Abstract
We report the case of a 14-year-old boy who developed ischemic contracture of the heart after open heart surgery to correct complex congenital heart disease. Because he had no cardiac function, an extracorporeal, continuous-flow device was used to support him until he was transferred to our institution. Shortly after his arrival, an implantable, long-term left ventricular assist device was implanted. The univentricular pump provided total cardiac support for this critically ill patient. After normalization of end-organ function, the patient underwent successful orthotopic cardiac transplantation.
Key words: Adolescent, cardiac surgical procedures, congenital heart defects, Fontan procedure, heart-assist devices, heart transplantation, myocardial ischemia, univentricular support
In 1978, we reported the 1st use of an implantable left ventricular assist device (LVAD) as a bridge to transplantation.1 In that case, the patient—a 22-year-old man undergoing double valve replacement surgery—had ischemic contracture of the heart after the procedure. This condition resulted in the patient's having no function of his native heart. The univentricular support from the LVAD was successful in supplying adequate systemic circulatory support because the patient had low pulmonary vascular resistance, which allowed adequate filling of the device by passive flow through the pulmonary circulation. He was successfully bridged to transplantation after 7 days of support.
The patient described here also had ischemic contracture of the heart and loss of heart function that occurred after open heart surgery—in this case, for further correction of complex congenital heart disease. An extracorporeal, continuous-flow device was used to support him until he was transferred to our institution. We discuss the case history, the choice of implantable, long-term ventricular assist device, the surgical challenges created by this patient's cardiac structure, and his survival to successful orthotopic heart transplantation.
Case Report
In January 2003, a 14-year-old boy arrived at another medical center with progressive dyspnea and cyanosis. Although he had a nonidentical twin with normal cardiac anatomy, the patient had complex congenital heart disease. His morphologic diagnoses included situs solitus, levocardia, and tricuspid atresia, with a hypoplastic right ventricle and an intact ventricular septum. His pulmonary arteries and veins were normal. The patient had undergone a Blalock-Taussig shunt procedure at 5 days of age, a Fontan procedure at 18 months, and a modified Fontan procedure at 4 years (Fig. 1A). After those operations, the patient did reasonably well, although his growth was retarded. He progressed through school at the appropriate grade levels for his age.
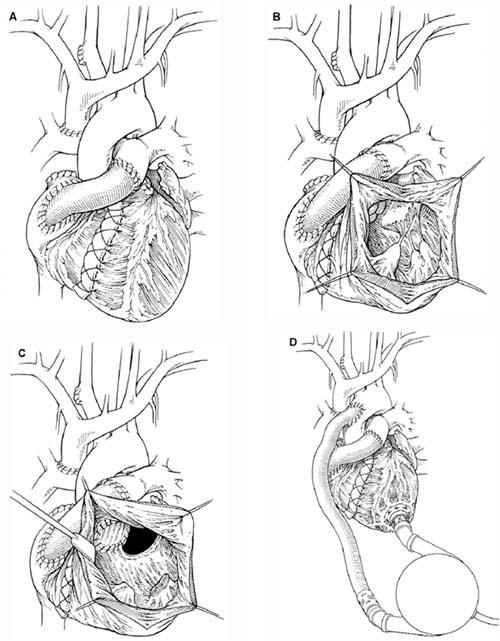
Fig. 1 A) The modified Fontan procedure. B) The nonfunctional right ventricle as seen through the ventricular cavity. C) After complete mitral valve excision, a pouch is constructed from the remaining viable myocardium and the left atrium to serve as a reservoir for the inlet cannula. D) The completed left ventricular assist device implantation. Drawings by Bill Andrews.
Cardiac catheterization performed at the admitting hospital revealed thrombosis of the right ventricle. After attempts at thrombolysis were unsuccessful, the patient was taken to the operating room. The thrombus was removed, the tricuspid valve resected, and the prior Fontan reconstructed by conduits from the superior vena cava to the right pulmonary artery and from the inferior vena cava to the main pulmonary artery. The right ventricle was also plicated. The Fontan circulation was intact and functional; nonetheless, the patient could not be weaned from cardiopulmonary bypass (CPB). The heart was small, contracted, and nonfunctional. Catheterization showed a thrombotic occlusion of the left coronary artery. Again, attempts at thrombolysis were unsuccessful. Left ventricular support was maintained with an extracorporeal Sarns pump (Sarns, Inc.; Ann Arbor, Mich). Heparin-induced thrombocytopenia and oliguric renal failure further complicated the postoperative course.
He was transferred more than 500 miles by Life Flight to our institution. Upon arrival, nitric oxide was administered to improve the transpulmonary Fontan gradient. An echocardiogram showed that the patient had no discernible cardiac function and that a large thrombus was present in the ventricular cavity. Support with the Sarns pump was marginal, and renal and hepatic failure were progressing. We determined that enhanced cardiac support was necessary for the boy's survival. Although he was small (body surface area, 1.90 m2) with a narrow habitus, we felt that the pneumatic HeartMate® IP LVAS (HeartMate implantable pneumatic left ventricular assist system, Thoratec Corp.; Pleasanton, Calif)—a flexible, versatile, and effective system—could still be implanted. The larger, less-resilient HeartMate vented-electric (VE) LVAS could not be placed in this patient.
He was taken to the operating room, and cardiopulmonary bypass (CPB) was initiated. The pulmonary flow was maintained via the previously placed cannulas. The right ventricle was not functional (Fig. 1B). The small left ventricle was necrotic and filled with a large thrombus. Aortic insufficiency was also present. We used a bovine pericardial patch to oversew the undersurface of the aortic valve. We removed the ventricular clot and more than half of the necrotic left ventricle. We then completely excised the mitral valve, and we fashioned the remaining left ventricle and the left atrium to create a single atrioventricular pouch for pulmonary venous return. The pouch served as a satisfactory though noncontractile reservoir for placement of the device's inlet cannula (Fig. 1C). The rest of the LVAS insertion procedure was performed in the standard fashion (Fig. 1D). Device function was initiated, and the patient was gradually weaned from CPB.
The patient was completely dependent on the device for circulatory support; the remnants of his heart served only as an inert blood conduit. He slowly recovered hepatic and renal function and attained normal functional status after 4 to 5 weeks of device sup-port. A suitable cardiac donor became available after 45 days of support. The bicaval technique was used for cardiac transplantation. The only remaining part of the native heart after transplantation was the back wall of the left atrium. The patient recovered and was transferred home. He has since returned to school and currently remains healthy more than 2 years after successful cardiac transplantation.
Comment
The low pulmonary vascular resistance in this patient facilitated successful univentricular support; however, the anatomic complexity of his congenital heart disease required an innovative approach for successful implantation of the ventricular assist device. The left ventricle was small and was further compromised by the myocardial necrosis that resulted from the intraoperative catastrophe. By excising the mitral valve, we were able to create an adequate pouch as an inert reservoir and passive conduit for pulmonary blood return. The patient's aortic insufficiency was significant and required a patch to be placed on the undersurface of the aortic annulus to allow successful device function. With this nonfunctional but anatomic connection, physiologic flows in excess of 6 L were achieved. The successful outcome was further aided by satisfactory support for more than 3 days with the extracorporeal, continuous-flow pump that had been implanted before the patient's transfer to our institution.
Acknowledgment
The authors wish to thank Pierantonio Russo, MD, from the Department of Cardiothoracic Surgery, University of Missouri, for referring this patient to us and for his participation in the patient's care.
Footnotes
Address for reprints: O.H. Frazier, MD, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345
E-mail: knowlin@heart.thi.tmc.edu


