Abstract
We present a case of aortic valve replacement combined with aortic root enlargement, performed on an achondroplastic dwarf with severe calcific aortic stenosis.
There are no data about the incidence of valvular diseases in achondroplastic patients. To our knowledge, this is the 1st time that an aortic valve replacement associated with an aortic root enlarging procedure has been performed in this kind of patient.
The aim of this report is to show that achondroplasia, in and of itself, is not a contraindication to aortic valve replacement.
Key words: Achondroplasia/surgery, airway obstruction, aortic valve stenosis, dwarfism/surgery, heart valve prosthesis
A chondroplasia is the most common type of chondrodysplasia and is inherited as an autosomal dominant trait. Eighty percent of cases are new mutations, and the likelihood of mutation increases with paternal age.1 Most of achondroplastic dwarfs have normal sexual development and good life spans.2
Case Report
On 6 July 2003, a 56-year-old achondroplastic woman, previously refused by several hospitals, was admitted to ours with a diagnosis of severe aortic stenosis.
According to her medical history, she had experienced dyspnea and angina for the preceding 10 months. During the 2 months preceding hospital admission, she had experienced an ingravescent symptomatology, despite intensive medical treatment. At the time of admission, she was in New York Heart Association (NYHA) functional class III–IV and Canadian Cardiovascular Society (CCS) functional class II–III.
Neither the patient's parents nor her brother was achondroplastic or showed any sign of cardiac disease.
Her habitus bore characteristic achondroplastic features: although the truncal length was proportioned, her limbs were very short and she had the typical facies of achondroplasia (a large head, small face, and saddle nose). She weighed 27 kg and was 100 centimeters tall (Fig. 1).
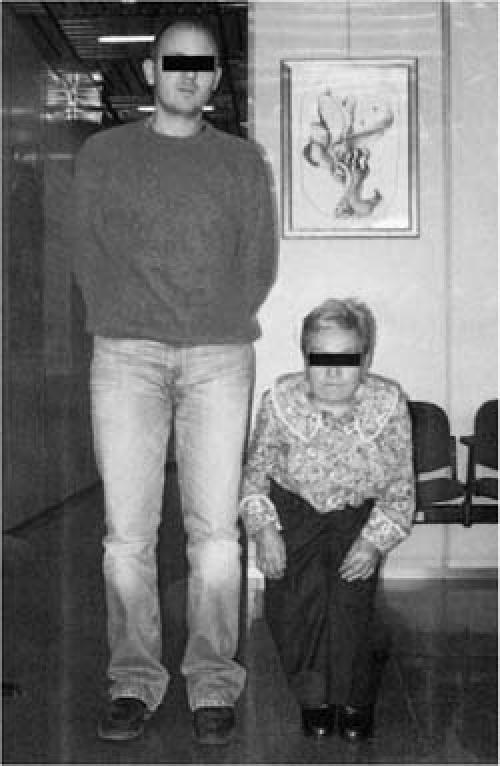
Fig. 1 The patient, standing next to one of her relatives.
A 12-lead electrocardiogram revealed sinus rhythm with signs of left ventricular hypertrophy. The results of the initial laboratory studies were substantially normal.
Chest radiography revealed poor expansion of the lung fields, possibly due to the patient's anatomic features. Total body computed tomography showed strong cervical and lumbar rotoscoliosis and severe bilateral coxofemoral osteoarthritis, which caused an abnormal orientation of the articular surfaces.
The echocardiographic examination showed a hypertrophic interventricular septum (15 mm) but well functioning left ventricle (ejection fraction, 0.55) and severe aortic stenosis, with a maximum transvalvular gradient of 144 mmHg and an aortic valve area of 0.5 cm2. The pulmonary pressure was 33 mmHg. Cardiac catheterization confirmed the presence of severe and calcific aortic stenosis, without coronary disease.
During the preoperative examinations, we performed a respiratory test that proved an obstructive deficit. Tracheobronchoscopy showed an abnormal tracheal curvature and a slight tracheal stenosis above the curvature. Such findings suggested the necessity of a specific pediatric tube for the intubation.
At operation, on 17 July 2003, we found a tricuspid, fibrous, and markedly calcific aortic valve in a very small aortic root. After valve removal, we extended the aortotomy through the middle of the noncoronary sinus of Valsalva along the subaortic space. The aortic annulus was 10 mm before enlargement by means of sewing an autologous pericardial patch into place on the aortic leaflet of the mitral valve, using a continuous 5-0 Prolene suture (Ethicon Inc., a Johnson & Johnson Co.; Somerville, NJ). Then a 16-mm mechanical Carbomedics valve (CarboMedics Inc., a Sorin Group Company; Austin, Tex) was placed by means of single 2-0 Ti-Cron (United States Surgical, a Tyco Healthcare Group LP Co.; Norwalk, Conn) U-shaped stitches with subannular pledgets; at the patch level, the pledgets were positioned externally. In closing the aortotomy, we sewed the elliptical, annulus-based pericardial patch along the cut aortic walls (Fig. 2) by means of 3 semicontinuous 4-0 Prolene sutures (Ethicon Inc.).
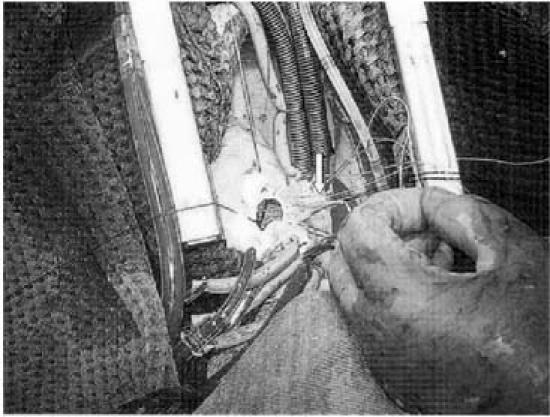
Fig. 2 A mechanical aortic valve prosthesis is implanted into an aortic annulus that has been enlarged with a pericardial patch.
Because of the very short and thin sternum (pectus carinatum), we did not use stainless steel stitches as usual to perform the sternal closure; we preferred 4 stitches of Vicryl 2.0 polyglactin suture (Ethicon, Inc.).
No problem was encountered during the institution of cardiopulmonary bypass (CPB), although we had to use pediatric cannulae both for the aorta (20F) and for the superior and inferior vena cavae (28F). The patient was monitored with a pediatric Swan-Ganz catheter (to measure pulmonary blood pressure, wedge pressure, and central venous pressure) and with a femoral catheter for systemic arterial blood pressure. Anesthetic management was carried out with no complication: both hemodynamic and hematologic values were normal.
The operative procedure required 119 minutes of CPB and 91 minutes of ascending aortic cross-clamp time under normothermia.
The patient was in the intensive care unit for 23 hours. Her postoperative course was complicated by atrial fibrillation, which required treatment with direct-current shock. Postoperative echocardiography showed a trans-prosthesis maximum gradient of 16 mmHg, with normal functioning of the new valve. The patient was discharged on the 8th postoperative day on daily oral anticoagulant therapy. Echocardiographic examinations, performed 1 and 6 months after the operation, showed a normally positioned and hemodynamically functioning valve. At the present time, 8 months after cardiac surgery, she is asymptomatic, in NYHA functional class I.
Discussion
Peculiar difficulties arise in the anesthesiologic management of patients with achondroplasia, the most common type of dwarfism. First, the unusual skeletal structure of such patients makes percutaneous arterial access difficult. Moreover, achondroplastic patients usually have severe respiratory defects.3,4 Therefore, a preoperative respiratory-function test appears to be extremely important. In the present case, in fact, this exam showed severe obstructive pulmonary disease. Last, but not least, intubation can be complicated by the abnormal anatomy of the upper respiratory tract.
The main problem in this patient with aortic stenosis was the very small size of the aortic root, which required aortic ring enlargement. The choice of a prosthesis that allowed only a mild degree of residual aortic stenosis was very important. In this patient, no available aortic valve prosthesis could provide a satisfactory orifice area, so the aortic root had to be enlarged by one of several known techniques.5–7
Footnotes
Address for reprints: Antonio Scafuri, MD, Department of Cardiac Surgery, Tor Vergata University of Rome, Via Oxford 81, 00133 Rome, Italy
E-mail: antonio.scafuri@fastweb.net.it
References
- 1.Strewler GJ. Osteonecrosis, osteosclerosis and other disorders of bone. In: Cecil RL, Wyngaarden JB, Smith LH, editors. Cecil Textbook of medicine. Vol I. 18th ed. Philadelphia: WB Saunders; 1988. p. 1435.
- 2.Balaguer JM, Perry D, Crowley J, Moran JM. Coronary artery bypass grafting in an achondroplastic dwarf. Tex Heart Inst J 1995;22:258–60. [PMC free article] [PubMed]
- 3.Krishnan BS, Eipe N, Korula G. Anaesthetic management of a patient with achondroplasia. Paediatr Anaesth 2003;13: 547–9. [DOI] [PubMed]
- 4.Walts LF, Finerman G, Wyatt GM. Anaesthesia for dwarfs and other patients of pathological small stature. Can Anaesth Soc J 1975;22:703–9. [DOI] [PubMed]
- 5.Nicks R, Cartmill T, Bernstein L. Hypoplasia of the aortic root. The problem of aortic valve replacement. Thorax 1970; 25:339–46. [DOI] [PMC free article] [PubMed]
- 6.Manouguian S, Seybold-Epting W. Patch enlargement of the aortic valve ring by extending the aortic incision into the anterior mitral leaflet. New operative technique. J Thorac Cardiovasc Surg 1979;78:402–12. [PubMed]
- 7.Rastan H, Koncz J. Aortoventriculoplasty: a new technique for the treatment of left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg 1976;71:920–7. [PubMed]


