Abstract
One of the least-understood areas in biology is the determination of the size of animals and their organs. In Drosophila, components of the insulin receptor phosphoinositide 3-kinase (PI3K) pathway determine body, organ, and cell size. Several biochemical studies have suggested that Akt/protein kinase B is one of the important downstream targets of PI3K. To examine the role of Akt in the regulation of organ size in mammals, we have generated and characterized transgenic mice expressing constitutively active Akt (caAkt) or kinase-deficient Akt (kdAkt) specifically in the heart. The heart weight of caAkt transgenic mice was increased 2.0-fold compared with that of nontransgenic mice. The increase in heart size was associated with a comparable increase in myocyte cell size in caAkt mice. The kdAkt mutant protein attenuated the constitutively active PI3K-induced overgrowth of the heart, and the caAkt mutant protein circumvented cardiac growth retardation induced by a kinase-deficient PI3K mutant protein. Rapamycin attenuated caAkt-induced overgrowth of the heart, suggesting that the mammalian target of rapamycin (mTOR) or effectors of mTOR mediated caAkt-induced heart growth. In conclusion, Akt is sufficient to induce a marked increase in heart size and is likely to be one of the effectors of the PI3K pathway in mediating heart growth.
One of the least-understood areas of biology is determination of the size of animals and their organs (23). Recent Drosophila genetic studies revealed that several molecules that are activated by insulin or insulin-like growth factors (IGFs) in mammals regulate organ and body size in flies (76). Hypomorphic mutations in Inr, which encodes the insulin/IGF receptor, results in smaller flies (12). Overexpression of activated or dominant negative phosphoinositide 3-kinase (PI3K), a target of the insulin/IGF receptor, increases or decreases the size of fly wings, respectively (42). A null mutation in chico, which encodes the Drosophila insulin receptor substrate protein, causes a reduction in the size and number of cells in each organ (8). In contrast, a mutation in ribosomal S6 kinase (S6K), a downstream effector of PI3K, results in flies that have a normal number of cells, but the cells are smaller than wild-type cells (46). Deletion of genes for IGFs or their receptors (6, 26, 44), insulin receptor substrates (5, 67, 78), or S6K (62) results in dwarfism in mice, suggesting the importance of the conserved insulin/IGF-PI3K pathway in body size determination in mammals.
Akt, also known as RAC-PK (protein kinase related to A and C kinases) (37) or protein kinase B (21), is one of the best-characterized targets of PI3K lipid products (11, 72). Mammals have three closely related genes that encode the isoforms Akt1, Akt2, and Akt3 (72). Akt2 and Akt3 show 81 and 83% amino acid identity, respectively, with Akt1. All of the Akt isoforms show a broad tissue distribution. Akt is activated by a multistep process via a variety of signals (11). In the early steps of this process, D3-phosphorylated phosphoinositides generated by PI3K bind the pleckstrin homology domain of Akt and translocate the kinase to the membrane. On the membrane, Thr308 of Akt is phosphorylated by phosphoinositide-dependent protein kinase 1 (PDK1) (2) and Ser473 is phosphorylated by a complex mechanism that may involve autophosphorylation (69). Phosphorylation of Thr308 and Ser473 is critical for activation of Akt, since mutations of Thr308 and Ser473 to alanine inhibit the insulin- or IGF1-induced activation of Akt. Conversely, mutations of both residues to aspartic acid, to mimic negative charges of phosphorylation, produce a constitutively activated form of Akt (1).
Akt is postulated to promote cell growth by regulating protein synthesis through other potential effectors (11, 71, 72). Glycogen synthase kinase 3 (GSK-3) is a well-defined direct target of Akt, and phosphorylation of GSK-3 by Akt reduces the kinase activity of GSK-3 (24, 28). GSK-3 phosphorylates the GTP-GDP exchange factor eIF2B and negatively regulates initiation factor eIF2-directed methionyl-tRNA binding to the 40S ribosome (20). Thus, phosphorylation of GSK by Akt might increase protein synthesis by increasing the efficiency of translation. Another potential downstream effector is S6K1. S6K1 is a physiological kinase for the ribosomal S6 protein, whose phosphorylation increases the rate of initiation of translation of mRNA by ribosomes (18, 68). A highly homologous gene, which encodes S6K2, has recently been identified. (62). Akt alone is sufficient to activate S6K1 in some cultured cell lines (4, 10), although it is likely that activation of S6K1 is not solely dependent on Akt (28).
Another potential effector of Akt is the mammalian target of rapamycin (mTOR) (55, 60, 61). Rapamycin, a lipophilic macrolide, is used as a potent immunosuppressant (58). In a search for molecules affected by rapamycin, TOR was identified in yeast (reviewed by Gingras et al. [29]). Purification and molecular cloning of mTOR revealed a 290-kDa protein that is highly related to yeast TOR. The mTOR protein contains a carboxy-terminal domain with homology to phosphatidylinositol kinases. Rapamycin binds with high affinity to FKBP12 (FK506-binding protein; molecular mass, 12 kDa), and this complex then binds to mTOR, inhibiting the function of mTOR. Recent findings reveal that mTOR controls a diverse set of downstream effectors that are important for cellular growth (59). These effectors include S6K1 and eukaryotic translation initiation factor 4E-binding proteins, which are also potential downstream effectors of Akt (4, 10, 30). Although overexpression of constitutively active Akt (caAkt) leads to a modest increase in mTOR kinase activity and moderately increases mTOR phosphorylation (55, 60, 61), an mTOR mutant protein possessing an alanine substitution at a putative Akt consensus phosphorylation site retains the ability to activate S6K1 after growth factor stimulation (61). Thus, the role of Akt in the regulation of mTOR kinase activity is not clear.
Akt regulates Drosophila cell and organ growth (74). In mammalian cells, Akt has been implicated in the control of cell cycle progression and protein synthesis, as well as in the regulation of other processes that influence growth, including cell survival and glucose metabolism (11). Akt-encoding genes are amplified or overexpressed in several forms of cancers (15, 48) and have been implicated in tumorigenesis in mice (33, 35). Body size was smaller in mice lacking Akt1 (14, 17). Expression of caAkt1 in mouse pancreatic β cells increased islet number and size, as well as individual β cell size (70). Akt has also been shown to increase cell size or protein synthesis in cultured cardiac myocytes (32, 47). GSK-3, which is inactivated by Akt, inhibits hypertrophy of cultured cardiac myocytes (32). Rapamycin was shown to inhibit agonist-induced cardiac myocyte hypertrophy (9, 57).
Previously, we have made and characterized transgenic mice expressing constitutively active PI3K (caPI3K) or kinase-deficient PI3K (kdPI3K) in the heart (63). Transgenic mice expressing caPI3K had larger hearts. In contrast, overexpression of kdPI3K reduced PI3K activity and resulted in smaller hearts. The amount of activated Akt was increased in caPI3K-expressing mice and was decreased in kdPI3K-expressing mice. To examine the role of Akt in the regulation of organ size in mammals, we have made transgenic mice expressing caAkt or kinase-deficient Akt (kdAkt) specifically in the heart.
MATERIALS AND METHODS
Generation of transgenic mice.
The cDNA insert for mouse Akt1(T308D/S473D) or Akt1K179M) was cloned into a SalI-digested α-myosin heavy chain (αMyHC) promoter construct (clone 26; a generous gift from J. Robbins) (31), and transgenic mice were generated as previously described (63). All of the aspects of the animal care and experimentation performed in this study were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
IGF1 injection into mice.
kdAkt transgenic mice or nontransgenic (NTg) mice were anesthetized with intraperitoneal injections of 2,2,2 tribromoethanol (Aldrich). IGF1 (0.5 mg/kg) or the same volume of saline was intravenously injected via a jugular vein. In preliminary experiments, IGF1 was injected into NTg mice for 5, 15, 30, or 60 min. Since Akt activity was highest in heart lysates from mice injected with IGF1 for 5 min (data not shown), hearts from NTg and kdAkt transgenic mice were harvested 5 min after the injection and rapidly frozen in liquid nitrogen.
Administration of rapamycin.
Rapamycin at 4 mg/kg/day was used in this study. The dose was determined on the basis of studies in which rapamycin was used as an immunosuppressant in mice (7, 13, 45). The solvent for rapamycin was 0.2% sodium carboxymethyl cellulose-0.25% polysorbate 80 in water. Rapamycin or solvent was intraperitoneally administered to NTg or caAkt transgenic mice from 3 weeks of age to 4 weeks of age. Mice were sacrificed at 4 weeks, heart weight (HW) was measured, and S6K1 activity was measured in heart lysates.
Protein preparation.
Hearts were removed after cervical dislocation and immediately frozen in liquid nitrogen. Heart lysates were obtained by homogenization in ice-cold buffer (1% NP-40, 10% glycerol, 137 mM NaCl, 20 mM Tris-HCl [pH 7.4], 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 mM β-glycerophosphate, 10 mM EDTA, 1 mM EGTA, 4 μg of aprotinin per ml, 4 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 4 μg of pepstatin per ml). The lysates were kept on ice for 15 min and cleared by centrifugation at 15,000 × g for 20 min at 4°C. Protein concentration was determined by the Bradford method (Bio-Rad).
Western blot analysis.
Cardiac tissue lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore). For analysis of transgene expression, the blots were probed with anti-Akt (1:1,000; New England Biolabs), followed by horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG; 1:10,000; Jackson). For analysis of S6 phosphorylation, the blots were probed with anti-phospho-S6 (1:5,000; gift from M. Birnbaum) (39) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10,000; Research Diagnostics; to confirm equal loading of protein), followed by horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG, respectively. For analysis of GSK-3β phosphorylation, 0.5 mg of lysates was immunoprecipitated by using 1 μg of anti-GSK-3β (Transduction Laboratory), separated by SDS-PAGE, blotted to PVDF membranes, and probed with anti-phospho-GSK-3β (1:1,000; New England Biolabs) or anti-GSK-3β (1:2,500), followed by horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG, respectively. The probed protein was then visualized by the enhanced chemiluminescence system (Amersham).
Akt kinase assay.
Akt kinase activity was measured by using an Akt-specific synthetic peptide, AKTide-2T, as a substrate (51). Heart tissue lysates prepared as described above (1 mg of protein) were immunoprecipitated with an anti-Akt N-terminal antibody (Santa Cruz). Immunoprecipitated enzyme was incubated in a reaction mixture of 20 mM HEPES (pH 7.4), 10 mM MgCl2, 1 μM protein kinase inhibitor (Sigma), 10 mM dithiothreitol (DTT), 5 μM ATP, 50 μM synthetic substrate peptide, and 3 μCi of [γ-32P]ATP at 30°C for 30 min. The reactions were terminated by adding stop solution containing 8 N HCl and 1 mM ATP. The reaction mixtures were spotted onto P81 phosphocellulose paper (Whatman) and washed five times with 180 mM phosphoric acid and once with 95% ethanol. Radioactivity was measured with a liquid scintillation counter.
S6K1 kinase assay.
Lysates (500 μg) were incubated with anti-S6K1 antibody (Santa Cruz) for 1.5 h and then incubated for an additional hour with 40 μl of a 50% slurry of protein A-Sepharose beads (Sigma) in phosphate-buffered saline. The beads were washed once with lysis buffer, once with buffer A (1 M NaCl, 0.1% NP-40, 10 mM Tris [pH 7.2], 1 mM sodium vanadate, 2 mM DTT, 4 μg of pepstatin per ml, 1 mM phenylmethylsulfonyl fluoride), once with ST buffer (150 mM NaCl, 50 mM Tris [pH 7.2]), and once with 20 mM HEPES-10 mM MgCl2. The kinase assays were performed by adding 30 μl of kinase buffer composed of 20 mM HEPES (pH 7.2), 10 mM MgCl2, 100 μg of bovine serum albumin per ml, 2 μM protein kinase inhibitor (rabbit sequence; Sigma), 30 μM β-mercaptoethanol, 50 μM ATP, 10 μCi of [γ-32P]ATP, and 2 μg of glutathione S-transferase (GST)-S6. Reaction mixtures were incubated for 20 min at 30°C, and reactions were stopped by adding 10 μl of 6× sample buffer. Samples were boiled for 5 min, and half of each sample was separated by SDS-12% PAGE and subjected to autoradiography. The other half of each sample was separated by SDS-7% PAGE, blotted onto a PVDF membrane, and probed with anti-S6K1 antibody.
PKCζ kinase assay.
The kinase activity of protein kinase Cζ (PKCζ) was measured by using a PKCζ-specific synthetic peptide (50). Heart tissue lysates prepared as described above (1 mg of protein) were immunoprecipitated with an anti-PKCζ antibody (Santa Cruz). Immunoprecipitated enzyme was incubated in a reaction mixture of 20 mM HEPES (pH 7.4), 10 mM MgCl2, 1 μM protein kinase inhibitor (Sigma), 10 mM DTT, 5 μM ATP, 50 μM synthetic substrate peptide, and 3 μCi of [γ-32P]ATP at 30°C for 30 min. GST-PKCζ (2 μg; gift from A. Toker) generated by a baculovirus system was used as a positive control for the reaction. The reactions were terminated by adding stop solution containing 8 N HCl and 1 mM ATP. The reaction mixtures were spotted onto P81 phosphocellulose paper (Whatman) and washed five times with 180 mM phosphoric acid and once with 95% ethanol. Radioactivity was measured with a liquid scintillation counter. Immunoprecipitated PKCζ or GST-PKCζ was separated by SDS-7% PAGE, blotted onto a PVDF membrane, and probed with the anti-PKCζ antibody.
Histological analysis.
Histological analysis of mouse hearts was performed as previously described (63).
Morphometric analysis of isolated cardiac myocytes.
Cardiac myocytes were enzymatically dissociated from mouse hearts in accordance with the previously published protocol, with minor modifications (79). The hearts from 10- to 12-week-old female transgenic or NTg mice were retrogradely perfused and enzymatically dissociated with 0.3% collagenase. The dissociated myocytes were plated on laminin-coated dishes. After 1 h of plating, unattached cells were removed by changing the medium. Photographs were taken under a microscope. The long axis was determined by measuring the distance between the two points farthest apart at the edge of the cell. A perpendicular line was drawn across the middle of the long axis, and the short axis was determined by measuring the distance between the two edges of the cell where the perpendicular line cut. The cell area was measured by tracing the edge of the cell and determining the area inside the outline. Morphometric analysis was performed with IPLab software (Scanalytics, Inc.). Cell volume was calculated by the following formula on the assumption that the cell is cylindrical in shape: cell volume = (short axis/2)2 × 3.142 × long axis.
The mean value for each mouse was calculated by using the measurements of 100 cells isolated from an individual mouse. Next, the mean value (± the standard error [SE]) for each experimental group was calculated on the basis of the mean values of the individual mice, and this value is presented.
Echocardiography.
Assessment of cardiac function using echocardiography was performed as previously described (63). The stroke volume and cardiac index were obtained by using the following formulas: stroke volume = (LV diastolic diameter − LV systolic diameter)3, where LV is the left ventricle, and cardiac index = (stroke volume × heart rate)/BW, where BW is body weight.
Statistical analysis.
Results are presented as the mean ± SE. Differences between the groups were compared by using the two-tailed unpaired Student t test. P < 0.05 was considered significant.
RESULTS
Creation of caAkt and kdAkt transgenic mice.
Akt(T308D/S473D), in which critical residues for activation of Akt are replaced by negatively charged amino acids, has been shown to have activity comparable to that of endogenous Akt stimulated with insulin (28). The αMyHC promoter was used to generate transgenic mice as described previously (63). In the atrium, this promoter is active in both embryonic and adult myocytes but in ventricular myocytes it becomes active mainly after birth (49, 53). To make transgenic mice expressing caAkt, the Akt(T308D/S473D) gene was cloned into the αMyHC promoter construct, and transgenic mice were produced. Six independently derived founders of caAkt were produced from 137 F0 mice screened by Southern blot analysis. All of the founders died within 6 months of birth. Three founders produced progenies. Progenies of two of the founders died within several weeks after birth. The progenies of the third founder survived to a mean age of 15 weeks, and this line was used for the subsequent analysis. In this line, most of the mice died after they showed signs of heart failure, such as labored respiration or reduced activity.
To generate transgenic mice expressing kdAkt in the heart, Akt(K179M), in which the critical ATP binding site was mutated, was cloned into the αMyHC promoter construct. Eight independently derived founders were produced from 63 mice screened by Southern blot analysis. Progenies from six founders expressed the transgene product in the heart as determined by Western blot analysis. Over a follow-up period of 1 year, three lines of kdAkt transgenic mice survived normally. Two lines died at a mean age of 11 weeks, and one line died at a mean age of 2 weeks. Kinase-deficient transgenic lines associated with lethality were lost during maintenance. One of the kdAkt transgenic lines that did not die was analyzed.
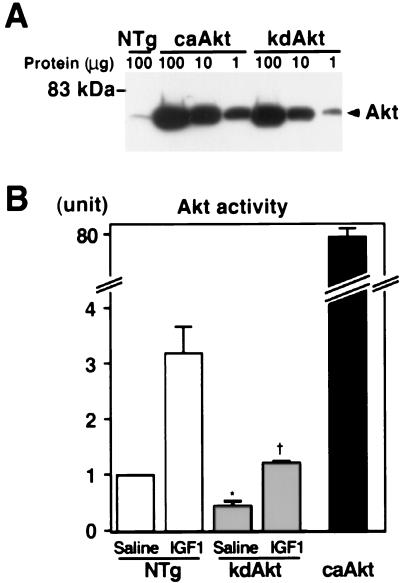
To compare the amount of transgene product with the endogenous Akt, cardiac tissue lysates from transgenic mice were serially diluted, separated by SDS-PAGE, and probed with anti-Akt antibody. In caAkt transgenic mice, the amount of total Akt was about 400-fold greater than that in NTg mice (Fig. 1A). In the kdAkt transgenic line we analyzed, there was approximately 120 times more transgene product (kdAkt) than endogenous Akt in NTg mice (Fig. 1A). To confirm the activity of the transgene products, the heart tissue lysates were immunoprecipitated with an N-terminal anti-Akt antibody and subjected to an in vitro kinase assay using an Akt-specific peptide substrate (AKTide-2T) (51). In caAkt transgenic mouse hearts, total Akt activity was increased 80.4-fold ± 2.9-fold compared with that in NTg mouse hearts (Fig. 1B). Akt activity in kdAkt mice injected with saline was 45% ± 8% of that in NTg mice injected with saline. Injection of IGF1 (0.5 mg/kg) for 5 min increased Akt activity by 3.18-fold ± 0.47-fold in the hearts of NTg mice. Akt activity in the hearts of mice injected with IGF1 kdAkt was 35.2% ± 0.9% of that of NTg mice injected with IGF1. Thus, overexpression of the kdAkt molecule significantly attenuated the activation of endogenous Akt in transgenic mouse hearts.
FIG. 1.
Generation of Akt transgenic mice. (A) Expression of the transgene. To compare the amount of transgene with endogenous Akt, 1, 10, and 100 μg of cardiac tissue lysate from caAkt or kdAkt transgenic mice and 100 μg of protein from NTg mice were separated by SDS-PAGE and probed with an anti-Akt antibody. (B) Akt activity in the transgenic heart. One milligram of cardiac tissue lysate was immunoprecipitated, and the kinase activity was measured by an in vitro kinase assay. kdAkt transgenic mice or NTg mice were injected with saline or 0.5 mg of IGF1 per kg for 5 min to activate Akt. Each group represents three or four hearts. Total Akt activity was markedly increased in the heart tissue of caAkt mice. Akt activity was significantly decreased in kdAkt mice under both basal and IGF1-stimulated conditions. Symbols: ∗, P < 0.05 versus saline-injected NTg mice; †, P < 0.05 versus IGF1-injected NTg mice.
Activation of potential downstream targets in Akt transgenic mice.
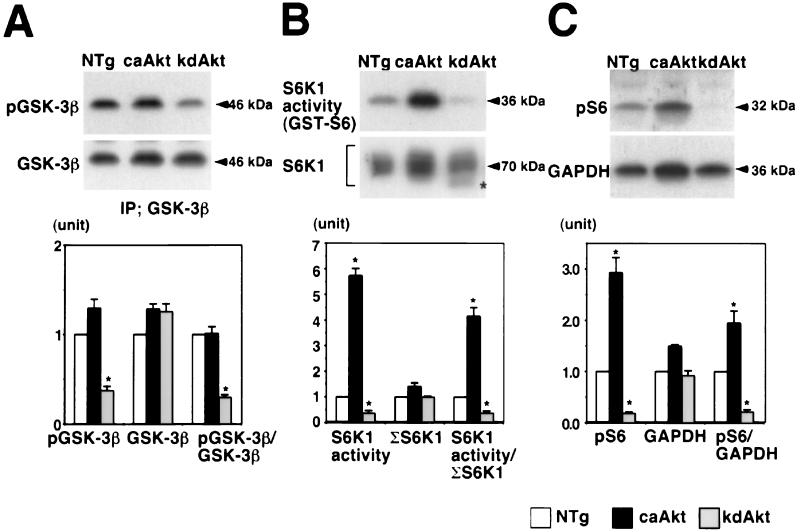
Next, we examined the activation of potential downstream targets of Akt. Representative Western blots and results of kinase assays are shown at the top and middle of each panel of Fig. 2, and the results of quantitative densitometry of four animals in each group are shown at the bottom of each panel. GSK-3β is one of the best-characterized targets of Akt in cultured cells (24, 28). Phosphorylation of GSK-3β in heart tissue was assessed by measuring the amount of GSK-3β phosphorylated at Ser9 by using a phosphospecific antibody. In caAkt mice, the amounts of phosphorylated GSK-3β and total GSK-3β and the ratio of phosphorylated GSK-3β to total GSK-3β were not significantly different from those of NTg mice (Fig. 2A). In kdAkt mice, the amount of phosphorylated GSK-3β was decreased to 37% ± 5% of that in NTg mice (Fig. 2A, top) and the phosphorylated GSK-3β/total GSK-3β ratio was 30% ± 3% of that of NTg mice (Fig. 2A, bottom). Unlike previous studies using cultured cell lines (24, 28), high Akt activity was not sufficient to phosphorylate GSK-3β at Ser9 in the intact heart. We also measured the amount of phosphorylated GSK-3β in the hearts of NTg mice injected with IGF1. Hearts were harvested 5 min after the injection. The ratio of phosphorylated GSK-3β to total GSK-3β was significantly increased 2.1-fold ± 0.2-fold in the heart tissue of NTg mice injected with IGF1 compared with that in untreated NTg mice (n = 3 for each group; data not shown). Thus, it is unlikely that GSK-3β was already maximally phosphorylated at baseline. In contrast, Akt seems to be necessary for GSK-3β activation in the intact heart, consistent with previous reports (28, 66).
FIG. 2.
Activation of GSK-3β and S6K1 in Akt transgenic mouse hearts. (A) Phosphorylation of GSK-3β in the heart tissue of Akt transgenic mice. GSK-3β was immunoprecipitated and probed with an anti-phospho-GSK-3β (Ser9) antibody (top) or anti-GSK-3β antibody (middle). In caAkt mice, the amount of phosphorylated GSK-3β normalized to total GSK-3β was not different from that in NTg mice. In kdAkt mice, the amount of phosphorylated GSK-3β normalized to total GSK-3β was significantly decreased compared with that in NTg mice (bottom). (B) S6K1 activity in the heart tissue of Akt transgenic mice. S6K1 activity was measured by an immune complex kinase assay using GST-S6 as a substrate (top). The amount of immunoprecipitated (IP) S6K1 was analyzed by Western blotting (middle). S6K1 activity normalized to total S6K1 was increased in the hearts of caAkt mice and decreased in the hearts of kdAkt mice (bottom). Faster-migrating bands were obtained from kdAkt mice, which suggests that S6K1 was less phosphorylated (middle, ∗).(C) Phosphorylation of ribosomal S6 protein in the heart tissue of Akt transgenic mice. Phosphorylation of ribosomal S6 protein was examined by Western blotting using an anti-phospho-S6 antibody (top). The amount of protein loading was normalized to the amount of GAPDH (middle). The amount of phosphorylated S6 normalized to GAPDH was increased 1.8-fold in caAkt mice, and it was decreased to 36% of that in NTg mice in kdAkt mice (bottom). Each group represents four hearts. ∗, P < 0.05 versus NTg mice.
S6K1 phosphorylates 40S ribosomal protein S6, which results in increased protein synthesis. S6K1 has been shown to be downstream of PI3K (18). Akt alone is sufficient for the activation of S6K1 when overexpressed in some cultured cells (4, 10). We measured the activity of S6K1 in heart lysates by an immune complex kinase assay using GST-S6 as a substrate (Fig. 2B, top). S6K1 activity was increased 5.7-fold ± 0.2-fold in caAkt mice, whereas in hearts from kdAkt mice it was decreased to 36% ± 11% of that in NTg mice (Fig. 2B, bottom). Western blotting with this S6K1 antibody produces broad bands at around 70 kDa due to the presence of S6K1 phosphorylation at multiple sites (Fig. 2B, middle). The total amount of S6K1 was estimated by adding the densitometric scores of these bands. The total amount of S6K1 was not significantly different among the three groups. Faster-migrating bands were observed in kdAkt mice, which suggests that S6K1 was less phosphorylated (Fig. 2B, asterisk). The ratio of S6K1 activity to the total S6K1 protein (Fig. 2B, bottom) was 4.2-fold ± 0.3-fold higher in caAkt mice but was decreased to 35% ± 10% of that of NTg mice in kdAkt mice. These results suggest that Akt plays an important role in the regulation of S6K1 activity in the intact heart.
To confirm our in vitro S6K1 activity measurements, the amount of the phosphorylated form of the ribosomal S6 protein in heart tissue was measured by using an antibody specific for phosphorylated S6 protein (Fig. 2C, top). Loading of equal amounts of protein was confirmed by probing the membrane with an anti-GAPDH antibody (Fig. 2C, middle). The amount of phosphorylated S6 was increased by 2.9-fold ± 0.3-fold in caAkt mice and decreased to 18% ± 3% of that of NTg mice in kdAkt mice. The amount of phosphorylated S6 normalized by the amount of GAPDH was increased 1.9-fold ± 0.2-fold in caAkt mice and decreased to 21% ± 5% of that of NTg mice in kdAkt mice (Fig. 2C, bottom). These results confirmed that S6K1 activity was increased in caAkt mice and decreased in kdAkt mice.
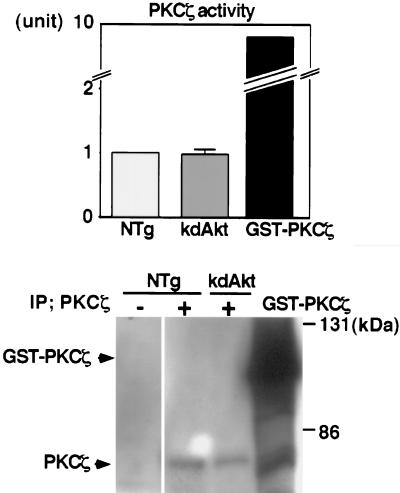
PDK1 phosphorylates Thr308 of Akt (2). It is possible that overexpression of the kdAkt transgene competitively inhibits phosphorylation and activation of the other targets of PDK1. To examine this possibility, we measured PKCζ activity in the heart tissue lysates of NTg mice and kdAkt mice (Fig. 3). PKCζ is a substrate of PDK1 (19) and is not likely to be downstream of Akt (41). The results of kinase assays from each group (three animals in each) are shown at the top, and representative Western blotting of immunoprecipitated PKCζ is shown at the bottom. The kinase activity of baculovirus-generated PKCζ (GST-PKCζ) was about 10-fold greater than that of immunoprecipitated PKCζ in heart tissue lysates from NTg mice (Fig. 3, top). Under the same assay conditions, PKCζ activity in the heart tissue of kdAkt mice was not different from that in NTg mice (Fig. 3, top). The amount of PKCζ was not different between NTg mice and kdAkt mice (Fig. 3, bottom). Thus, it is unlikely that suppression of S6K1 activity and GSK-3β phosphorylation in kdAkt mice resulted from the sequestration of PDK1 by overexpression of kdAkt.
FIG. 3.
PKCζ activity in kdAkt transgenic mice. PKCζ activity in the heart tissue of NTg mice or kdAkt transgenic mice was measured by an in vitro kinase assay using a synthetic peptide as a substrate (top). PKCζ was immunoprecipitated from 1 mg of heart tissue lysate. GST-PKCζ was used as a positive control for the kinase assay. PKCζ activity in the hearts of kdAkt transgenic mice was not different from that in the hearts of NTg mice. Each group represents three hearts. Immunoprecipitated (IP) PKCζ or GST-PKCζ was separated by SDS-PAGE, blotted onto a PVDF membrane, and probed with an anti-PKCζ antibody (bottom).
Postmortem analysis of Akt transgenic mice.
The HW/BW ratio is a well-established index of cardiac hypertrophy. In caAkt mice, the HW/BW ratio was significantly increased 2.2-fold ± 0.0-fold at an age of 2 weeks and by 2.2-fold ± 0.0-fold at a mean age of 14 weeks compared with that of NTg mice (Table 1). All of the founders and progenies of other lines of caAkt mice showed similar increases in the HW/BW ratio (data not shown). The HW, BW, and HW/BW ratio of kdAkt mice were not significantly different from those of NTg mice (Table 1). Thus, expression of caAkt induced a marked increase in heart size.
TABLE 1.
BWs and HWs of Akt transgenic and NTg micea
| Age (wks) and mice | No. of mice | BW (g) | HW (mg) | Lung wt (mg) | Liver wt (mg) | HW/BW ratio | Lung wt/BW ratio | Liver wt/BW ratio |
|---|---|---|---|---|---|---|---|---|
| 2 | ||||||||
| NTg | 7 | 9.0 ± 0.3 | 53.7 ± 2.3 | 100.5 ± 5.2 | 371 ± 25 | 5.98 ± 0.11 | 11.19 ± 0.46 | 41.1 ± 2.1 |
| caAkt | 6 | 7.7 ± 0.2 | 100.3 ± 4.1b | 132.2 ± 3.5b | 353 ± 11 | 13.02 ± 0.42b | 17.23 ± 0.73b | 45.6 ± 2.1 |
| kdAkt | 6 | 8.5 ± 0.4 | 8.5 ± 3.3 | 97.9 ± 7.0 | 366 ± 2.0 | 6.05 ± 0.22 | 10.99 ± 0.26 | 41.3 ± 0.9 |
| 14 | ||||||||
| NTg | 15 | 22.9 ± 0.5 | 104.3 ± 2.1 | 134.3 ± 5.2 | 1,075 ± 33 | 4.57 ± 0.08 | 5.88 ± 0.16 | 47.0 ± 1.2 |
| caAkt | 13 | 21.6 ± 0.4 | 213.1 ± 4.6b | 185.8 ± 5.0b | 1,050 ± 29 | 9.88 ± 0.22b | 8.62 ± 0.25b | 48.6 ± 0.9 |
| kdAkt | 4 | 23.1 ± 0.9 | 107.9 ± 5.6 | 125.3 ± 3.1 | 1,049 ± 67 | 4.67 ± 0.09 | 5.46 ± 0.27 | 46.0 ± 4.7 |
The values shown are means ± SE. In the 2-week-old groups, both male and female mice were used for analysis. In the 14-week-old groups, only female mice were used.
P < 0.05 versus NTg mice.
Pathological analysis of Akt transgenic mice.
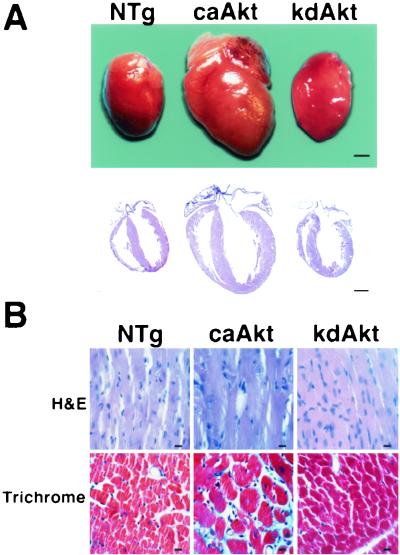
Postmortem pathological analysis was performed by using hearts from 3- to 4-month-old mice. In caAkt mice, there was a proportional increase in the size of all chambers of the heart and the thickness of the ventricular walls (Fig. 4A). Marked hypertrophy of the myocytes was evident upon histopathological analysis in caAkt mice (Fig. 4B). Extensive interstitial fibrosis was also observed (blue on Masson's trichrome stain). In contrast, heart size, ventricular wall thickness, and chamber sizes were not different between NTg and kdAkt mice (Fig. 4A). Myocyte hypertrophy was not observed in kdAkt transgenic mice. Since myocyte cell death due to apoptosis can affect myocyte cell number in the heart, we examined the presence of apoptosis by using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay and a DNA laddering assay. Apoptosis of myocytes was not observed in either caAkt or kdAkt transgenic mice (data not shown).
FIG. 4.
Pathological analysis of Akt transgenic mice. (A) Macropathological analysis of Akt transgenic mice. In caAkt mice, there was a marked increase in size in all of the chambers and in ventricular wall thickness. The proportion of the sizes of the chambers was maintained. In kdAkt mice, heart size was not different from that in NTg mice. Bars represent 1 mm. (B) Histopathological analysis of Akt transgenic mice. Hypertrophy of myocytes was evident in caAkt mice. Extensive interstitial fibrosis was also observed (blue on Masson's trichrome stain). Histology was normal in kdAkt mice. H&E, hematoxylin and eosin stain; trichrome, Masson's trichrome stain. Bars represent 10 μm.
Cell size analysis of cardiac myocytes from Akt transgenic mice.
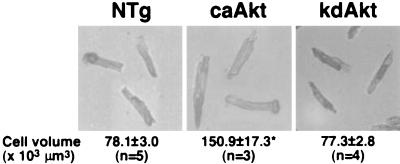
The size of an organ is determined by coordinated regulation of cell growth (increase in cell size), cell proliferation (increase in cell number), and cell death (23). The size of myocytes was directly measured by using isolated adult cardiac myocyte preparations (Table 2 and Fig. 5). Three- to 4-month-old female mice were used for this analysis. In caAkt mice, the long axis was increased 1.3-fold ± 0.1-fold and the short axis was increased 1.2-fold ± 0.0-fold. The cell area was also increased 1.5-fold ± 0.1-fold. The calculated cell volume of caAkt mice, based on the assumption that cardiac myocytes are cylindrical, was increased 1.9-fold ± 0.2-fold. Thus, the increase in HW (2.0-fold ± 0.0-fold) was associated with a comparable increase in cell size, suggesting that Akt increased heart size mainly through an increase in cell size. In kdAkt mice, the size of myocytes was not different from that in NTg mice.
TABLE 2.
Morphometric analysis of isolated cardiac myocytesa
| Mice | No. | Length (μm) of:
|
Long/short axis ratio | Cell area (μm2) | Cell vol (103 μm3) | |
|---|---|---|---|---|---|---|
| Long axis | Short axis | |||||
| NTg | 5 | 122.9 ± 1.2 | 28.4 ± 0.6 | 4.63 ± 0.17 | 2,499 ± 49 | 78.1 ± 3.0 |
| caAkt | 3 | 163.0 ± 10.2b | 34.2 ± 0.9b | 5.14 ± 0.18 | 3,845 ± 256b | 150.9 ± 17.3b |
| kdAkt | 4 | 115.4 ± 4.1 | 29.2 ± 0.2 | 4.18 ± 0.56 | 2,440 ± 88 | 77.3 ± 2.8 |
The mean value for each mouse was calculated from 100 cells. The mean value (± SE) for each group was then calculated on the basis of the mean values of individual mice.
P < 0.05 versus NTg mice.
FIG. 5.
Analysis of Akt transgenic mouse cell size. Cardiac myocyte size was determined by using isolated adult myocytes. The mean value for each mouse was calculated by using the measurements of 100 cells isolated from that mouse. Next, the mean value (± SE) for each experimental group was calculated on the basis of the mean values of the individual mice, and this value is presented. The cell volume was increased 1.9-fold ± 0.2-fold in caAkt transgenic mice. n, number of hearts examined.
Assessment of LV function by echocardiography.
Since hemodynamic loading status is one of the determinants of heart size, we assessed the LV dimensions and contractile function of Akt transgenic mice by using M-mode echocardiography at the ages of 2 and 14 weeks (Table 3). At 2 weeks old, there was a significant increase in wall thickness of both the anterior and posterior LV walls (1.4-fold ± 0.0-fold and 1.4-fold ± 0.0-fold, respectively) of caAkt mice. The LV diastolic and systolic diameters of caAkt mice were not different from those of NTg mice. Fractional shortening, an echocardiographic index of LV contractile function, of caAkt mice was normal (Table 3). HW was increased 1.9-fold ± 0.1-fold at 2 weeks of age (Table 1). A marked increase in HW in the absence of cardiac dysfunction suggests that the heart size increase in caAkt mice was not a secondary response to the changes in hemodynamic loading status.
TABLE 3.
Echocardiographic data for NTg and Akt transgenic micea
| Age (wks) and mice | No. of mice | BW (g) | Heart rate (beats/min) | Diastolic wall thickness (mm)
|
LV diam (mm)
|
% Fractional shortening | Stroke vol (mm3) | Cardiac index (mm3/min/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Anterior | Posterior | Diastolic | Systolic | |||||||
| 2 | ||||||||||
| NTg | 7 | 9.0 ± 0.3 | 420 ± 34 | 0.5 ± 0.0 | 0.5 ± 0.0 | 2.7 ± 0.1 | 1.3 ± 0.1 | 53 ± 4 | 18.4 ± 2.0 | 870 ± 144 |
| caAkt | 7 | 8.1 ± 0.3 | 359 ± 14 | 0.7 ± 0.0b | 0.7 ± 0.0b | 2.6 ± 0.1 | 1.3 ± 0.1 | 49 ± 3 | 14.6 ± 1.2 | 666 ± 96 |
| kdAkt | 5 | 8.5 ± 0.6 | 380 ± 24 | 0.5 ± 0.0 | 0.5 ± 0.0 | 2.7 ± 0.2 | 1.3 ± 0.2 | 50 ± 5 | 18.9 ± 3.5 | 805 ± 133 |
| 14 | ||||||||||
| NTg | 8 | 22.3 ± 0.7 | 285 ± 64 | 0.6 ± 0.0 | 0.6 ± 0.0 | 3.4 ± 0.1 | 1.2 ± 0.1 | 65 ± 6 | 37.3 ± 5.2 | 476 ± 121 |
| caAkt | 4 | 20.9 ± 1.5 | 217 ± 38 | 0.9 ± 0.1b | 0.9 ± 0.1b | 3.8 ± 0.2b | 2.4 ± 0.1b | 37 ± 3b | 42.3 ± 7.6 | 441 ± 110 |
| kdAkt | 4 | 23.0 ± 2.6 | 220 ± 12 | 0.6 ± 0.1 | 0.6 ± 0.1 | 3.5 ± 0.4 | 1.3 ± 0.2 | 56 ± 6 | 39.9 ± 6.4 | 390 ± 152 |
The results presented are means ± SE. In the 2-week-old group, both male and female mice were used for analysis. In the 14-week-old group, only female mice were used.
P < 0.05 versus NTg mice.
At 14 weeks old, an increase in the thickness of both the anterior and the posterior LV walls was evident in caAkt mice (1.5-fold ± 0.0-fold and 1.5-fold ± 0.0-fold, respectively). The LV systolic diameter was also significantly increased (2.0-fold ± 0.2-fold), while the LV diastolic diameter was minimally increased (1.1-fold ± 0.0-fold). Fractional shortening of caAkt mice was significantly decreased to 37% ± 3%. The cardiac index represents the volume of blood that is pumped out of the heart per minute. Although fractional shortening was reduced in caAkt mice, the cardiac index was not different from that of NTg mice. The reduced fractional shortening in caAkt mice might be an adaptive response to keep the cardiac index within the normal range. Alternatively, it is also possible that the reduced fractional shortening is the manifestation of cardiomyopathy in older caAkt mice, since extensive fibrosis was observed on histological analysis and most of the mice died after they showed signs of heart failure. In kdAkt mice, all echocardiographic parameters were normal (Table 3).
Crossbreeding of PI3K transgenic mice and Akt transgenic mice.
Akt is postulated to be one of the most important downstream effectors of PI3K. In Caenorhabditis elegans, an activated allele of Akt suppresses the daur arrest phenotype caused by a loss-of-function mutation in age-1 (a homolog of mammalian PI3K) (54). Although significant information about the biochemical relationship between PI3K and Akt in mammalian cells has been provided (72), genetic evidence that Akt functions as a downstream effector of PI3K in intact mammalian tissues is lacking. We have previously made and characterized transgenic mice expressing caPI3K or kdPI3K in the heart (63). To genetically determine the relationship between PI3K and Akt in heart growth, we crossed PI3K transgenic mice with Akt transgenic mice and examined heart size (Fig. 6).
FIG. 6.
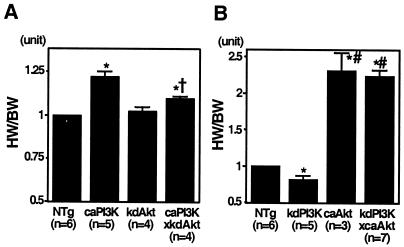
Genetic interaction of PI3K and Akt in the regulation of organ size. (A) The HW/BW ratios of double-transgenic mice expressing both the caPI3K and kdAkt transgenes were significantly lower than those of caPI3K mice (1.10 ± 0.01 versus 1.22 ± 0.03; P = 0.0096; NTg mice = 1.00). (B) The HW/BW ratios of double-transgenic mice having both kdPI3K and caAkt were similar to those of caAkt mice (2.24 ± 0.08 versus 2.31 ± 0.25; P = 0.7438; NTg mice = 1.00). n, number of mice. Symbols: ∗, P < 0.05 versus NTg mice: †, P < 0.05 versus caPI3K mice; #, P < 0.05 versus kdPI3K mice.
To examine if Akt is necessary for the PI3K-induced overgrowth of the heart, kdAkt mice were crossed with caPI3K mice (Fig. 6A). The HW/BW ratio of caPI3K mice was increased compared with that of NTg mice by 1.22-fold ± 0.03-fold. The HW/BW ratio of double-transgenic mice expressing both the caPI3K and kdAkt transgenes was significantly lower than that of caPI3K mice (1.10 ± 0.01 versus 1.22 ± 0.03; P = 0.0096; NTg mice = 1.00), suggesting that Akt activation is required for PI3K-induced hypertrophy. In order to determine whether Akt could suppress the decrease in heart size induced by the inhibition of PI3K activity, caAkt mice were crossed with kdPI3K mice (Fig. 6B). The HW/BW ratio of kdPI3K mice was significantly decreased compared with that of NTg mice. The HW/BW ratio of double-transgenic mice having both kdPI3K and caAkt was similar to that of caAkt-expressing mice (2.24 ± 0.08 versus 2.31 ± 0.25; P = 0.7438; NTg mice = 1.00), suggesting that the caAkt mutant protein circumvented cardiac growth retardation induced by kdPI3K. These data are consistent with the notion that Akt is one of the main downstream effectors of PI3K in organ growth.
Effect of rapamycin on the HW of caAkt mice.
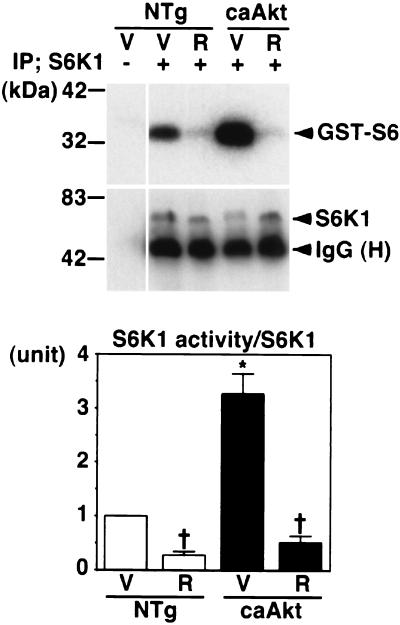
The caAkt transgene may induce overgrowth of the heart through mTOR or the effectors of mTOR, such as S6K1 or eukaryotic translation initiation factor 4E-binding protein 1 (4, 10, 30). To examine this possibility, we treated NTg mice or caAkt mice with rapamycin, a specific inhibitor of mTOR (Table 4) (25). Vehicle or rapamycin (4 mg/kg/day) was administered to NTg or caAkt mice from 3 weeks of age to 4 weeks of age. After that, mice were sacrificed and HW was measured. S6K1 activity was measured to monitor the effect of rapamycin. At 3 weeks of age, the HW/BW ratio of caAkt mice was already increased 2.0-fold ± 0.0-fold compared with that of NTg mice (Table 4). Rapamycin at 4 mg/kg significantly reduced S6K1 activity in the hearts of NTg mice at 4 weeks of age (Fig. 7). Rapamycin, at the dose used in this study, did not affect the HW/BW ratio of NTg mice (Table 4). Rapamycin reduced S6K1 activity in the hearts of caAkt mice to a level similar to that of rapamycin-treated NTg mice (Fig. 7). The HW/BW ratio of rapamycin-treated caAkt mice was significantly smaller than that of vehicle-treated caAkt mice (8.44 ± 0.12 versus 9.96 ± 0.15; P < 0.0001; Table 4). The HW of rapamycin-treated 4-week-old caAkt mice was comparable to that of 3-week-old caAkt mice (137.0 ± 4.1 g versus 134.0 ± 7.7 g; P = 0.9532). This result suggests that caAkt-induced overgrowth of the heart was, at least in part, dependent on mTOR or targets of mTOR.
TABLE 4.
Effect of rapamycin on HWs of NTg and caAkt transgenic micea
| Mice and age (wks) | Treatment | No. of mice | BW (g) | HW (g) | HW/BW ratio |
|---|---|---|---|---|---|
| NTg | |||||
| 3 | 4 | 15.0 ± 0.2 | 71.0 ± 1.0 | 4.73 ± 0.09 | |
| 4 | Vehicle | 4 | 17.8 ± 0.7 | 83.2 ± 3.5 | 4.76 ± 0.10 |
| 4 | Rapamycin | 4 | 17.3 ± 1.1 | 79.6 ± 2.8 | 4.63 ± 0.14 |
| caAkt | |||||
| 3 | 3 | 14.0 ± 1.0 | 134.0 ± 7.7 | 9.62 ± 0.22 | |
| 4 | Vehicle | 4 | 17.6 ± 1.1 | 175.1 ± 9.4b | 9.96 ± 0.15b |
| 4 | Rapamycin | 5 | 16.2 ± 0.4 | 137.0 ± 4.1 b,c | 8.44 ± 0.12 b,c |
The values shown are means ± SE.
P < 0.05 versus NTg mice treated with vehicle or rapamycin.
P < 0.05 versus vehicle-treated caAkt mice.
FIG. 7.
Effect of rapamycin on S6K1 activity in caAkt mice. NTg mice or caAkt mice were treated with vehicle (V) or rapamycin (R). S6K1 activity in heart tissue was measured by an immune complex kinase assay using GST-S6 as the substrate (top). The amount of immunoprecipitated (IP) S6K1 was analyzed by Western blotting (middle). The S6K1 activity/S6K1 protein ratio is shown at the bottom. Each group represents four mice. S6K1 activity was increased in the hearts of vehicle-treated caAkt mice compared with that in the hearts of vehicle-treated NTg mice. Rapamycin significantly reduced S6K1 activity in the hearts of NTg mice. S6K1 activity in the hearts of rapamycin-treated caAkt mice was comparable to that in the hearts of rapamycin-treated NTg mice. Symbols: ∗, P < 0.05 versus vehicle-treated NTg mice; †, P < 0.05 versus vehicle-treated mice of the same genotype. IgG (H), immunoglobulin heavy chain.
DISCUSSION
In order to determine the role of Akt in the regulation of organ size in mammals, we have perturbed the activity of Akt specifically in the heart by expressing caAkt or kdAkt in transgenic mice. The caAkt transgene caused heart enlargement associated with an increase in myocyte size. The intercross of kdPI3K transgenic mice and caAkt transgenic mice showed that Akt is likely to be one of the important downstream effectors of PI3K in mediating organ growth. Furthermore, rapamycin significantly attenuated caAkt-induced overgrowth of the heart, suggesting that Akt promoted heart growth through mTOR or effectors of mTOR.
To examine the role of Akt in intact mammalian tissue, we overexpressed caAkt or kdAkt in transgenic mouse hearts. By using this strategy, it is possible that the highly overexpressed molecule could affect a biological process in which the endogenous counterpart is not normally involved. Alternatively, cardiac hypertrophy may be induced secondary to the nonspecific toxic effect of the transgene (34). However, we think the marked increase in heart size observed in caAkt mice is likely due to the increase in Akt kinase activity for the following reasons. First, systolic function of the heart, assessed by the fractional shortening of echocardiography, was preserved in 2-week-old mice even though HW was increased 1.9-fold. Second, caAkt-induced overgrowth of the heart was significantly attenuated by rapamycin, a specific inhibitor of mTOR. Third, Akt has been shown to increase cell size and protein synthesis in cultured cardiac myocytes (32, 47). Fourth, a 200-fold beta-adrenergic receptor increase in the heart modulates contractile function in a specific manner without causing cardiomyopathy in transgenic mice (56).
There is one Akt-encoding gene in drosophila, and deletion of the Akt-encoding gene is associated with smaller cells (74). In this study, inhibition of Akt activity with a kinase-deficient mutant was not associated with a decrease in heart size. It is likely that residual Akt activity in the hearts (45% of that in NTg mice) was sufficient to preserve normal organ growth. Body size was smaller in mice lacking Akt1 (14, 17). Insulin resistance and a diabetes mellitus-like syndrome were observed in mice lacking Akt2 (16). Growth retardation was not observed in these mice, possibly due to the compensation by other Akt-encoding genes. Heart-specific deletion of an Akt-encoding gene(s) is needed to determine if Akt is necessary to promote heart growth in a cell-autonomous manner.
Several studies have found that kdAkt mutants work in a dominant negative manner (27, 36, 38, 80). However, several other studies have found that kdAkt does not inhibit Akt activation (40, 73, 75). In this experiment, kdAkt overexpression inhibited the activation of endogenous Akt under basal and IGF-stimulated conditions. kdAkt also effectively attenuated caPI3K-induced overgrowth of the heart. The mechanism of action of mutant kdAkt proteins may be different in different cells and tissues.
In this study, an increase in total Akt activity was not associated with an increase in the amount of phosphorylated GSK-3β in caAkt mice. This is not consistent with previous observations derived from experiments using cultured cell lines (24, 28). The same caAkt did not phosphorylate GSK-3β in mammary epithelium in transgenic mice (35). GSK-3β phosphorylation in intact tissue may be regulated by a mechanism different from that which operates in proliferating cultured cell lines. Alternatively, continuous expression of the transgene in the intact tissue may modulate the activity of potential downstream effectors in a different way from growth factor stimulation of cultured cells for a short period of time. GSK-3β phosphorylation was decreased in kdAkt mice, which is consistent with a previous study in which kdAkt inhibited insulin-induced inactivation of GSK-3 (66).
caAkt was sufficient to activate S6K1 in this study. S6K1 activation by membrane-targeted Akt has been previously reported (4, 10). However, the Akt(T308D/S473D) mutant protein did not activate S6K1 (4, 28). Akt does not appear to directly phosphorylate S6K1 (3), and the intermediates between Akt and S6K1 are unknown. The mechanism of S6K1 activation in caAkt mice needs further investigation. Since rapamycin significantly attenuated caAkt-induced overgrowth of the heart and S6K1 is one of the effectors of mTOR, S6K1 may be one of the candidates for mediation of the growth-promoting effects of Akt in caAkt mice.
In our study, expression of kdAkt reduced S6K1 activity in the heart. A dominant interfering mutant form of Akt was shown to inhibit S6K1 activation (40), while another dominant negative mutant form of Akt did not inhibit insulin-induced S6K1 activation (28). Since overexpression of Akt may sequester PDK1, which is essential for S6K1 activation, it is possible that the kdAkt transgene attenuated S6K1 activity by preventing it from accessing PDK1 (72, 77). To examine this possibility, we measured PKCζ activity in the hearts of kdAkt mice and found that PKCζ activity was not different from that in NTg mice. Thus, it is unlikely that suppression of S6K1 activity and GSK-3β phosphorylation in kdAkt mice resulted from the sequestration of PDK1 by overexpression of kdAkt. The 64% reduction in S6K1 activity in the hearts of kdAkt mice did not disturb developmental growth. It is likely that residual S6K1 activity was sufficient to maintain normal heart growth.
Growth hormone is an endocrine promoter of IGF-1 production. Patients with acromegaly, a condition of growth hormone excess, are associated with marked cardiac hypertrophy and reduced cardiac function (22). Cardiovascular mortality is increased in patients with acromegaly (52). Autopsies of hearts from acromegaly patients showed myocyte hypertrophy and fibrosis (43). These observations are similar to the findings in caAkt transgenic mice. We speculate that Akt may be involved in the pathogenesis of cardiomyopathy in acromegaly patients. We did not observe cardiomyopathic changes in caPI3K transgenic mice, probably due to the lower Akt kinase activity compared with that of caAkt (see below).
In our caAkt mice, the increase in HW (2.0-fold ± 0.0-fold) was associated with a comparable increase in cell volume (1.9-fold ± 0.2-fold), suggesting that Akt increased heart size by increasing cell size. This is in agreement with a previous study, in which overexpression of Akt resulted in increased cell size, but not cell number, in the Drosophila wing (74). However, an increase in heart size occurs postnatally, when the myocytes are postmitotic (64, 65), and the promoter used to generate transgenic mice in this study is active mainly after birth in the ventricles (49, 53). Therefore, interpretation of the present findings on the heart may be different from those on other mitotically competent organs, where there is a potential role for Akt in the regulation of cell number.
We previously reported that heart-specific expression of caPI3K in transgenic mice increased HW 1.2-fold ± 0.0-fold. Total Akt activity, measured by an in vitro kinase assay, was increased 2.2-fold ± 0.2-fold in caPI3K mice (Shioi et al., unpublished observation). In caAkt mice, HW was increased 2.0-fold ± 0.0-fold and total Akt activity was increased 80.4-fold ± 2.9-fold. Thus, the increase in heart size in caPI3K and caAkt mice appeared to be correlated with the increase in Akt activity in the heart. In addition, the mutant kdAkt protein attenuated the caPI3K induced overgrowth of the heart and the mutant caAkt protein circumvented cardiac growth retardation induced by a mutant kdPI3K protein. These results suggest that Akt is an important downstream effector of PI3K in promoting heart growth in the intact animal.
Acknowledgments
We thank J. Robbins for the αMyHC promoter DNA clone, S. Sehgal (Wyeth-Ayerst) for rapamycin, M. Birnbaum for the phospho-S6 antibody, A. Toker for GST-PKCζ, L. Zhou and K. Converso for assistance in echocardiography, J. Hampe for assistance in cell size measurement, and C. M. Yballe and H. Aoki for helpful discussions.
This work was supported in part by grant GM 41890 and a SCOR Grant on Atherosclerosis to L.C.C. and NIH grants AG 61716 and HL 65742 to S.I.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., M. T. Kozlowski, Q. P. Weng, N. Morrice, and J. Avruch. 1997. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 8:69-81. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., E. Blazek, and P. K. Vogt. 2001. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. USA 98:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki, E., M. A. Lipes, M. E. Patti, J. C. Bruning, B. R. Haag, R. S. Johnson, and C. R. Kahn. 1994. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186-190. [DOI] [PubMed] [Google Scholar]
- 6.Baker, J., J. P. Liu, E. J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73-82. [PubMed] [Google Scholar]
- 7.Blazar, B. R., P. A. Taylor, D. C. Snover, S. N. Sehgal, and D. A. Vallera. 1993. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. J. Immunol. 151:5726-5741. [PubMed] [Google Scholar]
- 8.Bohni, R., J. Riesgo-Escovar, S. Oldham, W. Brogiolo, H. Stocker, B. F. Andruss, K. Beckingham, and E. Hafen. 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97:865-875. [DOI] [PubMed] [Google Scholar]
- 9.Boluyt, M. O., J. S. Zheng, A. Younes, X. Long, L. O'Neill, H. Silverman, E. G. Lakatta, and M. T. Crow. 1997. Rapamycin inhibits alpha 1-adrenergic receptor-stimulated cardiac myocyte hypertrophy but not activation of hypertrophy-associated genes. Evidence for involvement of p70 S6 kinase. Circ. Res. 81:176-186. [DOI] [PubMed] [Google Scholar]
- 10.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 11.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. Akt/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965-1014. [DOI] [PubMed] [Google Scholar]
- 12.Chen, C., J. Jack, and R. S. Garofalo. 1996. The Drosophila insulin receptor is required for normal growth. Endocrinology 137:846-856. [DOI] [PubMed] [Google Scholar]
- 13.Chen, H., S. Qi, D. Xu, J. Wu, and P. Daloze. 1996. The immunosuppressive effect of rapamycin on mouse small bowel transplantation. Transplantation 61:523-526. [DOI] [PubMed] [Google Scholar]
- 14.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng, J. Q., B. Ruggeri, W. M. Klein, G. Sonoda, D. A. Altomare, D. K. Watson, and J. R. Testa. 1996. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA 93:3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw 3rd, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2. (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 17.Cho, H., J. L. Thorvaldsen, Q. Chu, F. Feng, and M. J. Birnbaum. 2001. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 18.Chou, M. M., and J. Blenis. 1995. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr. Opin. Cell Biol. 7:806-814. [DOI] [PubMed] [Google Scholar]
- 19.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 20.Clemens, M. G. 1996. Protein kinases that phosphorylate eIF2 and eIF2B, and their role in eukaryotic cell translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Coffer, P. J., and J. R. Woodgett. 1991. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201:475-481. [Erratum, 205:1217, 1992.] [DOI] [PubMed] [Google Scholar]
- 22.Colao, A., P. Marzullo, C. Di Somma, and G. Lombardi. 2001. Growth hormone and the heart. Clin. Endocrinol. 54:137-154. [DOI] [PubMed] [Google Scholar]
- 23.Conlon, I., and M. Raff. 1999. Size control in animal development. Cell 96:235-244. [DOI] [PubMed] [Google Scholar]
- 24.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 25.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeChiara, T. M., A. Efstratiadis, and E. J. Robertson. 1990. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345:78-80. [DOI] [PubMed] [Google Scholar]
- 27.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 28.Dufner, A., M. Andjelkovic, B. M. Burgering, B. A. Hemmings, and G. Thomas. 1999. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol. Cell. Biol. 19:4525-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingras, A.-C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 30.Gingras, A. C., S. G. Kennedy, M. A. O'Leary, N. Sonenberg, and N. Hay. 1998. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt (PKB) signaling pathway. Genes Dev. 12:502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulick, J., A. Subramaniam, J. Neumann, and J. Robbins. 1991. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J. Biol. Chem. 266:9180-9185. [PubMed] [Google Scholar]
- 32.Haq, S., G. Choukroun, Z. B. Kang, H. Ranu, T. Matsui, A. Rosenzweig, J. D. Molkentin, A. Alessandrini, J. Woodgett, R. Hajjar, A. Michael, and T. Force. 2000. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland, E. C., J. Celestino, C. Dai, L. Schaefer, R. E. Sawaya, and G. N. Fuller. 2000. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat. Genet. 25:55-57. [DOI] [PubMed] [Google Scholar]
- 34.Huang, W. Y., J. Aramburu, P. S. Douglas, and S. Izumo. 2000. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat. Med. 6:482-483. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson, J., J. Jin, R. D. Cardiff, J. R. Woodgett, and W. J. Muller. 2001. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol. Cell. Biol. 21:2203-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang, B. H., M. Aoki, J. Z. Zheng, J. Li, and P. K. Vogt. 1999. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones, P. F., T. Jakubowicz, F. J. Pitossi, F. Maurer, and B. A. Hemmings. 1991. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701-713. [DOI] [PubMed] [Google Scholar]
- 39.Kimball, S. R., L. M. Shantz, R. L. Horetsky, and L. S. Jefferson. 1999. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 274:11647-11652. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura, T., W. Ogawa, H. Sakaue, Y. Hino, S. Kuroda, M. Takata, M. Matsumoto, T. Maeda, H. Konishi, U. Kikkawa, and M. Kasuga. 1998. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol. Cell. Biol. 18:3708-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotani, K., W. Ogawa, M. Matsumoto, T. Kitamura, H. Sakaue, Y. Hino, K. Miyake, W. Sano, K. Akimoto, S. Ohno, and M. Kasuga. 1998. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 18:6971-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leevers, S. J., D. Weinkove, L. K. MacDougall, E. Hafen, and M. D. Waterfield. 1996. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15:6584-6594. [PMC free article] [PubMed] [Google Scholar]
- 43.Lie, J. T. 1980. Pathology of the heart in acromegaly: anatomic findings in 27 autopsied patients. Am. Heart J. 100:41-52. [DOI] [PubMed] [Google Scholar]
- 44.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-I) and type 1 IGF receptor (Igf-Ir). Cell 75:59-72. [PubMed] [Google Scholar]
- 45.Luo, H., H. Chen, S. Qi, D. Loh, P. Daloze, A. Veillette, D. Xu, and J. Wu. 1998. De novo-developed T cells have compromised response to existing alloantigens: using Ld-specific transgenic 2C T cells as tracers in a mouse heart transplantation model. J. Immunol. 161:73-82. [PubMed] [Google Scholar]
- 46.Montagne, J., M. J. Stewart, H. Stocker, E. Hafen, S. C. Kozuma, and G. Thomas. 1999. Drosophila S6 kinase: a regulator of cell size. Science 285:2126-2129. [DOI] [PubMed] [Google Scholar]
- 47.Morisco, C., D. Zebrowski, G. Condorelli, P. Tsichlis, S. F. Vatner, and J. Sadoshima. 2000. The Akt-glycogen synthase kinase 3β pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J. Biol. Chem. 275:14466-14475. [DOI] [PubMed] [Google Scholar]
- 48.Nakatani, K., D. A. Thompson, A. Barthel, H. Sakaue, W. Liu, R. J. Weigel, and R. A. Roth. 1999. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J. Biol. Chem. 274:21528-21532. [DOI] [PubMed] [Google Scholar]
- 49.Ng, W. A., I. L. Grupp, A. Subramaniam, and J. Robbins. 1991. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ. Res. 68:1742-1750. [DOI] [PubMed] [Google Scholar]
- 50.Nishikawa, K., A. Toker, F. J. Johannes, Z. Songyang, and L. C. Cantley. 1997. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 272:952-960. [DOI] [PubMed] [Google Scholar]
- 51.Obata, T., M. B. Yaffe, G. G. Leparc, E. T. Piro, H. Maegawa, A. Kashiwagi, R. Kikkawa, and L. C. Cantley. 2000. Peptide and protein library screening defines optimal substrate motifs for Akt/PKB. J. Biol. Chem. 275:36108-36115. [DOI] [PubMed] [Google Scholar]
- 52.Orme, S. M., R. J. McNally, R. A. Cartwright, P. E. Belchetz, et al. 1998. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J. Clin. Endocrinol. Metab. 83:2730-2734. [DOI] [PubMed] [Google Scholar]
- 53.Palermo, J., J. Gulick, M. Colbert, J. Fewell, and J. Robbins. 1996. Transgenic remodeling of the contractile apparatus in the mammalian heart. Circ. Res. 78:504-509. [DOI] [PubMed] [Google Scholar]
- 54.Paradis, S., and G. Ruvkun. 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12:2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson, R. T., P. A. Beal, M. J. Comb, and S. L. Schreiber. 2000. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J. Biol. Chem. 275:7416-7423. [DOI] [PubMed] [Google Scholar]
- 56.Rockman, H. A., R. A. Hamilton, L. R. Jones, C. A. Milano, L. Mao, and R. J. Lefkowitz. 1996. Enhanced myocardial relaxation in vivo in transgenic mice overexpressing the β2-adrenergic receptor is associated with reduced phospholamban protein. J. Clin. Investig. 97:1618-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadoshima, J., and S. Izumo. 1995. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ. Res. 77:1040-1052. [DOI] [PubMed] [Google Scholar]
- 58.Saunders, R. N., M. S. Metcalfe, and M. L. Nicholson. 2001. Rapamycin in transplantation: a review of the evidence. Kidney Int. 59:3-16. [DOI] [PubMed] [Google Scholar]
- 59.Schmelzle, T., and M. N. Hall. 2000. TOR, a central controller of cell growth. Cell 103:253-262. [DOI] [PubMed] [Google Scholar]
- 60.Scott, P. H., G. J. Brunn, A. D. Kohn, R. A. Roth, and J. C. Lawrence, Jr. 1998. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 95:7772-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekulic, A., C. C. Hudson, J. L. Homme, P. Yin, D. M. Otterness, L. M. Karnitz, and R. T. Abraham. 2000. A direct linkage between the phosphoinositide 3-kinase-Akt signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60:3504-3513. [PubMed] [Google Scholar]
- 62.Shima, H., M. Pende, Y. Chen, S. Fumagalli, G. Thomas, and S. C. Kozma. 1998. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 17:6649-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shioi, T., P. M. Kang, P. S. Douglas, J. Hampe, C. M. Yballe, J. Lawitts, L. C. Cantley, and S. Izumo. 2000. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 19:2537-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soonpaa, M. H., and L. J. Field. 1998. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ. Res. 83:15-26. [DOI] [PubMed] [Google Scholar]
- 65.Soonpaa, M. H., K. K. Kim, L. Pajak, M. Franklin, and L. J. Field. 1996. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271:H2183-H2189. [DOI] [PubMed] [Google Scholar]
- 66.Takata, M., W. Ogawa, T. Kitamura, Y. Hino, S. Kuroda, K. Kotani, A. Klip, A. C. Gingras, N. Sonenberg, and M. Kasuga. 1999. Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J. Biol. Chem. 274:20611-20618. [DOI] [PubMed] [Google Scholar]
- 67.Tamemoto, H., T. Kadowaki, K. Tobe, T. Yagi, H. Sakura, T. Hayakawa, Y. Terauchi, K. Ueki, Y. Kaburagi, S. Satoh, et al. 1994. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182-186. [DOI] [PubMed] [Google Scholar]
- 68.Thomas, G., and M. N. Hall. 1997. TOR signalling and control of cell growth. Curr. Opin. Cell Biol. 9:782-787. [DOI] [PubMed] [Google Scholar]
- 69.Toker, A., and A. C. Newton. 2000. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274. [DOI] [PubMed] [Google Scholar]
- 70.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7:1133-1137. [DOI] [PubMed] [Google Scholar]
- 71.Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki, and T. Kadowaki. 1998. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273:5315-5322. [DOI] [PubMed] [Google Scholar]
- 72.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 73.van Weeren, P. C., K. M. de Bruyn, A. M. de Vries-Smits, J. van Lint, and B. M. Burgering. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J. Biol. Chem. 273:13150-13156. [DOI] [PubMed] [Google Scholar]
- 74.Verdu, J., M. A. Buratovich, E. L. Wilder, and M. J. Birnbaum. 1999. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1:500-506. [DOI] [PubMed] [Google Scholar]
- 75.Wang, Q., R. Somwar, P. J. Bilan, Z. Liu, J. Jin, J. R. Woodgett, and A. Klip. 1999. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19:4008-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinkove, D., and S. J. Leevers. 2000. The genetic control of organ growth: insights from Drosophila. Curr. Opin. Genet. Dev. 10:75-80. [DOI] [PubMed] [Google Scholar]
- 77.Williams, M. R., J. S. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. R. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]
- 78.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. Bonner-Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900-904. [DOI] [PubMed] [Google Scholar]
- 79.Wolska, B. M., and R. J. Solaro. 1996. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am. J. Physiol. 271:H1250-H1255. [DOI] [PubMed] [Google Scholar]
- 80.Zimmermann, S., and K. Moelling. 1999. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741-1744. [DOI] [PubMed] [Google Scholar]