Abstract
Chylothorax is a rare but serious complication of cardiac surgery, with a poor prognosis unless treated properly. We report the case of 66-year-old woman who developed chylothorax after coronary artery bypass grafting. The chylothorax was successfully treated in 8 days by means of subcutaneous octreotide administration and a diet that contained medium-chain triglycerides. Octreotide, a long-acting somatostatin analog, is an effective and safe agent for the treatment of postoperative chylothorax and may reduce the need for reoperation.
Key words: Chylothorax/drug therapy, coronary artery bypass grafting, iatrogenic disease, octreotide/therapeutic use, postoperative complications, somatostatin
Chylothorax is a rare but serious complication of thoracic surgical procedures.1–3 After cardiac procedures, such as coronary artery bypass grafting (CABG), it is even rarer. Treatment for chylothorax includes conservative measures (total parenteral nutrition, pleural drainage, and pleurodesis) and surgery (thoracic duct ligation). Surgery may be required if drainage persists despite conservative treatment. However, surgery for chylothorax can lead to a longer hospital stay, added morbidity, failure of the procedure, and high costs. Although alternatives to surgery for this condition remain controversial, conservative management may be viewed favorably when major surgical intervention is contraindicated.
We present the case of an adult patient who developed chylothorax after CABG.
Case Report
In April 2004, a 66-year-old woman was admitted to our clinic with dyspnea and chest pain. Twelve days earlier, she had undergone CABG for triple-vessel disease. A median sternotomy had been followed by bypass with the saphenous vein and the left internal mammary artery as conduits. On physical examination, no sounds were audible in the middle and lower zones of the left thorax. Chest radiography revealed a left-sided pleural effusion and mediastinal shift through the contralateral side (Fig. 1A). A pleural catheter was inserted, and 2,500 mL of chylous fluid was drained by thoracentesis. Laboratory analysis of the pleural fluid revealed a total cholesterol level of 96 mg/dL; triglyceride, 349 mg/dL; glucose, 99 mg/dL; protein, 4,169 mg/dL; and lactate dehydrogenase, 634 IU/L, without bacterial growth. On the basis of these findings, a diagnosis of chylothorax was made.
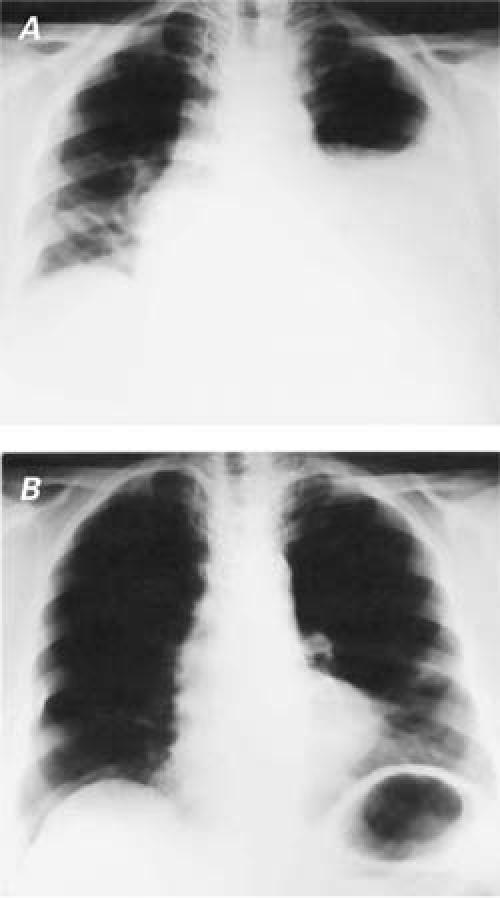
Fig. 1 Chest radiographs show A) pleural effusion and a mediastinal shift to the contralateral side and B) reexpansion of the lung 3 months after octreotide therapy.
Because of continuous, extensive drainage, the decision was made to administer subcutaneous octreotide (Sandostatin ampule; Novartis Pharmaceuticals; East Hanover, NJ) 100 μg every 8 hours. Additionally, an oral diet that contained medium-chain triglycerides (MCT) was begun. The chylous fluid drainage, which had amounted to 650 mL/day on the day before octreotide therapy, decreased to 100 mL/day on the 6th day after the initiation of octreotide therapy (Fig. 2). After 8 days of therapy, the octreotide was discontinued because of the satisfactory response, and the pleural catheter was removed on the 10th day—after pleural drainage had become serous and had decreased to 50 mL/day. The patient was discharged on the 12th day of treatment, with instructions to continue the diet that contained MCT. After 3 months of follow-up, the patient was on a normal diet, and her chest radiograph was normal (Fig. 1B). At her 14-month follow-up, the patient was doing well, without recurrence of chylothorax.
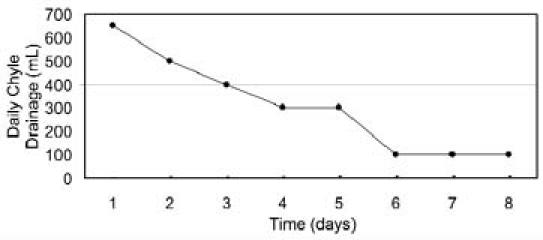
Fig. 2 Graph shows daily chylous output during octreotide treatment.
Discussion
Persistent postoperative chylothorax is a significant life-threatening complication, with an incidence of 0.3% to 1.5% in cardiothoracic procedures.1,4–6 The thoracic duct or the adjacent small lymphatic channels can be injured during esophagectomy, mediastinal exenteration, or pediatric cardiac operations. Lymphatic leaks have occurred after CABG; these may arise, in particular, as a consequence of internal thoracic artery harvesting in adult patients. In our department, chylothorax has been observed in 1 of 1,065 (0.09%) patients who underwent CABG.
Conventional treatment of chylothorax includes pleural drainage, total parenteral nutrition, and early duct ligation via a transthoracic surgical approach. Minimally invasive techniques such as video-assisted thoracoscopic surgery (VATS), introduced in the early 1990s, have replaced conventional surgical approaches and have been used successfully in clipping of the thoracic duct and in the application of fibrin glue.6–8
Common aggressive surgical approaches include transthoracic and transabdominal duct ligation; pleuroperitoneal shunt should be reserved for excessive chylous leaks (of 1 to 2 weeks' duration, with output greater than 1,000 mL/day).2 Surgical outcomes are not always satisfactory2,9 and are associated with significant complication rates.1 Because chylothorax leads to critical losses of fluid, lymphocytes, proteins, coagulation factors, and antibodies, and because patients who develop chylothorax generally are in poor physical condition, noninvasive, conservative treatment may be valuable in this population.10 Cerfolio and colleagues1 have reported reoperation in 34 of 47 patients diagnosed with chylothorax. In 23 of the 34 (68%), injury to the thoracic duct was discovered at reoperation. Complications occurred in 18 of the 34, and 3 of those required a 2nd reoperation.
Few agents other than octreotide have been used for conservative treatment of postsurgical lymphatic leak.4,11 OK-432 (Su-strain treated Streptococcus pyogenes) causes chemical pleurodesis and has been used in only 1 patient with success.4 Intravenous etilefrine hydrochloride decreases chyle output by a sympathomimetic effect on smooth muscle fibers of the main lymphatic vessels. It has been used in 10 patients and was successful in 8 of them.11 The other 2 patients required reoperation, after inadequate decreases in chyle output. This agent was stopped in 1 patient due to interactions with other sympathomimetic drugs.)
Octreotide is a long-acting somatostatin analog. It acts directly on vascular somatostatin receptors and minimizes lymph fluid excretion.12,13 Moreover, by increasing splanchnic arteriolar resistance and decreasing gastrointestinal blood flow, octreotide indirectly reduces lymphatic flow.12,13 Some authors have mentioned yet another mechanism: octreotide blocks pancreatic and biliary secretion by inhibiting serotonin and other gastrointestinal peptides.14
Recently, some investigators have reported success with somatostatin and its analog in treating chylothorax after cardiac operations in pediatric patients.15,16 There are only a few reports of its use in adults.17,18
We have used octreotide in our adult patients with the same positive results, attaining a significant decrease in chyle output within 6 days of the onset of therapy. To the best of our knowledge, no failure or recurrence in the treatment of chylothorax with octreotide therapy has been reported in the literature. Potential adverse effects of octreotide therapy are fluid retention, hyponatremia, stomachache, headache, nausea, vomiting, tympanites, and epistaxis.13 No adverse effects occurred in our patient. Surgical intervention was avoided. We used an MCT diet, because MCT is transported directly into the portal system, bypassing the lymphatic pathways, thus diminishing lymphatic flow through the thoracic duct.3 Octreotide administration may be used as the initial conservative therapy for moderate chylous drainage. A surgical approach has been suggested in adults after a traumatic event if the daily loss of chyle over a 5-day period exceeds 1,000 mL/day.1,6,7,17–19 Thoracoscopic surgery may be the 1st choice; in the event of failure, open surgery may be performed in the same session.
In conclusion, octreotide is an effective, noninvasive, and safe agent, and should be considered for treatment of chylothorax that occurs in an adult after cardiac surgery. Because it may reduce the need for surgery, further studies with increased numbers of patients are recommended to evaluate these results.
Footnotes
Address for reprints: Dalokay Kilic, MD, Department of Thoracic Surgery, Baskent University Faculty of Medicine, Adana Teaching and Medical Research Center, Dadaloglu Mah. 39. Sokak No: 6, Yuregir, 01250 Adana, Turkey
E-mail: dalokay7@yahoo.com
References
- 1.Cerfolio RJ, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361–6. [DOI] [PubMed]
- 2.Valentine VG, Raffin TA. The management of chylothorax. Chest 1992;102:586–91. [DOI] [PubMed]
- 3.Allen EM, van Heeckeren DW, Spector ML, Blumer JL. Management of nutritional and infectious complications of postoperative chylothorax in children. J Pediatr Surg 1991; 26:1169–74. [DOI] [PubMed]
- 4.Nakano A, Kato M, Watanabe T, Kawai N, Ota H, Hattori T, et al. OK-432 chemical pleurodesis for the treatment of persistent chylothorax. Hepatogastroenterology 1994;41: 568–70. [PubMed]
- 5.Nguyen DM, Shum-Tim D, Dobell AR, Tchervenkov CI. The management of chylothorax/chylopericardium following pediatric cardiac surgery: a 10-year experience. J Card Surg 1995;10(4 Pt 1):302–8. [DOI] [PubMed]
- 6.Kumar S, Kumar A, Pawar DK. Thoracoscopic management of thoracic duct injury: Is there a place for conservatism? J Postgrad Med 2004;50:57–9. [PubMed]
- 7.Wurnig PN, Hollaus PH, Ohtsuka T, Flege JB, Wolf RK. Thoracoscopic direct clipping of the thoracic duct for chylopericardium and chylothorax. Ann Thorac Surg 2000;70: 1662–5. [DOI] [PubMed]
- 8.Graham DD, McGahren ED, Tribble CG, Daniel TM, Rodgers BM. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg 1994;57:1507–12. [DOI] [PubMed]
- 9.Le Coultre C, Oberhansli I, Mossaz A, Bugmann P, Faidutti B, Belli DC. Postoperative chylothorax in children: differences between vascular and traumatic origin. J Pediatr Surg 1991;26:519–23. [DOI] [PubMed]
- 10.Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15–20. [PubMed]
- 11.Guillem P, Papachristos I, Peillon C, Triboulet JP. Etilefrine use in the management of post-operative chyle leaks in thoracic surgery. Int Cardiovasc Thorac Surg 2004;1:156–160. [DOI] [PubMed]
- 12.Nakabyashi H, Sagara H, Usukura N, Yoshimitsu K, Imamura T, Seta T, et al. Effect of somatostatin on the flow rate and triglyceride levels of thoracic duct lymph in normal and vagotomized dogs. Diabetes 1981;30:440–5. [DOI] [PubMed]
- 13.Bac DJ, Van Hagen PM, Postema PT, ten Bokum AM, Zondervan PE, van Blankenstein M. Octreotide for protein-losing enteropathy with intestinal lymphangiectasia. Lancet 1995;345:1639. [DOI] [PubMed]
- 14.Kahn CR, Shechter Y. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman's The Pharmacological basis of therapeutics, 8th ed. New York: Pergamon Press; 1990. p. 1489–90.
- 15.Pratap U, Slavik Z, Ofoe VD, Onuzo O, Franklin RC. Octreotide to treat postoperative chylothorax after cardiac operations in children. Ann Thorac Surg 2001;72:1740–2. [DOI] [PubMed]
- 16.Hamdan MA, Gaeta ML. Octreotide and low-fat breast milk in postoperative chylothorax. Ann Thorac Surg 2004; 77:2215–7. [DOI] [PubMed]
- 17.Kelly RF, Shumway SJ. Conservative management of postoperative chylothorax using somatostatin. Ann Thorac Surg 2000;69:1944–5. [DOI] [PubMed]
- 18.Gabbieri D, Bavutti L, Zaca F, Turinetto B, Ghidoni I. Conservative treatment of postoperative chylothorax with octreotide. Ital Heart J 2004;5:479–82. [PubMed]
- 19.Crosthwaite GL, Joypaul BV, Cuschieri A. Thoracoscopic management of thoracic duct injury. J R Coll Surg Edinb 1995;40:303–4. [PubMed]


