Abstract
While it is clear that cancer arises from the accumulation of genetic mutations that endow the malignant cell with the properties of uncontrolled growth and proliferation, the precise combinations of mutations that program human tumor cell growth remain unknown. The study of the transforming proteins derived from DNA tumor viruses in experimental models of transformation has provided fundamental insights into the process of cell transformation. We recently reported that coexpression of the simian virus 40 (SV40) early region (ER), the gene encoding the telomerase catalytic subunit (hTERT), and an oncogenic allele of the H-ras gene in normal human fibroblast, kidney epithelial, and mammary epithelial cells converted these cells to a tumorigenic state. Here we show that the SV40 ER contributes to tumorigenic transformation in the presence of hTERT and oncogenic H-ras by perturbing three intracellular pathways through the actions of the SV40 large T antigen (LT) and the SV40 small t antigen (ST). LT simultaneously disables the retinoblastoma (pRB) and p53 tumor suppressor pathways; however, complete transformation of human cells requires the additional perturbation of protein phosphatase 2A by ST. Expression of ST in this setting stimulates cell proliferation, permits anchorage-independent growth, and confers increased resistance to nutrient deprivation. Taken together, these observations define the elements of the SV40 ER required for the transformation of human cells and begin to delineate a set of intracellular pathways whose disruption, in aggregate, appears to be necessary to generate tumorigenic human cells.
Although oncogenic viruses are responsible for only a small portion of human cancers, the study of tumor virus-encoded proteins in experimental models of cellular transformation has revealed a number of critical intracellular signaling pathways that contribute to spontaneously arising cancers. In the case of acutely transforming retroviruses, acquired cellular genes are exploited by these viruses to transform infected cells (3). The antecedents of the oncogenes carried by the DNA tumor viruses are less clear. When rationalized functionally, it appears that the oncoproteins encoded by DNA tumor viruses have evolved to perturb host cell regulatory circuits in ways that favor viral replication. In uninfected cells, these same regulatory circuits regulate cell proliferation and survival.
Among the best studied of the DNA tumor virus-encoded oncoproteins are the large T antigen (LT) of simian virus 40 (SV40), the adenovirus E1A and E1B proteins, and the E6 and E7 proteins of certain strains of human papillomavirus (HPV). These viral proteins share the ability to bind and inactivate the pRB and p53 tumor suppressor proteins (12, 38, 48). Indeed, the study of these viral proteins greatly facilitated the elucidation at the molecular level of the pathways controlled by these two proteins. These two tumor suppressor pathways are also perturbed in the majority of human cancers through mutation and methylation of the genes controlling various components of these pathways (39, 67). In addition to perturbing the pRB and p53 pathways, these viral oncoproteins interact with and perturb the function of proteins that are components of yet other cellular regulatory circuits (1, 15, 16, 27, 37, 56).
In rodent cells, the coexpression of LT with an oncogenic allele of the H-ras gene suffices to transform normal fibroblasts to tumorigenicity (26, 43), and several investigators have delineated the functional domains of LT critical for the immortalization and transformation of various rodent cell lines (76, 77, 80, 90, 93). However, similar attempts to transform normal human cells have rarely led to transformed, tumorigenic cells, as the majority of the cells expressing these genes enter into an irreversible growth arrest or undergo apoptosis (64). These observations prompted some to propose that the limited life span of human cells presents a barrier to the progression of cells to a malignant growth state (51, 63). In consonance with this proposal, we recently demonstrated that introduction of the gene encoding the catalytic subunit of human telomerase (hTERT) together with the SV40 ER yielded immortalized human fibroblasts, kidney epithelial (human embryonic kidney [HEK]) cells, and human mammary epithelial cells (HMEC) and that these immortalized cells acquired a tumorigenic phenotype upon subsequent introduction of the H-ras oncogene (13, 21). These observations suggested that telomere maintenance plays an essential role in human cell transformation and that the differences in the susceptibility of human and rodent cells to transformation by oncogenes derive, in part, from the constitutive expression of telomerase, the enzyme that maintains telomeres, in rodent but not in human cells (33, 59).
These observations also suggested that the collaborative disruption of only a limited set of cellular pathways is sufficient for the tumorigenic transformation of human cells. More specifically, it appeared that perturbation of the pRB and p53 tumor suppressor pathways by LT in combination with ectopic expression of a mutant, constitutively active H-Ras oncoprotein and constitutive telomerase expression sufficed to transform these cells. However, the use of SV40-derived sequences in these experiments introduced the possibility that proteins encoded by these viral sequences affect pathways in addition to pRB and p53, the disturbance of which was also essential for transformation. For this reason, we initiated a series of experiments to dissect the properties of the genetic element that we previously termed LT (21). These characterizations demonstrate that perturbation of a fifth functional pathway contributes to the tumorigenic phenotype of human cells.
MATERIALS AND METHODS
Vectors.
To introduce the SV40 early region (ER) into cells, we used the ψ2:SV40 cell line that was produced by transfection with the pZIP-776-1 retroviral vector and stably produces a virus encoding the SV40 ER (29). To construct a retrovirus encoding a cDNA version of the LT mRNA, we inserted the BamHI fragment from the vector pSG5 (90) into pBABE-neo (47). The neomycin resistance-encoding gene was introduced by replacing the HindIII-ClaI fragment encoding the puromycin resistance-encoding gene with the neomycin resistance-encoding gene from pCI-neo (Promega, Madison, Wis.). The LT mutant forms K1, Δ434-444, and H42Q were also introduced into pBABE-neo by ligation of the respective sequences encoding these mutant forms from pSG5-based vectors (79, 90).
A retrovirus encoding the SV40 small t (ST) oncoprotein was constructed by amplifying the ST sequences by PCR using the vector pVU-Φ (30) and the oligonucleotides B5ST (5′ GGCGGATCCGCCACCATGGATAAAGTTTT 3′) and E3ST (5′AGGCGAATTCTTAGAGCTTTAAATCTCTG 3′). The resulting fragment was sequenced and introduced into pMIG (pMIG-ST) (81) and pBABE-zeo (13). ST deletion mutant forms were also produced by PCR amplification and sequencing, followed by introduction into pMIG, except for the ST 97,102 mutant form, which was produced by site-directed mutagenesis (QuikChange, Stratagene, La Jolla, Calif.). ST F88-174 was created by PCR amplification with oligonucleotides that introduced a FLAG epitope tag in frame with the coding sequence of ST corresponding to amino acids (aa) 88 to 174.
The hemagglutinin (HA)-tagged R24C CDK4 mutant form was constructed by inserting the BamHI-XbaI fragment of pCDNA3-HA-cdk4R24C into pBABE-hygro (69). The p53DD mutant form was removed from plasmid 572 (70) with BamHI and SmaI and inserted into pBABE-neo. pMIG-hTERT was produced by introducing the hTERT cDNA from pCI-hTERT-neo (9) into pMIG. The construction of pBABE-hygro-hTERT (9), pBABE-puro-H-Ras (68), and pBABE-puro-HA-cyclin D1 (25) has been described previously.
Virus production and cell lines.
Amphotropic retroviruses were produced by transfection of the 293T producer cell line with a retroviral vector and a vector encoding a replication-defective helper virus, pCL-Ampho (Imgenex, San Diego, Calif.), using Fugene 6 (Roche Molecular Biochemicals, Indianapolis, Ind.). Human BJ fibroblasts (population doubling 5 [PD5]) were infected with a virus encoding the SV40 LT or SV40 ER, followed by a vector encoding hTERT and finally by a vector specifying G12V H-ras. HEK cells expressing the SV40 ER were obtained from S. Bacchetti (78); hTERT and G12V H-ras were introduced as previously described (21). In addition, primary HEK cells from embryonic kidney tissue were infected (PD7) with a virus encoding the SV40 LT or SV40 ER, followed by vectors encoding hTERT and G12V H-ras. These newly derived HEK cells expressing the SV40 ER behaved identically to those previously generated by Stewart and Bacchetti (78). Wild-type or mutant ST was subsequently introduced by retroviral infection and selection for green fluorescent protein (GFP)-expressing cells with a fluorescence-activated cell sorter (FACS). After two rounds of infection, 70 to 90% of the cells expressed GFP; 50% of these GFP-expressing cells were retained by the FACS. The human papillomavirus type 16 (HPV-16) E6 and E7 viral oncoproteins were introduced into BJ or HEK cells by using retroviruses produced by the cell line PA317-16E6E7 (17). For BJ cells expressing cyclin D1, the R24C CDK4 mutant form, p53DD, hTERT, H-Ras, and ST, cells were infected with pBABE-puro-cyclin D1 and then serially infected with pBABE-hygro-R24C CDK4, pBABE-neo-p53DD, pMIG-hTERT, and pBABE-puro-Ras and finally with pBABE-zeo-ST. After each step, cell populations were purified by selection with hygromycin (100 μg/ml), neomycin (400 μg/ml), puromycin (0.5 μg/ml), or zeomycin (500 μg/ml), except for hTERT, for which no selection was employed.
The expression of these proteins was confirmed by immunoblotting with the following antibodies after separation by sodium dodecyl sulfate-7.5 to 15% gradient polyacrylamide gel electrophoresis: LT, Pab101; ST, Pab108 and Pab419; p53, Ab6 (Calbiochem, San Diego, Calif.); p53, Pab423; HA, HA-11 (Covance, Princeton, N.J.); cyclin D1, HD-11; CDK4, H-22; p21, C-19; pRB, 14001A (Becton Dickinson, San Jose, Calif.); actin, 6276 (Abcam, Cambridge, United Kingdom); and Ras, C20. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), except where noted otherwise. LT was expressed at a significantly higher level than ST when induced with retroviruses encoding the entire SV40 ER or by retroviruses encoding the LT and ST cDNAs. To obtain the LT and ST blots depicted, after electrophoretic transfer, blotting membranes were cut in half by using prestained markers as a guide and blotted separately for LT and ST. The expression of hTERT was confirmed by the telomere repeat amplification protocol (TRAP) as previously described (34).
Anchorage-independent growth and tumor formation.
Growth of cells in soft agar and in immunodeficient animals was performed as previously described (21). For tumorigenicity assays, 2 × 106 cells were injected subcutaneously (s.c.) in each experiment. Anchorage-independent (AI) colonies were counted with an automated colony counter (Gel-doc system; UVP, Inc.). In each experiment, 105 cells were seeded into 0.4% Noble agar. Cell lines derived from human tumor samples were used as controls and gave similar numbers of colonies under these conditions (21).
Proliferation assays.
BJ fibroblasts were maintained in a 4:1 mixture of Dulbecco modified Eagle medium to M199 supplemented with 15% heat-inactivated fetal calf serum (FCS). HEK cells were maintained in minimal Eagle medium alpha supplemented with 10% FCS. To generate proliferation curves, cells were plated in triplicate and counted either manually with a hemocytometer or in an automated fashion with a Z2 Particle Count and Size Analyzer (Beckman-Coulter, Miami, Fla.).
To test the growth of cells at a limiting glutamine concentration, glutamine-free medium was supplemented with dialyzed FCS and 0.375 mM glutamine, representing 10% of the usual glutamine concentration. Control cultures in which glutamine was supplemented to a standard glutamine concentration (3.75 mM) and dialyzed serum grew in a fashion indistinguishable from that of cells grown in standard medium.
For DNA content analysis, cells were labeled with 10 μM bromodeoxyuridine (BrdU) for 4 h, fixed in 70% ethanol, acid treated with 2 M HCl in phosphate-buffered saline supplemented with 0.5% FCS, and neutralized with 0.1 M sodium borate, pH 8.5. Labeled cells were then incubated with a monoclonal antibody (MAb) specific for BrdU, washed, incubated with a fluorescein-conjugated secondary MAb specific for murine immunoglobulin G, and stained with propidium iodide to measure DNA content. Cell cycle profiles of 10,000 cells were analyzed with a Becton Dickson FACStar flow cytometer in duplicate.
RESULTS
Role of SV40 ST as a participant in the transformation of human cells.
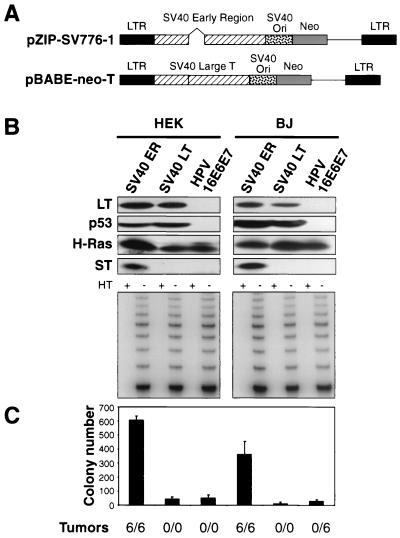
In our previously reported experiments, we introduced the SV40 ER into human BJ fibroblasts, HEK cells, and HMEC by using a retroviral vector (13, 21, 29). While this vector had been reported to express only the LT antigen (29), we noted that it had been constructed from a DNA fragment encompassing the entire ER of the SV40 genome and thus included an intron within the LT coding region that made possible the expression of other viral proteins from alternatively spliced transcripts (Fig. 1A). For this reason, we initiated a series of experiments to map and enumerate the functionally important domains of the SV40 ER required for tumorigenic transformation of human cells.
To facilitate this analysis, we constructed a new retrovirus encoding a cDNA version of the mRNA that, by omitting of the above-mentioned intron, encodes only the LT oncoprotein. This cDNA was introduced into a version of the pBABE retroviral vector carrying a neomycin resistance-encoding gene (Fig. 1A). We infected parallel cultures of early passage human BJ fibroblasts (PD5) and HEK cells (PD7) with this LT-encoding vector, with the previously used retrovirus transducing the entire SV40 ER (pZIP-SV776-1), or with a retroviral vector encoding HPV-16 oncoproteins E6 and E7 (HPV-16E6E7) (17). The latter vector was used to determine whether these two HPV proteins are able to substitute for LT through their known ability to inactivate pRB and p53. After infection and drug selection, the cells were serially infected with retroviral vectors encoding hTERT and with the G12V mutant form of the H-ras gene. The resulting polyclonal cell populations expressed similar amounts of the LT and H-Ras proteins and exhibited comparable levels of telomerase activity (Fig. 1B).
FIG. 1.
Tumorigenicity of cells expressing SV40 and HPV oncoproteins. (A) Schematic representation of the vector encoding the SV40 ER (pZIP-SV776-1) and the vector encoding only SV40 LT (pBABE-neo-T). The portion of pBABE-neo-T that encodes LT lacks the intron present in the SV40 ER. (B) Immunoblotting of cells expressing the SV40 ER or LT or HPV-16 E6 or E7. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels and immunoblotted with antibodies specific for LT (Pab101), p53 (Ab6), H-Ras (C20), and ST (Pab108 and Pab419). The level of LT expression is much greater than the level of ST expression. The LT exposure time was 1 min, while the ST exposure time was 30 min. TRAP assays were performed on 200 ng of total cellular protein. HT refers to heat-treated samples. An internal control band used to ensure PCR reactivity was present for each sample (data not shown). (C) AI growth and tumorigenicity of the indicated cell lines. To assess AI growth, 105 cells were plated in 0.4% Noble agar and colonies were counted 21 to 28 days after seeding. The mean and standard deviation of four experiments are shown. Tumor formation in immunodeficient mice was assessed by s.c. injection of 2 × 106 cells and is reported as the number of tumors identified/number of injection sites. LTR, long terminal repeat.
We then determined whether each of these cell populations was capable of AI growth and tumorigenic growth in immunodeficient hosts. In doing so, we reproduced earlier observations of others who had reported that human fibroblasts coexpressing HPV-16 E6 and E7, hTERT, and oncogenic Ras were unable to form colonies in an AI manner (Fig. 1C) (46). Unexpectedly, we also failed to find evidence of either AI growth or tumor formation in cells expressing the cDNA encoding LT together with hTERT and Ras (Fig. 1C). This behavior contrasted with the observed ability of the hTERT and ras genes, together with the SV40 ER, to induce the AI and tumorigenic phenotypes (Fig. 1C). These differences in outcome could not be attributed to the expression level of the SV40 LT oncoprotein, since these cells expressed amounts of LT protein similar to those seen in cells that had acquired the SV40 ER in combination with the hTERT and ras genes (Fig. 1B). Taken together, these observations indicated that the SV40 ER encodes an additional function essential for transformation beyond the functions encoded by the LT cDNA.
In fact, the SV40 ER is known to encode three distinct proteins as a consequence of alternative splicing of the ER primary transcript: the 708-aa LT antigen, the 174-aa ST antigen, and the 135-aa 17kT antigen (56, 58, 62, 73, 83, 92). All three of these proteins have the same 82-aa amino terminus. The polypeptide termed 17kT is formed from a splice product that results in the translation of a protein identical to the first 131 aa that specify LT, followed by four unique residues (ALLT) (92); ST is formed from a splice variant with sequences derived from an intron that encodes a unique carboxyl terminus, resulting in a polypeptide of 174 aa (58, 73, 83; reviewed in reference 62).
These observations prompted us to examine whether other SV40 ER proteins are expressed from the SV40 ER vector in addition to LT. To this end, we used two MAbs specific for epitopes present in the amino-terminal sequences common to the three proteins known to be encoded by the SV40 ER. As shown in Fig. 1B, cells created with the retroviral vector transducing the entire SV40 ER indeed expressed ST, while the cells expressing the vector encoding the LT cDNA or HPV-16 E6 and E7 failed, as expected, to do so. We note that LT is expressed much more abundantly than ST is and that we have not observed any expression of 17kT (data not shown). These observations suggested that the differences in AI growth and tumorigenicity between cells carrying the SV40 ER and those carrying the LT cDNA might be attributable to the ability of the ER vector to specify ST.
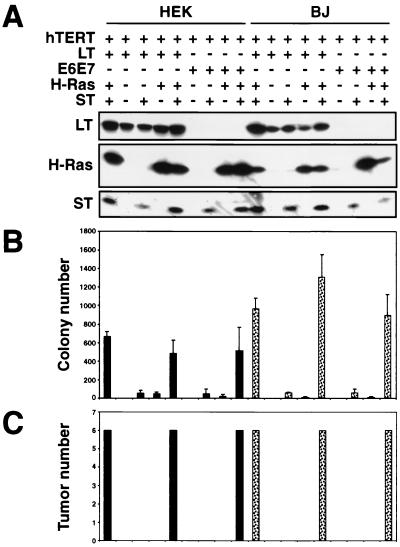
To test the possible contribution of ST to tumorigenicity, we constructed a retroviral vector that transduces an ST cDNA and introduced this vector into BJ and HEK cells already expressing hTERT, a cDNA version of LT, and Ras or appropriate control vectors in various combinations (Fig. 2A). Cells infected with this retrovirus expressed ST at levels similar to that observed in cells expressing the entire SV40 ER. We then determined the ability of these cells to grow in an AI manner and to form tumors in immunodeficient hosts. Indeed, we found robust AI colony growth when both ST and ras were coexpressed in combination with the cDNA version of LT and hTERT (Fig. 2B). In parallel with this result, tumors only formed after s.c. injection of BJ and HEK cells expressing cDNA versions of LT, hTERT, oncogenic Ras, and ST (Fig. 2C). We have obtained similar results by using primary HMEC expressing the same set of genetic elements (data not shown). BJ fibroblasts expressing hTERT and ST alone were immortal yet lacked the ability to grow in an AI manner and were unable to avoid the premature proliferative arrest induced by expression of oncogenic Ras (68) (data not shown), demonstrating that ST could not functionally substitute for LT in these types of transformation assays. These observations indicated that the actions of the LT oncoprotein, in combination with ras and hTERT expression, are insufficient to transform human cells and that the additional presence of ST is required for successful transformation.
FIG. 2.
Analysis of cell lines expressing combinations of the LT, ST, hTERT, H-Ras, and HPV proteins. (A) HEK or BJ cells expressing the indicated combinations of introduced proteins were analyzed by immunoblotting of 100 μg of total cell protein and separation on 7.5 to 15% gradient gels. All of the cell lines expressed hTERT, as assessed by TRAP assay (data not shown). Cell lines created with the SV40 ER are depicted in the first column of HEK and BJ cells. As shown, although LT is expressed at a higher level than ST, LT and ST introduced from distinct retroviral constructs were expressed at levels similar to those in cells infected with a retrovirus that transduces the entire SV40 ER. The LT exposure time was 1 min, while the ST exposure time was 30 min. (B) AI colony formation. The mean and standard deviation of three experiments are shown. (C) Tumor formation in immunodeficient mice. The numbers of tumors observed after six s.c. injections are depicted. In panels B and C, HEK cells are represented by black bars and BJ fibroblasts are represented by dotted bars.
These observations also appeared to explain the failure of the HPV-16 E6 and E7 oncogenes to transform cells in combination with ras and hTERT 46; Fig. 1C and 2B and C). Indeed, when ST was expressed in combination with HPV-16E6E7, hTERT, and oncogenic ras, vigorous colony growth was seen in soft agar (Fig. 2B) and tumors formed after cells were placed s.c. (Fig. 2C). These observations provided an initial indication that only those domains of the LT oncoprotein that are mimicked functionally by HPV-16E6E7 are essential to the tumorigenic transformation of these human cells.
Delineation of the domains of LT required to extend the cellular life span.
The above-described results indicated that coordinate expression of ST and LT is required for cell transformation but did not enumerate the number of distinct functions within the multifunctional LT protein that are required for this process. Indeed, several groups have identified at least three domains of LT required for the immortalization and transformation of primary and established rodent cells (76, 77, 80, 90, 93). To determine which of the many functions ascribed to LT are required for human cell transformation, we dissected the domains of LT that participate in the immortalization and transformation of human cells. We initially focused on the immortalization of primary cells, as previous work indicated that this step is a prerequisite for successful transformation (21, 50, 51).
Unlike murine cells, human cells must bypass two barriers to become immortalized: replicative senescence and crisis. Replicative senescence occurs upon proliferation of normal human cells and is characterized by an irreversible growth arrest but continued metabolic activity (66). This growth arrest can be avoided when LT is expressed in presenescent human cells (72, 78). Having circumvented senescence, such cells continue to proliferate for a period of time but ultimately enter a crisis characterized by widespread cell death (66, 72). This crisis, in turn, can be averted by additional expression of the hTERT protein, yielding immortalized cells (9, 22, 94).
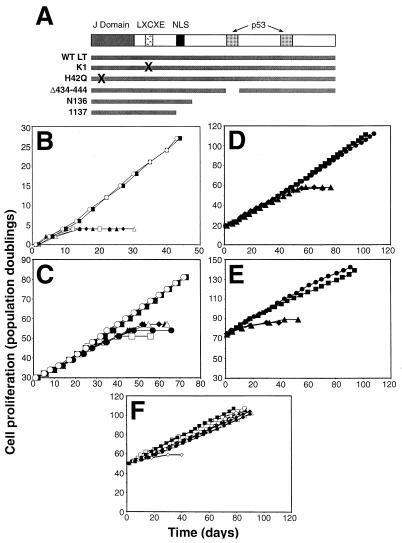
In order to determine the minimal structural elements of LT required to avoid entering senescence, we procured several well-characterized LT mutant forms and introduced these mutant forms into the previously used pBABE-neo retrovirus vector (Fig. 3A). These LT mutant forms could be divided into three categories: a mutant form that carries an alteration in the pRB consensus binding motif and is consequently unable to bind pRB family members (termed K1) (30), a mutant LT that contains an amino acid substitution at aa 42 that renders its J domain nonfunctional (termed H42Q) (79), and a group of mutant forms that contain deletions in the bipartite p53-binding domain of LT (termed Δ434-444, 1137, and N136) (32, 54, 57). The 82 amino-terminal amino acids of LT constitute a J domain that is related in sequence to the DnaJ family of molecular chaperones. This domain represents a third, functionally distinct, portion of LT, which has been reported to play an important role in some aspects of cell transformation (19, 76, 91, 93). Mechanistically, mutations in the sequences encoding the LT J domain alter the ability of LT to affect the phosphorylation state of pRB and pRB-related proteins p107 and p130 (91).
We tested these various LT mutant forms in early passage HEK cells (PD7), which proliferate for a limited number of PDs before entering into the state of replicative senescence. After introduction of each of these mutant forms into presenescent HEK cells, we were only able to obtain cell lines that avoided senescence through the expression of either the wild-type LT cDNA or the J domain mutant form H42Q. Conversely, mutant forms of LT that had lost either pRB or p53 binding did not protect against entrance into senescence (Fig. 3B).
FIG. 3.
Effects of LT mutant forms on replicative senescence and crisis. (A) Schematic representation of LT mutant forms. LXCXE, binding site for pRB family members; NLS, nuclear localization signal; X, amino acid substitutions that disrupt LT function; WT, wild type. Δ434-444 has a small in-frame deletion that eliminates p53 binding. (B) Effects of LT mutant forms on replicative senescence in HEK cells. HEK cells were infected with a control retrovirus (open squares) or with a retrovirus encoding an LT mutant form. Wild-type LT (filled squares), K1 (filled triangles), H42Q (open circles), Δ434-444 (open triangles), N136 (filled diamonds), and 1137 (filled circles) are shown. (C) Effects of LT mutant forms on replicative senescence in BJ cells. Symbols are the same as in panel B. (D) Effects of LT mutant forms on crisis in HEK cells. HEK cells expressing LT with (squares) or without (diamonds) hTERT or the LT H42Q mutant form with (circles) or without (triangles) hTERT are shown. (E) Effects of LT mutant forms on crisis in BJ cells. hTERT was introduced after the indicated cells had bypassed replicative senescence. Symbols are the same as in panel D. (F) Effects of LT mutant forms on immortalization in BJ fibroblasts expressing hTERT prior to introduction of the mutant forms. Symbols are the same as in panel B. Open diamonds represent the proliferation of BJ fibroblasts infected with only a control retrovirus.
We extended these observations by introducing these same LT mutant forms into BJ human fibroblasts. While these fibroblasts proliferate for approximately 60 to 80 PDs before entering the state of replicative senescence, we found results similar to those we had observed in HEK cells. Specifically, although BJ fibroblasts that had acquired the LT mutant proteins unable to bind to pRB or p53 exhibited a slightly extended life span; these cells eventually entered a state of replicative arrest (Fig. 3C). In contrast, ectopic expression of wild-type LT or the LT J domain mutant form H42Q permitted the BJ fibroblasts to avoid replicative senescence. These various observations are consistent with the observations of others (72), which indicated that human cells require inactivation of both the pRB and p53 tumor suppressor pathways in order to bypass replicative senescence completely. Moreover, these observations demonstrated that the J domain does not play an essential role in permitting HEK and BJ cells to avoid senescence.
While the introduction of wild-type LT or the LT J domain mutant form permitted cells to bypass replicative senescence, these BJ and HEK cells eventually entered crisis. Introduction of hTERT into HEK or BJ cells expressing either wild-type LT or the H42Q LT mutant form resulted in immortalization of these cells (Fig. 3D and E). Taken together, these observations indicate that the J domain of LT is not required for cells to circumvent either senescence or crisis. Moreover, these results indicated that expression of ST is not required for the immortalization of HEK or BJ fibroblasts expressing LT and hTERT.
In fact, the introduction of LT followed by hTERT is not the only strategy that is known to immortalize certain types of cells. Several groups have shown that the ectopic expression of hTERT is also sufficient to allow some human cell types, including fibroblast, retinal pigment epithelial, and endothelial cells, to bypass senescence and become immortalized in a single step (5, 11, 36, 82, 88). These observations suggested that there are two types of immortalized human cells: those human cells that have been immortalized by the expression of both LT and hTERT and those fibroblasts that have been immortalized by the expression of hTERT alone. Indeed, as expected, immortalization resulted when hTERT was introduced into BJ fibroblasts expressing each of the LT mutant forms before they reached replicative senescence (Fig. 3F).
Alterations required for resistance to ras-induced senescence.
We then determined whether cells immortalized by these two experimental strategies are equally susceptible to transformation by a ras oncogene. Expression of a ras oncogene induces a premature senescence-like growth arrest in normal human fibroblasts (68). This growth arrest appears to be dependent on the response of both the pRB and p53 tumor suppressor pathways to expression of the oncogenic allele of ras (55, 68), and avoidance of this senescence is clearly a prerequisite to the emergence of ras-transformed cells. When we introduced an oncogenic allele of H-ras into the above-described BJ fibroblasts expressing LT mutant forms, each of the resulting cell lines expressed comparable levels of Ras protein (Fig. 4A). In consonance with a prior report (85), expression of oncogenic Ras in human fibroblasts immortalized by hTERT alone resulted in a senescence-like growth arrest (Fig. 4B), indicating that immortalization induced by hTERT is not sufficient to render cells transformable by an introduced ras oncogene. The expression of ST and hTERT also failed to permit cells to avoid the premature proliferative arrest induced by high-level expression of the H-Ras oncoprotein (data not shown).
FIG. 4.
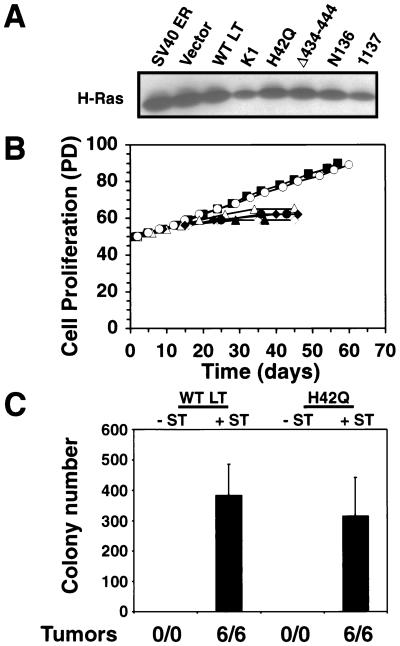
Effects of LT expression on resistance to ras-induced senescence. (A) Expression of H-Ras in BJ cells expressing the indicated LT mutant forms or a control vector and hTERT (data not shown). One hundred micrograms of total cell protein was analyzed. (B) Cell proliferation after introduction of H-Ras into BJ fibroblasts expressing a control vector (open diamonds), wild-type (WT) LT (filled squares), the K1 mutant form (filled triangles), the H42Q mutant form (open circles), the Δ434-444 mutant form (open triangles), the N136 mutant form (filled diamonds), and the 1137 mutant form (filled circles). (C) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of three experiments are shown. Tumor formation is reported as in Fig. 1.
Introduction of the ras oncogene induced a growth arrest in fibroblasts expressing LT mutant forms unable to bind to pRB (K1) or p53 (Δ434-444, N136, and 1137) (Fig. 4B). These observations indicated that both the pRB- and p53-inactivating functions of the LT protein are required to render hTERT-expressing cells resistant to ras-induced senescence. In addition, these observations are consistent with prior reports suggesting that ablation of both the pRB and p53 tumor suppressor pathways is necessary in order to bypass a ras-induced growth arrest (55, 68).
While the above-described experiments indicated that the inactivation of both p53 and pRB by LT is necessary to render cells resistant to ras-induced growth arrest, they did not indicate whether these ras-expressing cells had acquired the ability to grow in an AI manner or to form tumors. To address this point, we examined these two phenotypes in cells bearing the hTERT and ras genes together with wild-type LT or the H42Q J domain LT mutant form. While these cells were immortalized (Fig. 4B) and exhibited morphological features consistent with ras expression (data not shown), neither cell population was able to form colonies in soft agar or tumors in nude mice (Fig. 4C). These observations indicated that the pRB- and p53-inactivating functions of LT are indeed necessary for transformation in the presence of hTERT and ras but not sufficient. As anticipated, the subsequent introduction of ST enabled cells expressing wild-type LT or the H42Q J domain LT mutant form to grow in an AI fashion in soft agar and to form rapidly growing tumors (Fig. 4C).
Sufficiency of pRB and p53 disruption for transformation.
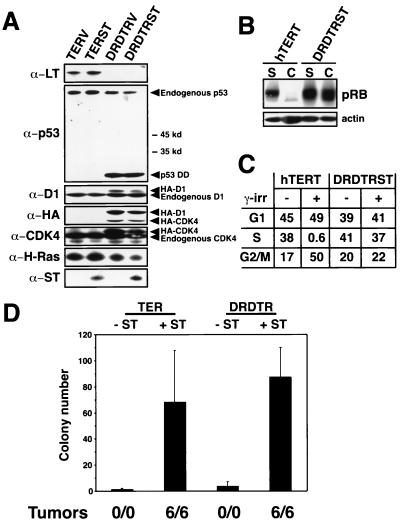
The above-described results did not preclude the possibility that an additional, uncharacterized function is exerted by LT that is essential for human cell transformation; such an essential but uncharacterized function might also be displayed by one of the HPV-encoded oncoproteins used in the transformation experiments described above. To address this possibility, we attempted to mimic the pRB- and p53-inactivating domains of LT by using mutant alleles of cellular genes that are known to disrupt the pRB and p53 pathways. To inactivate pRB functionally, we cointroduced into BJ fibroblasts a gene that expresses high levels of cyclin D1 constitutively together with the mutant R24C allele of the CDK4 gene (86), which encodes a kinase that is insensitive to inhibition by p16INK4A and p15INK4B, the major negative regulators of CDK4. In order to inactivate p53, we introduced into these cells a dominantly acting mutant allele of p53 (p53DD) that is known to disrupt tetramerization of the p53 protein (70). These three proteins were expressed in combination with hTERT, H-Ras, and ST (Fig. 5A).
As anticipated, coexpression of cyclin D1 and the R24C allele of CDK4 induced hyperphosphorylation of pRB even when cells reached confluence (Fig. 5B), confirming that these mutant forms acted to override the controls that normally govern pRB phosphorylation. Similarly, expression of p53DD conferred on these cells an increased resistance to the effects of gamma irradiation, one of the phenotypes known to result from the inactivation of p53 function (18). In BJ fibroblasts expressing only hTERT, treatment with 5 Gy induced a growth arrest by 24 h, as evidenced by a decrease in the percentage of cells traversing S phase from 38 to 0.6% (Fig. 5C) and an increase in the expression of p21 (data not shown). In contrast, identical exposure to gamma irradiation failed to induce a significant change in the cell cycle profile of cells expressing the p53DD mutant form (Fig. 5C). Only cells that expressed the combination of cyclin D1, the R24C CDK4 mutant form, p53DD, hTERT, H-Ras, and ST (DRDTRST) were able to grow in an AI fashion and form tumors in animals (Fig. 5D). Moreover, ST was needed for transformation when introduced into cells together with these cyclin D1, CDK4, and mutant p53 alleles, indicating that the requirement for ST is not specifically tied to the presence of LT in a cell.
FIG. 5.
Perturbation of the pRB and p53 tumor suppressor pathways. (A) Expression of indicated proteins in BJ cells expressing LT, hTERT, and ras with (TERST) or without (TERV) ST or in BJ cells expressing a combination of cyclin D1 (D1), the R24C CDK4 mutant form, hTERT, and ras with (DRDTRST) or without (DRDTRV) ST. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels, transferred nitrocellulose, and sequentially immunoblotted. The p53DD mutant form is a truncated form of p53, stabilizes endogenous p53, and migrates at approximately 28 kDa (70). Relative molecular mass (kilodaltons [kd]) is indicated. The D1 and R24C CDK4 proteins are HA epitope tagged and migrate more slowly than the endogenous forms of these proteins. Both the HA-tagged and endogenous forms of these proteins are indicated by arrowheads. All of the cell lines express hTERT, as assessed by TRAP assay (data not shown). (B) Effects of cyclin D1 and R24C CDK4 expression on pRB phosphorylation. Immunoblotting demonstrating pRB phosphorylation of cells growing exponentially (subconfluent [S]) or at confluence (C). BJ cells expressing only hTERT (hTERT) were used as a control. (C) Effects of gamma irradiation (γ-irr). Parallel cultures of BJ fibroblasts expressing only hTERT (hTERT) or DRDTRST were treated with 5 Gy. The percentage of cells in each phase of the cell cycle was determined by BrdU incorporation and propidium iodide staining 24 h after irradiation. (D) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of six experiments are shown. Tumor formation is reported as in Fig. 1.
These observations, as well as those obtained when we used HPV-16E6E7 (Fig. 2B and C), indicate that ST is required for transformation independently of how the pRB and p53 regulatory pathways are disrupted in cells. These observations also establish that while the LT oncoprotein may well perturb a number of other cellular pathways, its abilities to disrupt p53 and pRB function are both necessary and sufficient for its ability to collaborate with the other cointroduced genes to elicit the AI and tumorigenic cell phenotypes.
Host cell targets of ST.
The observations described above demonstrated the essential role played by ST in this cell transformation system. While sharing an N terminus with LT, ST has a unique C-terminal end. When assayed in vitro, this C-terminal portion of ST is known to bind to and inhibit the heterotrimeric cellular protein phosphatase 2A (PP2A) (52, 89). Serine- and threonine-specific PP2A is an abundant cellular enzyme that is composed of a catalytic C subunit bound to structural and regulatory A and B subunits. This enzyme exists in several forms, since at least two C and two A subunits have been described (reviewed in reference 44) and as many as 18 distinct type B subunits grouped into four families have been identified (reviewed in reference 28). The A and B subunits may confer substrate specificity on PP2A enzyme complexes that have been described (reviewed in references 28 and 44). ST is known to bind the A and C subunits of PP2A with high affinity, thereby displacing some, but not all, of the type B subunits (53, 75, 89). Specific cysteine residues located between aa 97 and 103 of ST form part of its PP2A-binding site, and alteration of these cysteines has been found to render ST incapable of binding PP2A (41, 49, 75).
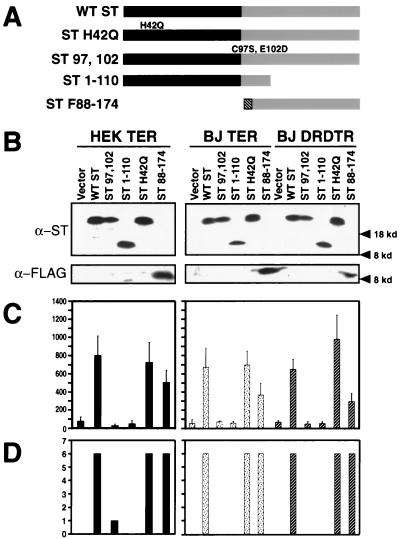
In order to determine whether the binding of ST to PP2A is essential to its ability to contribute to cell transformation, we created a series of ST mutant forms, which are depicted schematically in Fig. 6A. The effects of these mutations on the ability of ST to bind PP2A has been well described (40, 41, 49, 75). The ST 1-110 mutant form does not bind PP2A, while the ST H42Q mutant form contains a nonfunctional J domain (79). The ST 97,102 mutant form was created by replacing the cysteine and glutamic acid residues at positions 97 and 102 with serine and glutamine residues, respectively (ST 97,102). Each of these mutants retains the N-terminal epitopes recognized by MAbs Pab108 and 419, which were used in the analyses described here (23). In contrast, the ST F88-174 mutant form lacks the N-terminal portion of ST shared with LT. Since this mutant form also lacks the epitopes recognized by MAbs Pab108 and 419, we added an N-terminal FLAG epitope tag to this truncated ST to enable its identification.
We used retroviral vectors encoding each of these mutant forms or a control retrovirus conferring only drug resistance to introduce these versions of ST into three type of cells: BJ fibroblasts and HEK cells already expressing the cDNA version of LT, hTERT, and Ras (BJ TER and HEK TER) and BJ fibroblasts expressing the combination of cyclin D1, the R24C CDK4 mutant form, p53DD, hTERT, and ras genes (BJ DRDTR). When lysates prepared from these various cell populations were compared with one another, all of the ST mutant proteins were found to be expressed at comparable levels (Fig. 6B), as were the other ectopically expressed proteins (data not shown). When we tested these various cell populations for the ability to grow in an AI fashion (Fig. 6C) or to form tumors in animals (Fig. 6D), only ST mutant forms that retained the ability to bind PP2A (wild-type ST, H42Q ST, and ST F88-174) conferred tumorigenicity on these cells. We did not observe obvious differences in the ability of cells lacking a functional J domain to grow to a high density (data not shown), further suggesting that although a functional J domain is required for transformation in murine embryo fibroblasts (MEF) (79), it is dispensable for this type of human cell transformation. Thus, even subtle amino acid substitutions that disrupt the binding of ST to PP2A abolish the ability of ST to participate in the transformation process, making it highly likely that the critical targets of ST action are some of the PP2A enzyme complexes.
FIG. 6.
Mapping of the functional domain of ST required for transformation. (A) Schematic representation of ST mutant forms. Black bars represent the domain shared by LT and ST. The FLAG epitope tag is represented by the hatched region. WT, wild type. (B) Expression of ST mutant forms. One hundred micrograms of total cell protein was separated on 7.5 to 15% gradient gels and blotted with Pab108 and Pab419 or the M2 MAb specific for the FLAG epitope tag. Expression of the other introduced proteins was similar (data not shown). (C and D) AI growth and tumorigenicity of the indicated cell lines. The mean and standard deviation of three experiments are shown. Tumor formation is reported as in Fig. 1. Black bars represent HEK cells expressing LT, hTERT, and ras. Dotted bars represent BJ fibroblasts expressing LT, hTERT, and ras. Hatched bars represent BJ fibroblasts expressing the combination of cyclin D1, the R24C CDK4 mutant form, the p53DD mutant form, hTERT, and ras. kd, kilodaltons.
Effects of ST on cell proliferation.
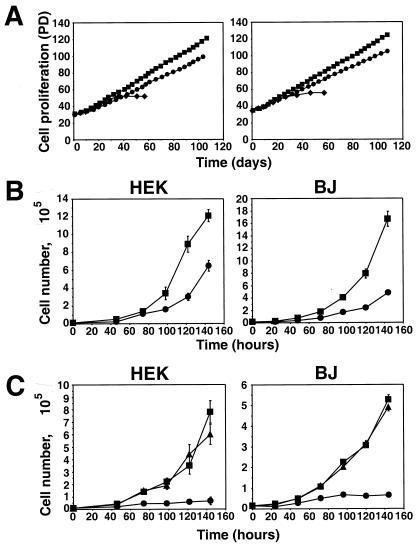
Because PP2A is a widely acting phosphatase and is likely to have numerous phosphoprotein substrates (reviewed in reference 44), we deferred attempts to uncover, at the biochemical level, the signaling pathway perturbed by ST. Instead, we attempted to learn some of the cell physiologic consequences of ST action. In order to determine whether the ability of hTERT to immortalize these BJ fibroblasts and HEK cells is altered by the presence of ST, we compared the proliferation rate of cell populations expressing ST with that of cell populations that had acquired only a control vector. As seen in Fig. 7A, expression of ST did not affect the ability of hTERT to immortalize cells expressing LT; hence, we could find no evidence that ST is able to affect immortalization or the cellular life span. However, when introduced into either BJ or HEK cells expressing LT, hTERT, and ras, the expression of ST had a significant effect on the their rate of proliferation (Fig. 7A and B). Specifically, cells expressing ST had a doubling time 33% shorter than that of control cells lacking ST expression, resulting in accumulation of two to three times as many cells after 6 days (Fig. 7B). The effect of ST was similar whether cells were grown under normal (10% FCS or low-serum (0.5% FCS) conditions (data not shown). Furthermore, when we analyzed the size of cells expressing ST, we found that they were consistently 15% smaller than cells expressing a control vector (data not shown). These observations confirmed that expression of ST leads to accelerated cell cycle transit and thus an increase in cell proliferation.
FIG. 7.
Effects of ST on cell proliferation. (A) ST does not affect immortalization. Proliferation of HEK or BJ cells expressing LT, hTERT, and ras with (squares) or without (circles) ST. For comparison, HEK or BJ cells expressing only LT are also depicted (diamonds). PD is defined as in Fig. 3. (B) Effects of ST on proliferation. HEK and BJ cells were grown in medium supplemented with the normal concentration of glutamine (3.75 mM). Symbols are as in panel A. (C) Effects of ST on cell proliferation at a limiting glutamine concentration (0.375 mM). Cells expressing LT, hTERT, and ras with (squares) or without (circles) ST and cells expressing LT, hTERT, and ST without ras (triangles) are shown. In panels B and C, the mean and standard deviation of three experiments are shown.
This observed enhanced rate of proliferation could not, on its own, explain the effects of ST on cell transformation. For example, cells lacking ST grew poorly when grown without anchorage to a solid substrate (Fig. 2B). Furthermore, cells expressing LT, hTERT, and H-Ras but lacking ST did not form tumors, even after 6 months of observation (Fig. 2C). Since AI growth conditions in vitro and s.c. growth in vivo may induce cellular stress and cell death by anoikis (65), we investigated the response of cells expressing ST to stimuli known to induce cellular stress. These stressors included amino acid deprivation, hypoxia, gamma irradiation, and exposure to oxygen radicals. Upon exposure to hypoxia (0.1% O2), oxygen radicals (dilute 30 μM hydrogen peroxide), or gamma irradiation (up to 12 Gy), we observed no difference in the ability of cells expressing ST to resist these insults compared with that of parallel cultures of cells expressing a control vector (data not shown).
A quite different outcome was observed when we stressed BJ and HEK cells with nutrient starvation by making the amino acid glutamine limiting (Fig. 7C). Under conditions in which glutamine was supplemented at 10% of the concentration normally used, the growth of cells expressing LT, hTERT, and ras but lacking ST slowed considerably compared with tht of parallel cultures grown under normal culture conditions, and their doubling time increased from 28 to 68 h. In contrast, cells expressing LT, hTERT, ras, and ST showed relative resistance to the proliferation-suppressing effects of this nutrient starvation, as evidenced by the small increase in their doubling time from 19 to 28 h. This effect of ST on cell proliferation was also observed in the absence of H-Ras expression, indicating that the expression of ST was not simply allowing cells to tolerate constitutive H-Ras signaling (Fig. 7C). Furthermore, this effect of ST on cell proliferation at limiting glutamine concentrations was not enhanced by the additional stresses created by either hypoxia (0.1% oxygen) or a low (0.5%) serum concentration (data not shown). Flow cytometric analysis of cells grown in the presence of BrdU revealed that under these nutrient-limiting conditions, the expression of ST did not markedly alter the cell cycle phase distribution of these cells, nor did any appreciable number of cells become apoptotic (Table 1 and data not shown), indicating that cells continued to transit the cell cycle, albeit more slowly. If cultured without further glutamine supplementation, ST-expressing cells eventually exhibited widespread cell death; supplementation of such cultures with further glutamine allowed the cells to continue to proliferate unabated (data not shown). Taken together, these observations suggest that ST may collaborate in the transformation of human cells by inactivating the controls that control cell proliferation in response to limiting nutrient conditions. It is clear, moreover, that the nutrient starvation used here to challenge cells may represent only one of a large class of physiologic stresses that operate to suppress cell proliferation, and thus transformation, in the absence of ST action.
TABLE 1.
Cell cycle distribution of HEK cells or BJ fibroblasts expressing LT, hTERT, ras, and a control vector or ST under normal and limiting glutamine conditionsa
| Cell type and presence of ST | Glutamine concn (mM) | G1 | S | G2/M |
|---|---|---|---|---|
| HEK | ||||
| − ST | 0.375 | 43 | 24 | 33 |
| 3.75 | 45 | 26 | 29 | |
| + ST | 0.375 | 41 | 23 | 36 |
| 3.75 | 43 | 21 | 36 | |
| BJ | ||||
| − ST | 0.375 | 52 | 18 | 30 |
| 3.75 | 52 | 17 | 32 | |
| + ST | 0.375 | 45 | 16 | 39 |
| 3.75 | 43 | 24 | 33 |
The values shown, unless indicated otherwise, are percentages of cells in each phase of the cell cycle. Results of an experiment representative of three experiments are shown.
DISCUSSION
The present observations indicate that the actions of hTERT and oncogenic H-Ras, together with three SV40-encoded functions, are sufficient for the transformation of human fibroblasts and HEK cells. The operational definition of transformation used here implies the ability of cells to form tumors in immunocompromised mice without showing the additional phenotypes of invasiveness or metastatic ability. At least two of these necessary viral functions—those affecting pRB and p53—can be replaced by mutant alleles of cellular genes. Indeed, our success in replacing LT with a combination of mutant cellular genes that selectively disrupt pRB and p53 function indicates that all of the other functions exerted by LT are not required for the induction of tumorigenic growth of human cells.
Previous studies have identified and characterized the elements of the SV40 ER required for the immortalization and transformation of various types of rodent cells. While the experimental transformations of human, mouse, and rat fibroblasts with SV40 share many fundamental features, several differences in the contribution of specific elements of the SV40 ER to human and rodent cell immortalization and transformation are apparent. Although some of these discrepancies likely represent differences in the experimental protocols used by various research groups, it is clear that rodent cells are much more susceptible to transformation than are human cells (42, 64). Identifying and understanding these species-specific differences in the functioning of the various SV40 ER oncoproteins will help elucidate the molecular mechanisms that lead to immortalization and transformation during the formation of human cancers.
In particular, the frequency of spontaneous immortalization upon extended passage in vitro differs considerably between rodent and human cells. Rodent cells are readily immortalized, in contrast to most types of human cells, which virtually never undergo spontaneous immortalization in culture (87). Paralleling this difference in the rate of spontaneous immortalization, several studies have demonstrated that expression of the bipartite p53-binding domain of LT alone is sufficient to extend the life span of most primary MEF (79, 80, 93). These findings are consistent with observations that implicate the p19ARF-MDM2-p53 pathway as a critical determinant of the murine cell life span (24, 31). In contrast, our observations obtained by using human fibroblasts and HEK cells (Fig. 3) confirm a previous report (71) that the binding and sequestration of both pRB and p53 by LT are necessary to allow human cells to bypass replicative senescence in the absence of telomerase expression. Even though most murine cells express constitutive telomerase activity (59) and maintain extremely long telomeres (35), this difference in telomere biology between human and rodent cells fails to explain the additional requirement for the inactivation of the pRB pathway for human cell immortalization. Although Conzen and Cole have reported that expression of LT mutant forms containing internal deletions of the pRB-binding site or of the J domain immortalized primary MEF with poor efficiency (8), we and others (71) have never obtained immortalized human cells expressing the LT K1 mutant form that is unable to bind pRB. In consonance with prior studies using MEF (8), we found that ST does not play a significant role in the immortalization of human cells.
The transformation of both primary human and murine cells by SV40 LT requires the inactivation of both the pRB and p53 pathways (21, 76, 77, 80, 90, 93). In addition, prior studies have suggested that the J domain of LT plays an important role in the transformation of MEF (76, 90). Expression of LT J domain mutant forms in MEF abrogates the ability of such cells to grow to a high density or to grow under limiting serum conditions compared to cells expressing wild-type LT yet did not affect the ability of such cells to grow in an AI manner in soft agar (79). Since the J domain was dispensable for these phenotypes in MEF doubly deficient for the pRB-related proteins p107 and p130, the J domain appears to alter the ability of LT to inactivate these pRB-related proteins (79). Moreover, the J domain of ST is functional since expression of ST can complement LT J domain mutant forms to transform MEF (45). However, in the experimental model of human cell transformation presented here, by expressing a mutant ST lacking a functional J domain (ST H42Q or ST F88-174) in combination with cyclin D1, the R24C CDK4 mutant form, p53DD, hTERT, and ras genes, we were able to transform primary fibroblasts to a tumorigenic state without introduction of a J domain (Fig. 6), indicating that an intact LT J domain is not required for immortalization, growth in soft agar, or the formation of tumors in human cells. Although the reason for this discrepancy between human and murine cell transformations remains obscure, it is possible that the relative contribution of each of the pRB-related proteins to suppression of transformation differs between MEF and human fibroblasts or that the particular combination of transforming elements used here permits transformation without the requirement for a functional J domain.
These observations raise the question of whether perturbation of the pathways examined here (involving the pRB, p53, PP2A, hTERT, and Ras proteins) is also sufficient for the transformation of these and other types of human cells. We have accumulated three lines of evidence that persuade us that the experimental disruption of these pathways does, indeed, suffice to convert normal human cells to tumorigenicity. First, we introduced these genes by retroviral vectors, ensuring that polyclonal populations were produced (21, 95); this substantially reduced the possibility that rare, secondary transforming mutations occurring in still unknown genes are required to act, in concert with the introduced genes, to achieve transformation. Importantly, the populations of tumor cells that emerge from these manipulations and, indeed, from tumor-bearing animals retain evidence of polyclonality (13, 21). Second, when transformed cells that have formed tumors in a host are subsequently reimplanted in a second host animal, these cells form tumors with kinetics that are identical to those of cells that have never been implanted in a host mouse (13, 21). Such observations make it highly unlikely that the tumorigenic phenotype of these cells depends on the acquisition of an additional, mutant allele during their passage in vivo. Finally, we have demonstrated that many of the transformed HEK cells lack evidence of widespread genomic instability, which might yield a plethora of secondary mutations that would confound the genetic characterization of the tumorigenic cells studied here. Instead, these transformed HEK cells were largely diploid, with no evidence of microsatellite instability or translocations (95). Taken together, these various observations provide persuasive evidence that these five alterations are sufficient to achieve the tumorigenic transformation observed by us (13, 21) and others (60).
The identification of ST as an essential participant in the transformation assay has permitted us to begin to enumerate the major pathways that contribute to human cell transformation and explains the previously observed inability of the HPV-16 E6 and E7 oncoproteins to transform human fibroblasts when expressed in combination with hTERT and oncogenic H-Ras (46; Fig. 2C and D). ST has long been known to play a role in transformation by SV40. SV40 mutants that are incapable of producing ST show a diminished ability to transform rodent cells (61, 74). In addition, De Ronde et al. (10) showed that similar SV40 mutants were unable to transform human fibroblasts when focus-forming ability was gauged but could transform murine fibroblasts, albeit less efficiently than wild-type SV40. This dependence on ST was more apparent in assays performed when cells were at a high density (93) or when LT was expressed at lower levels (2). More recently, others have demonstrated that human mesothelial cells expressing both SV40 LT and ST are more easily transformed by subsequent exposure to carcinogens than are cells expressing only LT (4). Indeed, transgenic mice expressing both LT and ST under the control of the mouse mammary tumor virus promoter developed a broader spectrum of epithelial cancers than did mice expressing only LT (7). While these studies investigated the role of ST in the context of LT expression, the data presented here demonstrate that the expression of ST is also required to complement the disruption of the pRB and p53 pathways even if these pathways are disrupted through other genetic means. Taken together, these observations demonstrate the important contribution of ST, ostensibly through its perturbation of cellular PP2A, to the transformation of human cells.
The present observations raise the question of whether alterations of PP2A function also occur during the pathogenesis of spontaneously arising human tumors. Recently, two groups have identified a subset of lung cancers, breast cancers, and malignant melanoma that show loss of heterozygosity at chromosome 11q22-24, which contains the genetic locus specifying the PP2A β56 A subunit (6, 84). In approximately 15% of such tumors, further mutations or deletions affecting conserved amino acids in the surviving β56 allele were identified that would be predicted to perturb PP2A function by disrupting the interaction of this PP2A subunit with the other two subunits of the PP2A holoenzyme. These observations suggest that alterations in PP2A can play an important role in the development of spontaneously arising human cancers. However, these tumors represent only a small proportion of human cancers and it remains to be determined whether systematic efforts to survey the array of PP2A subunit genes in human tumor genomes will reveal frequent disruptions of the normal signaling regulated by this enzyme complex.
Expression of ST induces increased cell proliferation in cells immortalized with LT and hTERT (Fig. 5). In addition, using cells expressing or lacking constitutive H-Ras signaling, we showed that ST induces cell proliferation independently of the activity of the H-Ras signaling pathway. While cells that express only LT, hTERT, and Ras are immortal, these cells double three times more slowly in vitro than do corresponding cells that also express ST, and these ST-negative cells do not form tumors, even after 6 months of observation postimplantation. Consistent with these observations, transient expression of ST in CV-1 monkey cells also stimulated cell growth, correlated with an increase in the phosphorylation of the ERK (extracellular signal-regulated protein kinase) and MEK (mitogen-activated protein/ERK kinase) kinases in vitro (75).
The stimulation of cell proliferation induced by the presence of ST cannot explain the full contribution of ST to cell transformation. In particular, we observed the growth-stimulatory effects of ST when cells were grown in the absence of anchorage to a solid substrate (Fig. 2B) or when they were grown under conditions of amino acid deprivation (Fig. 7C). Comparable conditions are likely to exist when cells are placed experimentally into host animals to assess tumorigenicity and may also mimic conditions that nascent tumor cells face early in the formation of a spontaneously arising tumor. Taken together, these observations suggest that ST disrupts one or more cellular regulatory checkpoints that limit cell proliferation when cells encounter suboptimal growth conditions.
ST mutant forms previously shown to disrupt PP2A binding also fail to cooperate to transform human cells (Fig. 6). While it remains formally possible that a second host cell protein shares the same binding site on ST, these observations suggest that ST must interact with PP2A in order to collaborate in the transformation process. However, the precise biochemical alterations that result from this interaction remain elusive. Genetic deletion of PP2A in eukaryotic cells results in cell death (14, 20); thus, complete inhibition of PP2A is not possible. Furthermore, since PP2A represents a large and complex family of enzymes, the resulting holoenzymes created through the combinatorial associations of its various alternative subunits may be responsible for the dephosphorylation and regulation of hundreds of distinct cellular phosphoprotein substrates. For these reasons, it is clear that further investigation is required to identify the substrate proteins whose dephosphorylation by PP2A must be inhibited by ST in order for cell transformation to occur.
We speculate that perturbation of the five pathways described here will be found in most, if not all, types of human tumors. If so, many of the genetic lesions found in human tumor cell genomes may one day be rationalized in terms of their effects on these five pathways. Clearly, these pathways do not elicit two changes that are frequently associated with human tumor cells: those that destabilize the genome and thereby accelerate the rate of tumor pathogenesis and those that confer the phenotypes of invasiveness and metastasis. Furthermore, since successful transformation required H-Ras expression at levels 10- to 20-fold higher than those seen in human tumor samples (13), the overexpressed Ras oncoprotein may function in a manner that is qualitatively similar to the actions of physiologic Ras levels, merely permitting the rapid growth of tumors in the 3- to 6-week time span of these experiments. Alternatively, the high levels of H-Ras expression used here may act in a fashion that is qualitatively different from that of the Ras oncoprotein in human tumors; by virtue of its higher levels of expression, it may activate additional downstream signaling pathways whose activation contributes to the observed tumorigenic growth.
The increased availability of cultures of primary human epithelial, mesenchymal, neuroectodermal, and hematopoietic cells from various tissues will facilitate the construction of a library of human tumor cells by genetic strategies similar to that used here. Further replacement of the genetic elements used in the present studies with mutant genes found in particular types of human cancers will create models that will increasingly mimic the phenotypes of naturally arising human tumor cells and will permit investigation of the additional changes required to allow cells to invade and metastasize. Finally, the availability of genetically defined human tumor cells will facilitate the testing and validation of therapies in which cancer cell responses to specific therapies will be rationalized in terms of the genetic and biochemical lesions introduced into such cells.
Acknowledgments
S.K.D. and M.W.B. contributed equally to this work.
We thank D. Galloway for the gift of retroviral producer cell lines expressing the HPV-16 E6 and E7 oncoproteins, J. Pippas and T. van Dyke for LT mutant forms, and M. Oren for the p53DD mutant form.
This work was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award (W.C.H.), grants from the National Cancer Institute (K01 CA94223 [W.C.H.], K08 HL04463 [S.K.D.], R01 CA63113 [J.A.D.], P01 CA50661 [J.A.D.], and R01 CA78461 [R.A.W.]), a Dunkin Donuts Rising Star Award (W.C.H.), and the G. Harold and Leila Y. Mathers Charitable Foundation (R.A.W.). J.A.D. is a Scholar of the Leukemia and Lymphoma Society. R.A.W. is an American Cancer Society Research Professor and a Daniel K. Ludwig Cancer Research Professor.
REFERENCES
- 1.Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. [DOI] [PubMed] [Google Scholar]
- 2.Bikel, I., X. Montano, M. E. Agha, M. Brown, M. McCormack, J. Boltax, and D. M. Livingston. 1987. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell 48:321-330. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, J. M., and R. A. Weinberg (ed.). 1996. Molecular oncology. Scientific American, Inc., New York. N.Y.
- 4.Bocchetta, M., I. Di Resta, A. Powers, R. Fresco, A. Tosolini, J. R. Testa, H. I. Pass, P. Rizzo, and M. Carbone. 2000. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc. Natl. Acad. Sci. USA 97:10214-10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 6.Calin, G. A., M. G. di Iasio, E. Caprini, I. Vorechovsky, P. G. Natali, G. Sozzi, C. M. Croce, G. Barbanti-Brodano, G. Russo, and M. Negrini. 2000. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19:1191-1195. [DOI] [PubMed] [Google Scholar]
- 7.Choi, Y. W., I. C. Lee, and S. R. Ross. 1988. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol. Cell. Biol. 8:3382-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conzen, S. D., and C. N. Cole. 1995. The three transforming regions of SV40 T antigen are required for immortalization of primary mouse embryo fibroblasts. Oncogene 11:2295-2302. [PubMed] [Google Scholar]
- 9.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ronde, A., C. J. Sol, A. van Strien, J. ter Schegget, and J. van der Noordaa. 1989. The SV40 small t antigen is essential for the morphological transformation of human fibroblasts. Virology 171:260-263. [DOI] [PubMed] [Google Scholar]
- 11.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, D. N. Louis, R. A. Weinberg, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also evade a p16ink4a-enforced lifespan limit become immortal while retaining normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyson, N., K. Buchkovich, P. Whyte, and E. Harlow. 1989. Cellular proteins that are targetted by DNA tumor viruses for transformation. Princess Takamatsu Symp. 20:191-198. [PubMed] [Google Scholar]
- 13.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. R., T. Myles, J. Hofsteenge, and B. A. Hemmings. 1999. Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J. Biol. Chem. 274:24038-24046. [DOI] [PubMed] [Google Scholar]
- 15.Fanning, E., and R. Knippers. 1992. Structure and function of simian virus 40 large tumor antigen. Annu. Rev. Biochem. 61:55-85. [DOI] [PubMed] [Google Scholar]
- 16.Flint, J., and T. Shenk. 1997. Viral transactivating proteins. Annu. Rev. Genet. 31:177-212. [DOI] [PubMed] [Google Scholar]
- 17.Foster, S. A., G. W. Demers, B. G. Etscheid, and D. A. Galloway. 1994. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J. Virol. 68:5698-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 19.Gjoerup, O., H. Chao, J. A. DeCaprio, and T. M. Roberts. 2000. pRB-dependent, J domain-independent function of simian virus 40 large T antigen in override of p53 growth suppression. J. Virol. 74:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotz, J., A. Probst, E. Ehler, B. Hemmings, and W. Kues. 1998. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Cα. Proc. Natl. Acad. Sci. USA 95:12370-12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumor cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 22.Halvorsen, T. L., G. Leibowitz, and F. Levine. 1999. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol. Cell. Biol. 19:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey, D. M., and A. J. Levine. 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 5:2375-2385. [DOI] [PubMed] [Google Scholar]
- 25.Hinds, P. W., S. Mittnacht, V. Dulic, A. Arnold, S. I. Reed, and R. A. Weinberg. 1992. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70:993-1006. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa, T., and H. E. Ruley. 1988. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc. Natl. Acad. Sci. USA 85:1519-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huibregtse, J. M., and S. L. Beaudenon. 1996. Mechanism of HPV E6 proteins in cellular transformation. Semin. Cancer Biol. 7:317-326. [DOI] [PubMed] [Google Scholar]
- 28.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jat, P. S., C. L. Cepko, R. C. Mulligan, and P. A. Sharp. 1986. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol. Cell. Biol. 6:1204-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalderon, D., and A. E. Smith. 1984. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology 139:109-137. [DOI] [PubMed] [Google Scholar]
- 31.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 32.Kierstead, T. D., and M. J. Tevethia. 1993. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J. Virol. 67:1817-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 34.Kim, N. W., and F. Wu. 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 25:2595-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipling, D., and H. J. Cooke. 1990. Hypervariable ultra-long telomeres in mice. Nature 347:400-402. [DOI] [PubMed] [Google Scholar]
- 36.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 37.Kubbutat, M. H., and K. H. Vousden. 1998. New HPV E6 binding proteins: dangerous liaisons? Trends Microbiol. 6:173-175. [DOI] [PubMed] [Google Scholar]
- 38.Levine, A. J. 1990. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology 177:419-426. [DOI] [PubMed] [Google Scholar]
- 39.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 40.Martin, R. G., V. P. Setlow, C. A. Edwards, and D. Vembu. 1979. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell 17:635-643. [DOI] [PubMed] [Google Scholar]
- 41.Mateer, S. C., S. A. Fedorov, and M. C. Mumby. 1998. Identification of structural elements involved in the interaction of simian virus 40 small tumor antigen with protein phosphatase 2A. J. Biol. Chem. 273:35339-35346. [DOI] [PubMed] [Google Scholar]
- 42.McCormick, J. J., D. G. Fry, P. J. Hurlin, T. L. Morgan, D. M. Wilson, and V. M. Maher. 1990. Malignant transformation of human fibroblasts by oncogene transfection or carcinogen treatment. Prog. Clin. Biol. Res. 340D:195-205. [PubMed]
- 43.Michalovitz, D., L. Fischer-Fantuzzi, C. Vesco, J. M. Pipas, and M. Oren. 1987. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J. Virol. 61:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 45.Montano, X., R. Millikan, J. M. Milhaven, D. A. Newsom, J. W. Ludlow, A. K. Arthur, E. Fanning, I. Bikel, and D. M. Livingston. 1990. Simian virus 40 small tumor antigen and an amino-terminal domain of large tumor antigen share a common transforming function. Proc. Natl. Acad. Sci. USA 87:7448-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 47.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munger, K., M. Scheffner, J. M. Huibregtse, and P. M. Howley. 1992. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 12:197-217. [PubMed] [Google Scholar]
- 49.Mungre, S., K. Enderle, B. Turk, A. Porras, Y. Q. Wu, M. C. Mumby, and K. Rundell. 1994. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J. Virol. 68:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newbold, R. F., and R. W. Overell. 1983. Fibroblast immortality is a prerequisite for transformation by EJ c-HA-ras oncogene. Nature 304:648-651. [DOI] [PubMed] [Google Scholar]
- 51.Newbold, R. F., R. W. Overell, and J. R. Connell. 1982. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature 299:633-635. [DOI] [PubMed] [Google Scholar]
- 52.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60:167-176. [DOI] [PubMed] [Google Scholar]
- 53.Pallas, D. C., W. Weller, S. Jaspers, T. B. Miller, W. S. Lane, and T. M. Roberts. 1992. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J. Virol. 66:886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peden, K. W., A. Srinivasan, J. M. Farber, and J. M. Pipas. 1989. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology 168:13-21. [DOI] [PubMed] [Google Scholar]
- 55.Peeper, D. S., J. H. Dannenberg, S. Douma, H. te Riele, and R. Bernards. 2001. Escape from premature senescence is not sufficient for oncogenic transformation by Ras. Nat. Cell Biol. 3:198-203. [DOI] [PubMed] [Google Scholar]
- 56.Pipas, J. M., and A. J. Levine. 2001. Role of T antigen interactions with p53 in tumorigenesis. Semin. Cancer Biol. 11:23-30. [DOI] [PubMed] [Google Scholar]
- 57.Pipas, J. M., K. W. Peden, and D. Nathans. 1983. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol. Cell. Biol. 3:203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prives, C., and Y. Beck. 1977. Characterization of SV40 T antigen polypeptides synthesized in vivo and in vitro. INSERM-EMBO 69:175-188. [Google Scholar]
- 59.Prowse, K. R., and C. W. Greider. 1995. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA 92:4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich, J. N., C. Guo, R. E. McLendon, D. D. Bigner, X. F. Wang, and C. M. Counter. 2001. A genetically tractable model of human glioma formation. Cancer Res. 61:3556-3560. [PubMed] [Google Scholar]
- 61.Rubin, H., J. Figge, M. T. Bladon, L. B. Chen, M. Ellman, I. Bikel, M. Farrell, and D. M. Livingston. 1982. Role of small t antigen in the acute transforming activity of SV40. Cell 30:469-480. [DOI] [PubMed] [Google Scholar]
- 62.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 63.Sager, R. 1991. Senescence as a mode of tumor suppression. Environ. Health Perspect. 93:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sager, R., K. Tanaka, C. C. Cau, Y. Ebina, and A. Anisowicz. 1983. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc. Natl. Acad. Sci. USA 80:7601-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz, M. A. 1993. Signaling by integrins: implications for tumorigenesis. Cancer Res. 53:1503-1506. [PubMed] [Google Scholar]
- 66.Sedivy, J. M. 1997. Can the ends justify the means?: telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA 95:9078-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sellers, W. R., and W. G. Kaelin. 1997. Role of the retinoblastoma protein in the pathogenesis of human cancer. J. Clin. Oncol. 15:3301-3312. [DOI] [PubMed] [Google Scholar]
- 68.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16ink4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro, G. I., C. D. Edwards, M. E. Ewen, and B. J. Rollins. 1998. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 18:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shay, J. W., O. M. Pereira-Smith, and W. E. Wright. 1991. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196:33-39. [DOI] [PubMed] [Google Scholar]
- 72.Shay, J. W., W. E. Wright, and H. Werbin. 1991. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1072:1-7. [DOI] [PubMed] [Google Scholar]
- 73.Shenk, T. E., J. Carbon, and P. Berg. 1976. Construction and analysis of viable deletion mutants of simian virus 40. J. Virol. 18:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sleigh, M. J., W. C. Topp, R. Hanich, and J. F. Sambrook. 1978. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell 14:79-88. [DOI] [PubMed] [Google Scholar]
- 75.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 76.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srinivasan, A., K. W. Peden, and J. M. Pipas. 1989. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J. Virol. 63:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart, N., and S. Bacchetti. 1991. Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology 180:49-57. [DOI] [PubMed] [Google Scholar]
- 79.Stubdal, H., J. Zalvide, K. S. Campbell, C. Schweitzer, T. M. Roberts, and J. A. DeCaprio. 1997. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol. Cell. Biol. 17:4979-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson, D. L., D. Kalderon, A. E. Smith, and M. J. Tevethia. 1990. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology 178:15-34. [DOI] [PubMed] [Google Scholar]
- 81.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11:281-288. [DOI] [PubMed] [Google Scholar]
- 82.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 83.Volckaert, G., J. Feunteun, L. V. Crawford, P. Berg, and W. Fiers. 1979. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J. Virol. 30:674-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang, S. S., E. D. Esplin, J. L. Li, L. Huang, A. Gazdar, J. Minna, and G. A. Evans. 1998. Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282:284-287. [DOI] [PubMed] [Google Scholar]
- 85.Wei, S., and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 86.Wölfel, T., M. Hauer, J. Schneider, M. Serrano, E. Wölfel, E. Klehmann-Hieb, E. De Plaen, T. Hankeln, K. H. Meyer zum Büschenfelde, and D. Beach. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytotoxic T lymphocytes in a human melanoma. Science 269:1281-1284. [DOI] [PubMed] [Google Scholar]
- 87.Wright, W. E., and J. W. Shay. 2000. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat. Med. 6:849-851. [DOI] [PubMed] [Google Scholar]
- 88.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 89.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zalvide, J., and J. A. DeCaprio. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol. Cell. Biol. 15:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zalvide, J., H. Stubdal, and J. A. DeCaprio. 1998. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol. Cell. Biol. 18:1408-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zerrahn, J., U. Knippschild, T. Winkler, and W. Deppert. 1993. Independent expression of the transforming amino-terminal domain of SV40 large T antigen from an alternatively spliced third SV40 early mRNA. EMBO J. 12:4739-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu, J., P. W. Rice, L. Gorsch, M. Abate, and C. N. Cole. 1992. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J. Virol. 66:2780-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu, J., H. Wang, J. M. Bishop, and E. H. Blackburn. 1999. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc. Natl. Acad. Sci. USA 96:3723-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimonjic, D. B., M. W. Brooks, N. Popescu, R. A. Weinberg, and W. C. Hahn. 2001. Derivation of human tumor cells in vitro without widespread genomic instability. Cancer Res. 61:8838-8844. [PubMed]