Abstract
ErbB2/Neu destabilizes the cyclin-dependent kinase (Cdk) inhibitor p27 and increases expression of cyclin D1. Therefore, we studied the roles of p27 and cyclin D1 in ErbB2-mediated mammary epithelial cell transformation. Overexpression of ErbB2 or cyclin D1 in p27+/− primary murine mammary epithelial cells resulted in increased proliferation, cyclin D1 nuclear localization, and colony formation in soft agar compared to those in p27+/+ cells. In contrast, ErbB2- or cyclin D1-overexpressing p27−/− cells displayed reduced proliferation, anchorage-independent growth, Cdk4 activity, cyclin D1 expression, and cyclin D1 nuclear localization compared to wild-type cells. A cyclin D1 mutation in its nuclear export sequence (T286A) partially rescued nuclear localization of cyclin D1 in p27−/− cells but did not increase proliferation or Cdk4 kinase activity. Overexpression of E2F1, however, increased proliferation to the same degree in p27+/+, p27+/−, and p27−/− cells. Mammary glands from MMTV (mouse mammary tumor virus)-neu/p27+/− mice exhibited alveolar hyperplasia, enhanced proliferation, decreased apoptosis, and accelerated tumor formation compared to MMTV-neu/p27+/+ glands. However, MMTV-neu/p27−/− glands showed decreased proliferation, cyclin D1 expression, and Cdk4 activity, as well as markedly prolonged tumor latency, compared to MMTV-neu/p27+/+ glands. These results suggest that p27+/− mammary epithelium may be more susceptible to oncogene-induced tumorigenesis, whereas p27-null glands, due to severely impaired cyclin D1/Cdk4 function, are more resistant to transformation.
The cell cycle is tightly regulated to control cell growth. Loss of cell cycle control can result in pathological processes such as cellular transformation (reviewed in reference 47). The cyclins and their catalytic partners, the cyclin-dependent kinases (Cdks), are instrumental in driving the cell cycle forward. Cyclin D/Cdk4 complexes phosphorylate the retinoblastoma gene product (pRb) early in the G1 phase of the cell cycle, while cyclin E/Cdk2 complexes phosphorylate pRb in late G1. When phosphorylated, pRb releases its suppression of E2F transcription factors, which can then induce transcription of S phase-specific genes and thus facilitate the G1-to-S transition. In this manner, cyclin/Cdk complexes are pivotal in regulating the progression of the cell cycle (reviewed in references 48 and 49).
Cyclin/Cdk complexes are regulated by a group of proteins known as Cdk inhibitors (CKIs). These include the Cip/Kip proteins (p21Cip1/Waf1, p27Kip1, and p57Kip2) and the Ink proteins (p15, p16Ink4a, p18, and p19). p27Kip1 has classically been regarded as a cell cycle inhibitor based on its potent inhibitory activity of cyclin E/Cdk2 (42) and the observation that its forced expression results in G1 arrest (reviewed in reference 49). However, p27 is also required for assembly and function of cyclin D1/Cdk4 complexes during early G1 (7, 8, 48, 55), suggesting that p27 may play a dual role, permitting early G1 progression (via assembly of cyclin D/Cdk4) and restraining late G1 progression (via repression of cyclin E/Cdk2). This is supported by the observation that mammary glands from p27−/− female mice (17, 25, 38) are underdeveloped compared to wild-type glands, while mammary glands from p27+/− mice are hyperproliferative and hyperplastic (35). Cyclin D1/Cdk4 activity and nuclear localization of cyclin D1 are severely impaired in p27−/− mammary cells, and the stability of cyclin D1 is reduced in the absence of p27 (7, 35). Thus, not surprisingly, the hypoplasia of p27−/− mammary glands mirrors what is observed in glands from cyclin D1-deficient mice (15, 50). In contrast, cyclin D1 in the mammary gland is required for Neu- or Ras-induced breast cancers (65), and its overexpression in the mammary epithelia of transgenic mice results in ductal hyperplasia (59). Furthermore, genetic studies of p27/cyclin D1 double-deficient mice demonstrate that p27 and cyclin D1 cooperate in vivo to regulate cell cycle control (19, 58).
Overexpression of cyclin D1 has been observed in human breast cancers (20, 22, 60). Reduced p27 protein levels are also seen in many breast cancers, and this reduction in p27 protein is associated with poor patient prognosis (6, 43, 57). Although they are rare, mutations of the p27 gene have also been reported (18, 56). Overall, these data are consistent with studies performed with mice demonstrating that p27 gene haploinsufficiency is associated with accelerated tumor formation: p27+/− mice treated with gamma irradiation or chemical carcinogens develop multiple tumors at an increased rate compared to wild-type mice (16). Notably, the remaining p27 allele in these tumors remained intact, implying the lack of a selective pressure in tumors to completely lose p27 function. Although p27−/− mice develop lung, gonadal, and intestinal tumors at an increased frequency compared to wild-type mice, mammary tumors were not reported in p27−/− mice (16). In addition, homozygous deletions of p27 have not been observed in human breast tumors. These observations suggest that loss of one p27 allele but not both may be permissive for breast tumorigenesis.
Levels of cyclin D1 and p27 are influenced to a large extent by mitogenic signals (1, 2, 8, 12, 24, 27, 28, 31, 33, 61, 62). In this study we have explored the link between p27 and mitogenic signals induced by ErbB2, a member of the ErbB family of transmembrane receptor tyrosine kinases which also includes the epidermal growth factor receptor (ErbB1), ErbB3, and ErbB4 (references 40 and 64 and references therein). Binding of specific ligands to the extracellular domains of ErbB1, ErbB3, and ErbB4 results in the formation of homodimeric and heterodimeric kinase-active complexes into which ErbB2 is recruited as a preferred partner (40, 64). MMTV (mouse mammary tumor virus)-neu transgenic mice, which overexpress c-Neu (the rat homolog of human ErbB2) in mammary epithelium, develop hyperplastic glands and focal mammary carcinomas (21). Approximately 25% of human breast tumors overexpress ErbB2 RNA and protein and/or exhibit gene amplification at the erbB2 locus (44, 53). Furthermore, treatment of ErbB2-overexpressing breast tumor cells with bivalent antibodies against the ectodomain of ErbB2 or ErbB kinase inhibitors can interfere with growth of ErbB2-overexpressing tumor cells (26, 29). These observations imply that increased activity or expression of ErbB2 may be a critical step in mammary epithelial cell transformation and tumor progression.
Activation of the ErbB2/Neu tyrosine kinase increases cyclin D1 expression (28), while decreasing p27 stability (29, 63). The stability of p27 is controlled, at least in part, by its phosphorylation at threonine 187 by Cdk2. Phosphorylation of T187 results in polyubiquitinylation and proteosomal degradation of p27 (46). The reduced p27 protein levels and elevated cyclin D1 expression accelerate cell cycle progression through G1, potentially explaining the dysregulated proliferation in ErbB2-overexpressing tumor cells. In fact, inhibition of ErbB2 with ErbB2 antibodies or small-molecule ErbB kinase inhibitors upregulates p27, decreases cyclin D1 protein levels, and induces cell cycle arrest of human breast cancer cells that express high levels of the proto-oncogene. Growth inhibition was blocked by antisense p27 or forced expression of cyclin D1, implying that both p27 and cyclin D1 are pivotal for ErbB2-mediated tumor cell growth (26, 29).
It has been observed that the complete absence of p27 results in loss of cyclin D1/Cdk4 activity, while the loss of one p27 allele results in accelerated mammary cell proliferation and reduced apoptosis (35). If indeed cyclin D1 is critical for the transformation of breast epithelial cells induced by ErbB2 or other proto-oncogenes, one would expect that this event will be blocked in p27−/− but not in p27+/− mammary cells. Therefore, to address the requirement of p27 for cyclin D1-mediated transformation in the breast, we have tested whether p27 gene dosage alters ErbB2/Neu- and cyclin D1-induced mammary cell transformation in culture and in transgenic mice. We report that transformation of p27+/− cells is accelerated compared to that of wild-type cells, while p27−/− cells are resistant to transformation. Furthermore, MMTV-neu/p27+/− mice develop mammary gland tumors at an accelerated rate compared to MMTV-neu/p27+/+ mice, whereas mammary tumor latency is significantly prolonged in MMTV-neu/p27−/− mice.
MATERIALS AND METHODS
Isolation and culture of PMECs.
All mice were derived from p27+/− P1 founders on a mixed C57BL/6J × 129Sv/J background (25) (a gift from Andrew Koff, Memorial Sloan Kettering Cancer Center, New York, N.Y.). Mice were genotyped by PCR analysis of genomic DNA as previously described (35). Ten inguinal mammary glands per genotype were harvested from 6-week-old siblings and digested at 37°C for 4 h in 100 U of hyaluronidase per ml and 3 mg of collagenase A per ml (Sigma, St. Louis, Mo.) in phosphate-buffered saline (PBS) (pH 7.4). The cell suspension was plated on dishes coated with growth factor-reduced Matrigel (Becton Dickinson, Mansfield, Mass.) in primary mammary epithelial cell (PMEC) medium (serum-free Dulbecco's modified Eagle's medium [DMEM]-F12 [50:50; GibcoBRL], 5 ng of epidermal growth factor [Clonetics] per ml, 5 ng of 17-β estradiol per ml, 5 ng of progesterone per ml, and 50 ng of insulin [Clonetics] per ml) and cultured at 37°C in 5% CO2.
Construction of pBabe retroviruses and PMEC infection.
All constructs were prepared in pBabe retroviral vectors (34). A myc epitope-tagged wild-type human erbB2 construct in pcDNA3.1 (Invitrogen) was provided by Cheryl Guyer (Vanderbilt University). The insert was excised from pcDNA3.1 using external HindIII and XbaI sites and blunt end ligated into pBabe at the EcoRI site. The cDNA construct encoding mouse cyclin D1 was provided by Charles Sherr (St. Jude's Children's Hospital Research Foundation, Memphis, Tenn.) and subcloned into the EcoRI site of the pBabe vector. A mutation was generated in pBabe-cyclin D1, resulting in an A-to-G substitution to encode a threonine-to-alanine substitution at amino acid 286. This mutation was generated using the Quick-change PCR-mediated site-directed mutagenesis kit (Invitrogen) and the following primer pair: 5′-CACGTCGGTGGGCGCGCAGGCCAGACCAGC-3′ and 5′-GCTGGTCTGGCCTGCGCGCCCACCGACGTG-3′. The boldface nucleotide represents the introduced mutation site. Primer sequences were derived from the sequence under GenBank accession no. NM007631. The mutation resulted in the introduction of an additional BssHII restriction site. Clones were screened using BssHII restriction digestion and confirmed by sequence analysis. The plasmid pCMV5-E2F1, encoding mouse E2F1, was provided by Scott Hiebert (Vanderbilt University). The E2F1 insert was excised using external XbaI sites and blunt end ligated into pBabe at the SmaI site. Retroviruses were generated by cotransfection of 293T cells with retroviral constructs and the packaging vector pCL-Eco (39) by using FuGene transfection reagent (Roche Diagnostics) and 5 μg of each plasmid per 0.5 × 106 cells. 293T cells were cultured at 5% CO2, 37°C in DMEM (GibcoBRL) supplemented with 10% fetal calf serum. After 48 h, medium conditioned by transfected 293T cells was filtered and immediately added to PMECs. At 48 h following infection, PMECs were selected by using 1 μg of puromycin per ml for 72 h. Expression of virally encoded proteins was confirmed by Western analysis (see below). For colony formation in soft agar, PMECs were plated in triplicate 35-mm-diameter dishes within a layer of 0.8% agarose in DMEM-F12 without fetal calf serum. PMEC medium was layered on top of the polymerized agarose-PMEC mixture. Cultures were incubated for 14 days and photographed, and colonies measuring ≥50 μm were counted with an Omnicon 3800 Tumor Colony Analyzer (Biologics, Gainesville, Va.).
Western analysis.
Cultured PMECs and mammary glands or tumors were harvested and homogenized as described previously (30). Total protein (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Western analyses were performed as previously described (5) using the following antibodies: p27 (Transduction Laboratories, Lexington, Ky.); pRb and cyclin D1 (Pharmingen, San Diego, Calif.); p21, p57, cyclin E, cyclin A, cyclin D2, Cdk2, Cdk6, Cdk4, and proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology, Santa Cruz, Calif.); 9E10 (Sigma); and HER2 (Neomarkers). Mouse ascitic fluid against murine E2F1 was provided by Scott Hiebert. For cell fractionation experiments, PMECs were collected by trypsinization, and cytoplasmic and nuclear extracts were prepared as previously described (29). The efficiency of the cellular fractionation was confirmed by Western analysis using antibodies against actin (cytosol) or PCNA (nucleus).
Northern analysis.
Unsynchronized PMECs (106) were collected and lysed in Trizol (GibcoBRL). Total cellular RNA was harvested according to the manufacturer's instructions. Northern analyses were performed as described previously (36) using 106 cpm of an [α-32P]dCTP-labeled random primer full-length mouse cyclin D1 cDNA or full-length glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA.
Immunofluorescence microscopy.
Cells were grown on glass coverslips, fixed in 10% formalin, and blocked in PBS supplemented with 1% normal rabbit serum. Slides were incubated with a cyclin D1 polyclonal antibody (diluted 1:100 in PBS) (Santa Cruz Biotechnology) for 1 h at room temperature, followed by incubation with an anti-rabbit antibody conjugated to Cy3 fluorochrome (diluted 1:2,500 in PBS) (Molecular Probes, Inc.). Slides were counterstained with 50 ng of DAPI (4′,6′-diamidino-2-phenylindole) (Sigma) per ml.
BrdU and TUNEL analysis.
PMECs and mice were labeled with bromodeoxyuridine (BrdU) (Sigma) as previously described (35). Immunohistochemical detection of BrdU incorporation was performed using a monoclonal BrdU antibody (Zymed) according to the manufacturer's instructions. Detection of apoptosis in PMECs by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis was performed using the Apoptag detection kit (Intergen Co.) according to the manufacturer's instructions.
Fluorescence-activated cell sorter analysis.
Proliferating, unsynchronized PMECs were harvested by trypsinization, fixed in ice-cold methanol, and labeled with 50 μg of propidium iodide (Sigma) per ml as described previously (29). A total of 10,000 stained nuclei per sample were analyzed in a FACS/Calibur flow cytometer (Becton Dickinson). DNA histograms were modeled using Modfit-LT software (Verity, Topsham, Maine).
Immunoprecipitation and kinase assays.
Five hundred micrograms of total protein was used for immunoprecipitation as described previously (29) with polyclonal antibodies against Cdk4 or control immunoglobulin G (Santa Cruz Biotechnology). The precipitates were either utilized for in vitro kinase assays or resolved by SDS-PAGE and Western analysis. For kinase assays, the immune complexes were resuspended in ice-cold kinase buffer (35). Kinase reactions were performed in the presence of 5 μCi of [γ-32P]ATP (specific activity, 3,000 Ci/mmol; Amersham Pharmacia) for 45 min at 30°C as described previously (29).
Studies with MMTV-neu/p27 mice.
p27+/− mice were crossed with MMTV-neu mice, expressing the neu proto-oncogene (21) (Jackson Laboratories, Bar Harbor, Maine). MMTV-neu/p27+/− mice were intercrossed to generate MMTV-neu/p27+/−, MMTV-neu/p27+/−, and MMTV-neu/p27−/− female mice. Mice were genotyped for the MMTV-neu transgene by using PCR. Six-week-old mice of each genotype were supplemented with a 90-day-release estrogen (0.1 mg)-progesterone (10 mg) pellet (E/P pellets; Innovative Research of America), implanted subcutaneously between the scapulae. Mammary glands were harvested at 30-day intervals after starting estrogen-progesterone exposure. Mice were monitored weekly by palpation to determine the presence of breast tumors.
Histological analysis.
Mammary glands were harvested and immediately fixed in 10% formalin (VWR Scientific). Hematoxylin-stained whole-mount preparations of no. 4 mammary glands were prepared as previously described (35). Paraffin-embedded mammary glands were sectioned (5 μm), rehydrated, and stained with Mayer's hematoxylin and eosin B-phloxine (Sigma).
RT-PCR and sequencing.
RNA from each tumor and surrounding tissue was harvested using the RNeasy extraction kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. RNA was used for reverse transcription (RT) with oligo(dT) and avian myeloblastosis virus reverse transcriptase. PCR-based amplification of cDNA was performed using primer 1 (5′-CGGAACCCACATCAGGCC-3′) and primer 2 (5′-TTTCCTGCAGCAGCCTACGC-3′), generating a 625-bp product. These products were separated on a 2.5% agarose gel, excised, and subjected to automated DNA sequencing using the internal oligonucleotide 5′GTCAACTGCAGTCATTTCCT3′ (52).
RESULTS
ErbB2-mediated morphological changes and induced levels of cyclin D1 are impaired in p27-null cells.
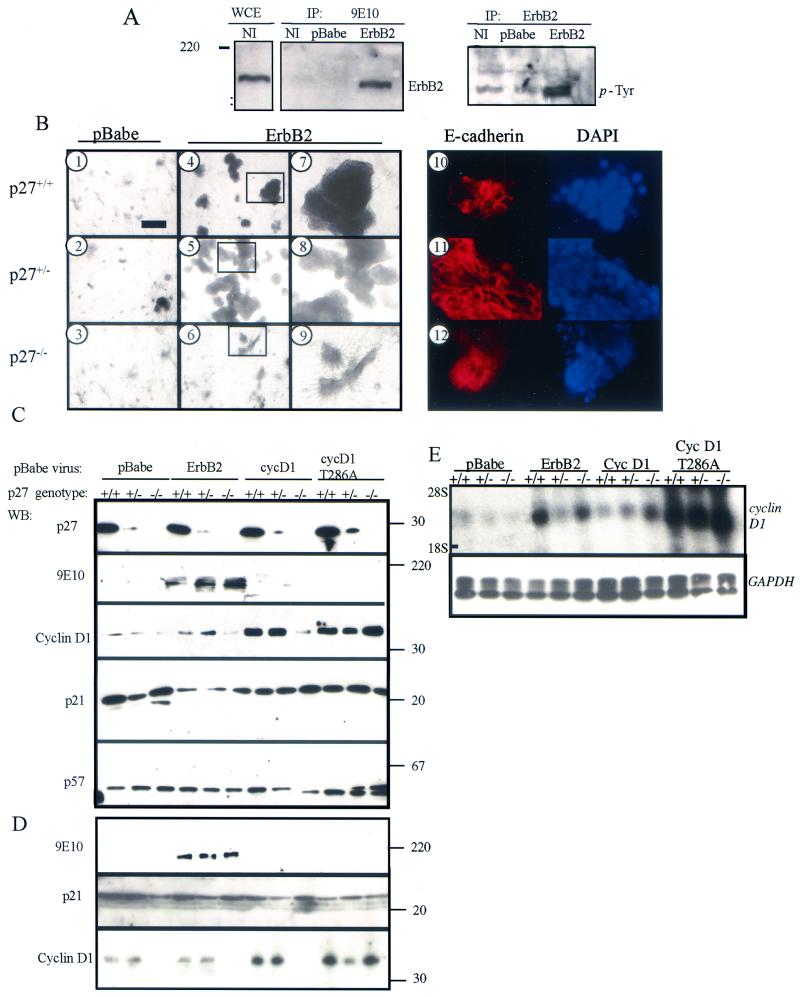
PMECs were harvested from female p27+/+, p27+/−, and p27−/− mice and infected in culture with a pBabe-derived retrovirus encoding myc-tagged ErbB2 or with an empty pBabe retrovirus. Expression of viral ErbB2 in PMECs was confirmed (Fig. 1A). Phosphotyrosine immunoblot analysis of ErbB2 precipitates demonstrated increased phosphorylated ErbB2 in pBabe-erbB2-infected cells. This result is consistent with the reported ability of wild-type Neu, the rat homolog of ErbB2, to multimerize and become activated in the absence of ligand when present at high concentrations in cells (45). A single-cell suspension of pBabe-erbB2-infected PMECs was cultured for 10 days in Matrigel (Fig. 1B). Wild-type or p27+/− PMECs infected with pBabe-erbB2 displayed increased morphological size of colonies compared to PMECs infected with empty pBabe (Fig. 1B, panels 4 and 5, compared to Fig. 1B, panels 1 and 2, respectively), with a loss of duct-like morphology (Fig. 1B, panels 7 and 8, compared to Fig. 1B, panels 1 and 2). p27−/−-erbB2 colonies were smaller than p27+/+- or p27+/+-erbB2 colonies (Fig. 1B, panels 3 and 6) and maintained branching structures (Fig. 1B, panel 9). The decrease in colony size of p27−/−-erbB2 PMECs correlated with a decrease in the average total number of cells per well ([0.6 ± 0.15] × 106) after 10 days in culture compared to p27+/+-erbB2 cells ([4.9 ± 0.6] × 106). p27+/−-erbB2 cells had an increase in cell number per well ([21.0 ± 3.3] × 106) compared to p27+/+-erbB2 cells. We examined E-cadherin expression in the organoid PMEC cultures grown in Matrigel by whole-mount immunohistochemistry. E-cadherin staining is apparent on the cellular membranes at areas of cell-cell contact in p27+/+, p27+/−, and p27−/− PMECs infected with pBabe-erbB2 (Fig. 1B, panels 10 to 12), consistent with a recent study by Muthuswamy et al. (37) in which overexpression of ErbB2 disrupted mammary acini organization without disrupting epithelial cell characteristics.
FIG. 1.
Overexpression of ErbB2 increases cyclin D1 expression in p27+/+ and p27+/− PMECs but not p27−/− PMECs. (A) Western analysis of whole-cell extracts (WCE), 9E10 immunoprecipitates (IP: 9E10), or ErbB2 immunoprecipitates (IP: ErbB2) from uninfected p27+/+ PMECs (NI) or p27+/+ PMECs infected with pBabe or pBabe-erbB2. Blots were probed with antibodies against ErbB2 (left panels) or phosphotyrosine (right panel). (B) PMECs infected with empty pBabe or pBabe-erbB2 were cultured for 10 days from a single-cell suspension embedded in growth factor-reduced Matrigel. Cultures were photographed at magnifications of ×100 (panels 1 to 6) and ×400 (panels 7 to 12). The photographs shown are representative of results obtained in three independent experiments. Panels 10 to 12, whole-mount immunohistochemical detection of E-cadherin in organoid cultures. DAPI-stained nuclei are shown to the right. (C) Western analysis of cell extracts harvested from PMEC monolayers infected with pBabe, pBabe-erbB2, pBabe-cyclin D1, or pBabe-cyclin D1(T286A). WB, primary antibodies used for Western blot analysis, listed at the left. The 9E10 antibody is against the myc epitope tag. Molecular masses are shown at right in kilodaltons. The results presented here are representative of results obtained in three independent experiments. (D) Western analysis of cell extracts harvested from PMEC organoid cultures infected with pBabe, pBabe-erbB2, pBabe-cyclin D1, or pBabe-cyclin D1(T286A). (E) Northern analysis of total cellular RNA harvested from infected PMECs. The cDNA probe used for hybridization is indicated (cyclin D1 and GAPDH). The positions of the 28S and 18S rRNAs are indicated.
We next examined the effect of increased ErbB2 activity on cyclin D1 protein levels in p27+/+, p27+/−, and p27−/− PMECs grown in monolayer (Fig. 1C) and in three-dimensional organoid culture (Fig. 1D). p27+/+- or p27+/−-erbB2 cells had moderately increased cyclin D1 protein levels compared to control p27+/+ or p27+/− PMECs, respectively. Cyclin D1 expression was also elevated in p27+/+- and p27+/−-cyclin D1 or -cyclin D1(T286A) (a mutation shown to prevent nuclear export and proteasomal degradation of cyclin D1 [3, 12, 13]) cells. Interestingly, infection with pBabe-erbB2 or -cyclin D1 did not increase cyclin D1 protein levels in p27−/− cells, but infection with pBabe-cyclin D1(T286A) resulted in a significant increase in the cyclin D1 protein levels in p27−/− cells. However, the levels of cyclin D1 mRNA were not lower in p27−/− cells infected with either ErbB2, cyclin D1, or cyclin D1(T286A) compared to p27+/+ or p27+/− infected cells (Fig. 1E), suggesting a posttranscriptional mechanism for the decreased levels of cyclin D1 in the absence of p27. The CKIs p21 and p57 were detectable at similar levels in cell suspensions from all three p27 genotypes (Fig. 1C and D), suggesting that there is little or no compensation by these proteins for the loss of p27.
Nuclear localization of cyclin D1 and Cdk4 activity is impaired in p27−/− mammary cells.
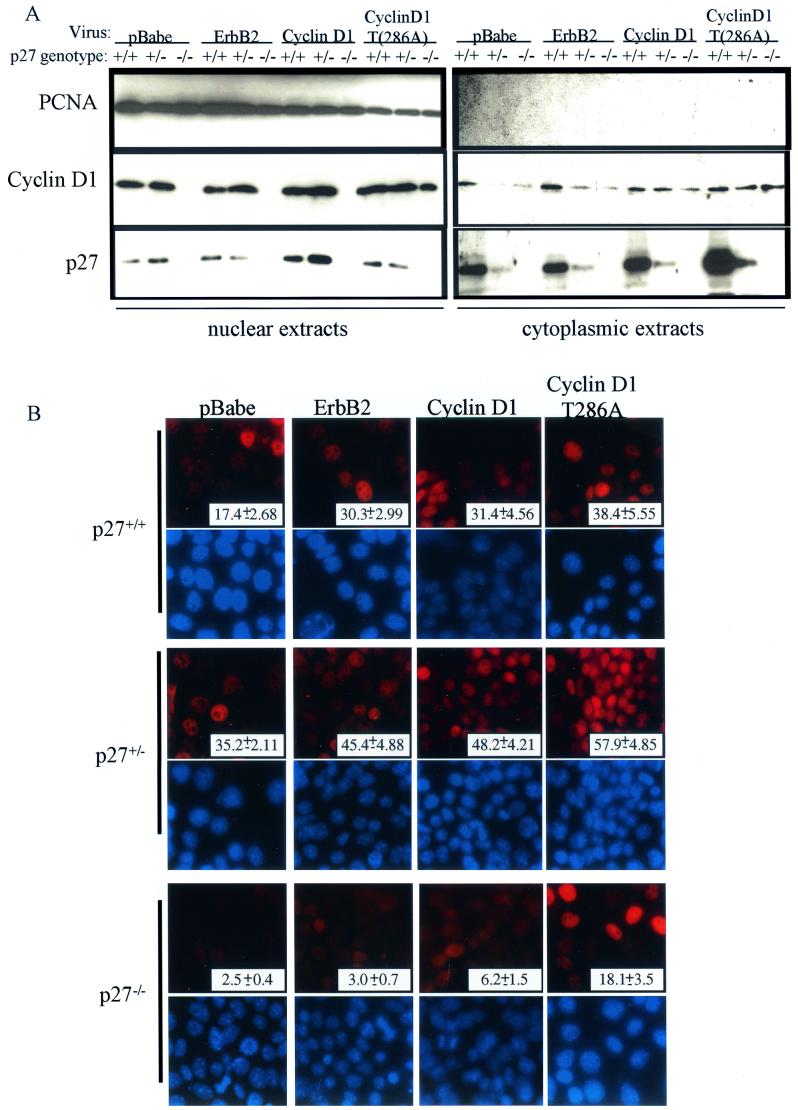
We determined the cellular distribution of cyclin D1 in p27+/+, p27+/−, and p27−/− PMECs overexpressing ErbB2, cyclin D1, or cyclin D1(T286A). By immunoblot analysis of cellular fractions, nuclear cyclin D1 was present in p27+/− and wild-type cells (Fig. 2A). However, cyclin D1 was undetectable in nuclear extracts of p27−/−-erbB2 or -cyclin D1 cells, even though it was present at low levels in the corresponding cytoplasmic extracts. Interestingly, p27−/−-cyclin D1(T286A) cells had nuclear accumulation of cyclin D1. These data were confirmed by immunofluorescence microscopy using cyclin D1 antibodies (Fig. 2B). It was found that 45.4, 48.2, and 57.9% of p27+/− cells infected with erbB2, cyclin D1, or cyclin D1(T286A) viruses , respectively, exhibited nuclear localization of cyclin D1, compared to 30.3, 31.4, and 38.4% of p27+/+ cells. In contrast, only 3.0, 6.2, and 18.1% of the p27−/− cells infected with pBabe-erbB2, -cyclin D1, or -cyclin D1(T286A), respectively, demonstrated cyclin D1 in the nucleus. Therefore, nuclear localization of cyclin D1 is impaired in the absence of p27 in mammary epithelial cells, similar to what is observed in CKI-deficient fibroblasts (7).
FIG.2.
Nuclear localization of cyclin D1 is impaired in p27−/− PMECs. (A) Western analysis of cytoplasmic and nuclear extracts from infected PMECs with antibodies against cyclin D1, p27, and PCNA. (B) Immunofluorescence analysis was used to detect cellular localization of cyclin D1 in infected PMECs. DAPI staining of nuclei is pictured directly below the corresponding cyclin D1 immunofluorescence. Magnification, ×400. Values shown represent the percentage of total nuclei that were positive for cyclin D1 staining. A total of 500 nuclei were counted per experimental condition.
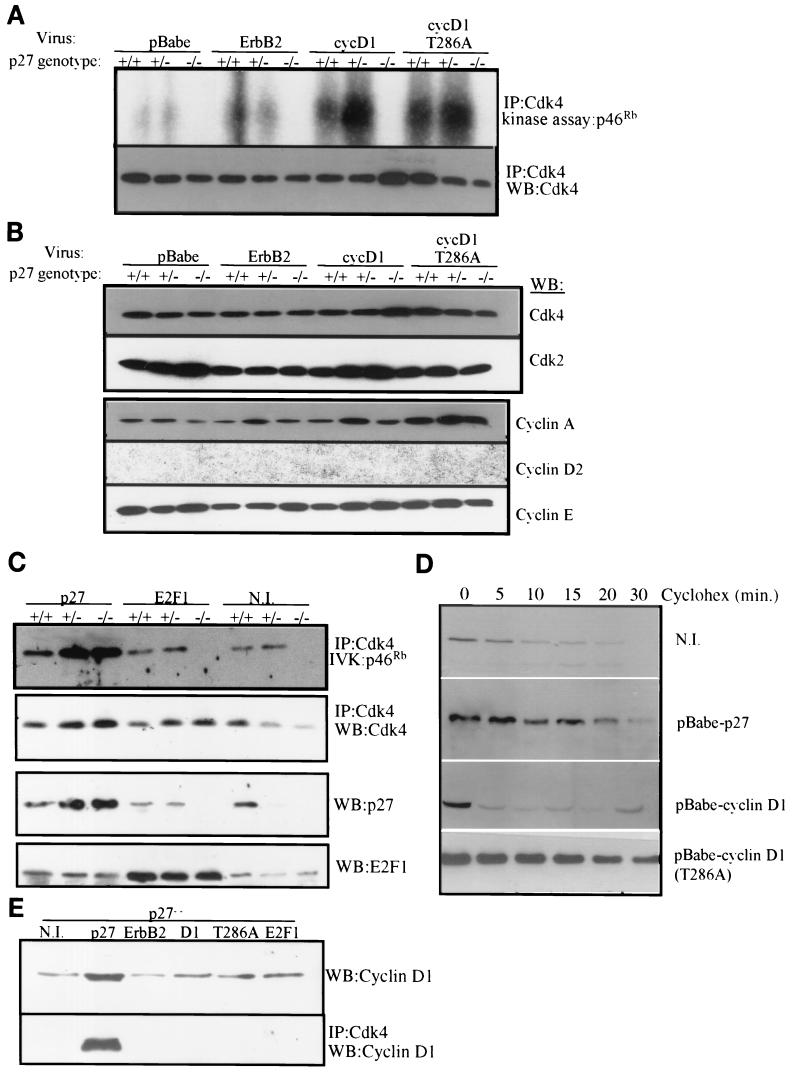
To determine the functional consequences of altered levels or localization of cyclin D1, we examined the ability of cellular Cdk4 to phosphorylate a 46-kDa fragment of pRb in vitro. Increased Cdk4 activity was observed in p27+/+- and p27+/−-erbB2, -cyclin D1, and -cyclin D1(T286A) cells compared to cells infected with empty pBabe (Fig. 3A). In contrast, Cdk4 activity was consistently lower in p27−/− cells infected with each of the three retroviruses than in wild-type and p27+/− cells. These differences in Cdk4 activity among the genotypes could not be explained by differences in the cellular levels of Cdk4, Cdk2, cyclin E, or cyclin A (Fig. 3B). Cyclin D2 was undetectable in all three genotypes. To confirm that the loss of p27 was limiting for Cdk4 activity, p27-null cells were infected with pBabe-p27 or -E2F1. Cdk4 activity against pRb was increased in all cells expressing exogenous p27, implying that restoration of p27 is sufficient to reestablish Cdk4 activity in p27−/− PMECs (Fig. 3C). In contrast, increased expression of E2F1 did not affect Cdk4 activity in p27−/− PMECs. p27−/− PMECs cultured in the presence of cycloheximide for increasing amounts of time were used to determine if the cyclin D1 protein half-life could be restored by introducing p27 expression (Fig. 3D). Although the cyclin D1 half-life was between 5 and 10 min in uninfected p27−/− cells, the cyclin D1 half-life increased to 20 to 30 min in these cells after infection with pBabe-p27. In contrast, overexpressed viral cyclin D1 in p27−/− cells exhibited a half-life similar to that of endogenous cyclin D1 of approximately <5 min (Fig. 3D). The cyclin D1(T286A) mutant displayed an increased stability, as previously reported (11-13). In addition to restoring cyclin D1 stability, reconstitution with ectopic p27 restored the assembly of cyclin D1 with Cdk4 as indicated by coimmunoprecipitation studies with Cdk4 antibodies (Fig. 3E). However, viral expression of ErbB2, cyclin D1, cyclin D1 (T286A), or E2F1 did not restore cyclin D1/Cdk4 complex formation in p27−/− PMECs (Fig. 3E).
FIG.3.
Activity of Cdk4 is impaired in p27−/− PMECs and is not restored by ErbB2 or cyclin D1 overexpression. (A) Cell extracts from infected PMECs were immunoprecipitated (IP) with an antibody against Cdk4. Immune complexes were divided in half and tested in an in vitro kinase assay with pRb as a substrate (upper panel) or used for Western blot analysis (WB) with a Cdk4 antibody (lower panel). Kinase reactions were performed in the presence of [γ-32P]ATP and then resolved by SDS-PAGE. (B) Whole-cell extracts from infected PMECs were subjected to Western blot analysis using the antibodies indicated at the right. (C) Whole-cell extracts from PMECs infected with pBabe-p27 or pBabe-E2F1 or from uninfected PMECs (N.I.) were immunoprecipitated with an antibody against Cdk4. Products were divided in half and used in an in vitro kinase reaction against pRb or in Western analysis for Cdk4 as described in panel A. Whole-cell extracts were used for Western analysis with p27 or E2F1 antibodies. (D) p27−/− PMECs infected with the indicated viruses were cultured in the presence of cycloheximide (Cyclohex) (1 μg/ml). Cell extracts were analyzed for cyclin D1 expression at various time points following cycloheximide administration. (E) Whole-cell extracts from p27−/− PMECs infected with the indicated viruses or from uninfected p27−/− PMECs (N.I.) were used for detection of cyclin D1 by Western analysis (top panel) or were immunoprecipitated with an antibody against Cdk4 and analyzed for coprecipitation of cyclin D1.
ErbB2- and cyclin D1-induced transformation in vitro is enhanced in p27-haploinsufficient mammary cells but abolished in p27-null cells.
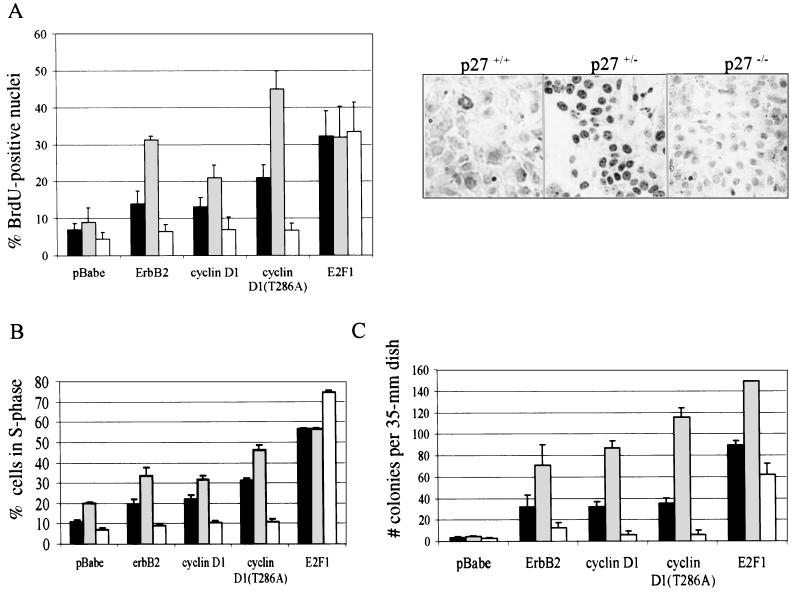
The effects of ErbB2 or cyclin D1 on transformation of p27+/+, p27+/−, or p27−/− cultured PMECs were investigated by BrdU incorporation, cell cycle distribution, and anchorage-independent colony formation. After a BrdU pulse, BrdU-labeled nuclei were increased in p27+/+ and p27+/− cells infected with pBabe-erbB2, pBabe-cyclin D1, or pBabe-cyclin D1(T286A) compared to cells infected with empty pBabe (Fig. 4A). For each retrovirus, however, BrdU incorporation was greater in p27+/− cells than in wild-type cells. In contrast, the percentage of BrdU-positive nuclei remained low in p27−/− cells expressing exogenous ErbB2, cyclin D1, or cyclin D1(T286A), similar to the low levels observed in p27−/− cells infected with control pBabe vector. However, the intrinsic ability to undergo DNA replication was not altered in p27−/− PMECs, since overexpression of E2F1, which can induce S phase progression in the absence of Cdk4/cyclin D1-mediated regulation of Rb, increased cellular proliferation to nearly equal levels in cells of each genotype. These data were consistent with the cell cycle distribution of retrovirally infected cells as measured by flow cytometry of propidium-labeled nuclei. Although both ErbB2 and cyclin D1 overexpression increased the percentage of cells in S phase for both p27+/+ and p27+/− PMECs, this increase was much larger in p27+/− cells than in wild-type cells. However, elevated expression of ErbB2 or cyclin D1 did not increase the proportion of cells in S phase in the p27−/− population (Fig. 4B). In contrast, infection with E2F1 markedly increased the proportion of p27−/− cells in S phase to levels comparable to those seen in p27+/+ and p27+/− cells. Finally, all pBabe-infected PMECs were unable to form colonies in soft agar under serum-free conditions. However, p27+/− PMECs formed more than twice as many colonies as p27+/+ PMECs when infected with pBabe-erbB2, -cyclin D1, or -cyclin D1(T286A) (Fig. 4C). Overexpression of ErbB2 or cyclin D1 did not promote substantial colony formation in p27−/− PMECs, whereas infection with E2F1 resulted in a significant increase of p27−/− colonies. These results suggest that p27+/− PMECs are more susceptible whereas p27−/− PMECs are more resistant to transformation by ErbB2 or cyclin D1.
FIG. 4.
ErbB2 increases proliferation and anchorage-independent growth in p27+/− cells but not in p27−/− cells. (A) Left panel, quantification of the percentage of nuclei from cultured, infected cells that were BrdU positive [(number of BrdU-positive nuclei/total number of nuclei) × 100], shown as the means from three experiments. Error bars represent standard deviations. Black bars, p27+/+ cells; gray bars, p27+/− cells; white bars, p27−/− cells. Right panels, immunohistochemical detection of BrdU-positive nuclei of pBabe-erbB2-infected PMECs. Samples shown are representative of three independent experiments. (B) Unsynchronized cells were stained with propidium iodide and then sorted using flow cytometry to determine the proportion of cells in each phase of the cell cycle. Results are reported as the percentage of the cell population in S phase and are presented as the averages from three independent experiments. Error bars represent the standard deviations. (C) Cells were grown in soft agar for 2 weeks. The number of colonies per 35-mm-diameter plate was counted with an automated colony counter. Each sample was analyzed in triplicate, and the experiment was repeated three times. The values shown are the averages. Error bars represent the standard errors of the means.
Neu-induced mammary transformation in vivo is modulated by p27 gene dosage.
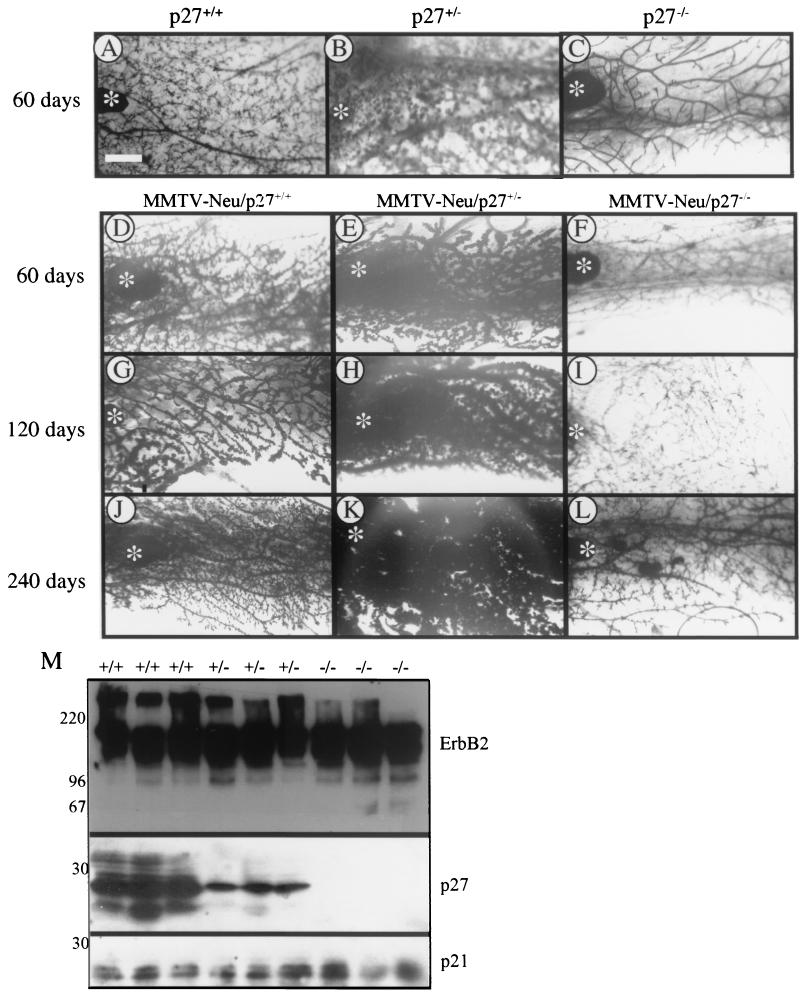
We generated MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mice by crossing p27+/− mice into a line of transgenic mice that express wild-type Neu, the mouse homolog of human ErbB2, under the control of the mammary epithelial cell-specific MMTV long terminal repeat promoter (21). Mammary gland development and preneoplastic changes in MMTV-neu/p27 mammary glands were monitored at 30-day intervals until 240 days. Expression of the MMTV-neu transgene enhanced the degree of epithelial content and areas of alveolar hyperplasia in all three p27 genotypes compared to age-matched (60-day) controls (compare Fig. 5A to C with D and E). These effects were most obvious in MMTV-neu/p27+/− glands, in which dark-staining epithelium filled the fat pad of the no. 4 inguinal mammary gland as early as 60 days of age (Fig. 5E, H, and K). In contrast, MMTV-neu/p27−/− mammary glands (Fig. 5F, I, and L) contained significantly less epithelium and less extensive alveolar budding than MMTV-neu/p27+/+ mammary glands (Fig. 5D, G, and J). Adipose tissue was abundant in MMTV-neu/p27−/− mammary glands. These morphological differences were not due to varying levels of transgene expression, as demonstrated by Western analysis to detect ErbB2 expression in a panel of mammary gland tissue extracts (Fig. 5M). Similarly, no pattern of altered p21 (Fig. 5M) or p57 (not shown) expression was observed.
FIG. 5.
MMTV-neu/p27+/− mammary glands exhibit increased lobuloalveolar hyperplasia. (A to L) Whole-mount hematoxylin staining of mammary glands from p27+/+, p27+/−, and p27−/− mice at 60 days of age (A to C) or MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mice taken at 60 days of age (D to F), 120 days of age (G to I), and 240 days of age (J to L). All mice were given slow-release estrogen-progesterone pellets, as indicated in Materials and Methods. Asterisks indicate locations of lymph nodes. Bar, 250 μm. (M) Western analysis of mammary gland lysates harvested from three independent MMTV-neu/p27+/+, p27+/−, and p27−/− mice each. The antibodies used are indicated at the right. Numbers at the left indicate molecular masses in kilodaltons.
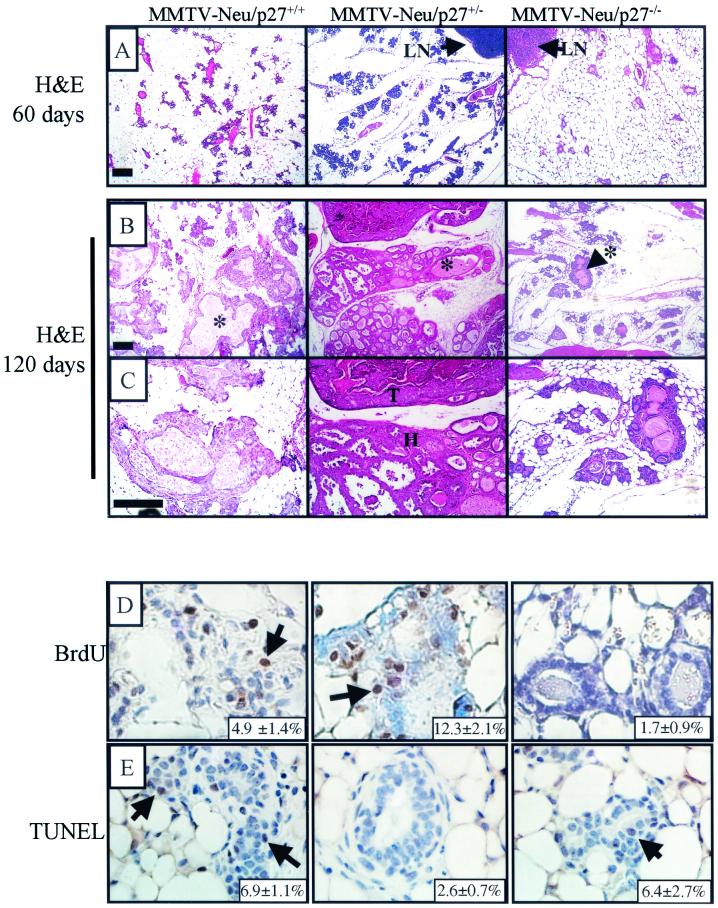
Hematoxylin- and eosin-stained sections of no. 4 mammary tissue harvested from 60- and 120-day old mice further demonstrated the decreased epithelial-to-stromal ratio and lack of secretion-filled alveolar structures in the MMTV-neu/p27−/− compared to MMTV-neu/p27+/+ or MMTV-neu/p27+/− glands (Fig. 6A to C). In contrast, extensive alveolar hyperplasia was apparent in MMTV-neu/p27+/+ and MMTV-neu/p27+/− mammary glands (Fig. 6B). We next studied whether the morphological changes observed reflected changes in rates of proliferation and/or apoptosis. To determine the level of proliferation in the glands, 120-day-old mice were pulsed with BrdU prior to harvesting of the no. 4 mammary gland (Fig. 6D). As determined by immunohistochemistry, the percentage of BrdU-positive nuclei was 2.7-fold higher in MMTV-neu/p27+/− than in MMTV-neu/p27+/+ mammary cells (P < 0.018). In contrast, the percentage of MMTV-neu/p27−/− mammary epithelial cells staining positive for BrdU was 2.9-fold lower than that observed in MMTV-neu/p27+/+ mammary glands (P < 0.004). We next used TUNEL analysis to determine the level of apoptosis in the mammary glands (Fig. 6E). At 120 days, there was a smaller percentage of apoptotic epithelial cells in MMTV-neu/p27+/− than in MMTV-neu/p27+/+ mammary glands (P < 0.0008). This suggests that the increased epithelial content in p27-haploinsufficient mammary glands may be due to both increased proliferation and reduced basal cell loss. The percentage of TUNEL-positive cells in MMTV-neu/p27−/− glands was similar to that observed in MMTV-neu/p27+/+ glands (P = 0.8).
FIG. 6.
Increased proliferation and decreased apoptosis in MMTV-neu/p27+/− mammary glands; decreased proliferation in MMTV-neu-p27−/− mammary glands. (A to C) Hematoxylin and eosin (H&E) staining of sections from MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mammary glands at 60 days (A) and 120 days (B and C). (D) Immunohistochemical detection of BrdU incorporation into the mammary glands of 120-day-old MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mice. Bar, 25 μm. (E) TUNEL analysis of mammary glands taken from 120-day-old MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mice. Bar, 50 μm. The average percentages of BrdU-positive and TUNEL-positive cells are indicated in the lower right corner of each panel.
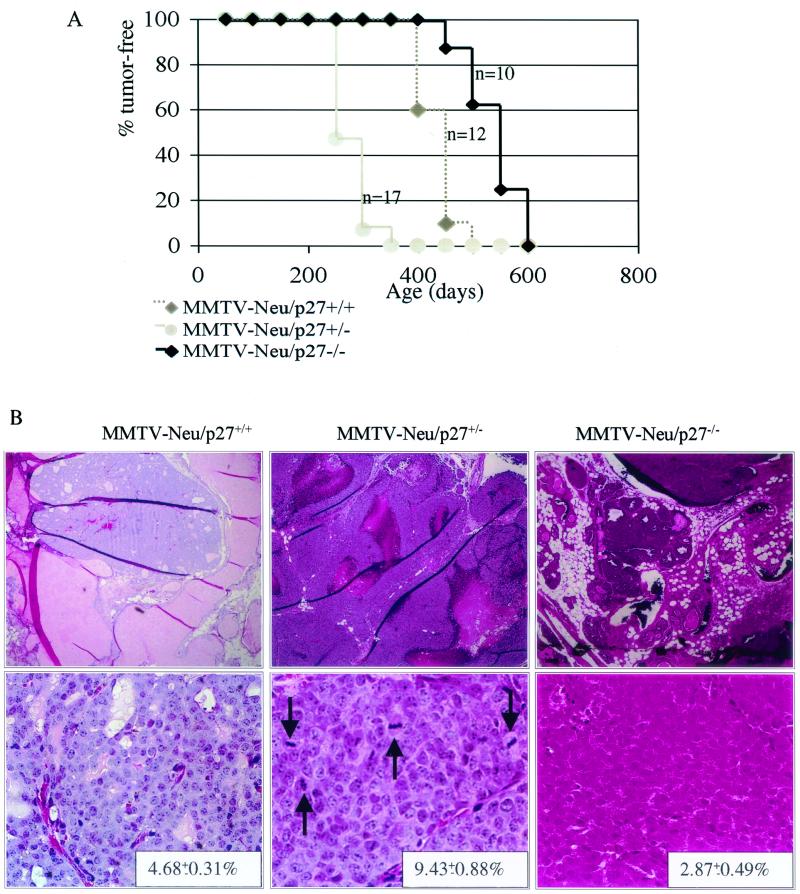
Finally, we compared the latency of Neu-induced mammary tumors as a function of p27 levels. MMTV-neu/p27+/− mice had a significantly shorter latency period for breast tumor formation than MMTV-neu/p27+/+ mice (average tumor latency, 217 and 483 days, respectively) (Fig. 7A). On average, neu-induced p27+/− tumors were larger than p27+/+ tumors (128 and 236 mm3, respectively). In contrast, MMTV-neu/p27−/− mice developed tumors after a longer average tumor latency than MMTV-neu/p27+/− mice (581 days). The p27+/− mice developed the greatest average number of tumors per mouse (4.3 ± 0.37), compared to p27+/+ mice (1.7 ± 0.21) and p27−/− mice (1.4 ± 0.19). Histologically, all tumors from p27+/+ and p27−/− mice were well-differentiated adenocarcinomas. However, p27+/− tumors were high-grade carcinomas. Large regions of necrosis were present only in p27+/− tumors, and large, heterogeneic nuclei were evident throughout p27+/− tumors. The mitotic index was highest in tumors arising in MMTV-neu/p27+/− mice (9.4 ± 0.9) and lowest in MMTV-neu/p27−/− tumors (2.9 ± 0.5; P < 0.002) (Fig. 7B).
FIG. 7.
Decreased tumor latency in MMTV-neu/p27+/− mice; increased tumor latency in MMTV-neu/p27−/− mice. (A) The mice remaining tumor free were examined until no mice were tumor free. (B) Hematoxylin and eosin staining of tumors taken from MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mice. Upper panels, photographs captured at a magnification of ×4 to visualize a wider field of mammary gland; lower panels, photographs of representative tumor tissue captured at a magnification of ×40. The pictures shown are representative for each sample group. Arrows indicate mitotic figures. Note larger tumor cell size, higher nuclear/cytoplasmic ratio, nuclear pleomorphism, and more prominent nucleoli in p27+/− tumors. The average mitotic index is shown in the lower right corner of each representative figure. Bars, 250 μm (upper panel) and 25 μm (lower panel).
Cyclin D1 content and Cdk4 kinase activity are markedly reduced in Neu-induced p27−/− tumors.
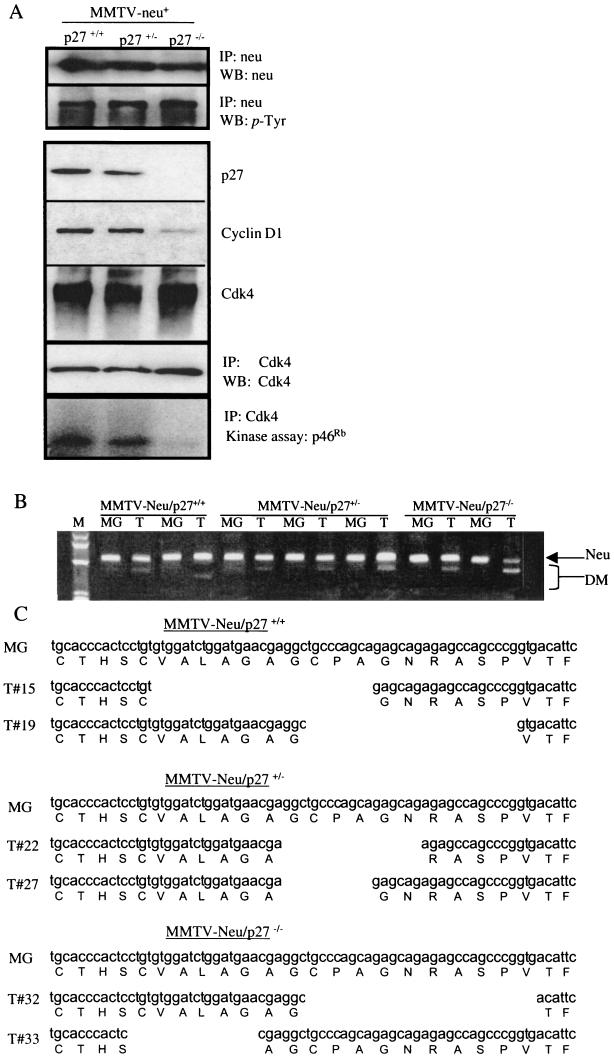
To determine the phosphorylation state of the neu proto-oncogene product, Neu immunoprecipitates from mammary gland lysates of each MMTV-neu/p27 genotype were subjected to phosphotyrosine immunoblot analysis (Fig. 8A). All samples analyzed contained phosphorylated Neu, regardless of their p27 status. This result strongly suggests that the increased tumor formation in p27+/− mammary glands or the decreased tumor formation in p27−/− mammary glands is not due to increased or decreased Neu kinase activity. We next examined cyclin D1/Cdk4 levels and kinase activity in MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− breast tumor extracts. Cdk4 levels were similar in tumors of all three genotypes (Fig. 8A). However, the in vitro kinase activity of Cdk4 immunoprecipitates was markedly decreased in MMTV-neu/p27−/− compared to MMTV-neu/p27+/+ and MMTV-neu/p27+/− tumor extracts. Steady-state cyclin D1 protein levels were also substantially diminished in MMTV-neu/p27−/− compared to MMTV-neu/p27+/+ or MMTV-neu/p27+/− tumors, potentially explaining the low mitotic index in p27-null tumors induced by Neu.
FIG.8.
Neu is constitutively phosphorylated, but Cdk4 activity is impaired in p27-null cells. (A) Extracts from MMTV-neu/p27+/+, MMTV-neu/p27+/−, and MMTV-neu/p27−/− mammary glands were subjected to immunoprecipitation (IP) with antibodies against Neu or Cdk4 or were used directly for Western analysis (WB). Immunoprecipitates were used for Western analysis or used directly for in vitro kinase assays, with p46Rb as a substrate. (B) RT-PCR using primers that flank the sequence encoding the transmembrane domain of Neu was performed on RNA harvested from normal mammary gland tissue (MG) or from tumors. The position of the expected band is indicated (Neu), as well as those of various deletion mutants (DM) that were excised and sequenced. (C) Sequence analysis of RT-PCR products. One sequence from normal mammary gland tissue per genotype is shown, as well as two representative sequences per genotype from the tumor-derived in-frame deletion mutants. Several out-of-frame mutants were identified (not shown).
Because Neu-induced mammary tumors display activating mutations of the neu transgene (4, 21), we determined if these mutations occurred in each of the three genotypes. Using RT-PCR analysis of RNA harvested from tumors and adjacent normal mammary tissue with primers that flank the sequence encoding the Neu transmembrane domain, we found that in addition to the band of the expected size, tumor RNA also generated bands of decreased size (Fig. 8B). This was observed in tumors derived from each p27 genotype. The bands were individually excised from the gel and sequenced, and it was found that the bands of the expected size, whether derived from normal mammary tissue or from tumor tissue, corresponded exactly to the published sequence for Neu (Fig. 8C and data not shown). However, the smaller RT-PCR products that were generated using tumor tissue revealed deletions in this transmembrane domain. The majority (66.7%) of these deletions generated in-frame mutations (Fig. 8C), while the remaining mutations were out of frame (sequences not shown). Deletion mutations were observed in each tumor examined regardless of p27 genotype.
DISCUSSION
The role of the Cdk inhibitor p27 as a haplosufficient tumor suppressor gene product has been previously established for many organs (reviewed above and in references 9, 41, and 54). Here, we extend this observation to breast tissue, demonstrating that p27+/− mammary epithelial cells are more susceptible to transformation by ErbB2/Neu. In fact, MMTV-neu mice develop breast tumors at a higher rate when in the context of only a single p27 allele. We also provide novel evidence that loss of both p27 alleles results in a decreased susceptibility to ErbB2-mediated transformation in the breast, in that ErbB2-induced tumor formation is substantially delayed in p27−/− mice. Thus, p27 appears to serve a complex role in breast tumorigenesis, as both a positive and negative regulator of the cell cycle. Our results suggest that in the absence of both p27 alleles, the ability of p27 to drive the cell cycle forward is lost, but in the presence of a single allele, the forward progression of the cell cycle is maintained, while p27 is unable to suppress the cell cycle. Since p27+/− mammary epithelial cells were highly susceptible to transformation but p27−/− cells were resistant to transformation, the levels of p27 present within the cell may determine p27 function within a given mammary epithelial cell.
The difference in p27 function in p27+/− versus p27−/− mammary glands may be due to the role of p27 in stabilizing cyclin D/Cdk4 complexes while inhibiting cyclin E/Cdk2 complexes (7, 48). The inability of cyclin D1 to associate with its catalytic partner Cdk4 in the absence of p27 has been shown previously in p27−/− mouse embryonic fibroblasts (7), as well as in p27−/− mammary glands (35). Although overexpression of ErbB2/Neu or cyclin D1 increased Cdk4 activity in wild-type and p27+/− mammary cells, the results presented here suggest that in the absence of p27, increased expression of ErbB2 or cyclin D1 could not increase Cdk4 activity from the diminished levels observed in p27−/− cells. Even in MMTV-neu/p27−/− mammary glands, cyclin D1 content and Cdk4 activity remained low (Fig. 8), consistent with the reported destabilization of cyclin D1 in the absence of p27 (7, 35). However, a mutant of cyclin D1 (T286A) that is resistant to proteasome-mediated degradation and nuclear exclusion (3, 12, 13) achieved high steady-state levels and nuclear localization in p27-null cells, confirming the role of p27 in counteracting the degradation of cyclin D1 in mammary cells. Despite robust levels of cyclin D1(T286A) in the nucleus, expression of this mutant did not induce Cdk4 activity (Fig. 3), mammary epithelial cell proliferation, or colony formation (Fig. 4). Because cyclin D/Cdk4 activity is required for progression through early G1 (31, 48, 55) and p27 is required for cyclin D1/Cdk4 activity (7, 8, 19, 58), entry into S phase may be blocked or delayed in p27-deficient cells. Since exogenous p27 expression was sufficient to reestablish cyclin D1/Cdk4 activity in p27−/− PMECs, these results suggest that Cdk4 activity is impaired due to the absence of p27, even when cells overexpress ErbB2 or cyclin D1. These data imply that (i) enhanced cyclin D1 stability does not compensate for the loss of p27 and (ii) p27 is required for nuclear D1/Cdk4 activity. However, it should be noted that MMTV-neu/p27−/− mammary glands eventually develop tumors after a lengthened latency, suggesting that ErbB2/Neu may eventually signal through a secondary p27/cyclin D/Cdk4-independent mechanism to induce tumor formation.
In contrast, Cdk4 activity is maintained in p27+/− glands, suggesting that at least one functional p27 allele is necessary for Cdk4 activity. In response to ErbB2 and cyclin D1 overexpression, p27+/− PMECs displayed heightened cyclin D1 nuclear localization and Cdk4 activity, consistent with the higher rate of proliferation and tumor formation in MMTV-neu/p27+/− glands. The mechanism(s) for increased nuclear localization of cyclin D1 in p27+/− cells requires further investigation. However, these results suggest the possibility that a threshold level of p27 might be required for the export of cyclin D1 from the nucleus. Nonetheless, the data presented show that loss of only one p27 allele preserves the permissive role of p27 in G1 progression by contributing to cyclin D1/Cdk4 activity but, importantly, that loss of only one p27 allele impairs the role of p27 as a cell cycle inhibitor.
An alternative hypothesis that may explain the delayed tumor latency in MMTV-neu/p27−/− mammary glands is that the decreased proliferative capacity of p27−/− mammary epithelial cells may result in a smaller stem cell population. Although stem cells have not been isolated from or identified in the mammary epithelium, their presence has been proven by the fact that transplantation of as few as 100 mammary epithelial cells can repopulate an entire mouse mammary gland (32). This reconstituted mammary gland retains the morphological and functional characteristics of a normal mouse mammary gland, suggesting that a pluripotent stem cell is responsible for the repopulation. Indeed, p27−/− PMECs can repopulate a reconstituted mouse mammary fat pad, albeit at a substantially reduced rate compared to p27+/+ or p27+/− PMECs (35). This suggests that stem cells are indeed present, but the proliferative capacity and/or the size of the stem cell population may be reduced in the absence of p27. However, this hypothesis is not exclusive to the idea that ErbB2 requires a threshold level of p27 to dysregulate proliferation in the mammary gland, since the data presented here, taken together with previous reports, establish a role for cyclin D1/Cdk4 (and therefore p27) activity in ErbB2-mediated breast tumor progression.
These studies underscore the observation that the ability of the mammary epithelium to proliferate strongly correlates with cyclin D1/Cdk4 activity (11, 15, 51, 65). This is consistent with the observations that cyclin D1 is often overexpressed in human breast cancers (22) and that cyclin D1 overexpression in the mouse mammary epithelium results in ductal hyperplasia (59). Given that cyclin D2 and cyclin D3 are not expressed in the mouse mammary epithelium even at times of maximal gland proliferation during mid-pregnancy (51), epithelial proliferation in the breast may be uniquely dependent on cyclin D1 expression to drive Cdk4 activity. This hypothesis is supported by the observation that cyclin D1-deficient mammary glands are hypoplastic (15, 51). Therefore, p27 may be specifically required within mammary epithelium to support the activity of cyclin D1/Cdk4. Indeed, the loss of p27 in the mammary gland also results in hypoplasia (35), suggesting that p27 and cyclin D1 cooperate to induce breast development. As many genes involved in mammary gland morphogenesis are often dysregulated during tumorigenesis, it is conceivable that p27 and cyclin D1 would play a prominent role in the progression of breast cancers, in addition to their function in development.
Recent evidence demonstrated that loss of cyclin D1 expression specifically protects mice from developing breast cancers induced by Neu or Ras (65). Thus, cyclin D1 is required for Neu-induced tumors (28). Since p27 is required for the stability of cyclin D1 and for activity of the cyclin D1/Cdk4 complex, this dependency of the Neu pathway on cyclin D1 may explain why Neu-induced tumors are delayed in the absence of p27 (Fig. 7). In the same study, other oncogenic pathways, specifically, those regulated by c-myc and Wnt-1, were able to induce the formation of breast tumors in the absence of cyclin D1. We would then predict that the loss of p27 may not prevent or delay c-myc- or Wnt-1- induced breast tumors.
MMTV-neu/p27+/− mammary glands displayed increased epithelial content compared to MMTV-neu/p27+/+ glands. The results presented here demonstrate that this is due to an increase in epithelial proliferation, as well as to a decrease in programmed cell death. This result is very intriguing, as p27+/− mammary glands have a decreased level of apoptotic cell death during postlactational involution (35). It is possible that both p27 alleles are required to initiate withdrawal from the cell cycle preceding apoptosis and/or terminal differentiation, events that would negate the transforming effect of the neu oncogene. Although the demonstration of such a role for p27 in mammary epithelial cell apoptosis would require further investigation, studies with oligodendrocytes indicate that accumulation of p27 is required for cell cycle arrest and terminal differentiation (14). Furthermore, adenoviral expression of p27 induced cell cycle arrest and triggered apoptosis in several human cancer cell lines, including breast cancer-derived cells (10, 23). A threshold level of p27 required for programmed cell death in the mammary gland would explain the reduced levels of apoptosis observed in the MMTV-neu/p27+/− hyperplasias.
In summary, we have demonstrated a dual role for p27 during tumorigenesis. The role of p27 as a tumor suppressor requires both p27 alleles, as loss of a single p27 allele resulted in an increased susceptibility to mammary tumor formation. We have provided intriguing evidence that both p27 alleles may also be required for apoptosis within the mammary epithelium. The role of p27 as a growth-inducing factor requires at least one p27 allele, since loss of both alleles results in mammary gland hypoplasia and delayed tumor formation, decreased cyclin D1 expression, and decreased nuclear localization of cyclin D1. These results convey the increasing complexity of p27 in cell cycle control and breast cancer and place p27 and cyclin D1 at a pivotal point in ErbB2-mediated tumorigenesis.
Acknowledgments
We are grateful to Andrew Koff for providing the p27-null mice and to Jean Simpson and Sandy Olson for histological and immunohistochemical analyses.
This work was supported by NIH training grant T32 CA09592 (to R.S.M.), a postdoctoral research fellowship from the Susan G. Komen Breast Cancer Foundation (to A.E.G.L.), NIH grant R01 CA80195 (to C.L.A.), and Vanderbilt-Ingram Comprehensive Cancer Center support grant CA68485.
REFERENCES
- 1.Aktas, H., H. Cai, and G. M. Cooper. 1997. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 17:3850-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. [DOI] [PubMed] [Google Scholar]
- 3.Alt, J. R., J. L. Cleveland, M. Hannink, and A. Diehl. 2000. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14:3102-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrechek, E. R., W. R. Hardy, P. M. Siegel, M. A. Rudnicki, R. D. Cardiff, and W. J. Muller. 2000. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc. Natl. Acad. Sci. USA 97:3444-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brantley, D. M., F. E. Yull, R. S. Muraoka, D. J. Hicks, C. M. Cook, and L. D. Kerr. 2000. Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech. Dev. 97:149-155. [DOI] [PubMed] [Google Scholar]
- 6.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3:227-230. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, M., V. Sexl, C. J. Sherr, and M. F. Roussel. 1998. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl. Acad. Sci. USA 95:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clurman, B. E., and P. Porter. 1998. New insights into the tumor suppression function of P27(kip1). Proc. Natl. Acad. Sci. USA 95:15158-15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, C., R. Wersto, M. Kim, E. Ohri, Z. Li, D. Katayose, S. J. Lee, J. Trepel, K. Cowan, and P. Seth. 1997. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene 14:2283-2289. [DOI] [PubMed] [Google Scholar]
- 11.Dickson, C., A. Creer, and V. Fantl. 2000. Mammary gland oncogenes as indicators of pathways important in mammary gland development. Oncogene 19:1097-1101. [DOI] [PubMed] [Google Scholar]
- 12.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehl, J. A., F. Zindy, and C. J. Sherr. 1997. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 11:957-972. [DOI] [PubMed] [Google Scholar]
- 14.Durand, B., M. L. Fero, J. M. Roberts, and M. C. Raff. 1998. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8:431-440. [DOI] [PubMed] [Google Scholar]
- 15.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 16.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando, A. A., M. Balbin, A. M. Pendas, F. Vizoso, G. Velasco, and C. Lopez-Otin. 1996. Mutational analysis of the human cyclin-dependent kinase inhibitor p27kip1 in primary breast carcinomas. Hum Genet. 97:91-94. [DOI] [PubMed] [Google Scholar]
- 19.Geng, Y., Q. Yu, E. Sicinska, M. Das, R. T. Bronson, and P. Sicinski. 2001. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl. Acad. Sci. USA 98:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillett, C., P. Smith, W. Gregory, M. Richards, R. Millis, G. Peters, and D. Barnes. 1996. Cyclin D1 and prognosis in human breast cancer. Int. J. Cancer 69:92-99. [DOI] [PubMed] [Google Scholar]
- 21.Guy, C. T., M. A. Webster, M. Schaller, T. J. Parsons, R. D. Cardiff, and W. J. Muller. 1992. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 89:10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall, M., and G. Peters. 1996. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv. Cancer Res. 68:67-108. [DOI] [PubMed] [Google Scholar]
- 23.Katayose, Y., M. Kim, A. N. Rakkar, Z. Li, K. H. Cowan, and P. Seth. 1997. Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res. 57:5441-5445. [PubMed] [Google Scholar]
- 24.Kerkhoff, E., and U. R. Rapp. 1997. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol. Cell. Biol. 17:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 26.Lane, H. A., I. Beuvink, A. B. Motoyama, J. M. Daly, R. M. Neve, and N. E. Hynes. 2000. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol. Cell. Biol. 20:3210-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271:20608-20616. [DOI] [PubMed] [Google Scholar]
- 28.Lee, R. J., C. Albanese, M. Fu, M. D'Amico, B. Lin, G. Watanabe, G. K. Haines III, P. M. Siegel, M. C. Hung, Y. Yarden, J. M. Horowitz, W. J. Muller, and R. G. Pestell. 2000. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell. Biol. 20:672-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenferink, A. E., D. Busse, W. M. Flanagan, F. M. Yakes, and C. L. Arteaga. 2001. ErbB2/neu kinase modulates cellular p27(Kip1) and cyclin D1 through multiple signaling pathways. Cancer Res. 61:6583-6591. [PubMed] [Google Scholar]
- 30.Lenferink, A. E., J. F. Simpson, L. K. Shawver, R. J. Coffey, J. T. Forbes, and C. L. Arteaga. 2000. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-alpha bigenic mice. Proc. Natl. Acad. Sci. USA 97:9609-9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J. Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medina, D. 1996. The mammary gland: a unique organ for the study of development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 1:5-19. [DOI] [PubMed] [Google Scholar]
- 33.Meyerson, M., and E. Harlow. 1994. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 14:2077-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muraoka, R. S., A. E. Lenferink, J. Simpson, D. M. Brantley, L. R. Roebuck, F. M. Yakes, and C. L. Arteaga. 2001. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J. Cell Biol. 153:917-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muraoka, R. S., S. E. Waltz, and S. J. Degen. 1999. Expression of hepatocyte growth factor-like protein is repressed by retinoic acid and enhanced by cyclic adenosine 3′,5′-monophosphate response element-binding protein (CREB)-binding protein (CBP). Endocrinology 140:187-196. [DOI] [PubMed] [Google Scholar]
- 37.Muthuswamy, S. K., D. Li, S. Lelievre, M. J. Bissell, and J. S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, and D. Y. Loh. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 39.Naviaux, R. K., E. Costanzi, M. Haas, and I. M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philipp-Staheli, J., S. R. Payne, and C. J. Kemp. 2001. p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 264:148-168. [DOI] [PubMed] [Google Scholar]
- 42.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 43.Porter, P. L., K. E. Malone, P. J. Heagerty, G. M. Alexander, L. A. Gatti, E. J. Firpo, J. R. Daling, and J. M. Roberts. 1997. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 3:222-225. [DOI] [PubMed] [Google Scholar]
- 44.Ross, J. S., and J. A. Fletcher. 1998. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells 16:413-428. [DOI] [PubMed] [Google Scholar]
- 45.Samanta, A., C. M. LeVea, W. C. Dougall, X. Qian, and M. I. Greene. 1994. Ligand and p185c-neu density govern receptor interactions and tyrosine kinase activation. Proc. Natl. Acad. Sci. USA 91:1711-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 47.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 48.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 49.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 50.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 51.Sicinski, P., and R. A. Weinberg. 1997. A specific role for cyclin D1 in mammary gland development. J. Mammary Gland Biol. Neoplasia 2:335-342. [DOI] [PubMed] [Google Scholar]
- 52.Siegel, P. M., D. L. Dankort, W. R. Hardy, and W. J. Muller. 1994. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol. Cell. Biol. 14:7068-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slamon, D. J., W. Godolphin, L. A. Jones, J. A. Holt, S. G. Wong, D. E. Keith, W. J. Levin, S. G. Stuart, J. Udove, A. Ullrich, et al. 1989. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707-712. [DOI] [PubMed] [Google Scholar]
- 54.Slingerland, J., and M. Pagano. 2000. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell. Physiol. 183:10-17. [DOI] [PubMed] [Google Scholar]
- 55.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7:135-146. [PubMed] [Google Scholar]
- 56.Spirin, K. S., J. F. Simpson, S. Takeuchi, N. Kawamata, C. W. Miller, and H. P. Koeffler. 1996. p27/Kip1 mutation found in breast cancer. Cancer Res. 56:2400-2404. [PubMed] [Google Scholar]
- 57.Tan, P., B. Cady, M. Wanner, P. Worland, B. Cukor, C. Magi-Galluzzi, P. Lavin, G. Draetta, M. Pagano, and M. Loda. 1997. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 57:1259-1263. [PubMed] [Google Scholar]
- 58.Tong, W., and J. W. Pollard. 2001. Genetic evidence for the interactions of cyclin D1 and p27(Kip1) in mice. Mol. Cell. Biol. 21:1319-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, T. C., R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt. 1994. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669-671. [DOI] [PubMed] [Google Scholar]
- 60.Weinstat-Saslow, D., M. J. Merino, R. E. Manrow, J. A. Lawrence, R. F. Bluth, K. D. Wittenbel, J. F. Simpson, D. L. Page, and P. S. Steeg. 1995. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat. Med. 1:1257-1260. [DOI] [PubMed] [Google Scholar]
- 61.Winston, J. T., S. R. Coats, Y. Z. Wang, and W. J. Pledger. 1996. Regulation of the cell cycle machinery by oncogenic ras. Oncogene 12:127-134. [PubMed] [Google Scholar]
- 62.Won, K. A., and S. I. Reed. 1996. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 15:4182-4193. [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, H. Y., B. P. Zhou, M. C. Hung, and M. H. Lee. 2000. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J. Biol. Chem. 275:24735-24739. [DOI] [PubMed] [Google Scholar]
- 64.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]
- 65.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]