Short abstract
Although gene therapy has huge potential for modern medicine, our enthusiasm for its powerful potential must not cloud our judgment about the dangers of using increasingly diverse, yet relatively untested, replicating viruses
Gene therapy is currently being studied in both the laboratory and the clinic in relation to many conditions, including cancer, heart disease, and autoimmune diseases. A few thousand patients have received genes in more than a thousand different clinical trials—overwhelmingly patients with cancer (two thirds of the trials), with most receiving non-replicative retroviruses or adenovirus as the vectors for the delivery of the new genes.w1
The use of viral vectors has now expanded from relatively safe, non-replicating viruses to the use of viruses that replicate more selectively in cancer cells than in normal cells (oncolytic viruses).1 The benefit of using these viruses is that as they replicate, they lyse their host cells. Cancer cells are ideal hosts for many viruses because they have the antiviral interferon pathway inactivated or have mutated tumour suppressor genes2,3 that enable viral replication to proceed unhindered. Adenovirus3,4 and herpes simplex virus,5 specifically mutated to replicate faster in cancer cells, are the main replicating human pathogenic viruses used in the clinic.3 To date, more than 250 patients have been treated with ONYX-015, a replicating adenovirus.
Before the Helsinki protocols were approved, only a handful of studies had used live viruses injected into solid tumours. Currently, laboratory (and some clinical) studies are using many different viruses (such as Newcastle disease virus, reovirus, poliovirus, vesicular stomatitis virus, measles,6 and vaccinia7), selected for their ability to actively replicate in cancer cells.8,9 Some of these viruses are pathogens in humans, some also in other species. Newcastle disease virus, for example, causes fatal disease in chickens.10
An argument for the use of these viruses is that some have shown long term safety as immunogens in humans. However, the dosage used for immunisation and that being used for gene therapy by intravenous or intratumoral injection is quite different. Measles vaccine (Priorix, GSK), for example, is used as an immunogen in humans at a dose of about 103 pfu in the measles, mumps, and rubella vaccine (MMR vaccine),w2 and in mice experiments a dose of 106-107 pfu is used, which is at least a 1000-fold increase.2
Newcastle disease virus is already in phase I clinical trials,11 with about 170 patients having been treated.w3 Surprisingly, no particular containment facilities have been described for this type of work despite virus detected in the urine up to three weeks after the first treatment.12
In the United States, researchers who want to conduct human studies with biological materials or viruses have to file an “investigational new drug” application with the Food and Drug Administration. Such application has to be supported by toxicity data from animal studies to justify the route, dose, and schedule of administration in humans. The researchers also have to demonstrate that the material is free of other harmful contaminants.w4 However, whether the shed virus is genetically identical to the injected virus has not been investigated. This is very important as the genome of RNA viruses mutates rapidly.
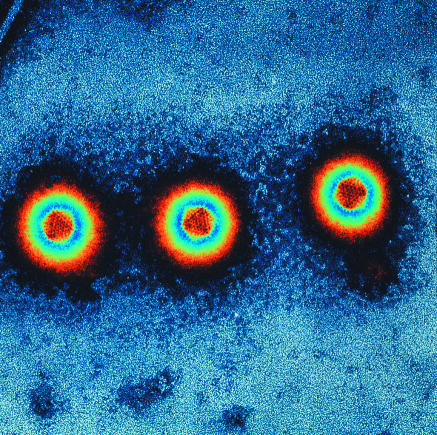
Figure 1.
Mutated adenoviruses are widely used in oncolytic cancer gene therapy
Credit: MIKE MILLER/SPL
The use of oncolytic viruses has a key limitation in that they are highly immunogenic. The host immune response limits their effectiveness to local sites of injection and possibly to a single or a few administrations. Kaufman and colleagues have suggested that, for longer lasting effects, viruses should be further engineered to induce T cell memory in the host to cancer antigens13 or with genes to express therapeutic molecules such as cytokines, pro-drug activating enzymes, and anti-angiogenic factors. Adding these and other features has been termed “arming” the viruses.8 A potential side effect of potent anticancer immunotherapy is autoimmune disease, as many antigens expressed in tumour cells are also expressed in normal cells. Melanoma gene therapy with vaccinia virus has led to vitiligo in some patients due to the expression of identical antigens in melanoma cancer cells and normal melanocytes.13-15 At least 19 patients have been treated with vaccinia virus.13 w5 Whether oncolytic viruses in other cancers might elicit other types of autoimmune diseases has not been investigated. Regardless of whether a replicative virus is armed, its safety and genetic variability and capability for recombination should be properly assessed. Recently the FDA has called for a workshop to discuss this, and hopefully new guidelines will became available.w6
Whether replicating armed viruses8 will be able to modify the immune response of the host and become highly pathogenic is not known and may not be answerable in currently used animal models. Some oncolytic viruses only replicate in partly “humanised” transgenic mice or in immunodeficient mice grafted with human tumours that do not reproduce the complexities of the human immune system.16 We suggest that an appropriate testing system would use immunodeficient mice reconstituted with human bone marrow,17 in which human tumours can be transplanted and these oncolytic viruses tested. Such an approach, although expensive, would ensure at least proper assessment of changes in immune parameters, which cannot be done in the currently used models.
Hermiston and Kuhn expressed the challenges of such arming, stating that: “The mechanisms of each of the various classes of gene-based therapeutics when used as monotherapies may be clear, but their potential interactions within the context of a replicating virus are not easily discerned. These interactions will either synergize to increase, or conflict to decrease patient benefit.”8
The arming of replicating viruses, particularly with immunomodulatory genes, can pose unforeseen consequences—one example being IL-4 producing, replication-competent ectromelia viruses (mousepox) in mice.18 Even a genetically resistant mouse strain became susceptible to acute symptoms of mousepox infection, causing high mortality; also, mice immunised with the wild-type virus succumbed to infection by the recombinant virus. Despite these original studies being halted, the armed virus is now being used as a biological warfare model to develop more potent antiviral drugs.19
Different viruses have developed different mechanisms for immune evasion, including the expression of cytokine and cytokine receptor homologue genes.20-27 How these immune evasion mechanisms may interact with the arming gene(s) cannot be predicted, and whether they may affect virus tropism, recombination, and propagation needs to be carefully assessed before use in clinical trials.
The use of a variety of oncolytic viruses has recently been reviewed.7,9,28 As an unsettling portent, some authors predict: “For the future we are heading towards developing selective replicating viruses that can avoid immune clearance, thereby enabling systemic administration.”9
In view of the expected pandemic arising from avian influenza virusw7 and the knowledge that species adaptation can occur relatively quickly, is it safe to consider the use of viruses from other species, breaking all natural and tropism barriers by intravenous or intratumoral administration in humans?
The use of replicating viruses poses new and unpredictable risks not only to the individual treated but also to the population as a whole as these viruses may spread in the environment and also potentially recombine with other wild-type viruses.29 Oncolytic viruses do not fall within the guidelines for genetically modified organisms, although when armed they will. Specific guidelines are urgently needed to cover the clinical application of such replicating oncolytic viruses both at local and international levels. Furthermore, because of the biological limitations of the animal models described earlier, we need to have more discussion about how preclinical testing for safety should be carried out.
Cancer is indeed a terrible disease demanding aggressive, ingenious, and imaginative approaches. However, the balance of risk and benefit must always be of prime consideration, not only for the patients but now also for the rest of the population.
Summary points
The introduction of therapeutic genes has been hailed as a potentially powerful medical intervention
Researchers first started with viruses that infect humans, but are now forging ahead with viruses that do not normally use humans as a host
The safety of using replicating viruses—particularly after genetic modification—and the possible environmental implications of this work need urgent assessment
Supplementary Material
 Additional references (w1-w7) are at bmj.com
Additional references (w1-w7) are at bmj.com
We thank Klaus Cichutek, Gill Adams, David Gould, Bruce Kidd, Rod Flower, and Robin Weiss for their comments.
Contributors and sources: YC and NL have longstanding research interests in gene therapy in autoimmunity and cancer. LL is an immunologist and has interest in the application of biomedical research.
Funding: Arthritis Research Campaign.
Competing interests: None declared.
References
- 1.Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 2005;65: 9991-8. [DOI] [PubMed] [Google Scholar]
- 2.Myers R, Greiner S, Harvey M, Soeffker D, Frenzke M, Abraham K, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther 2005;12: 593-9. [DOI] [PubMed] [Google Scholar]
- 3.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther 2005;12: 437-45. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Xue SA, Hallden G, Francis J, Yuan M, Griffin BE, et al. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res 2005;65: 1523-31. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa T, Chiocca EA. Comparative analyses of transgene delivery and expression in tumors inoculated with a replication-conditional or -defective viral vector. Cancer Res 2001;61: 5336-9. [PubMed] [Google Scholar]
- 6.Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther 2002;9: 961-6. [DOI] [PubMed] [Google Scholar]
- 7.Thorne SH, Bartlett DL, Kirn DH. The use of oncolytic vaccinia viruses in the treatment of cancer: a new role for an old ally? Curr Gene Ther 2005;5: 429-43. [DOI] [PubMed] [Google Scholar]
- 8.Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther 2002;9: 1022-35. [DOI] [PubMed] [Google Scholar]
- 9.Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther 2004;11: 643-64. [DOI] [PubMed] [Google Scholar]
- 10.Alexander D, Allan W. Newcastle disease virus pathotypes. Avian Pathol 1974;3: 269-74. [DOI] [PubMed] [Google Scholar]
- 11.Lorence RM, Pecora AL, Major PP, Hotte SJ, Laurie SA, Roberts MS, et al. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr Opin Mol Ther 2003;5: 618-24. [PubMed] [Google Scholar]
- 12.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol 2002;20: 2251-66. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest 2005;115: 1903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA 1999;96: 2982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 2003;198: 569-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 2004;64: 4919-26. [DOI] [PubMed] [Google Scholar]
- 17.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med 1990;172: 1055-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol 2001;75: 1205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins SJ, Jackson RJ, Fenner F, Beaton S, Medveczky J, Ramshaw IA, et al. The efficacy of cidofovir treatment of mice infected with ectromelia (mousepox) virus encoding interleukin-4. Antiviral Res 2005;66(1): 1-7. [DOI] [PubMed] [Google Scholar]
- 20.Alcami A, Smith GL. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today 1995;16: 474-8. [DOI] [PubMed] [Google Scholar]
- 21.Gooding LR. Virus proteins that counteract host immune defenses. Cell 1992;71(1): 5-7. [DOI] [PubMed] [Google Scholar]
- 22.Cortes PL, Cardona CJ. Pathogenesis of a Marek's disease virus mutant lacking vIL-8 in resistant and susceptible chickens. Avian Dis 2004;48(1): 50-60. [DOI] [PubMed] [Google Scholar]
- 23.Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schonrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol 2004;173: 3383-91. [DOI] [PubMed] [Google Scholar]
- 24.Sabourdy F, Casteignau A, Gelfi J, Deceroi S, Delverdier M, Messud-Petit FL. Tumorigenic poxviruses: growth factors in a viral context? J Gen Virol 2004;85(Pt 12): 3597-606. [DOI] [PubMed] [Google Scholar]
- 25.Mokros T, Rehm A, Droese J, Oppermann M, Lipp M, Hopken UE. Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. J Biol Chem 2002;277: 45122-8. [DOI] [PubMed] [Google Scholar]
- 26.Breen EC, Gage JR, Guo B, Magpantay L, Narazaki M, Kishimoto T, et al. Viral interleukin 6 stimulates human peripheral blood B cells that are unresponsive to human interleukin 6. Cell Immunol 2001;212: 118-25. [DOI] [PubMed] [Google Scholar]
- 27.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, et al. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 1992;69: 597-604. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus:teaching new tricks to an old dog. Mol Therapy 2005;11: 180-95. [DOI] [PubMed] [Google Scholar]
- 29.Yunus AS, Krishnamurthy S, Pastey MK, Huang Z, Khattar SK, Collins PL, et al. Rescue of a bovine respiratory syncytial virus genomic RNA analog by bovine, human and ovine respiratory syncytial viruses confirms the “functional integrity” and “cross-recognition” of BRSV cis-acting elements by HRSV and ORSV. Arch Virol 1999;144: 1977-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.