests suggested that the patient was in a hypocoagulable state, while TEG results suggested an abnormal hypercoagulable state. Ultimately, a thrombus was found surrounding a cannula of the LVAD, and the patient was successfully treated with antithrombin and antiplatelet therapy.5 Their results support our observation that TEG may be a more useful (if not superior) method for monitoring coagulation than are standard coagulation blood tests.6
Our anticoagulation regimen included 2 antiplatelet agents: aspirin and dipyridamole. Dipyridamole was used because of good outcomes reported with its application in patients with total artificial hearts.7 We monitored responsiveness to antiplatelet therapy using the PFA-100 assay to rule out refractoriness to platelet inhibition.
Although impractical for routine clinical use, coagulation-related peptides showed that a hypercoagulable state was present in our patient, primarily during the first 7 postoperative days (Fig. 1). During this time, the thrombin-antithrombin and prothrombin fragment F-1.2 levels were at their maximum values, D-dimer levels were rising sharply, and the MA on TEG was >60 mm. To counterbalance this tendency toward a hypercoagulable state, we substituted bivalirudin for heparin, beginning on postoperative day 4. It is important to recognize this hypercoagulable state early in the postoperative period, because it implies the need for an early, aggressive anticoagulation regimen.
Bleeding is the most common complication in LVAD patients. It necessitates reoperation in 60% of such patients and is associated with high morbidity and mortality rates.1 Accordingly, anticoagulation therapy is usually avoided during the early postoperative period. However, as with our patient, the early postoperative period can also be a paradoxically vulnerable period in which the patient is in a hypercoagulable state; therefore, an anticoagulation regimen may be warranted. Reilly and associates2 reported that all of their LVAD patients who developed ventricular thrombi did so within the 1st postoperative week. Subsequent strokes occurred in 88% of these patients.2
In our patient, we used bivalirudin, a direct antithrombin inhibitor, because of concerns about positive PF-4 platelet antibodies detected in the ELISA test (although later results from a serotonin assay were negative). Direct antithrombin inhibitors can be used in similar circumstances and for long periods. Bivalirudin was effective in our patient, and we encountered no bleeding complications other than epistaxis.
Our patient also had reduced ATIII levels, most likely a result of ATIII consumption by the clotting process. The ATIII levels had to be replenished on postoperative day 2. An important advantage of using bivalirudin instead of heparin is that there is no need to monitor or supplement ATIII levels for therapeutic effect.
While the patient was receiving warfarin, there was no major bleeding complication or thromboembolic event. We kept the INR within the target range of 2.5 to 3.5, and it appears that this INR range is sufficiently protective against clotting complications in HeartMate II patients.
In summary, the anticoagulation protocol that we used for the 1st successful HeartMate II implantation in the United States included the use of thromboelastographic monitoring and specific clotting markers for treatment optimization. We found that early, aggressive anticoagulation treatment, including the use of an antithrombin regimen as needed, was safe and successful. We also found that maintaining the target INR within a range of 2.5 to 3.5 was effective for preventing clotting complications in this patient.
Footnotes
Address for reprints: Arthur W. Bracey, MD, Department of Pathology, St. Luke's Episcopal Hospital, Room P-125E, 6720 Bertner Ave. Houston, TX 77030
E-mail: abracey@msn.com
Dr. Amir is now at the Department of Cardiology, Lady Davis Hospital, Carmel Medical Center, 7 Michal Street, Haifa, 34362 Israel.
References
- 1.Goldstein DJ, Beauford RB. Left ventricular assist devices and bleeding: adding insult to injury. Ann Thorac Surg 2003; 75(6 Suppl):S42–7. [DOI] [PubMed]
- 2.Reilly MP, Wiegers SE, Cucchiara AJ, O'Hara ML, Plappert TJ, Loh E, et al. Frequency, risk factors, and clinical outcomes of left ventricular assist device-associated ventricular thrombus. Am J Cardiol 2000;86:1156–9, A10. [DOI] [PubMed]
- 3.Zeerleder S, Mauron T, Lammle B, Wuillemin WA. Effect of low-molecular weight dextran sulfate on coagulation and platelet function tests. Thromb Res 2002;105:441–6. [DOI] [PubMed]
- 4.von Kier S, Smith A. Hemostatic product transfusions and adverse outcomes: focus on point-of-care testing to reduce transfusion need. J Cardiothorac Vasc Anesth 2000;14(3 Suppl 1):15–21; discussion 37–8. [PubMed]
- 5.Fries D, Innerhofer P, Streif W, Schobersberger W, Margreiter J, Antretter H, Hormann C. Coagulation monitoring and management of anticoagulation during cardiac assist device support. Ann Thorac Surg 2003;76:1593–7. [DOI] [PubMed]
- 6.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88:312–9. [DOI] [PubMed]
- 7.Copeland JG, Arabia FA, Smith RG, Sethi GK, Nolan PE, Banchy ME. Arizona experience with CardioWest Total Artificial Heart bridge to transplantation. Ann Thorac Surg 1999;68:756–60. [DOI] [PubMed]


