Abstract
Protein phosphatase 2A (PP2A) is a multimeric serine/threonine phosphatase that carries out multiple functions. Although numerous observations suggest that PP2A plays a major role in downregulation of the mitogen-activated protein (MAP) kinase pathway, the precise mechanisms are unknown. To clarify the role of PP2A in growth factor (insulin, epidermal growth factor [EGF], and insulin-like growth factor 1 [IGF-1]) stimulation of the Ras/MAP kinase pathway, simian virus 40 small t antigen was expressed in Rat-1 fibroblasts which overexpress insulin receptors. Small t antigen is known to specifically inhibit PP2A by binding to the A PP2A regulatory subunit, interfering with the ability of PP2A to bind to its cellular substrates. Overexpressed small t protein was coimmunoprecipitated with PP2A and inhibited cellular PP2A activity but did not inhibit protein phosphatase 1 (PP1) activity. Insulin, IGF-1, and EGF stimulation also inhibited PP2A activity. Growth factor-stimulated Ras, Raf-1, MAP kinase, and mitogen-activated extracellular-signal-regulated kinase kinase (MEK) activities were elevated in small-t-antigen-expressing cells. Furthermore, Shc tyrosine phosphorylation and its association with Grb2 were also elevated in small-t-antigen-expressing cells. Expression levels of Shc, Ras, MEK, or MAP kinase and phosphorylation of insulin, EGF, and IGF-1 receptors were not altered. Interestingly, we found that PP2A associated with Shc in the basal state and dissociated in response to insulin and EGF and that this dissociation was inhibited by 65% in small-t-antigen-expressing cells. In addition, we found that PP2A associates with the phosphotyrosine-binding domain (PTB domain) of Shc and that phosphorylation of tyrosine 317 of Shc was required for PP2A-Shc dissociation. We conclude (i) that PP2A negatively regulates the Ras/MAP kinase pathway by binding to Shc, inhibiting tyrosine phosphorylation; (ii) that the Shc-PP2A association is mediated by the Shc PTB domain but the interaction is independent of phosphotyrosine binding, indicating a new molecular function for the PTB domain; (iii) that growth factor stimulation, or small-t-antigen expression, causes dissociation of the PP2A-Shc complex, facilitating Shc phosphorylation and downstream activations of the Ras/MAP kinase pathway; and (iv) that this defines a new mechanism of small-t-antigen action to promote mitogenesis.
Protein phosphorylation plays a key role in many cellular processes, including signal transduction pathways (23, 32). The phosphorylation state of a target protein is regulated by opposing kinases and phosphatases (23). Protein phosphatase 2A (PP2A) is a major cytoplasmic serine/threonine phosphatase that plays an important role in the regulation of cell growth and a diverse set of cellular proteins, including metabolic enzymes, ion channels, hormone receptors, and kinase cascades (14, 30, 50). It is ubiquitously expressed and accounts for a significant fraction of serine/threonine phosphatase activity in most tissue and cell types (14, 30, 50).
Native PP2A exists primarily as a heterotrimer composed of a 36-kDa catalytic subunit (C), a 64-kDa scaffolding subunit (A or PR65), and one of a variety of regulatory subunits (B or R) (14, 30, 50). The C subunit is always associated with the A subunit. A number of variable B subunits can bind to this dimeric core (16, 21, 29), and these B subunits affect enzymatic activity and substrate specificity of PP2A (25, 28, 34, 40). Thus, PP2A heterotrimers, containing different B subunits, have different specific activities and target different protein substrates (25, 28, 34, 40). In this way, different holoenzyme assemblies can dephosphorylate distinct substrates in different cellular compartments, and the diverse functions of PP2A in cellular signaling are likely due to the presence of an assortment of holoenzyme assemblies.
Interestingly, PP2A is a target for proteins expressed by simian virus 40 (SV40), including small t antigen (small t) (31, 41). Small t associates with intracellular PP2A during SV40 infection (31, 41), and PP2A is the only cellular protein known to associate with SV40 small t. It has been reported that small t and the B subunit of PP2A bind to the same region of the A subunit; thus, small t associates with PP2A by displacing the cellular B subunits, resulting in inhibition of phosphatase activity (27, 59). Although small t is not transforming by itself, efficient transformation of quiescent cells requires the expression of small t, as well as large tumor antigen, the major transforming protein of SV40 (7, 26). Small t appears to have a mitogenic role during transformation by SV40 (48). Consistent with this idea, overexpression of SV40 small t in mammalian cells activates growth factor-stimulated signaling pathways involving phosphatidylinositol (PI) 3-kinase (53), protein kinase Cζ (PKCζ) (53) and mitogen-activated protein (MAP) kinase (51, 53).
Regulation of PP2A also involves association with other proteins in addition to the core phosphatase subunits (14, 30, 50). Several molecules can form stable complexes with PP2A, including protein kinases, cytoskeletal and/or structural proteins, and others (14, 30, 50) and, in these complexes, PP2A can modulate the phosphorylation state and function of the associated proteins (1, 30, 33, 42, 50, 52, 58). This indicates that the assembly of protein-protein complexes is a general mechanism for regulation of PP2A action and that a likely determinant for directing PP2A function is its association with other proteins in multiprotein signaling assemblies.
Numerous observations suggest that PP2A plays an important role in downregulation of the Ras/MAP kinase pathway (30, 50). PP2A can dephosphorylate and inactivate mitogen-activated extracellular-signal-regulated kinase kinase (MEK) and MAP kinase in vitro (3, 51), while an inhibitor of PP2A, okadaic acid, activates these enzymes (19, 49). Transient expression of SV40 small t activates MEK and MAP kinase in a PP2A-dependent fashion (51, 53), but the precise role of PP2A in the Ras/MAP kinase pathway is unresolved. Recently, several observations have suggested that PP2A is involved in insulin signal transduction. Insulin stimulates phosphorylation and inactivation of PP2A in vitro (10) and in vivo (5, 54), and tumor necrosis factor alpha-induced insulin resistance is accompanied by an elevation in PP2A activity (6). However, the precise role of PP2A in insulin signaling is unknown.
In the present study, we assessed the role of PP2A in growth factor stimulation of the Ras/MAP kinase pathway. The results indicate that in the basal state, PP2A associates with Shc through the Shc phosphotyrosine-binding domain (PTB domain), and that insulin, insulin-like growth factor 1 (IGF-1), or epidermal growth factor (EGF) treatment or small t expression causes dissociation of this complex with enhanced Shc phosphorylation. Thus, growth factor-stimulated Shc phosphorylation and downstream signaling were increased in small-t-expressing cells. As such, the data suggest that PP2A can negatively regulate growth factor signaling by binding to Shc preventing its phosphorylation. These results provide an additional level of control for Shc phosphorylation and a new mechanism for PP2A downregulation of the Ras/MAP kinase pathway.
MATERIALS AND METHODS
Materials.
Porcine insulin was kindly provided by Eli Lilly (Indianapolis, Ind.). Anti-small t antigen antibody was from BD PharMingen (San Diego, Calif.). Phospho-specific MAP kinase antibody, phospho-specific MEK antibody, anti-MEK antibody, and anti-Akt antibody were from New England Biolabs (Beverly, Mass.). Polyclonal anti-Shc antibody, anti-Grb2 antibody, anti-Raf-1 antibody, polyclonal anti-PP2A C-subunit antibody, and antiphosphotyrosine antibody (4G10) were from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Anti-PP2A A-subunit antibody; anti-PP1 antibody, anti-FLAG antibody, anti-hemagglutinin (anti-HA) antibody, anti-glutathione S-transferase (anti-GST) antibody, horseradish peroxidase (HRP)-linked anti-rabbit antibody, HRP-linked anti-mouse antibody, HRP-linked anti-goat antibody, and protein G-agarose were all from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-ERK-1 antibody, anti-Ras antibody, monoclonal anti-Shc antibody, and monoclonal anti-PP2A antibody were from Transduction Laboratories (Lexington, Ky.). Bromodeoxyuridine (BrdU) and glutathione-Sepharose beads were from Amersham Pharmacia Biotech (Piscataway, N.J.). Anti-BrdU antibody was from Oncogene Research Products (Cambridge, Mass.). Rhodamine-conjugated anti-mouse mouse immunoglobulin G antibody was from Jackson Immunoresearch Laboratories, Inc. (West Grove, Pa.). Okadaic acid and hygromycin B were from Calbiochem (San Diego, Calif.). SuperFECT was from Qiagen (Valencia, Calif.). Dulbecco modified Eagle medium and fetal calf serum were obtained from Life Technologies (Grand Island, N.Y.). [γ-32P]ATP was obtained from ICN (Costa Mesa, Calif.). XAR-5 film was obtained from Eastman-Kodak (Rochester, N.Y.). All other reagents and chemicals were purchased from Sigma (St. Louis, Mo.).
Generation of stable cell lines.
Rat-1 fibroblasts overexpressing human insulin receptors (HIRc B) were maintained as described previously (39). A plasmid encoding small t antigen, pCMV5-small t (51, 53), was a gift from Marc C. Mumby (University of Texas Southwestern Medical Center, Dallas). cDNA from pCMV5-small t was digested with HindIII and BamHI and subcloned into pcDNA3.1/Hygro (Invitrogen, Carlsbad, Calif.), which contains the hygromycin B phosphotransferase gene. The resultant plasmid, pcDNA3.1/Hygro-small t, was transfected into HIRc B cells by using SuperFECT according to the manufacturer's instructions. Stable cell lines were selected in 400 μg of hygromycin B/ml. Clonal cell lines were isolated by limiting dilution and then screened by immunoblotting with anti-small t antibody. Parental cells transfected with pcDNA3.1/Hygro alone (Hyg cells) were used as a control in our study.
Preparation of whole-cell lysates and immunoprecipitation.
Cells were lysed in solubilizing buffer (20 mM Tris, pH 7.5; 1 mM EDTA; 140 mM NaCl; 1% Nonidet P-40; 1 mM sodium vanadate; 50 mM sodium fluoride; 50 U of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride) for 30 min at 4°C. The cell lysates were centrifuged to remove insoluble material. For immunoprecipitaton, cell lysates were incubated with primary antibody for 6 h at 4°C and protein G-agarose for an additional 2 h. The immunoprecipitates were washed three times with solubilizing buffer, resuspended in Laemmli sample buffer containing 100 mM dithiothreitol, and heated for 5 min at 100°C.
Immunoblotting.
Whole-cell lysates and antibody immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon-P; Immobilon, Bedford, Mass.). Membranes were blocked and probed with specified antibodies. Blots were then incubated with HRP-linked second antibody, followed by chemiluminescence detection, according to the manufacturer's instructions (Pierce).
Ras activation assay.
Ras activity was determined by the method of Taylor and Shalloway (56). A cDNA encoding for a GST Ras-binding domain (RBD) fusion protein (GST-RBD) was kindly provided by Jeffrey E. Pessin (University of Iowa, Iowa City). Briefly, cells were starved for 24 h, stimulated with insulin for 3 min, with IGF-1 for 3 min, or with EGF for 1.5 min at 37°C and then lysed in lysis buffer (25 mM HEPES, pH 7.5; 1 mM EDTA; 150 mM NaCl; 10% glycerol; 1% Nonidet P-40; 0.25% deoxycholate; 25 mM sodium fluoride; 10 mM magnesium chloride; 1 mM sodium vanadate; 1 μg of leupeptin/ml, 50 U of aprotinin/ml; 1 mM phenylmethylsulfonyl fluoride). Clarified supernatants were incubated with the GST-RBD bound to glutathione-Sepharose beads (20 μg) for 1 h at 4°C. The Sepharose beads were washed three times with lysis buffer and analyzed by Western blotting with anti-Ras antibody.
Raf kinase assay.
Raf protein kinase activity was determined by an immunocomplex protein kinase cascade assay with exogenous substrates according to the manufacturer's specifications (Upstate Biotechnology, Inc.) as described elsewhere (18). Briefly, cells were starved for 24 h, stimulated with insulin for 3 min, with IGF-1 for 3 min, or with EGF for 1.5 min at 37°C and then lysed in lysis buffer (20 mM Tris, pH 7.5; 1 mM EDTA; 1 mM EGTA; 150 mM NaCl; 1% Triton X-100; 2.5 mM sodium pyrophosphate; 1 mM 2-glycerolphosphate; 1 mM sodium vanadate; 1 μg of leupeptin/ml; 50 U of aprotinin/ml; 1 mM phenylmethylsulfonyl fluoride). Clarified supernatants were incubated with anti-Raf antibody and protein G-Sepharose or with protein G-Sepharose alone for 2 h at 4°C. Samples were washed three times in lysis buffer and once with kinase dilution buffer (20 mM morpholinepropanesulfonic acid, pH 7.2; 2-glycerolphosphate; 5 mM EGTA; 1 mM sodium vanadate; 1 mM dithiothreitol). The washed precipitates were incubated with inactive fusion proteins GST-MEK1 (0.4 μg), GST-p42 MAP kinase (1.0 μg), 150 μM ATP, and 20 μM magnesium chloride for 30 min at 30°C with gentle agitation, after which a portion of each sample was incubated with 20 μg of myelin basic protein and 10 μCi of [γ-32P]ATP (3,000 Ci/mmol) for 10 min at 30°C with gentle agitation. Samples were transferred onto p81 phosphocellulose squares, washed five times in 0.75% phosphoric acid and once with acetone, and quantitated by liquid scintillation counting.
PP2A and PP1 phosphatase activity.
Phosphatase activity was measured by using para-nitrophenyl phosphate (p-NPP) as the substrate with the Phosphatase Assay Kit (Upstate Biotechnology) as described previously (55). Starved cells were pretreated with or without 1 μM okadaic acid for 40 min at 37°C, stimulated with 100 ng of insulin/ml for 20 min, 100 ng of IGF-1/ml for 20 min, or 10 ng of EGF/ml for 15 min at 37°C and then lysed in lysis buffer (50 mM HEPES, pH 7.5; 150 mM NaCl; 1 mM EGTA; 10% glycerol; 1.5 mM magnesium chloride; 1% Triton X-100; 1 μg of leupeptin/ml; 50 U of aprotinin/ml; 1 mM phenylmethylsulfonyl fluoride). Clarified supernatants were incubated with anti-PP2A antibody or anti-PP1 antibody and protein G-agarose for 2 h at 4°C. The immunoprecipitates were washed twice with lysis buffer and once with assay buffer (50 mM Tris, pH 7.0; 0.1 mM calcium chloride), resuspended in assay buffer containing 2.5 mM nickel chloride and 900 μg of p-NPP/ml, and incubated for 30 min at 30°C. The amount of para-nitrophenol produced was determined by measuring the absorbance at 405 nm.
Association of PP2A with GST fusion proteins.
The plasmid encoding the GST-SH2 (residues 378 to 471 of p52Shc) was obtained from Alan R. Saltiel (University of Michigan, Ann Arbor) and prepared as described previously (39). Plasmids encoding the GST-PTB (residues 1 to 215 of p52Shc) and GST-392 (residues 1 to 392 of p52Shc) were also prepared. Fusion proteins coupled with glutathione-Sepharose beads were incubated with cell lysates, and the precipitates were washed extensively and analyzed by Western blotting with anti-PP2A antibody.
Transient transfections.
The FLAG-tagged Shc expression vector, pRK5 Shc, was a generous gift from Edward Y. Skolnik (Skirball Institute, New York, N.Y.). Mutant Shc cDNA Y239/240F (tyrosines 239 and 240 to phenylalanines), Y317F (tyrosine 317 to phenylalanine), 3Y/F (all three tyrosines to phenylalanines), and S154P (serine 154 to proline) were generated by PCR with a mutagenic oligonucleotide and subcloned into pRK5 as previously described (38). The HA-tagged ERK 2 expression vector, pcDNA3 HA-ERK2 (15), was a gift from J. Silvio Gutkind (National Institutes of Health, Bethesda, Md.). Transient transfection into HIRc B cells was performed with SuperFECT in accordance with the manufacturer's instructions. After transfection, cells were allowed to grow for 24 h and then were serum starved for 24 h before the experiments as previously described (38).
BrdU incorporation.
Cells were grown on glass coverslips and rendered quiescent by starvation for 24 h. Serum-starved cells were incubated with BrdU plus various concentrations of insulin, IGF-1, or EGF for 16 h at 37°C. The cells were fixed in 3.7% formaldehyde in phosphate-buffered saline for 20 min and incubated with mouse monoclonal anti-BrdU antibody for 1 h. The cells were then stained by incubation with rhodamine-labeled donkey anti-mouse immunoglobulin G antibody for 1 h. After the coverslips were mounted, the cells were analyzed with an Axiophot fluorescence microscope (Carl Zeiss, Thornwood, N.Y.).
Statistics.
The unpaired Student's t test was used to determine the significance of any differences between two groups. A P value of <0.05 was considered significant.
RESULTS
Expression of small t in HIRc B cells and the association of small t with PP2A.
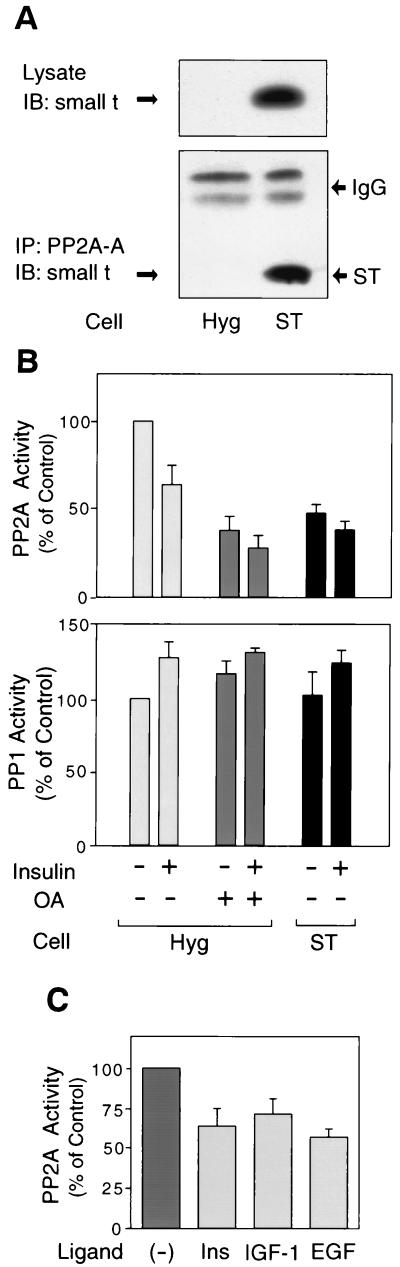
We transfected a small t expression plasmid into HIRc B cells to establish stable cell lines overexpressing small t (ST cells), as seen in Fig. 1A (upper panel). Parental cells transfected with pcDNA3.1/Hygro alone (Hyg cells) were used as controls. It has been reported that small t associates with the A subunit of PP2A, which interferes with the binding of the A subunit to the B subunit (27, 59). Thus, we examined whether expressed small t was associated with the endogenous PP2A A subunit. Cells were immunoprecipitated with antibody against the A subunit of PP2A (PP2A-A) and immunoblotted with anti-small t antibody. As shown in Fig. 1A (lower panel), small t was coimmunoprecipitated with anti-PP2A-A antibody in ST cells.
FIG. 1.
Expression of small t inhibits PP2A activity. (A) Control cells (Hyg) and cell lines overexpressing small t (ST) were lysed, and whole-cell lysates were analyzed by Western blotting with anti-small t antibody (upper panel). The same cell lysates were immunoprecipitated with antibody against the A subunit of PP2A, followed by immunoblotting with anti-small t antibody (lower panel). (B) Starved cells were pretreated with or without 1 μM okadaic acid (OA) for 40 min and then stimulated with 100 ng of insulin/ml for 20 min. Whole-cell lysates were prepared and assayed for PP2A activity (upper panel) and PP1 activity (lower panel) as described in Materials and Methods. (C) PP2A activity was assayed in cells stimulated with 100 ng of insulin/ml for 20 min, 100 ng of IGF-1/ml for 20 min or 10 ng of EGF/ml for 15 min. Data are presented as the percentage of phosphatase activity compared to unstimulated control cells and represent the mean ± the standard error (SE) of four independent experiments.
To evaluate the effect of small t expression on endogenous PP2A function, we measured PP2A activity by using p-NPP as the substrate. As a control to assess the specificity of any effects on PP2A, we also measured the activity of PP1, another major serine/threonine phosphatase. Cells were pretreated with or without 1 μM okadaic acid for 40 min and then stimulated with insulin, followed by immunoprecipitation with antibody against the catalytic subunit of PP2A (PP2A-C) or anti-PP1 antibody. It has been reported that okadaic acid is a specific inhibitor of PP2A at this concentration (17, 37). Consistent with this, pretreatment of Hyg cells with okadaic acid inhibited PP2A activity by 60% but did not affect PP1 activity (Fig. 1B). PP2A activity in ST cells was inhibited by ∼50% compared with Hyg cells, whereas PP1 activity was not affected (Fig. 1B). These results indicate that the expression of small t specifically inhibits PP2A activity. PP2A activity was inhibited after insulin, EGF, or IGF-1 stimulation (Fig. 1C), whereas insulin-stimulated PP1 activity was slightly increased (Fig. 1B).
Insulin-, IGF-1-, and EGF-stimulated MEK and MAP kinase phosphorylation are enhanced in small-t-expressing cells.
It has been reported that small t expression can augment MEK and MAP kinase activity and promote cell proliferation (51, 53). To assess this in our system, we measured MEK and MAP kinase phosphorylation in response to insulin, IGF-1, and EGF in ST cells. Cell lysates were analyzed by Western blotting with phospho-specific MEK antibody or phospho-specific MAP kinase antibody. Basal MEK and MAP kinase phosphorylation were unaffected by small t expression, and the levels of MEK and MAP kinase proteins were also unaltered. In addition, insulin-, IGF-1-, and EGF-stimulated MEK and MAPK phosphorylation were all enhanced by ∼2-fold (data not shown).
Insulin, IGF-1- and EGF-stimulated Ras and Raf-1 kinase activities are enhanced in small-t-expressing cells.
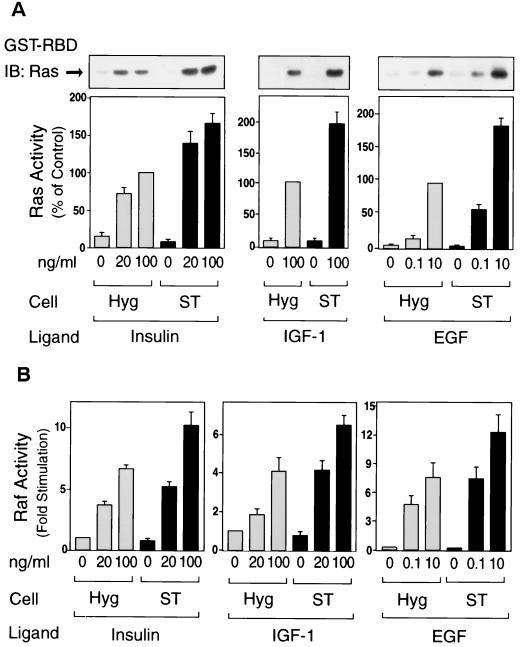
To further investigate the MAP kinase pathway, we assessed the upstream activators Ras and Raf-1 kinase (Fig. 2). To measure Ras activation, we utilized the specificity of the ability of Ras-GTP to bind to the Ras-binding domain of Raf-1 (RBD). This method has been shown to specifically reflect the amount of activated Ras (18, 56). Ras activity was stimulated by insulin, IGF-1, and EGF in a dose-dependent manner, and this effect was enhanced in ST cells, whereas basal Ras activity was unaffected (Fig. 2A). We also measured Raf-1 kinase activity and found similar results (Fig. 2B). Thus, insulin, IGF-1 and EGF all stimulated Raf-1 kinase activity in a dose-dependent manner, and these effects were increased in ST cells. Again, basal Ras and Raf-1 kinase activity (Fig. 2) and the cellular amounts of these proteins (data not shown) were unaffected. Taken together, these results suggest that PP2A acts upstream of Ras to stimulate the MAP kinase cascade.
FIG. 2.
Expression of small t enhances insulin-, IGF-1-, and EGF-stimulated Ras and Raf-1 kinase activity. Starved cells were stimulated with insulin (left panel) for 3 min, with IGF-1 (middle panel) for 3 min, or with EGF (right panel) for 1.5 min. (A) Whole-cell lysates were prepared and precipitated with the GST-RBD fusion protein. The precipitates were then analyzed by Western blotting with anti-Ras antibody as described in Materials and Methods (upper panel). Data are presented as the percentage of Ras activity when compared with maximally stimulated Hyg cells and represent the mean ± SE of three independent experiments. (B) Whole-cell lysates were prepared and assayed for Raf-1 protein kinase activity as described in Materials and Methods. Data are presented as the fold increase in Raf-1 kinase activity compared to unstimulated Hyg cells and represent the mean ± SE of four independent experiments.
Insulin-, IGF-1-, and EGF-stimulated tyrosine phosphorylation of Shc and its association with Grb2 are enhanced in small-t-expressing cells.
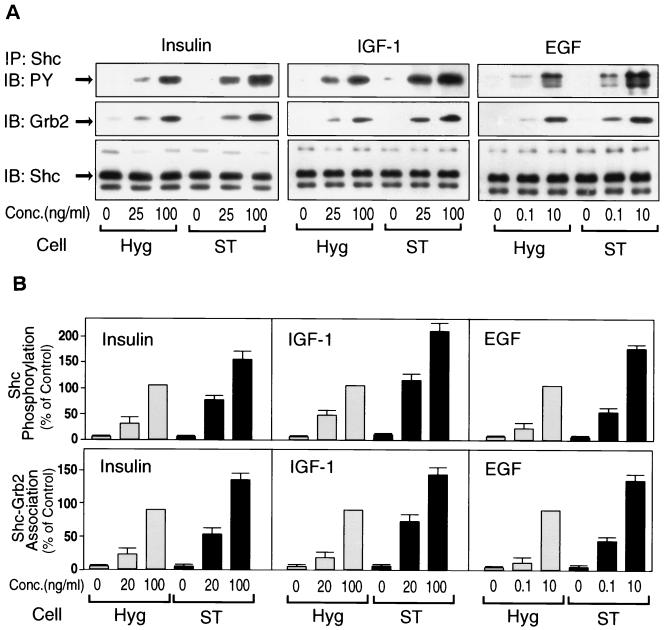
A major mechanism for growth factor stimulation of Ras activation involves tyrosine phosphorylation of the adapter protein Shc and subsequent recruitment of the Grb2-SOS complex. Thus, we examined tyrosine phosphorylation of Shc and its association with Grb2 in ST cells. Figure 3 shows that insulin stimulation caused Shc tyrosine phosphorylation and its association with Grb2, and this effect of insulin was enhanced in ST cells (left panel), whereas Shc protein expression was not altered (Fig. 3A, lower panel). Comparable results were observed for IGF-1 and EGF stimulation (Fig. 3A, middle and right panels). Figure 3B represents the graphical summary of four such experiments. Comparable results were observed in an additional ST clonal line.
FIG. 3.
Expression of small t enhances insulin-, IGF-1-, and EGF-stimulated tyrosine phosphorylation of Shc and its association with Grb2. (A) Starved cells were stimulated with insulin (left panel), IGF-1 (middle panel), or EGF (right panel) for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-Shc antibody, followed by immunoblotting with anti-phosphotyrosine antibody (upper panel), anti-Grb2 antibody (middle panel), or anti-Shc antibody (lower panel). (B) Data are expressed as the percentage of Shc phosphorylation levels (upper panel), and its association with Grb2 (lower panel) compared with maximally stimulated Hyg cells; the results represent the mean ± SE of four independent experiments.
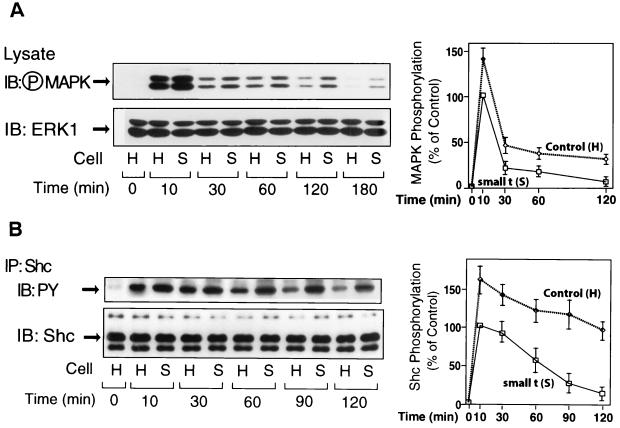
We also examined the time course of Shc and MAP kinase phosphorylation after insulin stimulation (Fig. 4). Phosphorylation of these proteins is maximal by 10 min, declining thereafter, and the levels of phosphorylation are greater in small t cells at all time points. Thus, activation of Shc and MAP kinase is both greater and more prolonged when PP2A function is inhibited by small t expression.
FIG. 4.
Expression of small t prolongs insulin-induced phosphorylation of MAP kinase and Shc. (A) Control cells (H) and cell lines overexpressing small t (S) were stimulated with insulin (100 ng/ml) for the indicated periods of time. Whole-cell lysates were analyzed by Western blotting with phospho-MAP kinase antibody (left upper panel) or anti-ERK1 antibody (left lower panel). (B) The same cell lysates were immunoprecipitated with anti-Shc antibody, followed by immunoblotting with anti-phosphotyrosine (PY) antibody (left upper panel) or anti-Shc antibody (left lower panel). Data are presented as the percentage of MAPK phosphorylation (⋄) (A, right panel) or of Shc phosphorylation (⋄) (B, right panel) compared to maximally stimulated control (□) cells and represent the mean ± SE of three independent experiments.
Small t expression did not affect tyrosine phosphorylation of the insulin, IGF-1, or EGF receptors (data not shown) and, therefore, the effect of small t expression to augment stimulation of the Ras/MAP kinase pathway appears to be at the step of Shc phosphorylation but downstream of the receptor.
Effect of transient expression of small t antigen on Shc and MAP kinase phosphorylation.
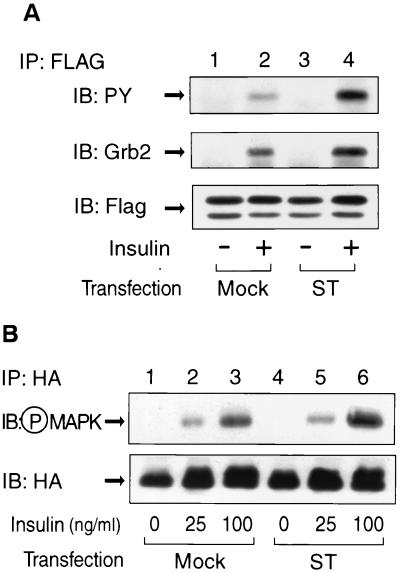
To assess the effect of PP2A on Shc phosphorylation, by using a different experimental approach, we coexpressed a pRK5 vector encoding FLAG-tagged Shc along with either pCMV5 vector encoding small t or a control empty vector in HIRc B cells. Cells were starved and stimulated with insulin and then immunoprecipitated with anti-FLAG antibody. The immunoprecipitates were analyzed by Western blotting with anti-phosphotyrosine antibody (Fig. 5A, upper panel) or anti-Grb2 antibody (Fig. 5A, middle panel). The expression of Flag-tagged Shc was comparable in all conditions, whereas insulin-stimulated FLAG-tagged Shc phosphorylation, as well as its association with Grb2, were enhanced in the small-t-transfected cells. In similar experiments shown in Fig. 5B, HA-tagged ERK2 was cotransfected with small t antigen, and insulin-stimulated MAP kinase phosphorylation was enhanced in the small-t-transfected cells.
FIG. 5.
Transient expression of small t enhances insulin-stimulated tyrosine phosphorylation of Shc, its association with Grb2 and MAP kinase phosphorylation. (A) pRK5 vector encoding FLAG-tagged Shc was transiently cotransfected with either pCMV5 vector encoding small t (ST, lanes 3 and 4) or control vector (mock, lanes 1 and 2) in HIRc B cells as described in Materials and Methods. Cells were starved and stimulated with 100 ng of insulin/ml (lanes 2 and 4) for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-FLAG antibody, followed by immunoblotting with anti-phosphotyrosine antibody (upper panel), anti-Grb2 antibody (middle panel), or anti-FLAG antibody (lower panel). (B) pcDNA3 vector encoding HA-tagged ERK2 was transiently cotransfected with either pCMV5 vector encoding small t (ST, lanes 4 to 6) or control vector (mock, lanes 1 to 3) in HIRc B cells. Cells were starved and stimulated with insulin for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-HA antibody, followed by immunoblotting with anti-phospho-MAP kinase antibody (upper panel) or anti-HA antibody (lower panel).
Insulin-, IGF-1-, and EGF-stimulated BrdU incorporation is enhanced in small-t-expressing cells.
An result of activation of the Ras/MAP kinase pathway is mitogenesis. To assess this event, we measured DNA synthesis by monitoring BrdU incorporation in Hyg and small-t-expressing cells in the basal state and after insulin, IGF-1, or EGF stimulation for 18 h. These data are summarized in Table 1. Basal BrdU labeling was unaffected by small t expression, whereas the stimulatory effects of all three growth factors were enhanced.
TABLE 1.
Expression of small t enhances insulin-, IGF-1-, and EGF-induced BrdU incorporationa
| Ligand | Concn (ng/ml) | Mean BrdU incorporation (%)b ± SE
|
|
|---|---|---|---|
| Hyg | Small t | ||
| Basal | 12.8 ± 1.1 | 13.1 ± 2.0 | |
| Insulin | 20 | 30.0 ± 5.2 | 39.5 ± 2.3* |
| 100 | 51.7 ± 5.3 | 62.3 ± 3.8** | |
| IGF-1 | 20 | 26.0 ± 3.6 | 40.5 ± 3.0** |
| 100 | 44.0 ± 3.7 | 54.0 ± 2.5 | |
| EGF | 0.1 | 29.1 ± 3.1 | 40.5 ± 4.0* |
| 10 | 44.1 ± 3.0 | 56.7 ± 4.7* | |
Cells were starved for 24 h and then incubated with insulin, IGF-1, or EGF for 16 h. BrdU incorporation was assayed as described in Materials and Methods. Data are expressed as the percentage of BrdU incorporation into cells and represent the mean ± the standard error of four independent experiments.
*, P < 0.05; **, P < 0.01 compared to Hyg cells.
Association of endogenous PP2A with Shc.
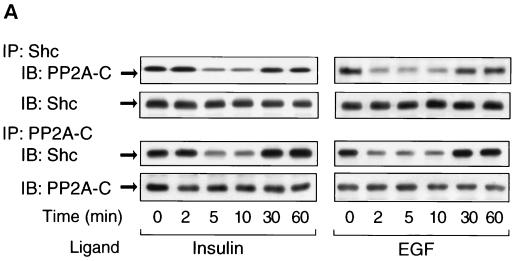
The present data show that inhibition of PP2A by small t expression can enhance Shc phosphorylation, suggesting the involvement of PP2A in Shc function. Since PP2A can form complexes with other proteins (14, 30, 50), we determined whether endogenous PP2A could associate in a protein complex with Shc in the presence or absence of growth factor stimulation (Fig. 6). Thus, cells were stimulated with insulin or EGF for the indicated times and immunoprecipitated with anti-Shc antibody. The immunoprecipitates were then analyzed by Western blotting with anti-PP2A-C antibody. As shown in Fig. 6A, PP2A coprecipitates with Shc in the basal state, and the magnitude of this association was decreased by 50 to 80% in the early time points after insulin or EGF stimulation, with full reassociation at 30 and 60 min. The same results were observed when the coimmunoprecipitation was performed with anti-PP2A-C antibody, followed by anti-Shc antibody immunoblotting.
FIG. 6.
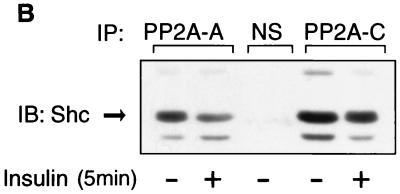
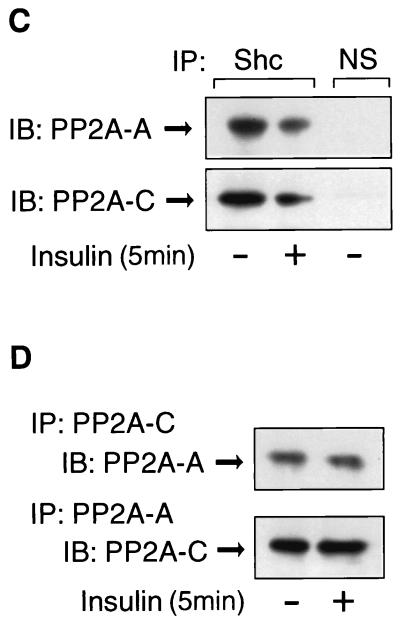
PP2A associates with Shc and dissociates upon insulin or EGF stimulation. (A) HIRc B cells were starved and stimulated with 100 ng of insulin/ml (left panel) or 10 ng of EGF/ml (right panel) for the indicated times. Whole-cell lysates were prepared and immunoprecipitated with anti-Shc antibody, followed by immunoblotting with anti-PP2A-C antibody (top panel) or anti-Shc antibody (second panel). The same lysates were immunoprecipitated with anti-PP2A-C antibody, followed by immunoblotting with anti-Shc antibody (third panel) or anti-PP2A-C antibody (bottom panel). (B to D) Cells were stimulated with 100 ng of insulin/ml for 5 min and immunoprecipitated with anti-Shc antibody, anti-PP2A-A antibody, anti-PP2A-C antibody, or nonimmune sera (NS). The immnoprecipitates were analyzed by Western blotting with anti-Shc antibody (B), anti-PP2A-A antibody (C and D, upper panel), or anti-PP2A-C antibody (C and D, lower panel).
To further verify the interaction between Shc and the PP2A heterodimer, Fig. 6B shows that Shc was coimmunoprecipitated with both anti-PP2A-A and anti-PP2A-C antibody and that these associations were decreased after insulin stimulation. Furthermore, both the A and C subunits of PP2A were detected in anti-Shc antibody immunoprecipitates, and this was decreased after insulin stimulation (Fig. 6C). Finally, the A and C subunits form stable complexes with each other that are unaffected by insulin treatment (Fig. 6D). Quantitation of the immunoprecipitation results show that the PP2A antibody precipitates ∼70% of cellular PP2A, and the Shc antibody precipitates 100% of cellular Shc. In addition, ∼30% of total cellular PP2A coprecipitates with Shc in the Shc antibody precipitates (data not shown).
In these various experiments we detected no signals in nonimmune precipitates, and we could not observe association of PP2A with insulin receptor substrate 1, another insulin-induced tyrosine phosphorylated protein (data not shown). All of these findings are consistent with a direct association between PP2A and Shc, which is modified by growth factor stimulation, a finding consistent with a role for PP2A in the control of Shc function.
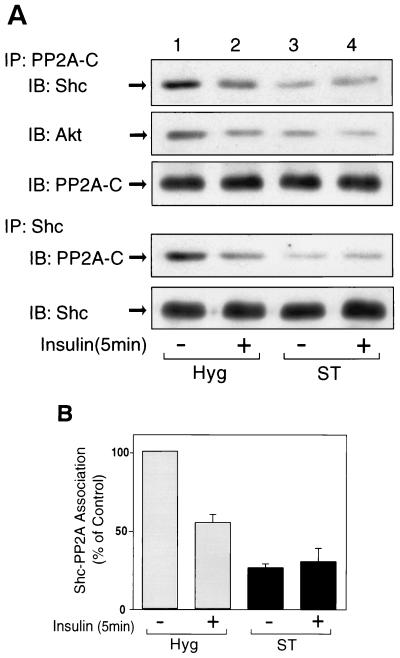
The association of PP2A with Shc is inhibited in small-t-expressing cells.
Since small t interferes with PP2A function by replacing the B subunit, which directs PP2A to its protein targets, and since PP2A associates with Shc, we next examined the effect of small t expression on the association between PP2A and Shc (Fig. 7). In control Hyg cells, PP2A associated with Shc in the basal state, and this was decreased by ∼50% after insulin stimulation (Fig. 7A, lanes 1 and 2). In contrast, in the small-t-expressing cells, the basal association was decreased by 60% and insulin had no further effect (Fig. 7A, lanes 3 and 4), a finding consistent with the idea that small t displaces the PP2A regulatory B subunit, inhibiting the ability of PP2A to associate with its cellular target (27, 59). Consistent with this, we found that in Hyg cells abundant PP2A B subunit was detected in PP2A C subunit precipitates, whereas no B subunit signal was observed in C subunit precipitates from small t cells, demonstrating that small t displaces the B subunit from the PP2A complex (data not shown). It has been reported that Akt associates with PP2A and is dephosphorylated by PP2A (4, 30). Along these lines, Akt was coprecipitated with anti-PP2A antibody, and this was also decreased by insulin stimulation and small t expression (Fig. 7A, second panel).
FIG. 7.
Expression of small t decreases the association of PP2A with Shc. (A) Hyg cells (lanes 1 and 2) and ST cells (lanes 3 and 4) were starved and stimulated with 100 ng of insulin/ml for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-PP2A-C antibody, followed by immunoblotting with anti-Shc antibody (top panel), anti-Akt antibody (second panel), and PP2A-C antibody (third panel). The same cell lysates were immunoprecipitated with anti-Shc antibody, followed by immunoblotting with anti-PP2A-C antibody (fourth panel) or anti-Shc antibody (bottom panel). (B) Data are presented as the percentage of the association of Shc with PP2A compared to unstimulated Hyg cells and represent the mean ± SE of four independent experiments.
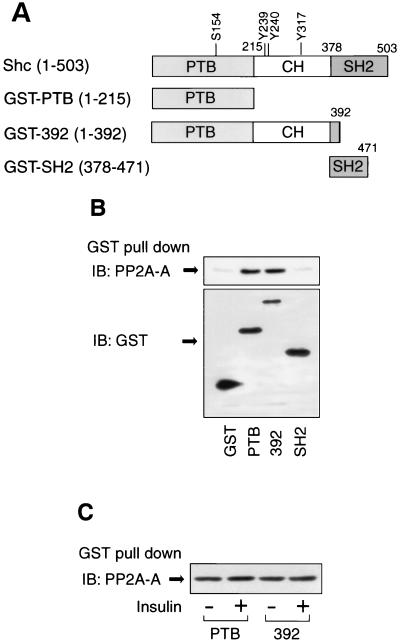
PP2A associates with the PTB domain of Shc.
To determine whether PP2A interacts with Shc directly and with which domain of Shc, we examined the association of PP2A with GST fusion proteins containing Shc domains as outlined in Fig. 8A. PP2A was precipitated with GST-PTB and GST-392, but not with GST alone or GST-SH2 (Fig. 8B), indicating that PP2A directly associates with Shc via the Shc PTB domain. Although PP2A can undergo tyrosine phosphorylation after growth factor stimulation (5, 10, 11, 54), these experiments were done in basal unstimulated cells so that PP2A exists in a relatively nonphosphorylated state. Therefore, it is unlikely that the role of the PTB domain in the Shc PP2A interaction is related to the ability of this domain to bind to phosphotyrosine residues.
FIG. 8.
Association of PP2A with GST fusion proteins containing Shc domains. (A) Schematic of Shc and constructs of GST fusion proteins. (B) PP2A associates with the PTB domain of Shc. Cell lysates from HIRc B cells were incubated with the indicated GST fusion proteins, and the precipitates were analyzed by Western blotting with anti-PP2A-A antibody as described in Materials and Methods (upper panel). The expression of GST fusion proteins were determined by Western blotting with anti-GST antibody (lower panel). (C) Cells were stimulated with or without 100 ng of insulin/ml for 5 min, and cell lysates were incubated with GST-PTB or GST-392; the precipitates were analyzed by Western blotting with anti-PP2A-A antibody.
Next, we compared the interactions between the Shc GST fusion proteins and PP2A in unstimulated and insulin-stimulated cells. As shown Fig. 8C, equal amounts of PP2A were precipitated with GST-PTB and GST-392, before or after insulin treatment, indicating that the dissociation of endogenous PP2A from Shc after insulin stimulation observed in vivo (Fig. 6 and 7) is due to the effects of insulin on Shc rather than on PP2A. Since, the GST-PTB precipitated PP2A equally from basal and insulin treated cells, it is mostly likely that the PTB domain interaction with PP2A does not involve phosphotyrosine binding.
Phosphorylation of tyrosine 317 of Shc is required for the dissociation of PP2A from Shc.
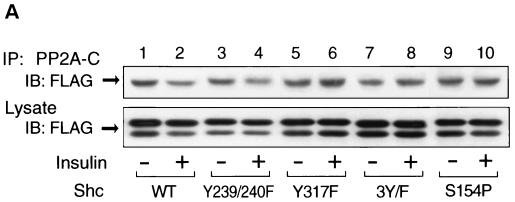
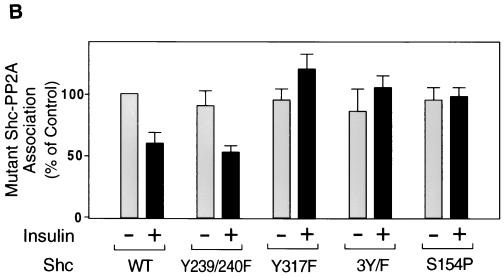
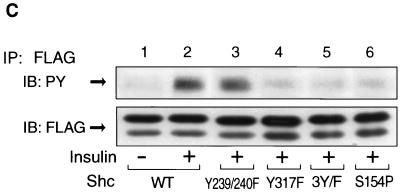
To address the role of tyrosine phosphorylation of Shc on the interaction with PP2A, we prepared Shc constructs with mutations at the various tyrosine residues and examined the interaction of these Shc mutants with PP2A. For this analysis, we utilized FLAG-tagged Shc constructs with mutations in tyrosine residues Y239/240F (tyrosines 239 and 240 to phenylalanines), Y317F (tyrosine 317, which is the Grb2 binding site [24, 43], to phenylalanine), and 3Y/F (all three tryrosines to phenylalanines). Each mutant Shc was transiently expressed in HIRc B cells. Starved cells were stimulated with insulin for 5 min and then immunoprecipitated with anti-PP2A-C antibody, followed by immunoblotting with anti-FLAG antibody. As shown in Fig. 9A, PP2A associated with all of the Shc constructs in the basal state. However, after insulin stimulation, PP2A dissociated from wild-type and Y239/240F Shc but did not dissociate from Y317F or 3Y/F. The expression levels of FLAG-tagged Shc were comparable (Fig. 9A, lower panel). Wild type and Y239/240F were tyrosine phosphorylated in response to insulin, whereas we could not observe tyrosine phosphorylation of Y317F or 3Y/F (Fig. 9C). These results indicate that the phosphorylation of tyrosine 317 of Shc is required for dissociation of Shc from PP2A.
FIG. 9.
Phosphorylation of tyrosine 317 is required for the dissociation of PP2A from Shc. (A) FLAG-tagged wild-type Shc (lanes 1 and 2), Y239/240F Shc (lanes 3 and 4), Y317F Shc (lanes 5 and 6), 3Y/F Shc (lanes 7 and 8), or S154P Shc (lanes 9 and 10) were transiently expressed in HIRc B cells as described in Materials and Methods. Cells were starved and stimulated with 100 ng of insulin/ml (lanes 2, 4, 6, 8, and 10) for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-PP2A-C antibody, followed by immunoblotting with anti-FLAG antibody (upper panel). The same lysates were analyzed by Western blotting with anti-FLAG antibody (lower panel). (B) Data are expressed as the percentage of the Shc-PP2A association levels compared to starved cells transfected with wild-type Shc and represent the mean ± SE of three independent experiments. (C) Phosphorylation of Shc mutants. The indicated Shc mutants were transiently expressed in HIRc B cells. Cells were starved and stimulated with 100 ng of insulin/ml (lanes 2 to 6) for 5 min. Whole-cell lysates were prepared and immunoprecipitated with anti-FLAG antibody, followed by immunoblotting with antiphosphotyrosine antibody (upper panel) or anti-FLAG antibody (lower panel).
In addition, to further examine the role of the PTB domain in the association with PP2A, we also constructed S154P (serine 154 to proline), which ablates the ability of the PTB domain to bind phosphotyrosines (38). As can be seen in Fig. 9A and B (lanes 9 and 10), S154P Shc binds to endogenous PP2A in the basal state. Insulin treatment did not decrease this association, and this is explained because this Shc protein with a disabled PTB domain cannot be phosphorylated after insulin stimulation (Fig. 9C, lane 6). These results also verify the idea that the ability of the Shc PTB domain to associate with PP2A does not involve the phosphotyrosine binding function of this domain.
DISCUSSION
The adapter protein Shc is a key signaling molecule transducing information from receptor tyrosine kinases to the Ras/MAP kinase pathway with subsequent mitogenic activation (35, 36, 44). In the present study, we describe a novel mechanism for Shc regulation involving the multimeric serine/threonine phosphatase PP2A (14, 30, 50). We find that PP2A forms a molecular complex with Shc, by interacting with the Shc PTB domain in a manner which does not rely on phosphotyrosine binding. Growth factor stimulation leads to phosphorylation of Shc, which then causes dissociation of the PP2A-Shc complex. Tyrosine phosphorylation at residue 317 of Shc protein appears to be critical for this event. Small t antigen expression facilitates mitogenesis and cell transformation (7, 13, 31, 48), and previous data indicate that small t antigen binds to PP2A by displacing the regulatory subunit that normally targets PP2A to its protein partners (27, 59). We find that expression of small t results in enhanced growth factor stimulation of Shc phosphorylation, leading to greater downstream activation of the Ras/MAP kinase pathway and higher rates of growth factor-stimulated mitogenesis. As such, these results indicate that PP2A can serve as an endogenous regulator of growth factor-mediated Shc phosphorylation. Furthermore, our results indicate that the interaction of small t with PP2A provides a new mechanism to help explain the mitogenic effects of small t antigen.
Several studies have shown that a major function of PP2A is downregulation of the Ras/MAP kinase pathway (2, 3, 22, 30, 50, 51, 53). However, the mechanisms of this effect are controversial (1, 2, 46, 53, 57). In EGF-treated adipocytes or PC12 cells, PP2A and an unidentified tyrosine phosphatase are responsible for MAP kinase inactivation, suggesting that PP2A may directly dephosphorylate MAP kinase (2). On the other hand, in small-t-transfected CV-1 cells, the activation of MEK and MAP kinase by inhibition of PP2A was dependent on PI 3-kinase and PKCζ (53). The role of PP2A in the regulation of Raf-1 is also unclear. Whereas small t did not affect Raf-1 activity in CV-1 cells (51), it has been reported that PP2A can associate with Raf-1 and positively regulates its activity in COS7 cells (1). These disparate results suggest that PP2A can act at multiple points in the Ras/MAP kinase cascade depending on the cell context. Since there are multiple targeting B subunits for PP2A, different forms of this trimeric protein may be responsible for its multiple actions, because each B subunit has different activity and substrate specificity and displays tissue- and cell type-specific distribution (25, 28, 29, 34, 40).
In the present study, we show that PP2A can physically associate with Shc protein in the basal state and that this interaction requires the Shc PTB domain. Since this requirement for the Shc PTB domain is necessary for the interaction of Shc with PP2A in the basal state and since PP2A is not tyrosine phosphorylated under these unstimulated conditions (5, 10, 11, 54), we conclude that the function of the PTB domain to associate with PP2A is independent of the phosphotyrosine-binding properties normally attributed to this domain. This conclusion is supported by the results of the transfection experiments we performed with the Shc S154P mutant, which contains a disabled PTB domain lacking phosphotyrosine-binding activity (38). The results showed that S154P Shc could associate with PP2A as well as wild-type Shc. That the phosphotyrosine binding function was ablated in S154P was shown by the fact that this Shc mutant was unable to bind to the insulin receptor (data not shown) and did not undergo insulin-stimulated tyrosine phosphorylation (Fig. 9C). Our results indicate that this interaction of PP2A with Shc is functionally important, keeping Shc protein in a relatively non-tyrosine-phosphorylated state. Since small t antigen displaces the B subunit of PP2A (27, 59) and since small t inhibited PP2A association with Shc, it is likely that it is the B subunit of PP2A which interacts with the Shc PTB domain. Further studies to determine the specific motifs within the PTB domain which are responsible for PP2A association will be of interest in the further characterization of this new PTB domain function.
The interaction of PP2A with Shc is dynamic, and the data show that stimulation of cells with either insulin, IGF-1, or EGF leads to dissociation of the PP2A-Shc complex. Exogenously expressed Shc can also associate with endogenous PP2A, and formation of this complex is also attenuated after ligand stimulation. By expressing various Shc mutants in these cells, we were able to further probe the determinants of ligand-mediated Shc-PP2A complex dissociation. Since GST fusion proteins containing the Shc PTB domain could bind to endogenous PP2A equally well from basal and insulin-stimulated cells, one can conclude that the dissociation of PP2A from Shc after ligand stimulation was due to a ligand-induced modification of Shc rather than of PP2A. Along these lines, we found that tyrosine phosphorylation of Shc was necessary for the dissociation between these two proteins. Using point mutants created at the three potential Shc phosphorylation sites (tyrosines 239, 240, and 317), we were able to show that Y239/240F Shc underwent tyrosine phosphorylation and PP2A dissociation, whereas Y317F and 3Y/F Shc did not, demonstrating that phosphorylation of tyrosine 317 was the critical event leading to dissociation of Shc from PP2A. Previously, it has been shown that the binding of Shc to the Grb2 SH2 domain is mediated by phosphorylation of tyrosine 317 (47). Consistent with this, Shc Y317F is unable to bind to Grb2, and expression of this mutant Shc leads to inhibition of insulin-stimulated MAP kinase (24) and cell transformation (43). Thus, it can be proposed that phosphorylation of tyrosine 317 alters the tertiary structure of Shc such that association of PP2A with the Shc PTB domain is impaired. Presumably, the dissociation of PP2A would then also enhance the ability of phosphorylated Shc to engage the Grb2 SH2 domain.
The data presented here clearly demonstrate that PP2A inhibits Shc phosphorylation and that the ability of small t antigen to prevent the interaction of PP2A with Shc enhances growth factor-mediated Shc phosphorylation; however, the studies do not elucidate the mechanism whereby PP2A modulates phosphorylation of Shc. Nevertheless, based on our data, several possibilities exist. First, since PP2A binds to non-tyrosine-phosphorylated Shc through the Shc PTB domain, it is possible that the presence of PP2A in this complex causes steric hindrance, inhibiting the access of the tyrosine kinase to Shc. Alternatively, it has been reported that, although PP2A predominantly is a serine/threonine phosphatase, it does possess some tyrosine phosphatase activity (9, 12, 20) and, therefore, it could directly dephosphorylate Shc. Another possibility has to do with the serine/threonine phosphorylation state of Shc itself. Evidence exists that Shc is phosphorylated on serine/threonine residues (8, 36, 45), and these could alter the structure of Shc, making it more accessible as a substrate for tyrosine kinases. In other words, when associated with Shc, PP2A would keep Shc in a relatively unphosphorylated state (on serine/threonine residues), but when not associated with Shc the serine/threonine phosphorylation state would increase, and this might make Shc a better substrate for tyrosine kinases. Finally, the phosphatase activity of PP2A could modify the phosphorylation and activity state of other tyrosine phosphatases and kinases which could directly affect tyrosine phosphorylation of Shc. Future studies to identify the precise mechanisms underlying this effect will be of interest.
Although our studies indicate that it is the effects of growth factors to cause phosphorylation of Shc at position 317, rather than the effects on PP2A, that mediate dissociation of the complex, our data also support the view that growth factor stimulation can directly modulate PP2A. Thus, we find that insulin, IGF-1 and EGF treatment can all inhibit PP2A activity, a finding consistent with previous reports that the C subunit of PP2A can undergo tyrosine phosphorylation mediated by p60v-src, p56lck, EGF receptors, and insulin receptors, in vitro or in vivo (5, 10, 11, 54). It has been proposed that tyrosine phosphorylation of the PP2A C subunit inhibits the catalytic activity of this protein (5, 10, 11, 54), a finding consistent with our results showing inhibition of PP2A activity by growth factor treatment.
Another important aspect of these studies relates to the effects of exogenously expressed small t antigen. We found that small t associated with PP2A, leading to an inhibition of PP2A activity. In small-t-expressing cells, formation of the PP2A Shc-complex was markedly inhibited, and this was associated with enhanced growth factor-stimulated Shc phosphorylation, as well as increased downstream signaling to Ras, Raf-1 kinase, MEK, and MAP kinase and enhanced mitogenesis. These results are consistent with the notion that small t associates with PP2A by displacing the regulatory B subunit. This would then interfere with the ability of PP2A to associate with its cellular protein targets, in this case Shc. This supports the view that Shc is a target of PP2A and that this interaction regulates Shc phosphorylation and downstream mitogenic signaling. Such a formulation is consistent with other reports in the literature. For example, it has been shown that casein kinase 2α can associate with and enhance PP2A activity and that this leads to inhibition of MEK activity (22). Furthermore, it has been shown that expression of small t can enhance MEK and MAP kinase activity (51, 53). Interestingly, some of the effects of small t to enhance MAP kinase activity are dependent on PI 3-kinase and PKCζ (53), suggesting that there may be more than one site of interaction of small t with the Ras/MAP kinase pathway. In any event, our results indicate that the ability of small t to bind to PP2A, displacing it from Shc, is an important mechanism underlying the effect of small t expression to promote mitogenesis and cell transformation.
In summary, our results show that PP2A forms a physical complex with Shc by interacting with the Shc PTB domain in a manner independent of phosphotyrosine binding. Growth factor stimulation leads to dissociation of the PP2A-Shc complex, and this is initiated by phosphorylation of the tyrosine 317 residue of Shc. The dissociation of the Shc-PP2A complex leads to enhanced downstream signaling to the Ras/MAP kinase pathway. We also show that small t antigen associates with PP2A and displaces PP2A from Shc. This relieves the normal inhibitory effect of PP2A on Shc phosphorylation, resulting in enhanced insulin, EGF, and IGF stimulation of Shc phosphorylation with increased signaling to Ras, Raf-1 kinase, MEK, and MAP kinase and enhanced mitogenesis. These studies support the view that endogenous PP2A can negatively regulate the Ras/MAP kinase pathway by binding to Shc and inhibiting Shc tyrosine phosphorylation. In addition, the ability of small t antigen to displace PP2A from Shc provides a molecular mechanism to help explain the transforming and mitogenic potentiation induced by small t expression.
Acknowledgments
We thank Marc C. Mumby, University of Texas Southwestern Medical Center, for providing pCMV5-small t; Jeffrey E. Pessin, University of Iowa, for providing the GST-RBD; Alan R. Saltiel, University of Michigan, for providing GST-SH2; Edward Y. Skolnik, Skirball Institute, for providing pRK5 Shc; J. Silvio Gutkind, National Institutes of Health, for providing pcDNA3 HA-ERK2; and Elizabeth Hansen for editorial assistance.
This work was supported by a research grant from the National Institutes of Health (DK 33651), the Veterans Administration San Diego Healthcare System, Research Service, and the Whittier Diabetes Institute.
REFERENCES
- 1.Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., N. Gomez, G. Moorhead, T. Lewis, S. M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283-295. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, N. G., J. L. Maller, N. K. Tonks, and T. W. Sturgill. 1990. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343:651-653. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. [DOI] [PMC free article] [PubMed]
- 5.Begum, N., and L. Ragolia. 1996. cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J. Biol. Chem. 271:31166-31171. [DOI] [PubMed] [Google Scholar]
- 6.Begum, N., L. Ragolia, and M. Srinivasan. 1996. Effect of tumor necrosis factor-alpha on insulin-stimulated mitogen-activated protein kinase cascade in cultured rat skeletal muscle cells. Eur. J. Biochem. 238:214-220. [DOI] [PubMed] [Google Scholar]
- 7.Bikel, I., X. Montano, M. E. Agha, M. Brown, M. McCormack, J. Boltax, and D. M. Livingston. 1987. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell 48:321-330. [DOI] [PubMed] [Google Scholar]
- 8.Burns, L. A., L. M. Karnitz, S. L. Sutor, and R. T. Abraham. 1993. Interleukin-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J. Biol. Chem. 268:17659-17661. [PubMed] [Google Scholar]
- 9.Cayla, X., K. Ballmer-Hofer, W. Merlevede, and J. Goris. 1993. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur. J. Biochem. 214:281-286. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., B. L. Martin, and D. L. Brautigan. 1992. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257:1261-1264. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J., S. Parsons, and D. L. Brautigan. 1994. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J. Biol. Chem. 269:7957-7962. [PubMed] [Google Scholar]
- 12.Chernoff, J., H. C. Li, Y. S. Cheng, and L. B. Chen. 1983. Characterization of a phosphotyrosyl protein phosphatase activity associated with a phosphoseryl protein phosphatase of Mr = 95,000 from bovine heart. J. Biol. Chem. 258:7852-7857. [PubMed] [Google Scholar]
- 13.Cicala, C., M. L. Avantaggiati, A. Graessmann, K. Rundell, A. S. Levine, and M. Carbone. 1994. Simian virus 40 small-t antigen stimulates viral DNA replication in permissive monkey cells. J. Virol. 68:3138-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, P. T. 1997. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem. Sci. 22:245-251. [DOI] [PubMed] [Google Scholar]
- 15.Crespo, P., N. Xu, W. F. Simonds, and J. S. Gutkind. 1994. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature 369:418-420. [DOI] [PubMed] [Google Scholar]
- 16.Csortos, C., S. Zolnierowicz, E. Bako, S. D. Durbin, and A. A. DePaoli-Roach. 1996. High complexity in the expression of the B' subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J. Biol. Chem. 271:2578-2588. [DOI] [PubMed] [Google Scholar]
- 17.Favre, B., P. Turowski, and B. A. Hemmings. 1997. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272:13856-13863. [DOI] [PubMed] [Google Scholar]
- 18.Fucini, R. V., S. Okada, and J. E. Pessin. 1999. Insulin-induced desensitization of extracellular signal-regulated kinase activation results from an inhibition of Raf activity independent of Ras activation and dissociation of the Grb2-SOS complex. J. Biol. Chem. 274:18651-18658. [DOI] [PubMed] [Google Scholar]
- 19.Gause, K. C., M. K. Homma, K. A. Licciardi, R. Seger, N. G. Ahn, M. J. Peterson, E. G. Krebs, and K. E. Meier. 1993. Effects of phorbol ester on mitogen-activated protein kinase kinase activity in wild-type and phorbol ester-resistant EL4 thymoma cells. J. Biol. Chem. 268:16124-16129. [PubMed] [Google Scholar]
- 20.Goris, J., C. J. Pallen, P. J. Parker, J. Hermann, M. D. Waterfield, and W. Merlevede. 1988. Conversion of a phosphoseryl/threonyl phosphatase into a phosphotyrosyl phosphatase. Biochem. J. 256:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix, P., R. E. Mayer-Jackel, P. Cron, J. Goris, J. Hofsteenge, W. Merlevede, and B. A. Hemmings. 1993. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J. Biol. Chem. 268:15267-71526. [PubMed] [Google Scholar]
- 22.Heriche, J. K., F. Lebrin, T. Rabilloud, D. Leroy, E. M. Chambaz, and Y. Goldberg. 1997. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276:952-955. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, T. 1995. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80:225-236. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara, H., T. Sasaoka, M. Ishiki, Y. Takata, T. Imamura, I. Usui, W. J. Langlois, T. Sawa, and M. Kobayashi. 1997. Functional importance of Shc tyrosine 317 on insulin signaling in Rat-1 fibroblasts expressing insulin receptors. J. Biol. Chem. 272:9581-9586. [DOI] [PubMed] [Google Scholar]
- 25.Kamibayashi, C., R. Estes, R. L. Lickteig, S. I. Yang, C. Craft, and M. C. Mumby. 1994. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 269:20139-20148. [PubMed] [Google Scholar]
- 26.Martin, R. G., V. P. Setlow, C. A. Edwards, and D. Vembu. 1979. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell 17:635-643. [DOI] [PubMed] [Google Scholar]
- 27.Mateer, S. C., S. A. Fedorov, and M. C. Mumby. 1998. Identification of structural elements involved in the interaction of simian virus 40 small tumor antigen with protein phosphatase 2A. J. Biol. Chem. 273:35339-35346. [DOI] [PubMed] [Google Scholar]
- 28.Mayer-Jaekel, R. E., H. Ohkura, P. Ferrigno, N. Andjelkovic, K. Shiomi, T. Uemura, D. M. Glover, and B. A. Hemmings. 1994. Drosophila mutants in the 55-kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J. Cell Sci. 107:2609-2616. [DOI] [PubMed] [Google Scholar]
- 29.McCright, B., A. M. Rivers, S. Audlin, and D. M. Virshup. 1996. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 271:22081-22089. [DOI] [PubMed] [Google Scholar]
- 30.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 31.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60:167-176. [DOI] [PubMed] [Google Scholar]
- 32.Pawson, T. 1995. Protein modules and signalling networks. Nature 373:573-580. [DOI] [PubMed] [Google Scholar]
- 33.Peterson, R. T., B. N. Desai, J. S. Hardwick, and S. L. Schreiber. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin-associated protein. Proc. Natl. Acad. Sci. USA 96:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price, N. E., and M. C. Mumby. 2000. Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry. 39:11312-11318. [DOI] [PubMed] [Google Scholar]
- 35.Pronk, G. J., A. M. de Vries-Smits, L. Buday, J. Downward, J. A. Maassen, R. H. Medema, and J. L. Bos. 1994. Involvement of Shc in insulin- and epidermal growth factor-induced activation of p21ras. Mol. Cell. Biol. 14:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pronk, G. J., J. McGlade, G. Pelicci, T. Pawson, and J. L. Bos. 1993. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J. Biol. Chem. 268:5748-5753. [PubMed] [Google Scholar]
- 37.Resjo, S., A. Oknianska, S. Zolnierowicz, V. Manganiello, and E. Degerman. 1999. Phosphorylation and activation of phosphodiesterase type 3B (PDE3B) in adipocytes in response to serine/threonine phosphatase inhibitors: deactivation of PDE3B in vitro by protein phosphatase type 2A. Biochem. J. 341:839-845. [PMC free article] [PubMed] [Google Scholar]
- 38.Ricketts, W. A., J. H. Brown, and J. M. Olefsky. 1999. Pertussis toxin-sensitive and -insensitive thrombin stimulation of Shc phosphorylation and mitogenesis are mediated through distinct pathways. Mol. Endocrinol. 13:1988-2001. [DOI] [PubMed] [Google Scholar]
- 39.Ricketts, W. A., D. W. Rose, S. Shoelson, and J. M. Olefsky. 1996. Functional roles of the Shc phosphotyrosine binding and Src homology 2 domains in insulin and epidermal growth factor signaling. J. Biol. Chem. 271:26165-26169. [DOI] [PubMed] [Google Scholar]
- 40.Ruediger, R., J. E. Van Wart Hood, M. Mumby, and G. Walter. 1991. Constant expression and activity of protein phosphatase 2A in synchronized cells. Mol. Cell. Biol. 11:4282-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rundell, K. 1987. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J. Virol. 61:1240-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito, T., H. Shima, Y. Osawa, M. Nagao, B. A. Hemmings, T. Kishimoto, and S. Hisanaga. 1995. Neurofilament-associated protein phosphatase 2A: its possible role in preserving neurofilaments in filamentous states. Biochemistry 34:7376-7384. [DOI] [PubMed] [Google Scholar]
- 43.Salcini, A. E., J. McGlade, G. Pelicci, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1994. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 9:2827-2836. [PubMed] [Google Scholar]
- 44.Sasaoka, T., D. W. Rose, B. H. Jhun, A. R. Saltiel, B. Draznin, and J. M. Olefsky. 1994. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J. Biol. Chem. 269:13689-13694. [PubMed] [Google Scholar]
- 45.Saxton, T. M., I. van Oostveen, D. Bowtell, R. Aebersold, and M. R. Gold. 1994. B cell antigen receptor cross-linking induces phosphorylation of the p21ras oncoprotein activators SHC and mSOS1 as well as assembly of complexes containing SHC, GRB-2, mSOS1, and a 145-kDa tyrosine-phosphorylated protein. J. Immunol. 153:623-636. [PubMed] [Google Scholar]
- 46.Sieburth, D. S., M. Sundaram, R. M. Howard, and M. Han. 1999. A PP2A regulatory subunit positively regulates Ras-mediated signaling during Caenorhabditis elegans vulval induction. Genes Dev. 13:2562-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skolnik, E. Y., C. H. Lee, A. Batzer, L. M. Vicentini, M. Zhou, R. Daly, M. J. Myers, Jr., J. M. Backer, A. Ullrich, M. F. White, et al. 1993. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 12:1929-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sleigh, M. J., W. C. Topp, R. Hanich, and J. F. Sambrook. 1978. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell 14:79-88. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda, Y., T. Kasahara, Y. Yamaguchi, K. Kuno, K. Matsushima, and N. Mukaida. 1997. Stimulation of interleukin-8 production by okadaic acid and vanadate in a human promyelocyte cell line, an HL-60 subline. Possible role of mitogen-activated protein kinase on the okadaic acid-induced NF-κB activation. J. Biol. Chem. 272:15366-15372. [DOI] [PubMed] [Google Scholar]
- 50.Sontag, E. 2001. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal 13:7-16. [DOI] [PubMed] [Google Scholar]
- 51.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 52.Sontag, E., V. Nunbhakdi-Craig, G. Lee, G. S. Bloom, and M. C. Mumby. 1996. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17:1201-1207. [DOI] [PubMed] [Google Scholar]
- 53.Sontag, E., J. M. Sontag, and A. Garcia. 1997. Protein phosphatase 2A is a critical regulator of protein kinase Cζ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 16:5662-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivasan, M., and N. Begum. 1994. Regulation of protein phosphatase 1 and 2A activities by insulin during myogenesis in rat skeletal muscle cells in culture. J. Biol. Chem. 269:12514-12520. [PubMed] [Google Scholar]
- 55.Takai, A., and G. Mieskes. 1991. Inhibitory effect of okadaic acid on the p-nitrophenyl phosphate phosphatase activity of protein phosphatases. Biochem. J. 275:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 57.Wassarman, D. A., N. M. Solomon, H. C. Chang, F. D. Karim, M. Therrien, and G. M. Rubin. 1996. Protein phosphatase 2A positively and negatively regulates Ras1-mediated photoreceptor development in Drosophila. Genes Dev. 10:272-278. [DOI] [PubMed] [Google Scholar]
- 58.Westphal, R. S., K. A. Anderson, A. R. Means, and B. E. Wadzinski. 1998. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science 280:1258-1261. [DOI] [PubMed] [Google Scholar]
- 59.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]