Abstract
Scatter factor/hepatocyte growth factor (SF/HGF) expression has been linked to malignant progression in glial neoplasms. Using two glioma cell lines, U373MG and SNB-19, we have demonstrated that SF/HGF stimulation allows cells to escape G1/G0 arrest induced by contact inhibition or serum withdrawal. SF/HGF induced effects on two mechanisms of cell cycle regulation: suppression of the cyclin-dependent kinase inhibitor p27 and induction of the transcription factor c-Myc. Regulation of p27 by SF/HGF was posttranslational and is associated with p27 nuclear export. Transient transfections of U373MG and SNB-19 with wild-type p27 and a degradation-resistant p27T187A mutant were insufficient to induce cell cycle arrest, and SF/HGF downregulation of p27 was not necessary for cell cycle reentry. Analysis of Cdk2 kinase activity and p27 binding to cyclin E complexes in the presence of exogenous wild-type p27 or p27T187A demonstrated that Cdk2 activity was not necessary for SF/HGF-mediated G1/S transition. Similarly, overexpression of dominant-negative forms of Cdk2 did not block SF/HGF-triggered cell cycle progression. In contrast, SF/HGF transcriptionally upregulated c-Myc, and overexpression of c-Myc was able to prevent G1/G0 arrest in the absence of SF/HGF. Transient overexpression of MadMyc, a dominant-negative chimera for c-Myc, caused G1/G0 arrest in logarithmically growing cells and blocked SF/HGF-mediated G1/S transition. c-Myc did not exert its effects through p27 downregulation in these cell lines. SF/HGF induced E2F1-dependent transcription, the inhibition of which did not block SF/HGF-induced cell cycle progression. We conclude that SF/HGF prevents G1/G0 arrest in glioma cell lines by a c-myc-dependent mechanism that is independent of p27, Cdk2, or E2F1.
Scatter factor (SF), also known as hepatocyte growth factor (HGF), is a multifunctional growth factor that is capable of acting as a potent mitogen, morphogen, motility factor, or angiogenic factor (6, 34, 59, 61, 66). Its signaling effects are mediated through a transmembrane receptor tyrosine kinase encoded by the c-Met proto-oncogene (7, 37). SF/HGF and c-Met are widely expressed and are thought to play a key role in normal mesenchymal (site of SF/HGF expression) and epithelial (site of c-Met expression) interactions (44, 46, 57). In neoplasia, SF/HGF-c-Met signaling is often aberrant. Constitutively active c-Met mutations underlie hereditary renal carcinoma; c-Met amplification occurs in multiple tumor types and carcinomas frequently possess an autocrine SF/HGF-c-Met loop that correlates with malignant progression (13, 40, 64).
Gliomas represent the most common form of primary central nervous system malignancy and are among the tumors most tightly linked with SF/HGF-c-Met signaling abnormalities. Multiple studies have demonstrated that human gliomas frequently coexpress SF/HGF and c-Met and that high levels of expression are associated with malignant progression. Additionally, experimental studies have demonstrated that SF/HGF gene transfer to glioma cell lines enhances tumorigenicity, tumor growth, and tumor-associated angiogenesis (18, 19, 24, 25, 31-33, 43, 51, 62, 63) and that blocking SF/HGF-c-Met signaling reverses these phenotypes in vivo (2).
The intermediary molecular events by which aberrant SF/HGF-c-Met signaling leads to malignant progression are slowly being elucidated. Recently, we demonstrated that SF/HGF-c-Met was able to activate Akt and protect glioma cell lines from apopototic death, both in vitro and in vivo (8). In the process of performing these experiments, we noted that the results of our in vitro screens depended upon plating density. When we plated glioma cells at low density, the principal effect of SF/HGF was antiapoptotic, while cells plated at higher densities experienced a mitogenic effect. From this observation, we hypothesized that SF/HGF signaling stimulated confluent cells to escape contact inhibition.
SF/HGF has previously been shown to release Mv1Lu mink epithelial cells that had been growth arrested via exposure to transforming growth factor β (TGF-β) by blocking association of the cyclin-depedent kinase inhibitor p27 with Cdk2-cyclin E complexes (56). Additionally, induction of p27 expression has been reported in glioma cell lines under conditions of contact inhibition, and loss of p27 expression is associated with poorer prognosis in patients suffering from glioma (14, 38) and other malignant tumors (27, 65). We therefore focused our efforts on elucidating the relationship between SF/HGF-mediated p27 regulation and release from growth arrest. Surprisingly, while SF/HGF dramatically downregulated p27 protein levels in the two glioblastoma cell lines examined, this effect proved independent of the SF/HGF-mediated growth response. Recently, several investigators have postulated the existence of a cell cycle pathway parallel to the retinoblastoma (Rb) pathway that is capable of activating cell cycle progression independently of Rb function (4, 38, 45). The proto-oncogene, transcription factor c-Myc, has been demonstrated to activate this pathway. Because c-myc is a delayed-early-response gene for SF/HGF signaling in hepatocytes (26, 52-54), it seemed plausible that c-Myc could be driving cell cycle progression under contact inhibition in gliomas. Our results support this conclusion and further demonstrate that SF/HGF-mediated G1/S transition occurs in these cells independent of p27, Cdk2, and E2F1.
MATERIALS AND METHODS
Cell culture and reagents.
U373MG and HEK-293 cell lines were purchased from American Type Culture Collection (Rockville, Md.) and grown in Dulbecco's modified essential medium (DMEM; Gibco-BRL, Rockville, Md.) supplemented with 10% fetal bovine serum (FBS; Gemini Bioproducts, Calabasas, Calif.), 20 mM HEPES (Mediatech, Herndon, Va.), and pencillin-streptomycin (Mediatech). U373-Neo and U373-SF cell lines have been previously described and were grown in the medium described above supplemented with 300 μg of Geneticin per ml (Gibco-BRL). SNB-19 cells were a gift of J. Rao, University of Illinois College of Medicine at Peoria, and were grown in DMEM-F12 (Gibco-BRL) supplemented with 10% FBS, 20 mM HEPES, and penicillin-streptomycin. All cells were grown at 37°C in a humidified incubator with 5% CO2. Recombinant human SF/HGF was provided as a gift from Genentech, Inc. (San Francisco, Calif.).
Vectors.
Adenovirus vectors encoding GFP, c-Myc-hemagglutinin (HA) and MadMyc-HA constructs were provided by B. Vogelstein, Johns Hopkins University School of Medicine, Baltimore, Md., and have been previously described (5, 17). Adenovirus constructs were propagated in HEK-293 cells as described for the pAdEasy adenoviral generation system (15). In vitro transductions were performed at a multiplicity of infection of 10. Transduction efficiency was judged at 24 h by expression of green fluorescent protein (GFP). pCS2, pCS2-p27, and pCS2-p27T187A constructs were provided by B. Clurman (Fred Hutchinson Cancer Center) and have been described previously (50). Full-length cDNA probes for c-Myc and p27 Northern blots were produced by restriction digests of pBS-c-Myc (gift of C. Dang, Johns Hopkins University School of Medicine) and pCEP4p27 (gift of B. Vogelstein) followed by gel purification (Qiaex gel extraction; Qiagen, Hilden, Germany). The dominant-negative (DN) form of Cdk2 (pCMV-Cdk2DN) (kindly provided by S. van den Heuvel) (58) (10), and the E2F1-DN expression vector pcDNA-E2F1(1-368)/RB- (379-792) and E2F1 promoter-luciferase reporter construct (pGL2AN/Luc) (kindly supplied by W. G. Kaelin) (35, 49) have been previously described. Target cells were transfected with Fugene-6 transfection reagent (Roche, Indianapolis, Ind.) according to manufacturer's specifications with a 3:1 ratio of transfection reagent volume to DNA mass. To judge the efficiency of transfection, cells were cotransfected with a pCS2 plasmid encoding GFP under the control of the cytomegalovirus (CMV) promoter at a ratio of 10:1 (target gene: GFP plasmid). Efficiency of transient transfection under the conditions of these experiments was found to be typically ≥80%.
Luciferase activity was assayed as described previously (20). Cells (2 × 10 5) grown in six-well plates were transfected with 3 μg of pGL2AN/Luc plasmid DNA or control plasmid DNA (pGL2). Twenty-four hours after transfection, cells were stimulated with SF/HGF (100 ng/ml) for an additional 24 h, and cell extracts were prepared with reporter lysis buffer (Promega, Madison, Wis.). Luminescence was measured in 20 μl of cell extract with a luciferase assay system kit (Promega). Activity was expressed as relative light units per milligram of cellular protein as determined by Coomassie protein assay (Pierce, Rockford, Ill.).
Antibodies.
Antibodies used for Western blotting, immunofluorescence, immunoprecipitation, and the kinase assay were obtained from the following sources: Transduction Laboratories (Lexington, Ky.), Kip1p27 (1:2,000, Western blot) and Cip1p21 (1:250, Western blot); Santa Cruz Biotechnology (Santa Cruz, Calif.), p27 (C-19; immunofluorescence, 1:100), actin (C-11; Western blot, 1:1,000), Cdk2 (M2; Western blot, 1:500; kinase assay, 1:50), Cdk4 (C-22, Western blot, 1:500), c-Myc (9E10; Western blot, 1:500), cyclin A (H-432; Western blot, 1:500), cyclin D1 (HD11; Western blot, 1:500), cyclin D2 (H-289; Western blot, 1:500), cyclin D3 (D-7; Western blot, 1:500), cyclin E (HE12; Western blot, 1:500; and C-19; immunoprecipitation, 1:50), and Rb (C-15; Western blot, 1:500); and Clontech (Palo Alto, Calif.), HA tag (Western blot, 1:1,000). Secondary antibodies conjugated to horseradish peroxidase were purchased from Jackson Immunoresearch Laboratories (West Grove, Pa.), and those conjugated to Alexa-fluor were purchased from Molecular Probes (Eugene, Oreg.).
Flow cytometry.
Cells growing as a monolayer on 100-mm-diameter tissue culture dishes (Corning, Corning, N.Y.) were trypsinized (Gibco-BRL), pelleted by centrifugation, resuspended in 1 ml of ice-cold phosphate-buffered saline (PBS), and fixed by the addition of 4 ml of ice-cold ethanol under gentle vortexing. Fixed cells were stored up to 1 week in 80% ethanol at −20°C until ready for labeling. Stored cells were collected by centrifugation, resuspended in 1 ml of PBS, and treated with 20 μg of DNase-free RNase (Boehringer-Mannheim, Mannheim, Germany) for 30 min at 37°C. Cells were labeled with propidium iodide (Sigma, St. Louis, Mo.) at a final concentration of 100 μg/ml for 10 min at room temperature. Analyses were performed on a FACscan (Becton-Dickinson, Fullerton, Calif.). Raw data were gated to remove doublets and cellular debris. The resultant cell cycle histograms were analyzed with CellQuest software (Becton-Dickinson), and the area under the curve was integrated for each peak.
Tritiated thymidine incorporation.
Cells were plated (100,000 cells/well) in six-well plates in medium containing 10% FBS and allowed to adhere overnight. Cells were then switched to fresh medium containing 0.1% FBS ± 100 ng of SF/HGF per ml. After 18 h, 5 μCi of [3H]thymidine was added to each well. Cells were incubated for an additional 6 h at 37°C, and then the medium was removed and each well was washed three times with PBS. Radiolabeled cells were treated three times in 10% trichloroacetate at 1 ml per well for 20 min at ambient temperature and then lysed in 0.2 M NaOH overnight at 37°C. The extent of radiolabeling was determined by subjecting an aliquot of the cell lysate to scintillation counting (LS 6500; Beckman, Fullerton, Calif.).
MTT assay.
Cells were plated at low density (2,000 cells per well) or high density (5,000 cells per well) in 96-well tissue culture plates in medium containing 10% FBS (full serum) or 0.1% FBS (low serum) supplemented with 100 ng of SF/HGF per ml or the equivalent amount of PBS. Seventy-two hours postplating, MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well at a final concentration of 1 mg/ml, and the cells were incubated for 2 h at 37°C. The medium was then removed, and the formazen reaction product was solubilized by addition of 100 μl of dimethyl sulfoxide (DMSO). A570 was measured with a microtiter plate reader (Dynatech, Alexandria, Va.). Absorbance values are presented as the mean of eight wells per treatment ± the standard error (SE).
Northern blot.
Total RNA was harvested from the cells by using Qiagen RNeasy kits according to manufacturer's recommendations. Ten micrograms of RNA per sample was denatured with deionized glyoxal, combined with RNA loading buffer, and run on a 1.0% agarose gel containing ethidium bromide for 3 h at 60 V. RNA was transferred to a nylon membrane (Nytran; Schleicher & Schuell) overnight in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Membranes were prehybridized for 4 h at 42°C and then hybridized overnight at 42°C in a rotating oven. Membranes were washed three times in 1× SSC-0.1% sodium dodecyl sulfate (SDS) at 50°C and exposed to a phosphorimager cassette (Fujifilm, Tokyo, Japan). Full-length cDNA probes were generated for c-Myc, p27, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with [32P]dCTP (Amersham-Pharmacia, Piscataway, N.J.) in conjunction with a random priming labeling kit (Boehringer-Mannheim) according to manufacturer's specifications.
Western blot.
SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting were performed by the method of Towbin et al. (55) with some modifications. Cells growing in a monolayer on 100-mm-diameter tissue culture dishes were washed with ice-cold PBS and then lysed by the addition of 300 μl of radioimmunoprecipitation assay (RIPA) buffer (1% Igepal, 0.5% sodium deoxycholate, and 0.1% SDS in PBS) containing fresh protease inhibitors (Calbiochem, San Diego, Calif.). Plates were scraped to recover cellular lysates that were passed through a 20-gauge needle to shear DNA, incubated on ice for 20 min, and centrifuged at 15,000 × g at 4°C for 15 min. Supernatant protein concentrations were determined for each sample with the Coomassie protein assay (Pierce) according the manufacturer's recommendations. Aliquots of 10 μg of total protein were combined with Laemmli loading buffer containing β-mercaptoethanol and heated at 100°C for 5 min. The proteins were separated on precast SDS-PAGE gels (Invitrogen, Carlsbad, Calif.) at 125 V for 90 min. Proteins were electrophoretically transferred to nitrocellulose with a semidry transfer apparatus at 50 mA per gel for 45 min (Amersham-Pharmacia). Membranes were incubated for 1 h in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBS/T) with 5% nonfat dried milk at room temperature. Incubation with primary antibody was performed overnight at 4°C in TBS/T-milk at the concentrations indicated above. Membranes were then washed three times with TBS/T, incubated with secondary antibody at 1:2,000 for 1 h in TBS/T-milk, washed three times with TBS/T, and then developed with an enhanced chemiluminescence (ECL) detection kit (Amersham-Pharmacia) per the manufacturer's instructions.
Immunoprecipitation.
Cyclin E immunoprecipitations were performed as previously described (9). Briefly U373MG cell monolayers were transiently cotransfected with pCS2-GFP, pCS2-p27, or pCS2-p27T187A as described above. Cells were then growth arrested in medium containing 0.1% FBS ± 100 ng of SF/HGF per ml for 24 h and then lysed on ice in a buffer containing 50 mM HEPES, 20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1% Triton X-100, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and complete protease inhibitor cocktail (Calbiochem). Lysates were homogenized and cleared by centrifugation at 10,000 × g for 10 min. Protein concentrations were determined in the supernatant as described above, and 100 μg of total protein was used for each immunoprecipitation. The volume of each tube was brought to 500 μl with lysis buffer, and 2 μg of cyclin E (c-19) antibody was added. The tubes were mixed continuously at 4°C for 1 h, and then 40 μl of protein A-agarose beads (Upstate Biotechnology, Lake Placid, N.Y.) was added to each tube, and the samples were mixed continuously for another hour. The beads were collected by centrifugation, washed three times with lysis buffer, heated to 100°C in Laemmli buffer, and subjected to Western blotting for p27 as described above.
Kinase assay.
Cdk2 kinase assays were performed as described previously (29). U373MG cell monolayers were transfected with p27 and arrested in medium with 0.1% FBS as described above for immunoprecipitation. Cells were lysed on ice in a buffer containing 50 mM HEPES (ph 7.5), 150 mM NaCl, 2.5 mM EGTA, 1 mM dithiothreitol (DTT), 0.1% Tween 20, 10% glycerol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM sodium orthovanadate, and complete protease inhibitor cocktail (Calbiochem). Lysates were cleared by centrifugation, and the protein concentration of the supernatant was determined. Each lysate (100 μg of total protein) was immunoprecipitated with 2 μg of Cdk2 (M2) antibody for 1 h at 4°C, followed by addition of 40 μl of protein A-agarose beads (Upstate Biotechnology) for an additional hour. Immune complexes were washed three times with lysis buffer and twice with 50 mM HEPES containing 1 mM DTT, and then were incubated for 30 min at 30°C in 30 μl of buffer containing 50 mM HEPES, 10 mM MgCl2, 1 mM DTT, 1 μg of histone H1 (Boehringer Mannheim, Indianapolis, Ind.), 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM NaF, 10 μCi of [γ-32P]ATP (100 μM ATP; Amersham-Pharmacia). After the incubation, 6× Laemmli loading buffer was added to each sample, the complexes dissociated by heating to 100°C for 5 min, and the reaction products were subjected to SDS-PAGE. The gel was subsequently dried, and the phosphorylated histone product was visualized by phosphorimaging (Fujifilm).
Immunofluorescence.
Cells were plated onto four-well chamber slides (Nalge-Nunc, Naperville, Ill.) in medium containing 10% FBS and allowed to adhere overnight. Fresh medium containing 0.1% FBS ± 100 ng of SF/HGF per ml was then substituted and the cells were incubated for an additional 24 h. Cells were fixed with methanol-acetone (1:1) for 2 min and then incubated for 1 h at room temperature with PBS containing 1.5% normal goat serum (Sigma). Cells were then incubated for 1 h at 4°C in PBS-goat serum containing rabbit polyclonal anti-human p27 antibody. Cells were washed three times with PBS and then incubated for 1 h with Alexa-conjugated mouse anti-rabbit secondary antibody at room temperature. Cells were washed three times with PBS, and the slides were coverslipped with Vectashield antifade solution with DAPI (Vector Laboratories, Burlingame, Calif.). Slides were observed with an Axiovision fluorescent microscope with fluorescein isothiocyanate (FITC) and DAPI filters.
Growth curves.
U373-SF and U373-Neo cell lines were plated at 10,000 cells per well in 24-well tissue culture dishes in medium supplemented with 10% FBS. On postplating days 1, 3, 4, 5, 6, and 7, four wells from each cell line were harvested by trypsinization, and the cell number was determined with a Coulter counter (Beckman-Coulter). The mean number of cells per well and the SE were generated for each cell line at each time point.
RESULTS
SF/HGF overcomes contact inhibition of U373MG cells.
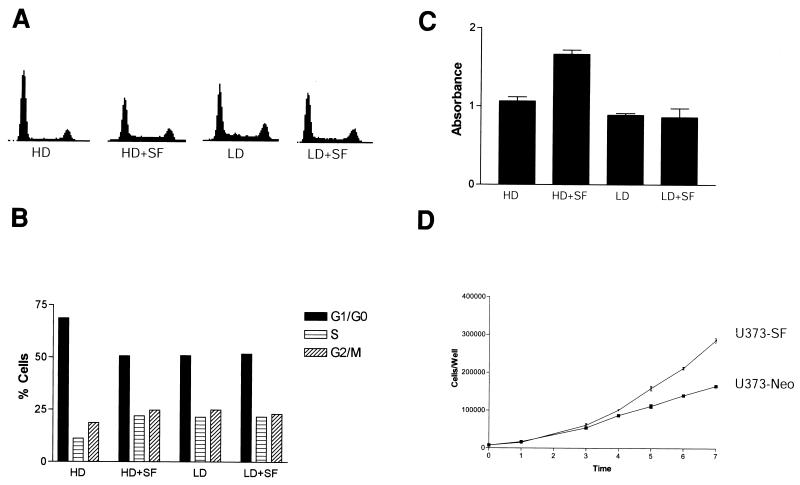
The U373MG cell line is a human malignant glioma cell line that expresses c-Met, but not SF/HGF (25). We have confirmed the glial origin of these cells by reverse transcriptase PCR for glial fibrillary acidic protein, a specific marker for glial cells. In cell culture, U373MG cells arrest in G1/G0 when grown to a confluent monolayer and express high levels of the cyclin-dependent kinase inhibitor p27 (data not shown). In contrast, when U373MG cells are grown to confluency in medium supplemented with 100 ng of SF/HGF per ml, G1/G0 arrest does not occur. SF/HGF reduced the G1/G0 fraction by 29.6% ± 4.3% compared to contact-inhibited cells, producing a cell cycle profile indistinguishable from cells in log growth phase (Fig. 1A and B). SF/HGF had no appreciable mitogenic effect on cells at lower confluencies that were already in log growth phase. Treatment with 10 ng of SF/HGF per ml was 50% as effective as 100 ng/ml, while the results of treatment with 1 ng of SF/HGF per ml were indistinguishable from those in controls (data not shown). MTT assays confirmed that SF/HGF was promoting cellular replication as opposed to solely redistributing cells in the cell cycle. SF/HGF at 100 ng/ml increased raw cell numbers by 64.3% (n = 8, P < 0.0001, t test) compared to control cells maintained for 72 h at confluency (Fig. 1C). Again, SF/HGF did not produce any mitogenic effect on sparsely plated cells. Finally, to replicate the effect of an SF/HGF-c-Met autocrine loop, a condition associated with malignant glioma progression in vivo, we examined growth curves of U373MG cells stably transfected with SF/HGF or control neomycin resistance expression plasmids. U373-SF cell lines continued to grow logarithmically even after the growth of control U373-Neo cell lines had plateaued due to contact inhibition (Fig 1D).
FIG. 1.
SF/HGF prevents contact inhibition in U373MG cells. U373MG cells plated in medium containing 10% FBS at high density (HD) or low density (LD) were treated for 24 h with 100 ng of SF/HGF per ml. Fluorescence-activated cell sorter analysis (A and B) demonstrated that SF/HGF reduced the fraction of cells in G1/G0 by 29.6% (n = 3 experiments, SE = 4.3%) compared to untreated controls when cells were grown at high density. SF/HGF had no effect on cell cycle distribution in cells grown at low density. (C) MTT assay demonstrated SF/HGF increased U373MG cell number by 64.3% (n = 8 per group, P < 0.001, t test) when cells were grown for 72 h at high density. SF/HGF produced little mitogenic effect on cells at low density. (D) Growth curves comparing U373 cells engineered to secrete human SF/HGF (U373-SF) with control transfected SF-negative U373 cells (U373-Neo).
SF/HGF overcomes serum starvation growth inhibition.
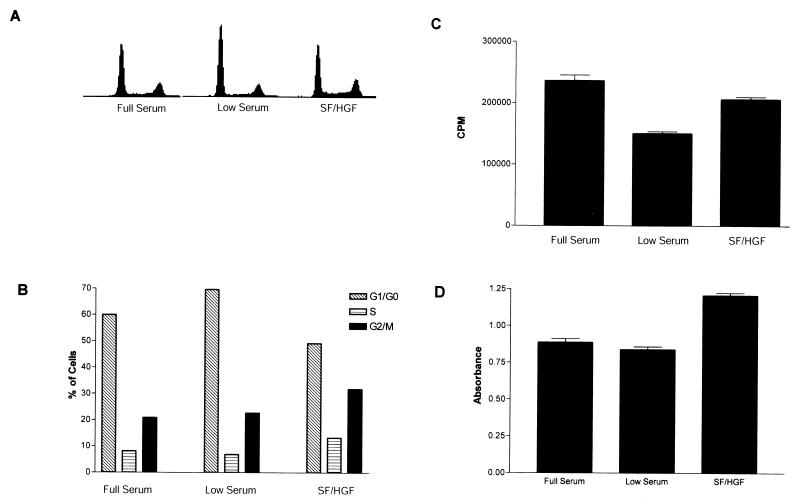
Contact inhibition and nutrient deprivation growth arrest are mediated by similar pathways. We therefore examined the ability of SF/HGF to reverse growth arrest induced by serum starvation. When U373MG cells in the log phase of growth were switched to medium containing 0.1% FBS for 24 h, they experienced growth inhibition associated with the accumulation of cells in G1/G0. SF/HGF completely blocked the effect of serum withdrawal (Fig. 2A and B), reducing the G1/G0 fraction by 25.3% (n = 3 experiments, SE = 5.1%) compared to that in growth-inhibited cells. Serum withdrawal did not result in an increased subdiploid fraction, indicating that U373MG cells were not undergoing apoptosis. Tritiated thymidine uptake also demonstrated that SF/HGF was able to restore DNA synthesis levels to those of cells growing in full serum (Fig. 2C). MTT assays again confirmed that SF/HGF was acting as a true mitogen, rather than stimulating DNA synthesis in the absence of cellular replication (Fig 2D).
FIG. 2.
SF/HGF overcomes serum starvation growth inhibition in U373MG cells. (A and B) Fluorescence-activated cell sorter analysis of U373MG cells grown in 0.1% FBS showed an increase in the G1/G0 fraction compared to cells in 10% FBS. Addition of 100 ng of SF/HGF per ml to U373MG cells for 24 h in low serum blocked serum starvation growth inhibition, reducing the G1/G0 fraction by 25.3% (n = 3 experiments, SE = 5.1%) compared to that of untreated controls. SF/HGF increased tritiated thymidine incorporation (C) in U373MG cells grown in low serum by 37.4% (n = 6 per group, SE = 2.7%, P < 0.001, t test) and MTT conversion (D) to formazen by 43.9% (n = 8 per group, SE = 0.2%, P < 0.001, t test)
SF/HGF selectively downregulates p27 and induces c-Myc.
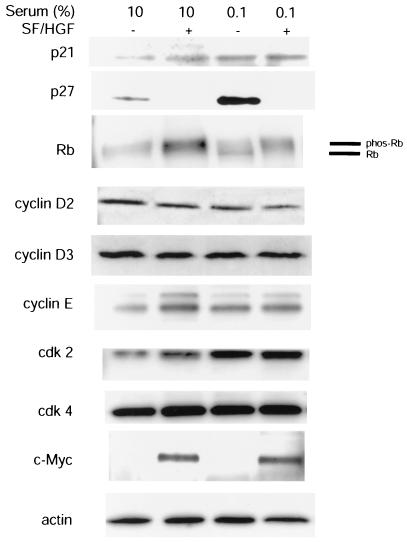
To determine candidate mechanisms for the SF/HGF-mediated mitogenic response on U373MG cells, we performed Western blots for known G1/S checkpoint proteins on cellular lysates from U373MG cells with growth inhibited by confluency or serum starvation (Fig. 3). Of the proteins examined, we noticed significant inhibition of p27 protein levels with SF/HGF stimulation. Corresponding to p27 inhibition was a molecular weight shift in the downstream Rb product to the phosphorylated state, consistent with loss of cyclin-dependent kinase inhibitory function. Mild increases in cyclin E levels were also seen with SF/HGF. In contrast, c-Myc was undetectable in quiescent cells, but prominent in SF/HGF-treated cells. In addition to the data shown in Fig. 3, cyclin D1 was examined, but was not expressed highly in this cell line. Cyclin A was also examined, but was not significantly altered with SF/HGF treatment. The tumor suppressor genes PTEN, TP53, p15INK4B, and p16INK4A are mutated and are not expressed in U373MG (3, 21, 42).
FIG. 3.
SF/HGF selectively alters p27 and c-Myc protein levels under conditions of contact inhibition and serum starvation. Treatment of U373MG cells growth arrested by contact inhibition in 10% FBS or by serum starvation in 0.1% FBS with 100 ng of SF/HGF per ml resulted in downregulation of the cyclin-dependent kinase inhibitor p27 and a corresponding shift in the phosphorylation state of the downstream Rb product. c-Myc protein levels were elevated in response to SF/HGF. Minor changes were seen in the gross levels of all other G1/G0 cell cycle checkpoint proteins examined.
SF/HGF regulates p27 levels posttranscriptionally.
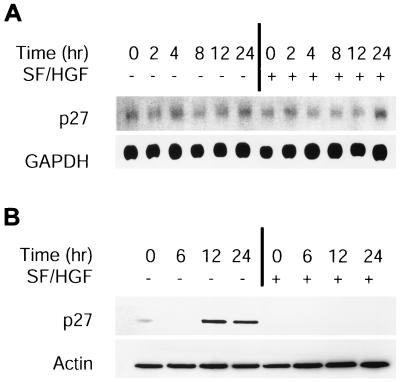
To determine if SF/HGF affects p27 levels transcriptionally, RNA was isolated from U373MG cells growing in medium containing 0.1% FBS supplemented with SF/HGF or the equivalent amount of PBS at time points up to 24 h following the switch from medium with 10% FBS lacking SF/HGF at 0 h. Northern blot analysis demonstrated no appreciable change in p27 mRNA, either with the switch to low-serum medium or with the addition of SF/HGF (Fig. 4A). In contrast, p27 protein levels determined by Western blotting were increased by 12 h following the switch to low-serum medium, while addition of SF/HGF blocked p27 accumulation (Fig. 4B). These results indicated that both p27 induction by serum starvation and p27 inhibition by SF/HGF were mediated posttranscriptionally.
FIG. 4.
SF/HGF regulates p27 by posttranscriptional mechanisms. Cells in the log growth phase were switched to 0.1% FBS ± 100 ng of SF/HGF per ml at 0 h. (A) Northern blot analysis for p27 mRNA revealed no significant changes in p27 transcript level over 24 h, induced either by the switch to low serum or the addition of SF/HGF. (B) In contrast, Western blotting for p27 protein levels showed a gradual accumulation of p27 protein following induction of growth arrest by serum starvation that was entirely blocked by SF/HGF.
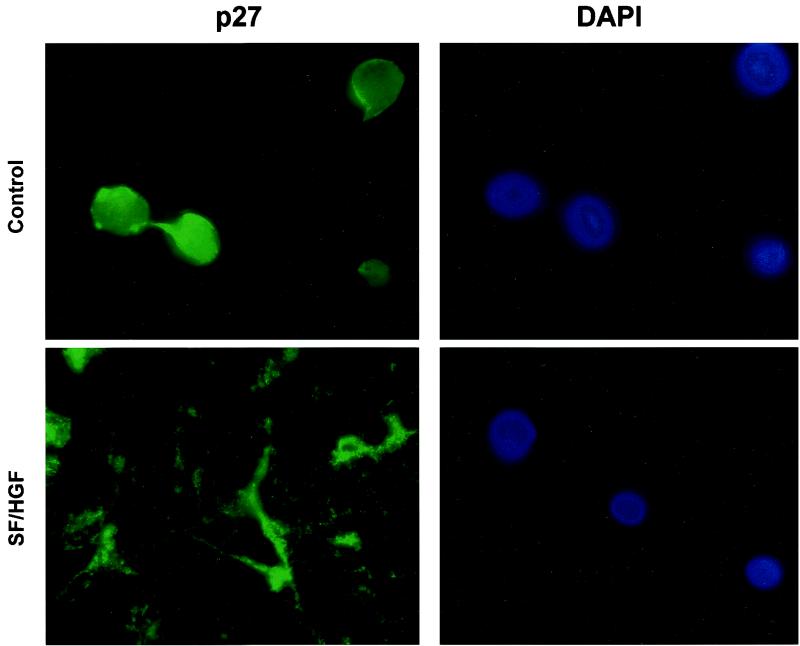
SF/HGF translocates p27 from the nucleus.
To confirm that SF/HGF was mediating a posttranscriptional change in p27, we examined U373MG cells with growth inhibited by either serum starvation (Fig. 5) or confluency (data not shown) in the presence or absence of SF/HGF. Under either condition of growth inhibition, in the absence of SF/HGF, p27 accumulated in the nucleus, its putative site of action acting as a cyclin-dependent kinase inhibitor. In contrast, with the addition of SF/HGF, p27 is primarily cytoplasmic, since cytoplasm is the site of proteasome-mediated degradation.
FIG. 5.
SF/HGF translocates p27 from the nucleus. Immunofluorescence micrographs of U373MG cells grown in 0.1% FBS in the absence of SF/HGF demonstrate that p27 colocalizes with DAPI in cell nuclei. Addition of 100 ng of SF/HGF per ml for 24 h results in p27 targeting to the cytoplasm. Detection of diminished p27 levels in SF/HGF-treated cells required increased exposure times relative to controls.
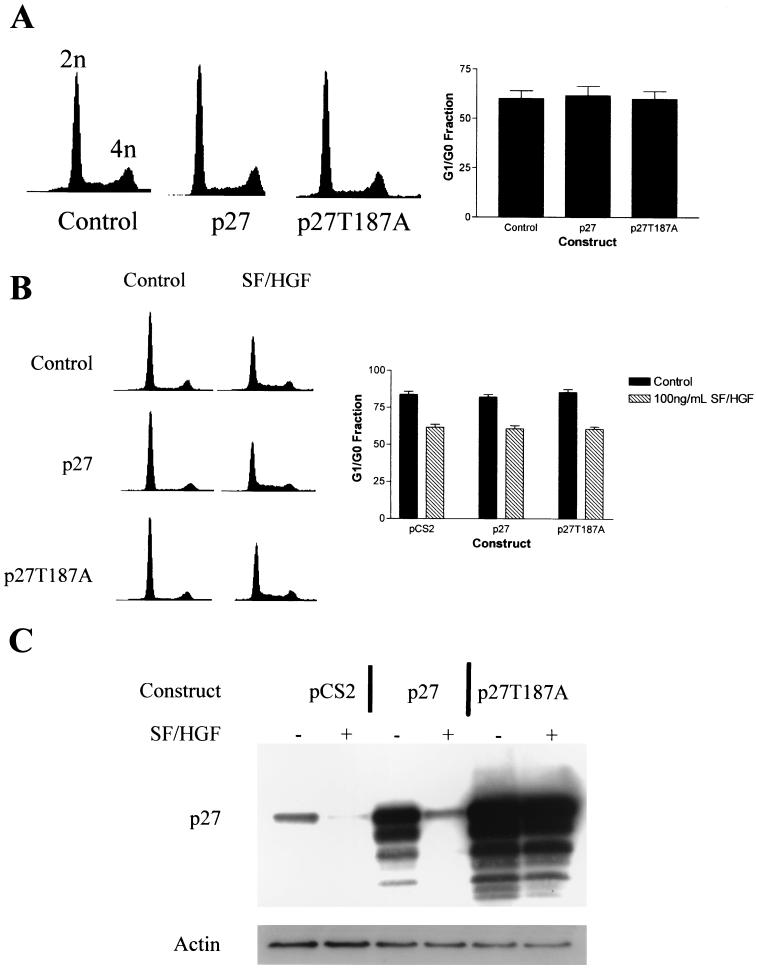
Increased p27 expression is neither sufficient for nor is its suppression necessary to overcome G1/G0 arrest in U373MG.
To test the hypothesis that changes in p27 levels were mediating the mitogenic effect of SF/HGF, we examined the effect of transgenic p27 expression on U373MG cell cycle profiles. Interestingly, expression of transgenic p27, even at levels several times those of native p27, did not change the cell cycle profile of U373MG cells in the log growth phase in 10% FBS (Fig. 6A and B), indicating that high levels of p27 expression were not sufficient to induce growth inhibition in this cell line. To determine if p27 suppression was necessary for the SF/HGF-mediated cell cycle response, we transfected U373MG cells with p27, p27T187A, or control plasmids. The p27T187A mutant retains the cyclin-dependent kinase inhibitory properties of wild-type p27, but is mutated at the targeting site for proteasome-mediated degradation, rendering it resistant to degradation (50). SF/HGF was unable to stimulate degradation of the p27T187A construct (Fig. 6C); however, its effect on the cell cycle was identical to that of control cells. From this, we concluded that overexpression of p27 is not sufficient to induce G1/G0 growth arrest in U373MG and that SF/HGF-mediated cell cycle progression can proceed even in the absence of p27 suppression. Additionally, the results of these experiments confirmed that SF/HGF was mediating p27 levels posttranslationally, because SF/HGF was able to almost completely suppress p27 expression in cells transfected with 5 μg of plasmid constitutively expressing wild-type p27 under control of the CMV promoter, while it was not able to alter intracellular p27 levels in cells transfected with the degradation-resistant p27T187A. Transfection with larger amounts of p27 plasmid (10 or 15 μg) was sufficient to overcome proteasome-mediated degradation, but still could not cause G1/G0 arrest (data not shown).
FIG. 6.
p27 is not required for growth arrest or SF/HGF-mediated mitogenic response. (A) Fluorescence-activated cell sorter analyses of U373MG cells transiently transfected with expression plasmids encoding wild-type p27 or a proteasome-resistant p27 mutant T187A demonstrate no effect of p27 on cells in the log growth phase in 10% FBS. Transfection efficiency as judged by cotransfection with a plasmid encoding GFP was greater than 80%. (B) Overexpression of p27 or p27T187A did not block SF/HGF-mediated mitogenesis in U373MG cells. (C) Western blot analysis of transfected cells demonstrates increased p27 protein following transient transfection. SF/HGF was able to downregulate exogenous wild-type p27, but not the degradation-resistant p27T187A mutant.
SF/HGF stimulation of cell cycle progression is Cdk2 independent.
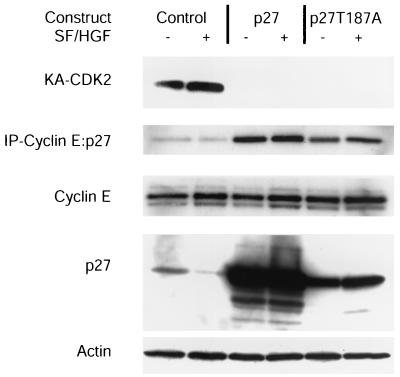
Since SF/HGF can mediate cell cycle progression in mink Mv1Lu cells by retargeting p27 away from cyclin E-Cdk2 complexes, we investigated whether SF/HGF retargeting of p27 was responsible for the SF/HGF-mediated release from cell cycle arrest. Immunoprecipitation experiments with a cyclin E antibody demonstrated a mild decrease in p27 associated with cyclin E complexes in response to SF/HGF stimulation; however, transfection with exogenous p27 or p27T187A clearly resulted in increased cyclin E-p27 complexes that SF/HGF could not downregulate (Fig. 7). Similarly, analysis of Cdk2 complex activity showed that overexpression of p27 or p27T187A completely blocked Cdk2 activity and that SF/HGF was unable to rescue this activity. Because SF/HGF was still able to mediate cell cycle progression, even in the presence of excess p27 or p27T187A, we concluded that neither p27 suppression nor Cdk2 kinase activity was required for SF/HGF-mediated cell cycle progression.
FIG. 7.
SF/HGF does not restore Cdk2 activity in the presence of exogenous p27. Cdk2 and Cyclin E complexes were immunoprecipitated (IP) from lysates of U373MG cells transiently transfected with p27 and p27T187A. Cdk2 complexes were subjected to in vitro kinase assays with histone H1 as a substrate (KA-CDK2). Cyclin E complexes were probed for p27 binding by SDS-PAGE immunoblotting. Expression of cyclin E, p27, and actin in the raw lysates was determined by SDS-PAGE immunoblotting. SF/HGF was unable to restore Cdk2 kinase activity or retarget p27 away from cyclin E complexes in the presence of exogenous p27 or p27T187A.
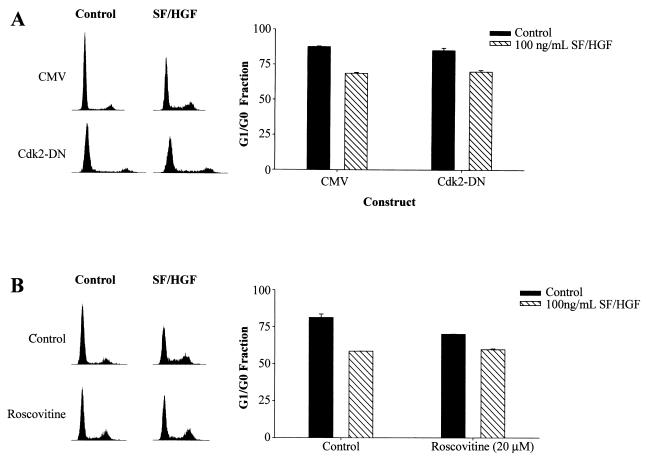
To further examine the Cdk2 independence of SF/HGF-mediated G1/S transition, dominant-negative Cdk2 (Cdk2-DN) (10, 58) and the specific Cdk2 inhibitor Roscovitine were used. U373MG cells transiently transfected with Cdk2-DN passed through G1/S in response to SF/HGF as efficiently as control transfected cells (Fig. 8A). Similarly, pretreatment with Roscovitine (20 μM), under conditions shown to completely inhibit Cdk2 kinase activity (23), did not block SF/HGF-mediated cell cycle progression (Fig. 8B).
FIG. 8.
SF/HGF stimulation of cell cycle progression is Cdk2 independent. (A) Addition of 100 ng of SF/HGF per ml to medium containing 0.1% FBS for 24 h reduced the G1/G0 fraction of U373MG cells transiently transfected with a control plasmid compared to that in cells grown in identical medium lacking SF/HGF (26.6%, SE = 1.3). Transient transfection of U373MG cells with dominant-negative Cdk2 (Cdk2-DN) did not inhibit SF/HGF-triggered cell cycle progression. The efficiency of transfection was >80% for both experiments, and the expression of transgenic Cdk2-DN was verified by Western blotting (not shown). (B) Similarly, pretreatment with Roscovitine (20 μM), a pharmacologic inhibitor of Cdk2, reduced the baseline G1/G0 fraction compared to the level in control cells, but did not block SF/HGF-induced cell cycle progression.
c-myc is a delayed-early-response gene for SF/HGF.
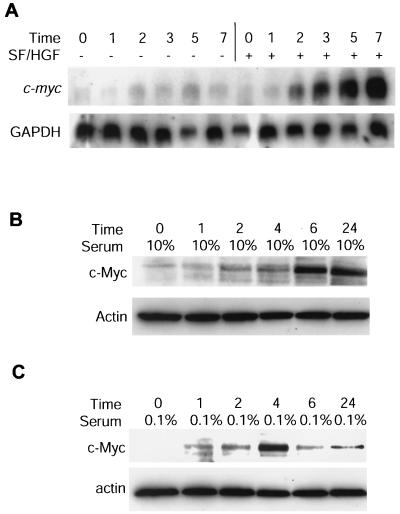
To examine the role of c-Myc in SF/HGF-mediated mitogenesis, we performed Northern blot analysis with RNA isolated from U373MG cells pretreated with 10 μM cycloheximide (Fig. 9A). SF/HGF induced a greater than fourfold increase in c-myc message, confirming c-myc is a direct target of SF/HGF signaling, rather than a factor activated as an epiphenomenon of proliferating cells. Similarly, c-Myc protein levels in U373MG cells arrested by contact inhibition or serum starvation rise within 24 h of addition of SF/HGF (Fig. 9B and C).
FIG. 9.
c-Myc is a delayed-early-response gene for SF/HGF. (A) U373MG cells were grown in 0.1% FBS for 24 h, pretreated with 10 μM cycloheximide, stimulated with 100 ng of SF/HGF per ml, and subjected to analysis by Northern blotting with full-length c-myc and GAPDH cDNA probes. (B and C) Western blot analysis for c-Myc protein levels following stimulation of U373MG cells with 100 ng of SF/HGF per ml arrested by contact inhibition in 10% FBS (B) or by serum starvation in 0.1% FBS (C).
c-Myc overexpression mimics SF/HGF mitogenicity.
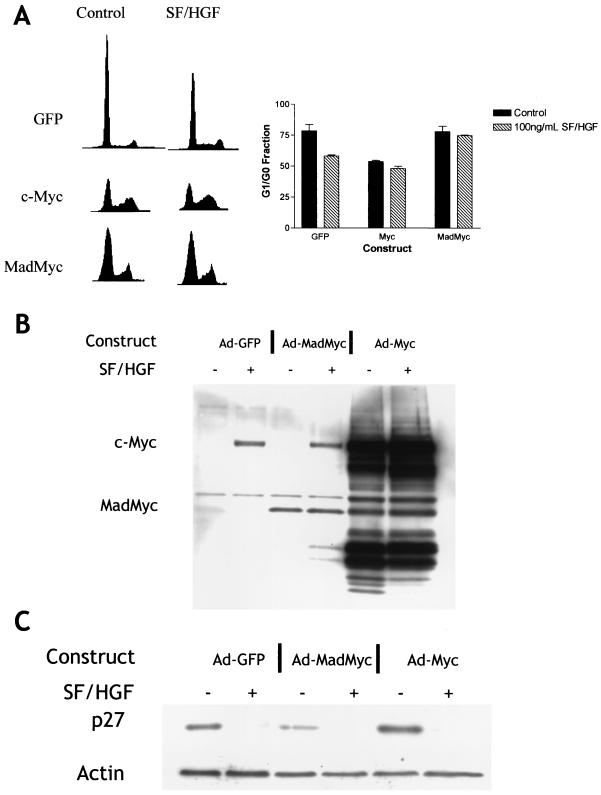
To determine if c-Myc is capable of mediating a mitogenic response in U373MG cells, we transduced U373MG cells with adenoviruses encoding GFP, c-Myc, or MadMyc (a dominant-negative form of Myc). Overexpression of c-Myc prevented U373MG cells growing in 0.1% FBS from accumulating in G1/G0, indicating U373MG cells are mitogenically responsive to c-Myc signaling (Fig. 10A). Conversely, overexpression of MadMyc completely blocked the SF/HGF-mediated mitogenic response, indicating that functioning c-Myc is necessary for SF/HGF-mediated cell growth (Fig. 10A). We also examined the effect of the c-Myc constructs on p27 levels (Fig. 10C). Western blots on U373MG lysates transduced with GFP, c-Myc, and MadMyc demonstrated that SF/HGF was able to efficiently stimulate degradation of p27, even when cell cycle progression was blocked in the case of the cells transduced with MadMyc. Similarly, overexpression of c-Myc in the absence of SF/HGF drove the cell cycle through G1/S without downregulating p27. The pathways by which SF/HGF promotes cell cycle entry and induces p27 degradation are therefore separate.
FIG. 10.
c-Myc induction is necessary and sufficient for SF/HGF-induced mitogenic response. (A) Fluorescence-activated cell sorter analyses of U373MG cells transduced with adenovirus (Ad) constructs encoding GFP, c-Myc, or the dominant-negative MadMyc chimera demonstrate that overexpression of c-Myc is sufficient to prevent G1/G0 block under conditions of serum starvation, while the effect of SF/HGF is blocked by the dominant-negative MadMyc. (B) Western blotting confirms expression of c-Myc (67 kDa) and MadMyc (30 kDa) constructs. A 30-kDa band in c-Myc-transduced cells represents a c-Myc degradation product, rather than cotransduction with MadMyc. (C) Downregulation of p27 occurs with addition of SF/HGF regardless of cell cycle response or the presence of the dominant-negative MadMyc.
SF/HGF-stimulated cell cycle progression is E2F1 independent.
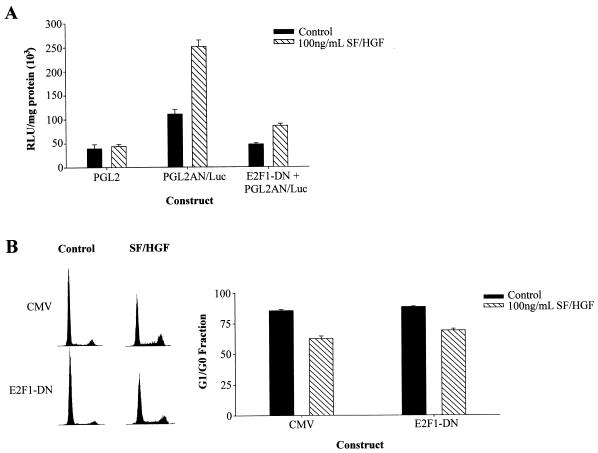
We asked if SF/HGF activates E2F1-dependent transcription and assessed its role in cell cycle progression. U373MG cells were cotransfected with an E2F1 promoter-luciferase reporter construct in conjunction with plasmids encoding either a dominant-negative form of E2F1 (E2F1-DN) under control of a CMV promoter or control plasmids carrying only the CMV promoter. SF/HGF effectively induced E2F1-dependent luciferase expression in control cells, and E2F1-DN effectively inhibited luciferase induction. Thus, SF/HGF induced E2F1 transcriptional activity, and the E2F1-DN effectively inhibited transcriptional activity under these experimental conditions (Fig. 11A). SF/HGF-mediated cell cycle progression was not inhibited in the presence of the E2F1-DN (Fig. 11B). The pathway downstream of SF/HGF stimulation, therefore, is not E2F1 dependent.
FIG. 11.
SF/HGF-stimulated cell cycle progression is E2F1 independent. U373MG cells were transiently transfected with plasmids encoding an E2F1 promoter-luciferase reporter construct in conjunction with either a dominant-negative form of E2F1 (E2F1-DN) under control of a CMV promoter or control plasmids carrying only the CMV promoter. Transcription of E2F1 target genes was measured by luciferase activity. (A) Stimulation with 100 ng of SF/HGF per ml efficiently induced luciferase activity in U373MG cells transfected with PGL2AN/Luc, indicating SF/HGF induces E2F1-dependent transcription. Cotransfection with dominant-negative E2F1 reduced luciferase activity nearly to levels exhibited by control transfected cells not stimulated by SF/HGF. (B) Overexpression of E2F1-DN was unable to block SF/HGF (100 ng/ml)-triggered cell cycle progression in cells grown in medium containing 0.1% FBS.
SF/HGF mediates identical mitogenic effects on SNB-19 cells.
To determine if SF/HGF's effects on the cell cycle are unique to U373MG cell lines, we examined its effects on a second glioma cell line, SNB-19. Like U373MG, SNB-19 cells express c-Met, but lack SF/HGF expression (data not shown). SF/HGF reduced the G1/G0 fraction by 21% in SNB-19 cells arrested under low-serum conditions and by 27% in cells arrested by contact inhibition. SF/HGF increased tritiated thymidine incorporation by 12.6% in serum-starved cells. Concomitantly, SF/HGF reduced p27 protein signal on Western blotting by >80% and upregulated c-myc mRNA fourfold as determined by Northern blotting in SNB-19 cells. Transient transfection of SNB-19 cells with p27 and p27T187A did not affect cell cycle distribution or the SF/HGF-induced mitogenic response. Overexpression of c-Myc in SNB-19 was able to substitute for SF/HGF in promoting cell cycle reentry and did not result in p27 degradation. Overexpression of MadMyc blocked SF/HGF mitogenicity in SNB-19 cells.
DISCUSSION
In this paper, we demonstrate that SF/HGF is able to prevent G1/G0 arrest in glioma cells induced by confluency or serum starvation. Our observation that the mitogenic effects of SF/HGF were associated with increased degradation of p27 is consistent with known mechanisms concerning contact and serum withdrawal-mediated growth arrest. Both situations result in G1/G0 arrest associated with increased p27 levels in multiple cell types corresponding to increased p27 half-life (11, 16, 39). The ability of growth factors such as platelet-derived growth factor (PDGF), insulin-like growth factor, and epidermal growth factor (EGF) to overcome G1/G0 block in fibroblasts correlates with their ability to induce p27 degradation (11), and constitutively active receptor tyrosine kinases effectively suppress p27 (65). Additionally, glioma cell lines have previously been shown to express increasing levels of p27 as they grow to confluency, and loss of p27 expression in vivo correlates with a poor clinical prognosis for this disease (14, 27, 38). Although transcriptional regulation of p27 by extracellular mitogens has been reported in glioma cell lines via the Akt target transcription factor FKHR (30), we saw no significant changes in p27 transcript levels and no phosphorylation of FKHR1 in response to SF/HGF in our cells (data not shown). Our observation that SF/HGF overcomes situational G1/G0 arrest in association with increased p27 breakdown was therefore in line with the dominant models regarding p27 regulation and cell cycle control.
We were suprised, therefore, that p27 expression levels, while clearly regulated by SF/HGF signaling, appear irrelevant to the mitogenic effect induced by SF/HGF. Expression of ectopic p27, even at levels several fold greater than those in wild-type cells, was ineffective at producing cell cycle arrest among logarithmically growing U373MG or SNB-19 cells. Additionally, SF/HGF efficiently mediated cell cycle progression even in cells transfected with a mutant p27 not susceptible to degradation in response to SF/HGF stimulation. Both constructs effectively bound to cyclin E in vivo and completely blocked Cdk2 kinase activity, indicating both constructs retained their cyclin-dependent kinase inhibitory activity and were being appropriately targeted within the cells.
SF/HGF releases mink Mv1Lu cells from TGF-β-mediated G1/G0 growth arrest by blocking p27 association with cyclin E-Cdk2 complexes in the absence of changes in total p27 levels (56), and extracellular signals such as TGF-β are able to redistribute p27 between different cyclin-dependent kinase complexes (41), raising the possibility that SF/HGF-induced p27 retargeting is more important than degradation in mediating cell cycle progression in these cells. Again, while SF/HGF reduced the amount of p27 complexed to cyclin E and increased Cdk2 activity in U373MG, it could not exert similar effects in cells transiently transfected to express p27 or p27T187A. SF/HGF could still induce cell cycle reentry in these cells, however, intimating that p27 was not functionally relevant in this cell line. These findings could be explained by the presence of a mutation in a p27-regulated pathway, rendering it constitutively active. However, the most likely candidate, Rb, is not known to be mutated in these cells, and we see an appropriate protein band on Western blot for these cell lines. Alternately, the loss of p15INK4B and p16INK4A expression inherent to U373MG may be sufficient to allow one or more of the cyclin-Cdk complexes to remain constitutively active and substitute for cyclin E-Cdk2 (3, 42). Overexpression of dominant-negative Cdk2 did not inhibit SF/HGF-mediated cell cycle progression, however, indicating that the transition is likely independent of these molecules.
Our observation that SF/HGF mitogenesis is mediated, at least in part, through c-Myc is consistent with separate pathways for p27 regulation and cell cycle progression. c-Myc is required for SF/HGF-mediated G1/S transition in primary hepatocytes (53). p27−/− and p27+/+ 3T3 fibroblasts stably transfected with a Myc-estrogen receptor (MycER) construct are stimulated equally well to enter S phase by 4-hydroxytamoxifen (4-OHT) (4), while both cell lines arrest under conditions of serum starvation (12). The dominant-negative MadMyc chimera has been shown to efficiently block cell cycle progression without altering p27 levels in 3T3 cells (5). Additionally, c-Myc is able to overcome G1/G0 arrest in Rat1 cells overexpressing p27 without downregulating p27 (60). c-Myc has also been shown to promote G1/S progression in the absence of cyclin E-Cdk2 activation in 3T3 cells (4) and by a mechanism that bypasses the “classical” pRb/E2F pathway in U2OS sarcoma cells (45). c-Myc can directly promote transcription of the E2F2 protein (47, 48). Similarly, SF/HGF activated E2F1 in our study, although E2F1-dependent transcription was not required for cell cycle progression. These observations, in conjunction with the results we present here, indicate that SF/HGF-mediated changes in c-Myc expression are sufficient to drive cell cycle progression in the absence of p27 changes, when p27 is not expressed, or when p27 is functionally irrelevant to cell cycle control.
At present, the c-Myc-responsive gene or genes that allow cells to escape G1/G0 arrest following SF/HGF stimulation are unknown. Vlach et al. postulated the existence of a c-Myc-regulated protein capable of sequestering p27 (60). Such a mechanism is not likely to function in our cells, since cell cycle progression occurs in U373MG and SNB-19, even in the absence of p27 retargeting from cyclin E-Cdk2. Similarly, while c-Myc regulation of the proteasome via Cul1 may play a role in p27 degradation, c-Myc overexpression drives cell cycle progression in these cell lines even in the absence of p27 degradation (36). The c-Myc target, Cdc25A, a positive regulator of Cdk2 function, is an unlikely candidate, because cell cycle progression was independent of Cdk2 activation. In certain cases, c-Myc can upregulate cyclin D or Cdk4; however, we do not see marked changes in the levels of these molecules in response to SF/HGF (17). Additionally, upregulation of Cdk4 and Cdk6 does not rescue c -myc−/− cells from growth inhibition, indicating c-Myc has Cdk4/6-independent effects on the cell cycle (28). The pRb/E2F-independent activation of G1/S transition described in U2OS cells was found to be cyclinE-Cdk2 dependent (45). This contrasts our finding of Cdk2-independent SF/HGF-mediated cell cycle progression in glioblastoma cells. This finding suggests the possibility of a Cdk2-independent cyclin A-dependent mechanism during S-phase entry and DNA synthesis.
While c-Myc is critical for release from G1/G0 arrest, additional genes are likely to be necessary for continuing cellular proliferation past this initial checkpoint. Phosphatidylinositol-3-kinase (PI-3-kinase) and Akt activation are generally needed in addition to c-Myc transcription to prevent c-Myc-induced apoptosis (22). SF/HGF is a potent activator of Akt in glioma cells (8), and U373MG and SNB-19 also lack expression of PTEN, a negative regulator of PI-3-kinase. SF/HGF can also activate the mitogenic mitogen-activated protein (MAP) kinase pathway and upregulates AP-1-dependent transcription in U373MG cells (1). Pharmacologic inhibitors of MAP kinase kinase (PD98059) and PI-3-kinase (wortmannin and LY249002) as well as transient transfection with dominant-negative forms of Akt did not block SF/HGF-mediated transition through G1/G0 in U373MG (data not shown), ruling out a role for those pathways in the initial cell cycle checkpoint; however, it is still possible activation of these pathways is important for prolonged proliferation.
In summary, SF/HGF prevents G1/G0 arrest in U373MG and SNB-19 glioma cell lines under conditions of contact inhibition or serum withdrawal. The possible mechanisms by which SF/HGF prevents arrest include downregulation of p27 by posttranslational mechanisms and induction of the c-Myc transcription factor. The cell cycle distributions of U373MG and SNB-19 were not affected by ectopic p27, nor did p27 overexpression block SF/HGF-mediated cell cycle progression, leading to the conclusion that p27 is not an effective regulator of the cell cycle in these cell lines. Similarly, SF/HGF-triggered cell cycle progression did not depend on Cdk2 or E2F1 activation. In contrast, SF/HGF cell cycle progression was dependent on c-Myc signaling. The mechanism of c-Myc-mediated progression is separate from p27 regulation or the activity of cyclin E-Cdk2 complexes and occurs parallel to E2F1 function.
Acknowledgments
This work was supported in part by grants from the National Institute of Neurologic Diseases and Stroke (R01 NS32148 [J.L.]) and from the National Cancer Institute (F32 CA83287 [K.A.W.]).
We thank Eleanor Carson-Walter for helpful discussions about methodology and for reviewing the manuscript. We thank Bruce Clurman, Chi Dang, William Kaelin, Sander van den Heuvel, and Bert Vogelstein for their gifts of biological reagents.
REFERENCES
- 1.Abounader, R., S. Ranganathan, B. Y. S. Kim, C. Nichols, and J. Laterra. 2001. Signaling pathways in the induction of c-met receptor expression by its ligand scatter factor/hepatocyte growth factor in human glioblastoma. J. Neurochem. 76:1497-1508. [DOI] [PubMed] [Google Scholar]
- 2.Abounader, R., S. Ranganathan, B. Lal, K. Fielding, A. Book, H. Dietz, P. Burger, and J. Laterra. 1999. Reversion of human glioblastoma malignancy by U1snRNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met gene expression. J. Natl. Cancer Inst. 91:1548-1556. [DOI] [PubMed] [Google Scholar]
- 3.Arap, W., R. Nishikawa, F. B. Furnari, W. K. Cavenee, and H.-J. S. Huang. 1995. Replacement of the p16/CDKN2 gene suppresses human glioma cell growth. Cancer Res. 55:1351-1354. [PubMed] [Google Scholar]
- 4.Beier, R., A. Burgin, A. Kiermaier, M. Fero, H. Karsunsky, R. Saffrich, T. Moroy, W. Ansorge, J. Roberts, and M. Eilers. 2000. Induction of cyclin E-cdk2 kinase activity, E2F-dependent transcription and cell growth by Myc are genetically separable events. EMBO J. 21:5813-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berns, K., E. M. Higmans, and R. Bernards. 1997. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene 15:1347-1356. [DOI] [PubMed] [Google Scholar]
- 6.Bladt, F., D. Rietmacher, S. Isenmann, A. Aguzzi, and C. Birchmeier. 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376:768-771. [DOI] [PubMed] [Google Scholar]
- 7.Bottaro, D. P., J. S. Rubin, D. L. Faletto, A. M. Chan, T. E. Kmiecik, and G. F. VandeWoude. 1991. Identification of the hepatocyte growth factor as the c-met proto-oncogene product. Science 251:802-804. [DOI] [PubMed] [Google Scholar]
- 8.Bowers, D. C., S. Fan, K. A. Walter, R. Abounader, J. A. Williams, E. M. Rosen, and J. Laterra. 2000. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 60:4277-4283. [PubMed] [Google Scholar]
- 9.Cheney, I. W., S. T. C. Neuteboom, M.-T. Vaillancourt, M. Ramachandra, and R. Bookstein. 1999. Adenovirus-mediated gene transfer of MMAC1/PTEN to glioblastoma cells inhibits S phase entry by the recruitment of p27Kip1 into cyclin E/CDK2 complexes. Cancer Res. 59:2318-2323. [PubMed] [Google Scholar]
- 10.Cheng, L., F. Rossi, W. Fang, T. Mori, and D. Cobrinik. 2000. Cdk2-dependent phosphorylation and functional inactivation of the pRB- related p130 protein in pRB(−), p16INK4A(+) tumor cells. J. Biol. Chem. 275:30317-30325. [DOI] [PubMed] [Google Scholar]
- 11.Coats, S., W. M. Flanagan, J. Nourse, and J. M. Roberts. 1996. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272:877-880. [DOI] [PubMed] [Google Scholar]
- 12.Coats, S., P. Whyte, M. L. Fero, S. Lacy, G. Chung, E. Randel, E. Firpo, and J. M. Roberts. 1999. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27Kip1-deficient cells. Curr. Biol. 9:163-173. [DOI] [PubMed] [Google Scholar]
- 13.DiRenzo, M. F., R. P. Narsimhan, M. Olivero, S. Bretti, S. Giordano, E. Medico, P. Gaglia, P. Zara, and P. M. Comoglio. 1991. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 6:1997-2003. [PubMed] [Google Scholar]
- 14.Fuse, T., M. Tanikawa, M. Nakanishi, K. Ikeda, T. Tada, H. Inagaki, K. Asai, T. Kato, and K. Yamada. 2000. p27Kip1 expression by contact inhibition as a prognostic index of human glioma. J. Neurochem. 74:1393-1399. [DOI] [PubMed] [Google Scholar]
- 15.He, T.-C., S. Zhou, L. T. DaCosta, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengst, L., and S. I. Reed. 1996. Translational control of p27Kip1 accumulation during the cell cycle. Science 271:1861-1864. [DOI] [PubMed] [Google Scholar]
- 17.Hermeking, H., C. Rago, M. Schuhmacher, Q. Li, J. F. Barrett, A. J. Obaya, B. C. O'Connell, M. K. Mateyak, W. Tam, F. Kohlhuber, C. V. Dang, J. M. Sedivy, D. Eick, B. Vogelstein, and K. W. Kinzler. 2000. Identification of CDK4 as a target of c-MYC. Proc. Natl. Acad. Sci. USA 97:2229-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose, Y., M. Kojima, M. Sago, T. Hayashi, H. Murakami, K. Shimazaki, K. Yoshida, and T. Kawase. 1998. Clinical importance of c-Met protein expression in high grade astrocytic tumors. Neurol. Med.-Chir. 38:851-859. [DOI] [PubMed] [Google Scholar]
- 19.Hirose, Y., M. Kojima, M. Sagoh, H. Murakami, K. Yoshida, K. Shimazaki, and T. Kawase. 1998. Immunohistochemical examination of c-Met protein expression in astrocytic tumors. Acta Neuropathol. 95:345-351. [DOI] [PubMed] [Google Scholar]
- 20.Hossain, M. A., C. M. Bouton, J. Pevsner, and J. Laterra. 2000. Induction of vascular endothelial growth factor in human astrocytes by lead. Involvement of a protein kinase C/activator protein-1 complex-dependent and hypoxia-inducible factor 1-independent signaling pathway. J. Biol. Chem. 275:27874-27882. [DOI] [PubMed] [Google Scholar]
- 21.Ishii, N., D. Maier, A. Merlo, M. Tada, Y. Sawamura, A.-C. Diserens, and E. G. V. Meir. 1999. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, and PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 9:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauffman-Zeh, A., P. Rodriquez-Viciana, E. Ulrich, C. Gilbert, P. Coffer, J. Downward, and G. Evan. 1997. Suppression of c-Myc induced apoptosis by Ras signaling through PI(3)K and PKB. Nature 385:544-548. [DOI] [PubMed] [Google Scholar]
- 23.Kim, S. G., S. N. Kim, H. S. Jong, N. K. Kim, S. H. Hong, S. J. Kim, and Y. J. Bang. 2001. Caspase-mediated Cdk2 activation is a critical step to execute transforming growth factor-beta1-induced apoptosis in human gastric cancer cells. Oncogene 20:1254-1265. [DOI] [PubMed] [Google Scholar]
- 24.Koochekpour, S., M. Jeffers, S. Rulong, G. Taylor, E. Klineberg, E. A. Hudson, J. H. Resau, and G. F. VandeWoude. 1997. Met and hepatoycte growth factor/scatter factor expression in human gliomas. Cancer Res. 57:5391-5398. [PubMed] [Google Scholar]
- 25.Laterra, J., E. M. Rosen, M. Nam, S. Ranganathan, K. Fielding, and P. Johnston. 1997. Scatter factor/hepatocyte growth factor expression enhances human glioblastoma tumorigenicity and growth. Biochem. Biophys. Res. Commun. 235:743-747. [DOI] [PubMed] [Google Scholar]
- 26.Ljubimova, J. Y., L. M. Petrovic, S. E. Wilson, S. A. Geller, and A. A. Demetriou. 1997. Expression of HGF, its receptor c-met, c-myc, and albumin in cirrhotic and neoplastic human liver tissue. J. Histochem. Cytochem. 45:79-87. [DOI] [PubMed] [Google Scholar]
- 27.Loda, M., B. Cukor, S. W. Tam, P. Lavin, M. Fiorentino, G. F. Draetta, J. M. Jessup, and M. Pagano. 1997. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3:231-234. [DOI] [PubMed] [Google Scholar]
- 28.Mateyek, M. K., A. J. Obaya, and J. M. Sedivy. 1999. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol. Cell. Biol. 19:4672-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J.-Y. Kato. 1994. D-type cyclin-depedent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medema, R. H., G. J. P. L. Kops, J. L. Bos, and B. M. T. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27Kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama, T., H. Kataoka, H. Tsubouchi, and M. Koono. 1995. Concomitant expression of hepatocyte growth factor (HGF), HGF activator and c-met genes in human glioma cells in vitro. FEBS Lett. 372:78-82. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, H.-W., A. Michel, D. Heckel, U. Fischer, M. Tonnes, L.-C. Tsui, S. Scherer, K. D. Zang, and E. Meese. 1997. Identification of an amplified gene cluster in glioma including two novel amplified genes isolated by exon trapping. Hum. Genet. 101:190-197. [DOI] [PubMed] [Google Scholar]
- 33.Nabeshima, K., Y. Shimao, S. Sato, H. Kataoka, T. Moriyama, H. Kawano, S. Wakisaka, and M. Koono. 1997. Expression of c-Met correlates with grade of malignancy in human astrocytic tumors: an immunohistochemical study. Histopathology 31:436-443. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, T., T. Nishizawa, M. Hagiya, T. Seki, M. Shimonishi, A. Sugimura, K. Tashiro, and S. Shimizu. 1989. Molecular cloning and expression of human hepatocyte growth factor. Nature 342:440-443. [DOI] [PubMed] [Google Scholar]
- 35.Neuman, E., E. K. Flemington, W. R. Sellers, and W. G. Kaelin, Jr. 1994. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol. Cell. Biol. 14:6607-6615. (Author's correction, 15:4660, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hagan, R. C., M. Ohh, G. David, I. M. D. Alboran, F. W. Alt, W. G. Kaelin, and R. DePinho. 2000. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 14:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park, M., M. Dean, K. Kaul, M. J. Braun, M. A. Gonda, and G. VandeWoude. 1987. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA 84:6379-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piva, R., I. Cancelli, P. Cavalla, S. Bortolotto, J. Dominguez, G. F. Draetta, and D. Schiffer. 1999. Proteasome-dependent degradation of p27/kip1 in gliomas. J. Neuropathol. Exp. Neurol. 58:691-696. [DOI] [PubMed] [Google Scholar]
- 39.Polyak, K., M.-H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 40.Prat, M., R. P. Narsimhan, T. Crepaldi, M. R. Nicotra, P. G. Natali, and P. M. Comoglio. 1991. The receptor encoded by the human c-met oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int. J. Cancer 49:323-328. [DOI] [PubMed] [Google Scholar]
- 41.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 42.Rich, J. N., M. Zhang, M. B. Datto, D. D. Bigner, and X.-F. Wang. 1999. Transforming growth factor-beta-mediated p15INK4B induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J. Biol. Chem. 49:35053-35058. [DOI] [PubMed] [Google Scholar]
- 43.Rosen, E. M., J. Laterra, A. Joseph, L. Jin, A. Fuchs, D. Way, M. Witte, M. Weinand, and I. D. Goldberg. 1996. Scatter factor expression and regulation in human glial tumors. Int. J. Cancer 67:248-255. [DOI] [PubMed] [Google Scholar]
- 44.Rosen, E. M., S. K. Nigam, and I. D. Goldberg. 1994. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J. Cell Biol. 127:1783-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoni-Rugiu, E., J. Falck, N. Mailand, J. Bartek, and J. Lukas. 2000. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol. Cell. Biol. 20:3497-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, C., F. Bladt, S. Goedcke, V. Brinkmann, W. Zschiesche, and M. Sharpe. 373. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373:699-702. [DOI] [PubMed]
- 47.Sears, R., G. Leone, J. DeGregori, and J. R. Nevins. 1999. Ras enhances Myc protein stability. Mol. Cell 3:169-179. [DOI] [PubMed] [Google Scholar]
- 48.Sears, R., K. Ohtani, and J. R. Nevins. 1997. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol. Cell. Biol. 17:5227-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sellers, W. R., J. W. Rodgers, and W. G. Kaelin, Jr. 1995. A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc. Natl. Acad. Sci. USA 92:11544-11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 51.Shiota, G., H. Kawasaki, and T. Nakamura. 1996. Coexpression of hepatocyte growth factor and its receptor (c-met oncogene) in HGL4 glioblastoma cells. Lab. Investig. 53:511-516. [DOI] [PubMed] [Google Scholar]
- 52.Skouteris, G. G., and C. H. Schroder. 1996. C-Myc and Max interactions in quiescent and mitogen-stimulated primary hepatocytes. Exp. Cell Res. 225:237-244. [DOI] [PubMed] [Google Scholar]
- 53.Skouteris, G. G., and C. H. Schroder. 1996. C-myc is required for the G0/G1-S transition of primary hepatocytes stimulated with a deleted form of hepatocyte growth factor. Biochem. J. 316:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tacchini, L., P. Dansi, E. Matteucci, and M. A. Desiderio. 2000. Hepatocyte growth factor signal coupling to various transcription factors depends on triggering of met receptor and protein kinase transducers in human hepatoma cells HepG2. Exp. Cell Res. 256:272-281. [DOI] [PubMed] [Google Scholar]
- 55.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsubari, M., J. Taipale, E. Tihonen, J. Keski-Oja, and M. Laiho. 1999. Hepatocyte growth factor releases mink epithelial cells from transforming growth factor β1-induced growth arrest by restoring Cdk6 expression and cyclin E-associated Cdk2 activity. Mol. Cell. Biol. 19:3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uehara, Y., O. Monowa, C. Mori, K. Shiota, J. Kuno, and T. Noda. 1995. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373:702-705. [DOI] [PubMed] [Google Scholar]
- 58.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 59.Vigna, E., L. Naldini, L. Tamagone, P. Longati, A. Bardelli, F. Maina, and P. M. Comoglio. 1994. Hepatocyte growth factor and its receptor, the tyrosine kinase encoded by the c-MET proto-oncogene. Cell. Mol. Biol. 40:597-604. [PubMed] [Google Scholar]
- 60.Vlach, J., S. Hennecke, K. Alevizopoulos, D. Conti, and B. Amati. 1996. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 15:6595-6604. [PMC free article] [PubMed] [Google Scholar]
- 61.Weidner, K. M., G. Hartmann, M. Sachs, and W. Birchmeier. 1993. Properties and functions of scatter factor/hepatocyte growth factor and its receptor c-Met. Am. J. Respir. Cell Mol. Biol. 8:229-237. [DOI] [PubMed] [Google Scholar]
- 62.Welch, W. C., P. L. Kornblith, G. K. Michalopoulos, B. E. Petersen, A. Beedle, S. M. Gollin, and R. H. Goldfarb. 1999. Hepatocyte growth factor (HGF) and receptor (c-met) in normal and malignant astrocytic cells. Anticancer Res. 19:1635-1640. [PubMed] [Google Scholar]
- 63.Wullich, B., H.-P. Sattler, U. Fischer, and E. Meese. 1994. Two independent amplification events on chromosome 7 in glioma: amplification of the epidermal growth factor receptor gene and amplification of the oncogene met. Anticancer Res. 14:577-580. [PubMed] [Google Scholar]
- 64.Yamashita, J., M. Ogawa, S. Yamashita, K. Nomura, M. Kuramoto, T. Saishoji, and S. Shin. 1994. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 54:1630-1633. [PubMed] [Google Scholar]
- 65.Yang, H.-Y., B. P. Zhou, M.-C. Hung, and M.-H. Lee. 2000. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J. Biol. Chem. 275:24735-24739. [DOI] [PubMed] [Google Scholar]
- 66.Zarnegar, R., and G. K. Michalopoulos. 1995. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J. Cell Biol. 129:1177-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]