Abstract
A 76-year-old woman presented with respiratory failure that was later determined to be a result of a right aortic arch with an aberrant left brachiocephalic artery. This vascular ring compressed the trachea, requiring operative intervention. A median sternotomy gave access for an aorta-to-left brachiocephalic artery bypass and division of the vascular ring. This is a unique case, because vascular rings rarely present in elderly patients with such acute life-threatening symptoms. To our knowledge, this is the oldest and heaviest patient ever reported with symptomatic presentation and one of only 4 patients over the age of 50. The current literature on vascular rings of the thoracic aorta in adults is reviewed.
Key words: Airway obstruction/diagnosis; brachiocephalic trunk/abnormalities; diagnosis, differential; dyspnea/etiology; tracheal diseases/diagnosis; tracheomalacia; vascular ring
Vascular rings are developmental abnormalities involving partial or complete encirclement of the trachea or esophagus by the aortic arch, which can cause pressure symptoms of respiratory distress or dysphagia. The most common anomaly is a double aortic arch, which is rarely found in adults because of its greater propensity to become symptomatic in infants and young children.1 The 2nd most common variant is a right aortic arch with aberrant left brachiocephalic artery and ligamentum arteriosum,1 as in our patient. Vascular rings are considered rare in adults and can be misdiagnosed as asthma in the adult population. Common presenting symptoms include dyspnea on exertion, recurrent pneumonia, bronchitis, stridor, and dysphagia in the event of concomitant esophageal compression. Symptomatic vascular rings require operative intervention. Relief of symptoms follows surgical correction, in most cases. Complete resolution of respiratory symptoms is more likely after correction of pulmonary artery slings and brachiocephalic artery compression than after correction of complete vascular rings.2
Case Report
A 76-year-old, morbidly obese woman (265 lbs; body mass index, 44) with a history of hypertension, congestive heart failure, chronic obstructive pulmonary disease, sleep apnea, diabetes, and chronic renal insufficiency presented at a community hospital with worsening shortness of breath and respiratory distress that required immediate intubation on admission. There was no history of shortness of breath. The cause of the patient's respiratory failure was thought to be congestive heart failure. Despite patient optimization, subsequent extubation attempts failed over the course of 2 weeks. A chest radiograph showed a widened mediastinum. A computed tomographic (CT) scan revealed a right-sided aortic arch and anomalous left brachiocephalic artery, compressing the trachea anteriorly and resulting in a vascular ring (Fig. 1). The vascular anomaly was determined to be the cause of the patient's presenting symptoms and respiratory distress, so surgical intervention was recommended. The patient was transferred, intubated, to our institution. Before surgery, CT angiography of the chest, with reconstructions, was performed at our institution, and this confirmed the presence of a right-sided aortic arch with left brachiocephalic artery traversing anterior to the trachea (Fig. 2).

Fig. 1 Frontal view. Illustration of a right-sided aortic arch with an anomalous left brachiocephalic artery compressing the trachea anteriorly, resulting in a vascular ring.
MPA = main pulmonary artery; LCCA = left common carotid artery; LPA = left pulmonary artery; LSA = left subclavian artery; RCCA = right common carotid artery; RPA = right pulmonary artery; RSA = right subclavian artery

Fig. 2 Computed tomographic angiograms (A and B) of the chest, with reconstructions, show a right-sided aortic arch with brachiocephalic artery traversing anterior to the trachea (arrow).
Preoperative echocardiography showed moderate left ventricular systolic dysfunction with severe apical hypokinesis and anterior septal hypokinesis. The estimated left ventricular ejection fraction was 0.37. No other significant cardiac abnormalities were seen.
The patient was taken to surgery, where a median sternotomy was performed with aorto–brachiocephalic bypass and division of the vascular ring. A side-biting clamp was placed on the aorta, and an end-to-side anastomosis was created between the aorta and a 10-mm Dacron graft. The graft was brought underneath the brachiocephalic vein. A side-biting clamp was placed on the brachiocephalic–carotid artery junction, which permitted perfusion to the carotid as well as the subclavian artery, as confirmed by adequate stump pressure and a left radial arterial line. The distal anastomosis was performed in end-to-side fashion between the Dacron graft and the brachiocephalic–carotid junction such that both the left subclavian and the carotid arteries would be perfused (Fig. 3). After the side-biting clamp was removed, distal flow was confirmed by Doppler visualization of both vessels. A vascular clamp was then placed at the junction of the aorta and the brachiocephalic artery, and another was placed 2 cm distally. The brachiocephalic artery was divided; after its division, the tight vascular ring was released and 3 cm of space was immediately created, releasing the compression of the trachea. Both of the brachiocephalic artery stumps were secured with 4.0 Prolene sutures with pledgets. In addition, a thick fibrous band, which enveloped the trachea, was released to further relieve tracheal stenosis. The operative course was unremarkable, and the patient was transferred to the intensive care unit for routine postoperative monitoring. Postoperative bronchoscopy revealed mild narrowing of the right mainstem bronchus with distal bronchomalacia and tracheomalacia at the level of the carina.
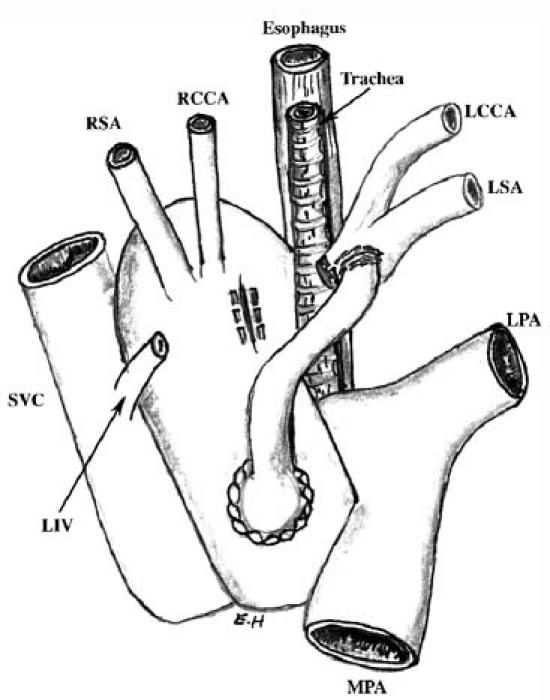
Fig. 3 Frontal view. Illustration of aorto–brachiocephalic bypass with end-to-side anastomosis and division of the vascular ring.
MPA = main pulmonary artery; LCCA = left common carotid artery; LIV = left innominate vein; LPA = left pulmonary artery; LSA = left subclavian artery; RCCA = right common carotid artery; RSA = right subclavian artery; SVC = superior vena cava
Discussion
Vascular rings were first reported in 1737 by Hommel,3 who described a double aortic arch. However, it was not until 1945 that Gross coined the term vascular ring, after performing the 1st successful division of a vascular ring, which had resulted from a double aortic arch.3 The incidence of vascular rings is less than 0.2% annually. They most commonly present in infants and children, with either respiratory or gastrointestinal symptoms, in approximately 93% and 41% of cases, respectively.1 To our knowledge, this is the oldest and heaviest patient ever reported with symptomatic presentation and 1 of only 4 patients over the age of 50.1 Dysphagia, rather than respiratory symptoms, is thought to be the most common presenting symptom of a vascular ring in adults.4 The exact mechanism whereby these vascular rings remain asymptomatic and then present later in life is poorly understood. However, a right-sided aortic arch occurs with a frequency of 0.1% in adults and is frequently associated with a complete vascular ring that may lead to symptomatic compression of the trachea and esophagus.1
The various forms of this anomaly occur very early in embryonic development, resulting from either abnormal or incomplete regression of one of the 6 embryonic branchial arches. Right aortic arch anomalies, as in our patient, occur when the left 4th branchial arch involutes and the right remains. In all vascular rings, the anomalous right aortic arch courses over the right mainstem bronchus and may descend on either the right or the left side of the thorax. In right aortic arch, the ring is completed by a left ligamentum arteriosum1 (Fig. 4). In patients with a double aortic arch, the left arch crosses over the left mainstem bronchus, joining the descending aorta, thereby completing the ring1 (Fig. 5).

Fig. 4 Left lateral view. Illustration of a right aortic arch with a left ligamentum arteriosum completing the vascular ring. (Adapted from Backer CL, Mavroudis C. Vascular rings. The child's doctor. Journal of Children's Memorial Hospital. Spring 1998.)
LC = left carotid artery; LSA = left subclavian artery; PA = pulmonary artery; R Arch = right aortic arch; RC = right carotid artery; RSA = right subclavian artery

Fig. 5 Left lateral view. Illustration of a double aortic arch. The left arch crosses over the mainstem bronchus, joining the descending aorta and completing the vascular ring. (Adapted from Backer CL, Mavroudis C. Vascular rings. The child's doctor. Journal of Children's Memorial Hospital. Spring 1998.)
L Arch = left aortic arch; LC = left carotid artery; LSA = left subclavian artery; PA = pulmonary artery; R Arch = right aortic arch; RC = right carotid artery; RSA = right subclavian artery
Respiratory symptoms may result from several possible mechanisms, one of which is direct aortic compression of the trachea, which causes tracheomalacia. Tracheomalacia is a weakness of the trachea that results in excessive narrowing of the tracheal lumen during expiration or some other increase in intrathoracic pressure. Tracheomalacia diminishes or disappears altogether after surgical relief of the external compression.1 Although no definitive time frame for resolution of tracheomalacia resulting from vascular ring compression exists in adults, the pediatric literature states that signs and symptoms of tracheal obstruction are relieved almost immediately, and that fewer than 10% of children have residual tracheomalacia. Symptoms may persist on occasion for 1 to 2 years and then resolve completely.5
Respiratory symptoms can also result from mechanical compression of the trachea by aortic dilatation, which can arise from either dynamic or static mechanisms. Fluid administration or exercise can cause dynamic dilatation of the aorta; exercise is known to exacerbate vague pre-existing symptoms, thereby leading patients to seek medical attention. During exercise, the diameter of the ascending aorta increases by approximately 1 mm or more for every 15-mmHg increase in systolic or diastolic pressure.1 Exercise can lead to a 3- to 6-mm circumferential airway narrowing.
Normal aging can result in aortic dilatation of approximately 0.1 cm per decade. Hypertension and atherosclerosis can enlarge the aorta and increase its tortuosity, with subsequent worsening of tracheal compression. The latter 2 mechanisms may account for the onset of respiratory symptoms in our patient this late in life. Finally, age-related changes of the thorax and vertebrae can limit the dimensions of the mediastinum, thereby worsening existing compression.1
Diagnostic evaluation of patients suspected of having a vascular ring usually begins with routine chest radiography. Typical signs suggesting a vascular ring include a widened mediastinal silhouette, presence of the aortic knob on the right, or evidence of tracheal compression. A barium swallow may reveal extrinsic indentation of the posterior wall of the esophagus caused by extrinsic vascular compression, which does not alter with peristalsis. A barium esophagram is the most accurate procedure for diagnosis of a vascular ring in children. Different projections and views are necessary to establish the location and the persistence of the filling defect produced by the vascular ring.5 Oftentimes, in children, the combination of chest radiograph and barium swallow is sufficient to confirm the presence of a vascular ring and to reveal whether the ring is the probable cause of the symptomatic presentation. This is followed by surgical intervention with intraoperative dissection and inspection for anatomic variables.6 However, CT scanning, magnetic resonance imaging, and angiography are used widely in adult patients for further delineation of the anatomy, to help determine the most appropriate operative approach. Computed tomographic and magnetic resonance scans can also delineate tracheoesophageal anatomy. Bronchoscopy is useful in the diagnostic evaluation of a patient, particularly an adult, who is suspected of having a vascular ring, because it effectively excludes other conditions that can be mistaken for a vascular ring—such as endobronchial lesions that present with obstructive symptoms. This study is usually not indicated in children, with the exception of older children who might require investigation of the tracheobronchial tree for the presence of foreign bodies or for extrinsic abnormalities that result in symptomatic presentation.5 Echocardiography is being used increasingly for diagnostic evaluation of vascular rings. This method is particularly helpful in younger patients to rule out the presence of an associated cardiovascular anomaly. Importantly, if a vascular ring is confirmed with less invasive studies and clinical history, more invasive studies are not necessarily required and are left to the discretion of the operating surgeon.
Surgical intervention is indicated in all patients who present with symptomatic vascular rings. The surgical approach to these lesions can be via either thoracotomy or sternotomy.
Although vascular rings are extremely rare in adults, our case illustrates that the differential diagnosis should include vascular ring in elderly patients who present with clinical signs and symptoms of tracheal or esophageal obstruction, especially if chest radiograms suggest the diagnosis. In our patient, the age-related changes of the aorta, combined with the patient's coexisting medical problems (in particular, hypertension, arteriosclerosis, and marked obesity), were most likely responsible for the late manifestation of compression symptoms. Approximately 5% of patients with vascular ring present with symptoms attributa-ble to the development of atherosclerosis and vascular rigidity, or tortuosity of the aorta, or possibly aneurysmal dilatation of the anomalous vessel, all of which worsen compression and lead to the onset of symptoms.6
Another interesting aspect of this case was that the chronic tracheal compression by the right-sided arch and aberrant brachiocephalic artery led to the development of tracheomalacia. In 1 series, it was demonstrated that tracheomalacia is not uncommon in adult patients who present with vascular rings due to progressive vascular enlargement. Operative intervention was performed to release the ring, and the trachea was simply observed postoperatively. The tracheomalacia either diminished or disappeared once the obstruction was relieved.6 However, due to the sporadic nature of symptomatic presentations of tracheomalacia, limited information is available regarding optimal therapeutic interventions and long-term prognosis in adult patients. Therefore, treatment decisions arise from clinical judgment. If tracheal symptoms persist, then tracheostomy, aortopexy, tracheal resection, or placement of an intraluminal stent are potential therapeutic options.4,6 In our patient, clinical judgment guided the decision to perform an early tracheostomy on the basis of these factors: the patient's tracheomalacia (resulting both from the vascular ring and from prolonged intubation before her transfer to our institution); her morbid obesity, accompanied by obstructive sleep apnea; her chronic obstructive pulmonary disease; and her history of repeated failed extubations and re-intubations over the course of 1 month, which had resulted in further injury to the trachea.
The acute presenting respiratory symptoms in our patient demonstrate the crucial need in such cases for airway control, which often necessitates intubation to prevent airway collapse. Furthermore, symptomatic vascular rings require operative intervention, which should not be unduly deferred due to comorbidities or advanced age, or pending extensive radiographic evaluation. In the appropriate candidate, surgery yields excellent operative results and a high probability of symptomatic improvement.
In conclusion, extrinsic compression by a vascular ring is extremely rare and can present with vague symptoms or with overt evidence of tracheal or esophageal obstruction. Our patient's respiratory failure was initially attributed to congestive heart failure, which resulted in prolonged intubation and several failed extubations, worsening the associated tracheomalacia. Therefore, a high degree of suspicion is often necessary to make the appropriate diagnosis. This rare congenital lesion should be included in the differential diagnosis when a patient presents with respiratory distress or dysphagia. Awareness of vascular rings as a potential cause of these symptoms in the elderly avoids misdiagnosis and treatment delay.
Footnotes
Address for reprints: Gregory R. Brevetti, MD, FACS, FACC, Division of Cardiothoracic Surgery, State University of New York–Health Science Center at Brooklyn, 450 Clarkson Avenue, Box 40, Brooklyn, NY 11203
E-mail: gbrevetti@downstate.edu
References
- 1.Grathwohl KW, Afifi AY, Dillard TA, Olson JP, Heric BR. Vascular rings of the thoracic aorta in adults. Am Surg 1999; 65:1077–83. [PubMed]
- 2.Stoica SC, Lockowandt U, Coulden R, Ward R, Bilton D, Dunning J. Double aortic arch masquerading as asthma for thirty years. Respiration 2002;69:92–5. [DOI] [PubMed]
- 3.Gross RE. Surgical relief for tracheal obstruction from vascular ring. N Engl J Med 1945;233:586–90. [DOI] [PubMed]
- 4.Adkins RB Jr, Maples MD, Graham BS, Witt TT, Davies J. Dysphagia associated with an aortic arch anomaly in adults. Am Surg 1986;52:238–45. [PubMed]
- 5.Backer CL, Ilbawi MN, Idriss FS, DeLeon SY. Vascular anomalies causing tracheoesophageal compression. Review of experience in children. J Thorac Cardiovasc Surg 1989; 97:725–31. [PubMed]
- 6.van Son JA, Julsrud PR, Hagler DJ, Sim EK, Pairolero PC, Puga FJ, et al. Surgical treatment of vascular rings: the Mayo Clinic experience. Mayo Clin Proc 1993;68:1056–63. [DOI] [PubMed]


