Abstract
Cripto-1 (CR-1), an epidermal growth factor-CFC (EGF-CFC) family member, has a demonstrated role in embryogenesis and mammary gland development and is overexpressed in several human tumors. Recently, EGF-CFC proteins were implicated as essential signaling cofactors for Nodal, a transforming growth factor β family member whose expression has previously been defined as embryo specific. To identify a receptor for CR-1, a human brain cDNA phage display library was screened using CR-1 protein as bait. Phage inserts with identity to ALK4, a type I serine/threonine kinase receptor for Activin, were identified. CR-1 binds to cell surface ALK4 expressed on mammalian epithelial cells in fluorescence-activated cell sorter analysis, as well as by coimmunoprecipitation. Nodal is coexpressed with mouse Cr-1 in the mammary gland, and CR-1 can phosphorylate the transcription factor Smad-2 in EpH-4 mammary epithelial cells only in the presence of Nodal and ALK4. In contrast, CR-1 stimulation of mitogen-activated protein kinase and AKT in these cells is independent of Nodal and ALK4, suggesting that CR-1 may modulate different signaling pathways to mediate its different functional roles.
Human cripto-1 (CR-1) (also known as teratocarcinoma-derived growth factor 1) was originally identified from an embryonal carcinoma library, and subsequent studies have implicated a role for CR-1 in early vertebrate development and carcinogenesis (33). In vivo, gene targeting experiments in mice have shown mouse cripto-1 (Cr-1) to be critical for early cell movements during gastrulation and cardiomyocyte formation (46). In addition, genetic studies in zebra fish have shown through epistasis experiments that CR-1 is an essential cofactor for signaling by the transforming growth factor β (TGF-β) family ligand Nodal during early vertebrate embryogenesis (35, 40). Postnatally, Cr-1 is expressed at a low level in all stages of the mammary gland development, and expression increases during lactation and pregnancy (21). Overexpression of Cr-1 in mouse mammary epithelial cells leads to their transformation in vitro, and when injected into mammary glands, produces ductal hyperplasia (6, 20). However, very little is known about the identity of the pathways that CR-1 stimulates in the adult or in cancer cells, and there is no evidence for the expression of Nodal postnatally. Activation of the ras/raf/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3′ kinase (PI3K)/AKT pathways by CR-1 has been demonstrated in mouse and human mammary epithelial cells and cervical carcinoma cells (10, 13, 19). However, the relationship between these pathways and the Nodal pathway is unclear.
CR-1 is the founding member of a family of proteins identified only in vertebrates, including human cryptic, human criptin (unpublished Human Genome Science, Inc., patent number S5981215), mouse cripto-1 (Cr-1/tdgf-1/cfc-2), mouse cryptic (cfc-1), chicken cripto, Xenopus FRL-1, and zebra fish one-eyed pinhead (oep) (2, 4, 7, 8, 12, 23, 41, 49). These proteins contain multiple domains consisting of a potential NH2-terminal signal peptide, a variant epidermal growth factor (EGF)-like domain, a cysteine-rich motif (CFC domain), and a short COOH terminus containing, in some cases, consensus sequences for glycosylphosphatidylinositol (GPI) attachment to the cell membrane (26, 33). The modified EGF-like domain corresponds to a region of approximately 40 amino acids containing 6 cysteine residues (32). Whereas the canonical EGF-like domain that is present in EGF-related peptides such as EGF, TGF-α, and heregulins contains three loops (A, B, C) as a result of possessing three intramolecular disulfide bonds, the variant EGF-like domain in the EGF-CFC proteins lacks the A loop, has a truncated B loop, and possesses a complete C loop. The presence of this unusual EGF-like domain could explain the observation that CR-1 does not directly interact with EGF receptor, erb B-2, erb B-3, or erb B-4 (4, 30). Although CR-1 does not directly interact with any of the erb B tyrosine kinase receptors, it is able to indirectly induce erb B-4 tyrosine transphosphorylation in several mouse and human mammary epithelial cell lines by facilitating either heterodimerization of erb B-4 with a novel CR-1 receptor or by indirectly stimulating erb B-4 tyrosine phosphorylation through a soluble src-like tyrosine kinase (3).
Evidence for a distinct receptor for CR-1 in mammalian cells came from studies demonstrating 125I-CR-1 binding to a distinct high-affinity, saturable receptor in several different human breast carcinoma cell lines that could not be competed by other EGF-like peptides such as EGF, TGF-α, amphiregulin, or HRGβ1 (19). In addition, chemical cross-linking of 125I-CR-1 to NMuMG mouse mammary epithelial or MDA-MB-453 human breast cancer cell membranes has identified two specific bands, of 130 and 60 kDa (3). It has been proposed that during embryogenesis Nodal and EGF-CFC proteins are inactive independently and together function through activation of an Activin type II (ActRIIA or ActRIIB) and type IB (ALK4/ActRIB) receptor complex (36). Activation of ALK4/ActRIB can in turn phosphorylate Smad-2 and Smad-3 signaling factors which bind to Smad-4 and then interact with FoxH1 to enhance transcription of target genes (1, 47). Recently, biochemical evidences have demonstrated that residues in the CFC domain of mouse Cr-1 are important for binding to ALK4 after expression in Xenopus embryos, and this interaction is necessary for Nodal binding to the ActRIIB/ALK4 receptor complex and for Smad-2 activation by Nodal (48). However, conclusive data demonstrating a direct interaction between human CR-1 and this family of receptors in mammalian cells are lacking, and there is no evidence for a Nodal/CR-1/ALK4 pathway in postnatal development.
In order to identify a CR-1 receptor from an adult tissue, we screened a human adult brain cDNA phage display library using human CR-1 protein as bait. We isolated several CR-1 binding clones with identity to an amino acid sequence in the extracellular domain (ECD) of ALK4. CR-1 was able to specifically bind to the ECD of ALK4 but not to the ECD of ActRIIA or ActRIIB type II receptors. Reverse transcription (RT)-PCR analysis revealed that Nodal is coexpressed with Cr-1 during mouse mammary gland development, suggesting that a Nodal/CR-1 signaling pathway could be important in the adult animal. In addition, we demonstrate that CR-1 can activate the Smad-2 pathway in mammary epithelial cells in a Nodal- and ALK4-dependent manner. In contrast, MAPK and AKT phosphorylation induced by CR-1 is independent of Nodal and ALK4, suggesting that CR-1 may modulate different signaling pathways to mediate its different functional roles.
MATERIALS AND METHODS
Cell culture, transfection, and growth factors.
K562 human erythroleukemia cells (American Type Culture Collection [ATCC], Manassas, Va.) were cultured in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum. Human 293 embryonal kidney cells (ATCC), mouse MC3T3-E1 (24) osteoblastic cells (kindly provided by M. Kuehn, National Cancer Institute [NCI], National Institutes of Health [NIH]), EpH-4 mouse mammary epithelial cells, and monkey kidney COS1 cells (ATCC) were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. EpH-4 cells were transfected with mouse Nodal cDNA (kindly provided by C. Meno, Osaka University, Osaka, Japan) as previously described (44). Total RNA was isolated and RT-PCR with Nodal-specific primers was performed as described below. Expression of Nodal protein in the cultured medium of Nodal stably transfected cells was assessed by Western blot analysis as previously described (44) using a rabbit polyclonal anti-Nodal antibody (1:1,000 dilution) kindly provided by M. Kuehn, NCI, NIH. Human recombinant EGF and human recombinant Activin A were purchased from Collaborative Research (Bedford, Mass.) and R&D Systems (Minneapolis, Minn.), respectively. Human CR-1ΔC and CR-1ΔC-Fc recombinant proteins were expressed in CHO cells and purified as previously described (37).
Expression vectors and reporter constructs.
The TGF-β-Activin responsive reporter construct, p3TP-Lux, was provided by J. Massague, Memorial Sloan-Kettering Cancer Center. The expression constructs containing the full-length and truncated Activin type I receptor ALK4-Flag- and ALK4-2--Flag-tagged cDNAs were provided by A. Klibanski, Massachusetts General Hospital, and the constructs containing ALK4-hemagglutinin (HA)- and ActRIIB-HA-tagged cDNAs were kindly provided by M. Whitman, Harvard Medical School. The cDNA encoding human CR-1 was cloned into the EcoRI site of pCI-neo (Promega, Madison, Wis.) as previously described (13). The cDNA encoding mouse Nodal was subcloned into the blunt SfiI site of the pEF/myc/cyto plasmid (Invitrogen, Carlsbad, Calif.) to yield the Nodal expression vector.
Biopanning of human brain phage display library against CR-1ΔC protein.
A T7 phage display library of human brain cDNA was obtained from Novagen (Madison, Wis.). CR-1ΔC (1 μg/well) was adsorbed to 96-well microtiter plates overnight at 4°C. Clones (107) of the T7 human brain phage display library were added to the wells for 1 h at room temperature. The plates were then washed several times with Tris-buffered saline containing 0.1% Tween 20. The bound phage was eluted with T7 elution buffer (Novagen). After the third panning, phage was plated on Luria broth-carbenicillin plates and phage plaques were transferred to nitrocellulose membranes. The membranes were incubated in duplicate in the presence or absence of 1 μg of CR-1ΔC per ml for 1 h at room temperature. After incubation with anti-CR-1 rabbit polyclonal antibody (Biocon, Frederick, Md.) and anti-rabbit horseradish peroxidase-conjugated secondary antibody, the plaques that specifically bound to CR-1ΔC were detected using enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech, Piscataway, N.J.). The positive plaques were picked and DNA was extracted. The purified inserts were sequenced with T7 Up and Down primers (Novagen) using a Big Dye Terminator Cycle Sequencing kit (Perkin Elmer, Wellesley, Mass.). The sequences were analyzed for sequence homology using the nucleotide basic local alignment search tool (BLAST) program.
Coimmunoprecipitation experiments and fluorescence-activated cell sorter (FACS) analysis in COS1 and 293 cells.
COS1 cells (6 × 105 cells in 60-mm-diameter plates) were transiently transfected with 2 μg of CR-1, ALK4-Flag, and dominant-negative ALK4-2-Flag expression vectors alone or with 1 μg of CR-1 plus 1 μg of ALK4-Flag or dominant-negative ALK4-2-Flag expression vector using Fugene 6 (Roche, Indianapolis, Ind.). Forty-eight hours after transfection, the cells were lysed as previously described (3). Eight hundred micrograms of total proteins was incubated with anti-Flag antibody gel matrix slurry (M2-agarose; Sigma, St. Louis, Mo.) for 2 h at 4°C and gel matrix-bound Flag-tagged receptors were eluted by competition with a 3×Flag peptide (Sigma). The eluted samples were run on a sodium dodecyl sulfate-4 to 20% polyacrylamide gel electrophoresis gel and transferred to Immobilon-P membranes. The membranes were probed with a 1:5,000 dilution of a rabbit polyclonal anti-CR-1 antibody (Biocon) overnight at 4°C and developed with ECL reagent (Amersham Pharmacia Biotech). The same blots were stripped and reprobed with a 1:1,000 dilution of a mouse monoclonal anti-Flag horseradish peroxidase-conjugated antibody (Upstate Biotechnology, Lake Placid, N.Y.). Alternatively, human 293 cells (6 × 106 cells in 100-mm-diameter plates) were transiently transfected with 8 μg of plasmid expressing ALK4-HA or ActRIIB-HA with Fugene 6 (Roche). Forty-eight hours after transfection, the cells were lysed and 1 mg of total cell lysates was immunoprecipitated with 1 μg of CR-1ΔC-Fc or LTβR-Fc prebound to protein A-Sepharose. The immunoprecipitated proteins were run on a 4 to 20% gel, transferred to nitrocellulose, and probed with anti-HA rabbit polyclonal antibody (Convance, Princeton, N.J.). To ensure that ALK4-HA and ActRIIB receptors were expressed in 293 cells, 40 μg of cell lysate was run on a 4 to 20% gel and probed with anti-HA rabbit polyclonal antibody (Convance).
For FACS analysis, human 293 cells were transiently transfected for 48 h with plasmid DNAs expressing ALK4-HA, ActRIIB-HA, or control vector as described above. Cells (3 × 105) were then incubated with 5 μg of CR-1ΔC-Fc or LTβR-Fc proteins per ml for 30 min and analyzed by FACS.
Enzyme-linked immunosorbent assay (ELISA) and competition assay.
CR-1ΔC (300 ng/well) was adsorbed to 96-well microtiter plates overnight at 4°C. The plates were blocked with 2% milk and incubated with the ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc recombinant protein (R&D Systems) at concentrations ranging from 1.6 μg/well to 12.5 ng/well for 1 h at room temperature. Dilutions (1:3,000) of anti-ALK4, anti-ActRIIA, or anti-ActRIIB goat polyclonal antibodies were added to the plates for 1 h at room temperature and then incubated with anti-goat immunoglobulin G conjugated to horseradish peroxidase (1:3,000) (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 1 h at room temperature. The plates were developed with 3,3′,5,5′-tetramethyl-benzidine (TMB) peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and the absorbance was read at 450 nm.
In another set of experiments, the ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc recombinant protein (100 ng/well) was adsorbed to 96-well microtiter plates and incubated overnight at 4°C. After blocking the plates in 2% milk, CR-1ΔC recombinant protein was added at concentrations ranging from 400 to 12.5 ng/well and incubated for 1 h at room temperature. The plates were incubated with anti-CR-1 rabbit polyclonal antibody (Biocon) at a 1:3,000 dilution for 1 h at room temperature and the ELISA was developed as described above.
For the competition assay, 100 ng of CR-1ΔC recombinant protein was preincubated for 45 min at room temperature with different concentrations of the ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc recombinant protein ranging from 2 to 0.062 μg. CR-1ΔC alone or preincubated with different concentrations of the ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc was added for 1 h at room temperature to plates preadsorbed overnight with the ECD of ALK4-Fc (100 ng/well) and blocked with 2% milk. The anti-CR-1 rabbit polyclonal antibody (1:3,000 dilution) was added for 1 h at room temperature. The plates were then developed with TMB peroxidase substrate. Kd was calculated using the Prism program.
Luciferase assay.
K562 cells (105 cells/well in 12-well plates) were transfected with 0.5 μg of different plasmid DNAs using X-TremeGENE Q2 Transfection reagent (Roche). The amount of DNA for each transfection was adjusted to 2 μg using pCI-neo control vector (Invitrogen). Renilla luciferase control reporter vector (pRL-TK) (Promega) was cotransfected in the cells to normalize for transfection efficiency. Four hours after transfection, complete medium was added to the wells and human Activin A recombinant protein (10 ng/ml) was added as a positive control. Twenty-four hours after transfection, the cells were lysed and luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega) according to the manufacturer's instructions.
RT-PCR analysis of Cr-1, Nodal, ALK4, ActRIIB, CK-18, and GAPDH during different stages of mouse mammary development and in MC3T3-E1 and EpH-4 WT cells.
Total RNA was prepared from different stages of BALB/c mouse mammary gland (kindly provided by B. Vondherhaar, NCI, NIH) and reverse transcribed to cDNA with Superscript II (Invitrogen) and random primers. cDNAs synthesized from 100 ng of RNA were amplified for 35 cycles of three steps of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. The conditions used for cytokeratin-18 (CK-18) were 30 cycles of three steps of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The primers used in each reaction were as follows: ALK4, 5′GTGGTGACGTGGCTGTGAAA3′ and 5′TTTGGAGCAATGTCTATGGT3′; ActRIIB, 5′ATGTGCCGTGGTGTCGTGGT3′ and 5′GACCTCCTGATCAGGGATAC3′; Cr-1, 5′ATTTGGACCCGTTGCTGGGAGAGA3′ and 5′CAGCTAGCATAAAAGTGGTCGTCA3′; Nodal, 5′ATTTGCCAGACAGAAGCCAAC3′ and 5′TCCTCCACAATCATGTCCTTG3′; CK-18, 5′ACTCGCTCCACCACCTTCTCCACCAACTAC3′ and 5′CCACAGAATTCGCAAGGATC3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′CCCTTCATTGACCTCAACTAC3′ and 5′CCACCTTCTTGATGTCATCAT3′. cDNAs from lactation days 7 and 15 were amplified using Nodal primers and the purified PCR products were sequenced and analyzed for sequence homology using the BLAST program. To analyze ALK4 expression, total RNA from MC3T3-E1 and EpH-4 wild-type (WT) cells was reverse transcribed to cDNA and the cDNAs were amplified using primers specific for ALK4.
Western blot analysis.
MC3T3-E1, EpH-4 WT, and EpH-4 N cells were seeded in 60-mm-diameter plates (8 × 105 cells/plate) and serum starved for 24 h. The cells were then stimulated with human Activin A, EGF, and CR-1ΔC recombinant proteins at different concentrations for various times. The cells were then lysed and 50 μg of protein was run on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gels and transferred to Immobilon-P membranes. The blots were incubated with specific anti-phospho Smad-2 (2 μg/ml) (Upstate Biotechnology), anti-phospho MAPK (1:1,000) (Biolab Laboratories, Beverly, Mass.), or anti-phospho AKT (serine 473) (1:1,000) (Biolab Laboratories) rabbit polyclonal antibodies overnight at 4°C. The bound antibodies were detected as previously described (3). To ensure equal loading of proteins the blots were stripped and reprobed with Smad-2/Smad-3 (2 μg/ml) (Upstate Biotechnology), total MAPK (1:1,000) (Biolab Laboratories), or total AKT (1:1,000) (Biolab Laboratories) rabbit polyclonal antibodies. In the experiments with dominant-negative ALK4-2-Flag receptor, EpH-4 WT and/or EpH-4 N cells (5 × 105 cells in 60-mm-diameter plates) were transiently transfected with different concentrations of ALK4-2-Flag or pCI-neo plasmid DNAs using Lipofectamine (Invitrogen) in serum-free medium. After 48 h, the cells were stimulated with EGF or CR-1ΔC recombinant protein and Western blot analysis for phospho Smad-2, phospho MAPK, or phospho AKT was performed. Densitometric analysis was performed using the Kodak 1D, 2.0, program.
RESULTS
Production of a bioactive fusion protein, CR-1ΔC.
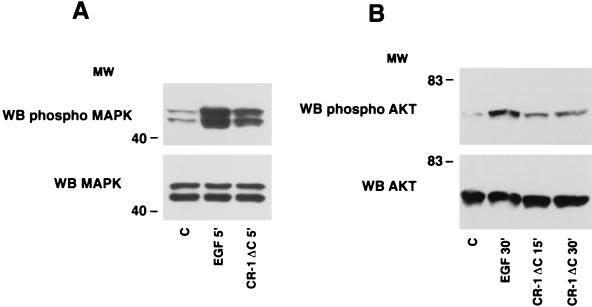
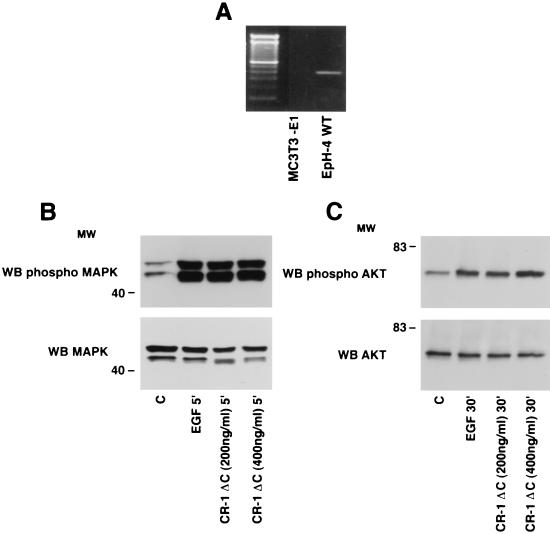
In vitro activities that were previously demonstrated for CR-1, including mediating MAPK, PI3K, and AKT phosphorylation (10, 13, 19), cell migration and branching (44), and inhibition of β-casein milk protein expression (10) in mammary epithelial cell lines, have been performed with a CR-1 peptide (p47 CR-1) (19) and with an Escherichia coli-produced recombinant human CR-1 protein (39). For expression cloning, we decided to produce a human CR-1 protein expressed in eukaryotic cells that would include appropriate posttranslational modifications, which are potentially important for high-affinity interactions with putative binding partners. As previously reported, we truncated the C terminus to remove the GPI linkage, thus generating a form of CR-1 that would be readily secreted (37). This protein is designated CR-1ΔC and was produced with and without an Fc tag. To verify that the protein is functionally active, we tested its ability to induce MAPK and AKT phosphorylation in EpH-4 mouse mammary epithelial cells. We have previously demonstrated that CR-1 can stimulate proliferation, migration, and branching morphogenesis in EpH-4 cells (44). CR-1ΔC stimulated a threefold increase in MAPK phosphorylation in EpH-4 cells after 5 min of incubation (Fig. 1A), as previously reported for active CR-1 protein, and stimulated a four- and fivefold increase in the phosphorylation of AKT after 15 and 30 min of incubation, respectively (Fig. 1B). Thus, the CR-1ΔC protein is a functionally active molecule.
FIG. 1.
MAPK and AKT activation by CR-1ΔC in EpH-4 cells. Serum-starved EpH-4 cells were stimulated with recombinant CR-1ΔC (200 ng/ml) or EGF (50 ng/ml) protein and analyzed by Western blot (WB) analysis. MW, molecular weight in thousands; C, control.
Identification of ALK4 as a candidate receptor for CR-1.
To identify a receptor for CR-1, we used CR-1ΔC as bait to screen a T7 human brain phage display cDNA library, which is known to express CR-1, for T7 phage clones that were expressing a ligand binding fragment of the putative CR-1 receptor on their surface. After the third biopanning, the isolated phage clones were plated and transferred to nitrocellulose membranes. The membranes were incubated in duplicate in the presence or absence of CR-1ΔC protein and clones that specifically bound CR-1 were detected using an anti-CR-1 rabbit polyclonal antibody. Thirty clones out of 98 were positive for apparent binding to CR-1ΔC and did not react with only the anti-CR-1 rabbit polyclonal antibody. DNA sequencing of the phage inserts revealed that 25 of 30 clones contained the same sequence, sharing identity with a region of the ECD of the human ALK4 (ActRIB) receptor protein (GenBank accession number Z22536) (43), a type I serine/threonine kinase receptor for Activin. The remaining clones did not share similarity with any known sequence and did not contain open reading frames. Among the 25 positive clones, only one clone contained a large insert of 1.1 kb, corresponding to amino acids 1 to 373 of the ECD and part of the transmembrane and cytoplasmic domains of the human ALK4 protein. All the remaining clones had smaller inserts ranging from 400 to 650 bp and corresponding to the ECD domain and part of the transmembrane domain.
CR-1ΔC binds ALK4, but not ActRIIB, expressed on the surface of mammalian epithelial cells.
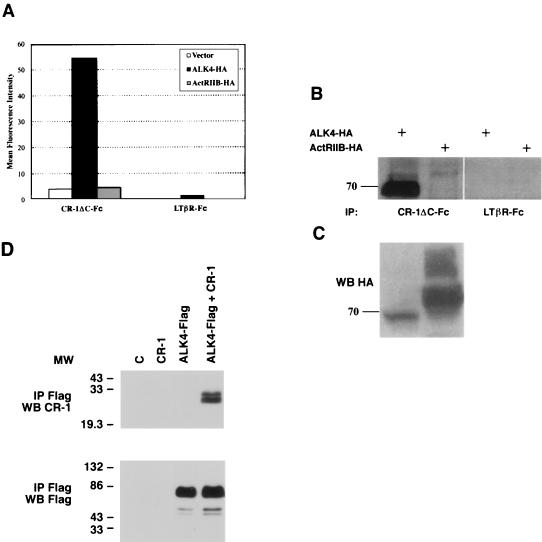
In order to determine whether CR-1ΔC could bind to ALK4 expressed on the surface of mammalian epithelial cells, human 293 kidney cells were transiently transfected with HA-tagged ALK4, ActRIIB, or a control vector. Binding of CR-1ΔC containing an Fc tag (CR-1ΔC-Fc) followed by an anti-Fc phycoerythrin-conjugated secondary antibody to the transfected cells was evaluated using FACS analysis. CR-1ΔC-Fc was able to specifically bind to 293 cells expressing ALK4-HA, whereas no binding was detected on cells transfected with ActRIIB-HA (Fig. 2A). An irrelevant control protein, LTβR-Fc, did not show any binding to the transfected 293 cells (Fig. 2A) (14). In addition, ALK4-HA, but not ActRIIB-HA, could be immunoprecipitated by CR-1ΔC-Fc from transfected 293 cells, as detected by Western blot analysis using an anti-HA rabbit polyclonal antibody, whereas the control LTβR-Fc protein did not associate with either receptor (Fig. 2B). Both ALK4-HA and ActRIIB-HA receptors were expressed in 293 cells as detected by Western blot analysis using an anti-HA rabbit polyclonal antibody (Fig. 2C). Furthermore, full-length CR-1 protein and Flag-tagged ALK4 coexpressed in COS1 cells coimmunoprecipitated with an anti-Flag monoclonal antibody (Fig. 2D). Immunoprecipitates were analyzed for the presence of CR-1 using an anti-CR-1 rabbit polyclonal antibody. Thus, CR-1 can associate with ALK4 when expressed together, and their interaction is relevant within the context of cell surface association.
FIG. 2.
Binding of CR-1 to ALK4 expressed on the cell surface of mammalian epithelial cells. (A) 293 cells transiently transfected with ALK4-HA, ActRIIB-HA, or vector alone were incubated in the presence of CR-1ΔC-Fc recombinant protein or LTβR-Fc control protein. Binding of CR-1ΔC-Fc or LTβR-Fc protein to 293 cells was analyzed by FACS analysis as described in Materials and Methods. (B) 293 cells transiently transfected with ALK4-HA or ActRIIB-HA expression vector were immunoprecipitated (IP) with CR-1ΔC-Fc or LTβR-Fc and analyzed by Western blot analysis using an anti-HA rabbit polyclonal antibody. (C) As control, cell lysates from ALK4-HA- and ActRIIB-HA-transfected 293 cells were analyzed by Western blot analysis using an anti-HA rabbit polyclonal antibody. (D) COS1 cells were transiently transfected with ALK4-Flag and/or CR-1 expression vectors and Flag-tagged proteins were immunoprecipitated with an anti-Flag mouse monoclonal antibody. The immunoprecipitated proteins were analyzed by Western blot (WB) with an anti-CR-1 rabbit polyclonal antibody or an anti-Flag mouse monoclonal antibody. MW, molecular weight in thousands.
Specific, saturable binding of CR-1ΔC to the ECD of ALK4.
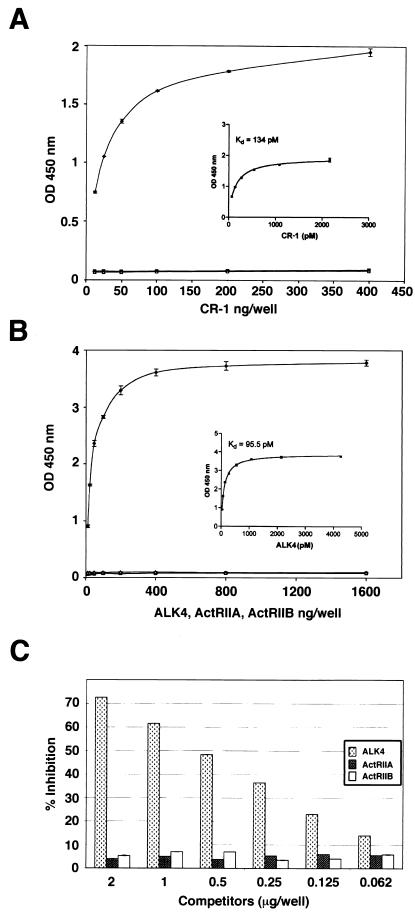
To evaluate if CR-1 can bind to ALK4 in a specific and saturable manner and whether this binding is direct between purified proteins, we determined whether CR-1ΔC could bind the ECD of ALK4 by using an ELISA assay. We immobilized the ECD of ALK4, ActRIIA, or ActRIIB to ELISA plates and reacted these with increasing concentrations of CR-1ΔC recombinant protein. CR-1ΔC binding to the immobilized ECD of ALK4 protein was saturable, with a Kd of 134 pM, whereas the ECD of ActRIIA or ActRIIB receptors failed to bind to CR-1ΔC, as expected (Fig. 3A). Likewise, in the reciprocal experiment the soluble ECD of ALK4 showed a specific, saturable binding to immobilized CR-1ΔC protein, with a Kd of 95.5 pM (Fig. 3B). In addition, CR-1 interaction with immobilized ALK4 was specifically competed in a dose-dependent manner by preincubation of CR-1ΔC with various concentrations of the ECD of ALK4, but not ActRIIA or ActRIIB (Fig. 3C). A 72% inhibition in the binding of CR-1ΔC to immobilized ECD of ALK4 was achieved when 100 ng of CR-1ΔC recombinant protein was preincubated with 2 μg of soluble ECD of the ALK4 protein. Thus, CR-1 binds directly to ALK4, without intermediate cofactors, and the binding is specific and saturable, suggesting a high-affinity interaction.
FIG. 3.
Binding of CR-1ΔC to the ECD of ALK4 (⧫), ActRIIA (▪), and ActRIIB (▵) in ELISA assay. (A) ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc (100 ng/well) was adsorbed to 96-well microtiter plates and incubated with various concentrations of CR-1ΔC recombinant protein (ng/100 μl). ELISA binding of soluble CR-1ΔC to immobilized proteins was detected as described in Materials and Methods. (B) CR-1ΔC (300 ng/well) was adsorbed to 96-well microtiter plates and incubated with various concentrations of the ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB-Fc recombinant protein (ng/100 μl). ELISA binding of soluble ECD of ALK4-Fc, ActRIIA, or ActRIIB to immobilized CR-1ΔC was detected as described in Materials and Methods. (C) One hundred nanograms of CR-1ΔC aloneor preincubated with different concentrations of ECD of ALK4-Fc, ActRIIA-Fc, or ActRIIB recombinant protein was added to plates that were preadsorbed with ECD of ALK4-Fc. ELISA binding of soluble CR-1ΔC to immobilized ECD of ALK4-Fc was detected as previously described. In panels A and B, the inserts show the Kd of each experiment. OD, optical density.
Activation of a TGF-β-Activin-3TP-Lux luciferase reporter construct by CR-1 in K562 cells.
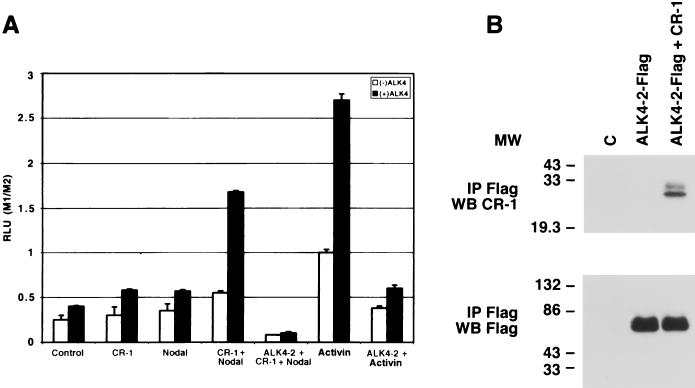
We investigated whether CR-1 can induce Nodal-dependent transcription from a TGF-β-Activin transcriptional response element in the 3TP luciferase reporter (28) in K562 human erythroleukemia cells. These cells have previously been used to delineate Activin signaling (38, 50). A 3TP-luciferase (3TP-Lux) reporter construct was transiently transfected into K562 cells together with CR-1 and/or Nodal expression plasmids in the absence or presence of ALK4-Flag. Renilla luciferase control reporter vector (pRL-TK) was cotransfected into the K562 cells to normalize for transfection efficiency. A small increase in the luciferase activity was observed in the K562 cells cotransfected with ALK4-Flag and CR-1 or ALK4-Flag and Nodal compared to the control vector-transfected cells. However, when ALK4-Flag, CR-1, and Nodal were introduced together into the K562 cells a fourfold increase in the luciferase activity was observed compared to control cells (Fig. 4A). In the absence of transfected ALK4-Flag, endogenous ALK4 in K562 cells was able to induce activation of the luciferase reporter gene by Activin A or CR-1 and Nodal, but to a lesser degree. To confirm that this CR-1 signaling was indeed mediated by ALK4, we used a dominant-negative isoform of ALK4 (ALK4-2-Flag) which contains an intact ECD but a truncated cytoplasmic domain and has previously been shown to inhibit Activin signaling (50). As predicted, induction of 3TP-Lux was significantly suppressed when the dominant-negative ALK4-2-Flag receptor was cotransfected together with ALK4, CR-1, and Nodal into K562 cells (Fig. 4A). Finally, ALK4-2-Flag was able to significantly interfere with endogenous or transiently transfected ALK4 in K562 cells treated with Activin A, suggesting that ALK4-2-Flag is able to interfere with WT receptor function as a dominant-negative receptor.
FIG. 4.
3TP dual-luciferase reporter assay in K562 cells transiently expressing ALK4-Flag, ALK4-2-Flag, CR-1, and/or Nodal. (A) Human K562 erythroleukemia cells were transfected with 0.5 μg of different plasmid DNAs together with Renilla luciferase control reporter vector (pRL-TK). Twenty-four hours after transfection, the cells were lysed and luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega). M1/M2, plasmid DNA/control reporter vector ratio; RLU, relative light units. (B) Coimmunoprecipitation of dominant-negative ALK4-2-Flag with CR-1 in COS1 cells. COS1 cells transiently transfected with ALK4-2-Flag and/or CR-1 cDNAs were immunoprecipitated (IP) with an anti-Flag mouse monoclonal antibody and analyzed by Western blot (WB) using an anti-CR-1 rabbit polyclonal antibody or an anti-Flag mouse monoclonal antibody. MW, molecular weight in thousands; C, control.
In order to test if CR-1 can interact with the truncated ALK4 receptor, COS1 cells were transfected with the human ALK4-2-Flag cDNA alone or in combination with the human CR-1 cDNA. CR-1 coimmunoprecipitated with the dominant-negative ALK4-2-Flag receptor (Fig. 4B), indicating that the interference in signaling by the dominant-negative receptor is due to blockade of downstream signaling and not to interference of CR-1's ability to bind to ALK4.
Nodal is expressed postnatally during mammary gland development.
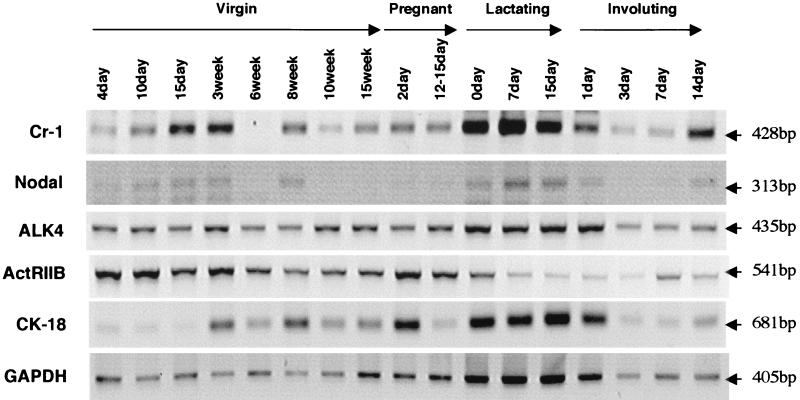
CR-1-dependent Nodal signaling is well documented in the embryo (35), but a nonembryonic role for Nodal has never been defined. Here we show the first documented postnatal expression of Nodal by RT-PCR analysis in different stages of mammary gland development (Fig. 5). We analyzed expression of Nodal, ALK4, and ActRIIB during different stages of mouse mammary development using RT-PCR and compared it with mouse Cr-1 expression. The 313-bp PCR products amplified with Nodal-specific primers from the lactating mammary gland (lactation days 7 and 15) showed 100% sequence homology to mouse Nodal (GenBank accession number X70514), as determined by sequence analysis. The expression of CK-18 and that of the housekeeping gene GAPDH were used to normalize for variances in expression of Cr-1, Nodal, ALK4, and ActRIIB (Fig. 5). CK-18, a specific marker for epithelial cells (42), was found to be upregulated in the postpubescent, pregnant, and lactating mammary gland during stages of increased epithelial growth, whereas GAPDH expression was more constant during these different stages. Cr-1 was expressed during all stages of mammary gland development. Apparently, higher levels of Cr-1 transcripts are expressed during lactation and in the virgin mammary gland at 2 and 3 weeks as the mouse enters puberty. These data are concordant with previous findings that were obtained by immunohistochemistry and in situ hybridization (16, 21). Expression of Nodal transcript was found in virgin, lactating, and involuting mammary glands, and to a lesser extent in the mammary glands from pregnant mice, following the same expression pattern exhibited by Cr-1. ALK4 was expressed at comparable levels during all the stages of mouse mammary development. In contrast, ActRIIB expression was found in the virgin mammary gland and during pregnancy, while it was barely detectable during lactation and the early stages of involution. The similar expression pattern exhibited by Cr-1 and Nodal in the mouse mammary gland suggests that these two signaling proteins may be operating together at comparable stages during mammary gland development.
FIG. 5.
RT-PCR for Cr-1, Nodal, ALK4, and ActRIIB in different stages of mouse mammary development. RT-PCR was performed using specific primers as described in Materials and Methods.
Activation of Smad-2 by CR-1ΔC in EpH-4 mouse mammary epithelial cells requires the presence of Nodal.
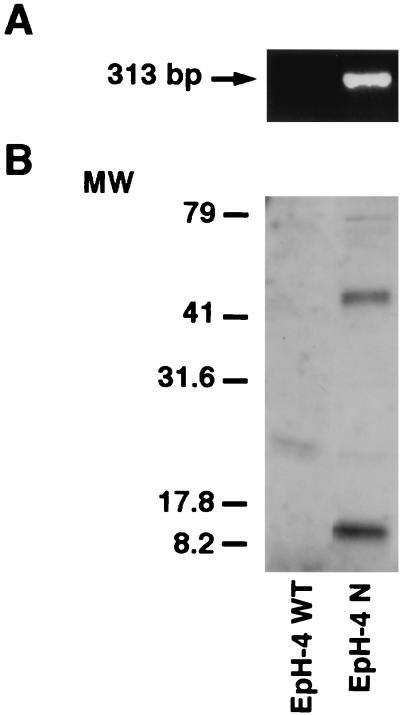
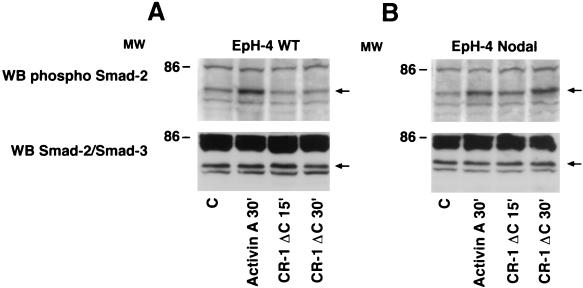
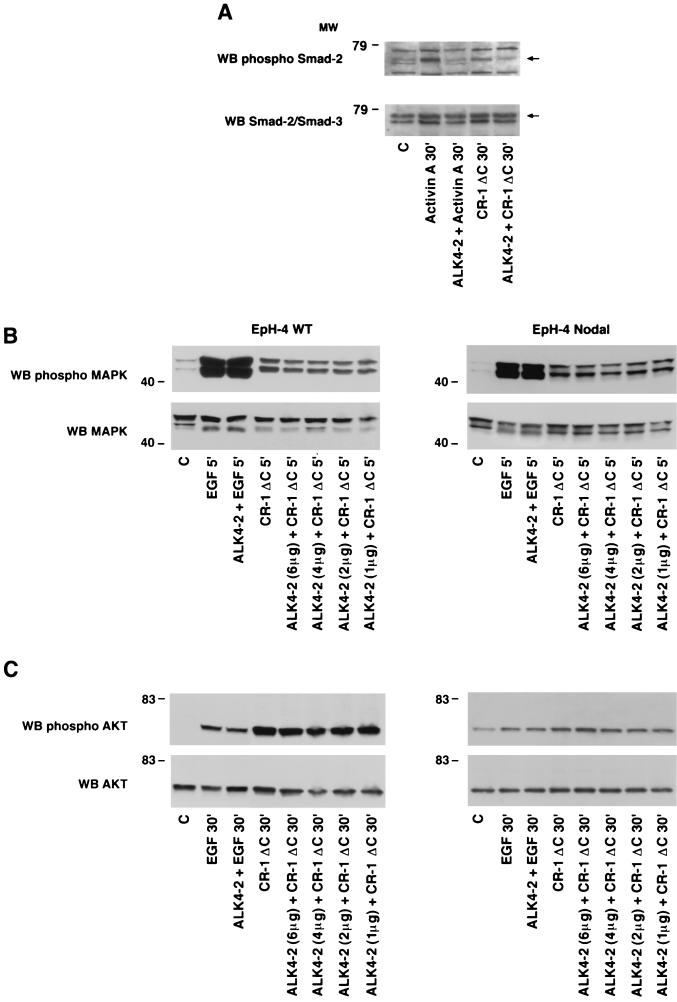
We evaluated whether CR-1 following binding to ALK4 could induce Smad-2 activation in EpH-4 mouse mammary epithelial cells. EpH-4 cells do not express Cr-1 or Nodal, but they express ALK4, as assessed by RT-PCR and Western blot analysis (data not shown; also see Fig. 6A and 9A). Therefore, these cells represent an appropriate model system in which we can assess the effect of CR-1 on the activation of Smad-2 in the absence or presence of expressed Nodal. EpH-4 WT cells were transfected with a mouse Nodal expression vector and a stable cell line overexpressing mouse Nodal was established (EpH-4 N), as assessed by RT-PCR and Western blot analysis for Nodal mRNA and protein, respectively (Fig. 6). Mature and precursor forms of Nodal were detected in the culture medium of EpH-4 Nodal transfected cells. Activation of Smad-2 was then evaluated in EpH-4 WT and EpH-4 N cells. EpH-4 WT and EpH-4 N cells were serum starved for 24 h and then stimulated with CR-1ΔC recombinant protein (400 ng/ml) for 15 and 30 min. CR-1ΔC was unable to activate Smad-2 in EpH-4 WT cells (Fig. 7A). In contrast, a two- and threefold increase in the phosphorylation of Smad-2 was detected in the EpH-4 N cells following 15 and 30 min of CR-1ΔC treatment (Fig. 7B), indicating that activation of Smad-2 by CR-1 is dependent on Nodal expression in EpH-4 cells. Activin A, which was used as positive control, induced a threefold increase in the activation of Smad-2 in both EpH-4 WT and EpH-4 N cells. In contrast to the Nodal dependence for Smad-2 activation by CR-1, CR-1ΔC stimulated MAPK and AKT phosphorylation equally well in both EpH-4 WT and EpH-4 N cells (Fig. 8B and C), indicating that Nodal is not required for CR-1 activation of these pathways.
FIG. 6.
RT-PCR and Western blot analysis for Nodal in EpH-4 WT and Eph-4 N cells. (A) RT-PCR with specific Nodal primers (313 bp) in EpH-4 WT and EpH-4 N cells. (B) Western blot analysis using a rabbit polyclonal anti-Nodal antibody in the culture medium of EpH-4 WT and EpH-4 N cells. MW, molecular weight in thousands.
FIG. 9.
MAPK and AKT phosphorylation by CR-1ΔC in MC3T3-E1 cells. (A) RT-PCR using specific primers for ALK4 (435 bp) in EpH-4 WT and MC3T3-E1 cells. A 100-bp DNA marker is shown in the first lane. (B and C) Serum-starved MC3T3-E1 cells were stimulated with CR-1ΔC (200 and 400 ng/ml) or EGF (50 ng/ml) recombinant protein and analyzed by Western blot (WB) analysis. MW, molecular weight in thousands; C, control.
FIG. 7.
Smad-2 phosphorylation by CR-1ΔC in EpH-4 WT and EpH-4 N cells. Serum-starved EpH-4 WT (A) and EpH-4 N (B) cells were stimulated for different times with CR-1ΔC (400 ng/ml) or Activin A (50 ng/ml) recombinant protein and analyzed by Western blot (WB) analysis. MW, molecular weight in thousands; C, control.
FIG. 8.
Smad-2, MAPK, and AKT phosphorylation by CR-1ΔC in EpH-4 WT and EpH-4 N cells transiently transfected with a dominant-negative ALK4-2-Flag receptor. EpH-4 WT and EpH-4 N cells transiently transfected with ALK4-2-Flag or pCI-neo plasmid were stimulated with CR-1ΔC, EGF, or Activin A recombinant protein and cell lysates were assessed by Western blot (WB) analysis. (A) EpH-4 N cells transfected with pCI-neo (4 μg) (lanes 1, 2, and 4) and ALK4-2 (4 μg) were stimulated with Activin A (50 ng/ml) and CR-1ΔC (400 ng/ml) and analyzed by Western blot analysis. (B and C) EpH-4 WT or EpH-4 N cells transfected with pCI-neo (6 μg) (lanes 1, 2, and 4) and ALK4-2 (6, 4, 2, and 1 μg) were stimulated with EGF (50 ng/ml) and CR-1ΔC (200 ng/ml) and analyzed by Western blot analysis. MW, molecular weight in thousands; C, control.
ALK4 is required by CR-1ΔC to activate Smad-2 but is not necessary for stimulation of MAPK and AKT.
Because Nodal is required by CR-1 for activation of Smad-2, but not MAPK or AKT, in EpH-4 cells, we asked if CR-1 is stimulating both of these pathways through the ALK4 receptor. We transiently expressed the dominant-negative ALK4-2-Flag receptor in EpH-4 WT or EpH-4 N cells in order to interfere with the endogenous ALK4 receptor. The transiently transfected cells were then stimulated with CR-1ΔC, Activin A, or EGF. ALK4-2-Flag significantly interfered with the capacity of CR-1ΔC to activate Smad-2 in EpH-4 N cells, suggesting that ALK4 mediates the ability of CR-1ΔC to phosphorylate Smad-2 in this cell line (Fig. 8A). In contrast, MAPK and AKT phosphorylation was stimulated by CR-1ΔC to similar levels in EpH-4 WT and EpH-4 N cells in the presence or absence of the dominant-negative ALK4-2-Flag receptor (Fig. 8B and C). EGF-induced phosphorylation of both MAPK and AKT was also not affected by expression of the dominant-negative ALK4-2-Flag receptor. All the membranes were reprobed with specific rabbit polyclonal anti Smad-2/Smad-3, MAPK, or AKT antibodies that recognize the unphosphorylated form of the proteins to ensure equal loading of the proteins on the gel. Finally, CR-1ΔC induced activation of both MAPK and AKT pathways in mouse MC3T3-E1 osteoblast cells (24) which do not express ALK4, as assessed by RT-PCR analysis (Fig. 9). Therefore, ALK4 is required by CR-1 for activation of Smad-2 in a Nodal-dependent context, whereas activation of MAPK and AKT by CR-1ΔC is independent of Nodal and ALK4 in EpH-4 mammary epithelial cells. This result indicates that CR-1 can signal through distinct pathways and possibly distinct receptors to mediate its biological effects
DISCUSSION
Using a phage display approach, ALK4 was cloned and identified as a receptor for human CR-1. Our results indicate that CR-1ΔC can bind to full-length or kinase-truncated ALK4 protein, but not to ActRIIA or ActRIIB. The demonstration of a human ALK4 and CR-1 interaction is consistent with previous genetic studies in zebra fish that have implied an interaction of the Activin type I and type II receptors in mediating CR-1 and Nodal signaling (36). In this context, zebra fish CR-1 (oep) is an essential component for the zebra fish Nodal-related genes, cyclops and squint, signaling pathway and functions as a coreceptor for Nodal-related proteins (15, 48, 49). Nodal-related proteins and oep subsequently signal through the downstream Activin type IIB and type I (ALK4) receptors that induce Smad-2 and Smad-4 activation. Mutations in oep, cyclops, and squint display a similar phenotype, suggesting that they may act in the same pathway, and the oep mutant phenotype can be rescued by expression of a full-length or secreted COOH-terminal truncated oep protein, overexpression of Activin, or activated ALK4 or Smad-2 (15). Recent results indicate that mouse Cr-1 is required for Nodal to bind to an ALK4-ActRIIB receptor complex and to induce Smad-2 phosphorylation in a heterologous Xenopus oocyte injection system (48). Our binding studies with ALK4 and CR-1 largely agree with those reported by Yeo and Whitman (48). In addition, Reissmann et al. (29) have also demonstrated that CR-1 is able to directly interact not only with ALK4 but also with the orphan receptor ALK7. In this regard, CR-1 can function as a binding molecule for Nodal and can enhance the responses to Nodal mediated by ALK4 and ALK7. Whereas Nodal requires CR-1 to interact with ALK4, Nodal binds directly to ALK7 without CR-1. From all these studies, EGF-CFC proteins appear to function as coreceptors during embryonal development, enhancing Nodal ligand binding to the type I-type II receptor complex.
We document here the first evidence of Nodal expression in an adult tissue. Specifically, we found expression of Nodal in the mammary gland during development coinciding with Cr-1 expression, suggesting that CR-1-dependent Nodal signaling may play a role in the development of this tissue. The pattern of Nodal expression is high during puberty and lactation and slightly less during pregnancy and decreases during early and mid involution. In addition, Nodal expression parallels Cr-1 expression in the mammary gland. While ALK4 and ActRIIB were expressed in the mammary gland, their expression did not completely parallel the expression of Cr-1 and Nodal, consistent with the idea that ALK4 and ActRIIB are also receptors for other TGF-β ligands, most notably Activin (25). The coexpression of CR-1 and Nodal in the mammary gland supports a role for CR-1 as coreceptor for Nodal, also in an adult tissue. However, it is possible that EGF-CFC proteins could exert their function independently from Nodal, also acting as soluble growth factors. Therefore, CR-1 could function as a coreceptor for Nodal or as a signaling molecule able to activate alternative signal transduction pathways in the adult tissues. In fact, there is evidence that GPI-linked coreceptors of TGF-β family members can also signal as soluble molecules (34, 45). For example, GFRα-1, a GPI-linked coreceptor for the TGF-β family member GDNF, can signal in multiple ways (34, 45).
Since our results support a postembryonic role for Nodal in the mammary gland, we examined whether CR-1 could stimulate Smad-2 phosphorylation in EpH-4 mammary epithelial cells in a Nodal- and ALK4-dependent manner. CR-1 can induce the specific phosphorylation of Smad-2 only in mammary epithelial EpH-4 cells which express mouse Nodal, suggesting that activation of Smad-2 by CR-1 in mammalian epithelial cells is a Nodal-dependent activity. In contrast, we demonstrate for the first time that CR-1 can activate ras/raf/MAPK and PI3K/AKT signaling pathways independently from Nodal and that the ability of CR-1 to mediate MAPK and AKT phosphorylation is not significantly affected by the addition of a dominant-negative ALK4 receptor to EpH-4 WT and EpH-4 N cells and is fully preserved in MC3T3-E1 cells lacking ALK4 expression. We obtained a slight decrease (10 to 15%) in MAPK signal following addition of the dominant-negative ALK4 receptor in EpH-4 WT cells. However, this phenomenon was only observed at the highest concentration of the titration and it is probably a nonspecific effect (Fig. 8B). Several recent studies have demonstrated that TGF-β family members can cross talk with and/or directly activate the MAPK pathway (27). For example, Hu et al. (17) showed that TGF-β induction of p21 in a human keratinocyte cell line was blocked with inhibitors of the MAPK cascade, whereas MAPK blockade did not affect TGF-β stimulated Smad phosphorylation. Therefore, while ALK4 and Nodal are not required for the ability of CR-1 to stimulate MAPK or AKT, CR-1 could potentially interact with another ALK or TGF-β receptor family member, or an unknown receptor, on mammary cells to promote MAPK and/or AKT activation. Since recently it has been shown that ALK7 can directly interact with CR-1, we cannot exclude the possibility that ALK7 mediates CR-1 ability to activate MAPK and AKT pathways. In this regard, expression of a constitutively activated ALK7 in PC12 rat pheochromocytoma cells results in the activation of MAPK and c-Jun-N-terminal kinase as well as Smad-2 and Smad-3 (18). Therefore, in MC3T3-E1 cells lacking ALK4 expression or in EpH-4 cells transfected with a dominant-negative ALK4-2 receptor, CR-1 could mediate MAPK/AKT activation through ALK7.
Recently, it has been shown that Nodal can signal in a CR-1-dependent and -independent manner. In this regard, Brennan et al. (5) elegantly demonstrated that Nodal can act independently of Cr-1 and Smad-2 to promote posterior cell fates in the early mouse embryo but requires Cr-1 signaling in the epiblast which leads to displacement of the anterior visceral endoderm. In addition, Nodal can also inhibit BMP signaling by a CR-1-independent mechanism (22, 48). Finally, Ding et al. (11) reported that while the Nodal and Cr-1 null mice share many phenotypic similarities, they differ in that Cr-1 mutant embryos form extra-embryonic mesoderm and express posterior markers such as T and Fgf8 which are not seen in Nodal mutants. These differences could be due to known or unknown EGF-CFC proteins that may compensate for one another in the mouse embryo or to the fact that Nodal signaling in the mouse is enhanced by EGF-CFC factors but not strictly dependent on them. Thus, while CR-1 and Nodal are mutually dependent for some activities, they may both have functional roles independent of one another.
In addition to patterning the early embryo, CR-1 plays a role in mammary gland development and CR-1 expression is upregulated in several different types of human tumors including human breast carcinomas (32). In this respect , overexpression of mouse or human CR-1 can lead to the in vitro transformation of NOG-8 and EpH-4 mouse mammary epithelial cells (6, 44). In addition, in mice that are overexpressing an MMTV-human CR-1 transgene in the mammary gland, there is an increase in ductal hyperplasias in nulliparous and multiparous female mice (C. Wechselberger, Y. Sun, C. Bianco, N. Khan, and D. S. Salomon, unpublished data). Since MAPK and AKT transduction pathways have been shown to induce cell growth and survival (9, 31), CR-1 could be involved in the pathogenesis of human cancer through inappropriate activation of these two signaling pathways in a Nodal- and ALK4-independent manner. Therefore, CR-1 might elicit various cellular responses by the utilization of different signal transduction pathways and/or through alternate receptors. Interestingly, the presence of two distinct CR-1 binding molecules is also suggested by our previous cross-linking studies (3). 125I-CR-1 cross-linking to mouse and human mammary epithelial cells yielded an affinity labeled product of 60 kDa, a size typical of TGF-β/Activin type I receptors. We were not able to detect a type II receptor, which has an expected size of 85 to 95 kDa, cross-linked to 125I-CR-1 protein. However, an affinity-labeled product with a molecular size of 130 kDa was detected. This protein might represent another CR-1 binding molecule distinct from ALK4 and potentially involved in regulating the activation of MAPK and AKT.
In summary, our experiments demonstrate that CR-1 can function as a coreceptor for Nodal and as an autonomous signaling molecule through a Nodal- and ALK4-independent pathway, suggesting a complex interplay among different signaling molecules.
Acknowledgments
This work was supported in part by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) to N.N.
We acknowledge Matthew Jarpe of Biogen for help in calculating the Kd and analyzing the kinetics of the ELISA binding. We thank Brenda Jones and Joy Kim for their excellent technical assistance.
REFERENCES
- 1.Attisano, L., C. Silvestri, L. Izzi, and E. Labbe. 2001. The transcriptional role of Smads and FAST (FoxH1) in TGFβ and activin signaling. Mol. Cell. Endocrinol. 180:3-11. [DOI] [PubMed] [Google Scholar]
- 2.Bamford, R. N., E. Rossler, R. D. Burdine, U. Saplakoglu, J. dela Cruz, M. Splitt, J. Towbin, P. Bowers, B. Marino, A. F. Schier, M. M. Shen, M. M. Muenke, and B. Casey. 2000. Loss of function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat. Genet. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 3.Bianco, C., S. Kannan, M. De Santis, M. Seno, C. K. Tang, I. Martinez-Lacaci, N. Kim, B. Wallace-Jones, M. E. Lippman, A. Ebert, C. Wechselberger, and D. S. Salomon. 1999. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J. Biol. Chem. 274:8624-8629. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, R., N. Normanno, W. J. Gullick, J. H. Lin, R. Harkins, D. Schneider, B. Jones, F. Ciardiello, M. G. Persico, F. Armenante, N. Kim, and D. S. Salomon. 1994. Identification and biological characterization of an epidermal growth factor-related protein: Cripto-1. J. Biol. Chem. 269:17320-17328. [PubMed] [Google Scholar]
- 5.Brennan, J., C. C. Lu, D. P. Norris, T. A. Rodriguez, R. S. Beddington, and E. J. Robertson. 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411:965-969. [DOI] [PubMed] [Google Scholar]
- 6.Ciardiello, F., R. Dono, N. Kim, M. G. Persico, and D. S. Salomon. 1991. Expression of cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 51:1051-1054. [PubMed] [Google Scholar]
- 7.Ciccodicola, A., R. Dono, S. Obici, A. Simeone, M. Zollo, and M. G. Persico. 1989. Molecular characterization of a gene of the EGF family expressed in undifferentiated human NTERA2 teratocarcinoma cells. EMBO J. 8:1987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colas, J. F., and G. C. Scoenwolf. 2000. Subtractive hybridization identifies chick-cripto, a novel EGF-CFC ortholog expressed during gastrulation, neurulation and early cardiogenesis. Gene 255:205-217. [DOI] [PubMed] [Google Scholar]
- 9.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 10.De Santis, M. L., S. Kannan, G. H. Smith, M. Seno, C. Bianco, N. Kim, I. Martinez-Lacaci, B. Wallace-Jones, and D. S. Salomon. 1997. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21ras- and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 8:1257-1266. [PubMed] [Google Scholar]
- 11.Ding, J., L. Yang, Y. T. Yan, A. Chen, N. Desai, A. Wynshaw-Boris, and M. M. Shen. 1998. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 395:702-707. [DOI] [PubMed] [Google Scholar]
- 12.Dono, R., L. Scalera, F. Pacifico, D. Acampora, M. G. Persico, and A. Simeone. 1993. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development 118:1157-1168. [DOI] [PubMed] [Google Scholar]
- 13.Ebert, A. D., C. Wechselberger, S. Frank, B. Wallace-Jones, M. Seno, I. Martinez-Lacaci, C. Bianco, M. De Santis, H. K. Weitzel, and D. S. Salomon. 1999. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthetase kinase 3 beta in human cervical carcinoma cells. Cancer Res. 59:4502-4505. [PubMed] [Google Scholar]
- 14.Ehrenfels, C. W., P. J. Carmillo, O. Orozco, R. L. Cate, and M. Sanicola. 1999. Perturbation of RET signaling in the embryonic kidney. Dev. Genet. 24:263-272. [DOI] [PubMed] [Google Scholar]
- 15.Gritsman, K., J. Zhang, S. Cheng, E. Heckscher, W. S. Talbot, and A. F. Schier. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97:121-132. [DOI] [PubMed] [Google Scholar]
- 16.Herrington, E. E., T. G. Ram, D. S. Salomon, G. R. Johnson, W. J. Gullick, N. Kenney, and H. L. Hosick. 1997. Expression of epidermal growth factor-related proteins in the aged adult mouse mammary gland and their relationship to tumorigenesis. J. Cell. Physiol. 170:47-56. [DOI] [PubMed] [Google Scholar]
- 17.Hu, P. P., X. Shen, D. Huang, Y. Liu, C. Counter, and X. F. Wang. 1999. The MEK pathway is required for stimulation of p21 (WAF1/CIP1) by transforming growth factor-beta. J. Biol. Chem. 274:35381-35387. [DOI] [PubMed] [Google Scholar]
- 18.Jornvall, H., A. Blokzijl, P. ten Dijke, and C. F. Ibanez. 2001. The orphan receptor serine/threonine kinase ALK7 signals arrest of proliferation and morphological differentiation in a neuronal cell line. J. Biol. Chem. 276:5140-5146. [DOI] [PubMed] [Google Scholar]
- 19.Kannan, S., M. De Santis, M. Lohmeyer, D. J. Reise II, G. H. Smith, N. Hynes, M. Seno, R. Brandt, C. Bianco, M. G. Persico, N. Kenney, N. Normanno, I. Martinez-Lacaci, F. Ciardiello, D. F. Stern, W. J. Gullick, and D. S. Salomon. 1997. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J. Biol. Chem. 272:3330-3335. [DOI] [PubMed] [Google Scholar]
- 20.Kenney, N. J., G. H. Smith, M. D. Johnson, K. Rosenberg, D. S. Salomon, and R. B. Dickson. 1997. Cripto-1 activity in the intact and ovariectomized virgin mouse mammary gland. Pathogenesis 1:57-71. [Google Scholar]
- 21.Kenney, N. J., R. P. Huang, G. R. Johnson, J. X. Wu, D. Okamura, W. Matheny, E. Kordon, W. J. Gullick, G. Plowman, G. H. Smith, D. S. Salomon, and E. D. Adamson. 1995. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol. Reprod. Dev. 41:277-286. [DOI] [PubMed] [Google Scholar]
- 22.Kiecker, C., F. Muller, W. Wu, A. Glinka, U. Strahle, and C. Niehrs. 2000. Phenotypic effects in Xenopus and zebrafish suggest that one-eyed pinhead functions as antagonist of BMP signalling. Mech. Dev. 94:37-46. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita, N., J. Minshull, and M. W. Kirschner. 1995. The identification of two novel ligands of the FGF receptor by a yeast screening method and their activity in Xenopus development. Cell 83:621-630. [DOI] [PubMed] [Google Scholar]
- 24.Macias-Silva, M., P. A. Hoodless, S. J. Tang, M. Buchwald, and J. L. Wrana. 1998. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273:25628-25636. [DOI] [PubMed] [Google Scholar]
- 25.Massague, J., S. W. Blain, and R. S. Lo. 2000. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295-309. [DOI] [PubMed] [Google Scholar]
- 26.Minchiotti, G., S. Parisi, G. Liguori, M. Signore, G. Lania, E. D. Adamson, C. T. Lago, and M. G. Persico. 2000. Membrane-anchorage of Cripto protein by glycosylphopshatidylinositol and its distribution during early mouse development. Mech. Dev. 90:133-142. [DOI] [PubMed] [Google Scholar]
- 27.Mulder, K. M. 2001. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 11:23-35. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan, R. P., F. Chen, W. Li, E. Vig, M. A. Harrington, H. Nakshatri, and Y. Chen. 2000. Repression of transforming-growth-factor-beta-mediated transcription by nuclear factor kappaB. Biochem. J. 348:591-596. [PMC free article] [PubMed] [Google Scholar]
- 29.Reissmann, E., H. Jornvall, A. Blokzijl, O. Andersson, C. Chang, G. Minchiotti, M. G. Persico, C. F. Ibanez, and A. H. Brivanlou. 2001. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 15:2010-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riese, D. J., II, and D. F. Stern. 1998. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays 20:41-48. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 32.Salomon, D. S., C. Bianco, A. D. Ebert, N. I. Khan, M. De Santis, N. Normanno, C. Wechselberger, M. Seno, K. Williams, M. Sanicola, S. Foley, W. J. Gullick, and M. G. Persico. 2000. The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer. Endocr. Relat. Cancer 7:199-226. [DOI] [PubMed] [Google Scholar]
- 33.Salomon, D. S., C. Bianco, and M. De Santis. 1999. Cripto: a novel epidermal growth factor (EGF)-related peptide in mammary gland development and neoplasia. BioEssays 21:61-70. [DOI] [PubMed] [Google Scholar]
- 34.Sanicola, M., C. Hession, D. Worley, P. Carmillo, C. Ehrenfels, L. Walus, S. Robinson, G. Jaworski, H. Wei, R. Tizard, A. Whitty, R. B. Pepinsky, and R. L. Cate. 1997. Glial cell line-derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc. Natl. Acad. Sci. USA 94:6238-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schier, A. F., and M. M. Shen. 2000. Nodal signaling in vertebrate development. Nature 403:385-389. [DOI] [PubMed] [Google Scholar]
- 36.Schier, A. F., and W. S. Talbot. 2001. Nodal signaling and the zebrafish organizer. Int. J. Dev. Biol. 45:289-297. [PubMed] [Google Scholar]
- 37.Schiffer, S., S. Foley, A. Kaffashan, X. Hrnonowski, A. Zichitella, C. Y. Yeo, K. Miatkowski, H. Adkins, B. Damon, M. Whitman, D. S. Salomon, M. Sanicola, and K. Williams. 2001. Fucosylation of Cripto is required for its ability to facilitate Nodal signaling. J. Biol. Chem. 276:37769-37778. [DOI] [PubMed] [Google Scholar]
- 38.Sehy, D. W., L. E. Shao, A. L. Yu, W. M. Tsai, and J. Yu. 1992. Activin A-induced differentiation in K562 cells is associated with a transient hypophosphorylation of RB protein and the concomitant block of cell cycle at G1 phase. J. Cell. Biochem. 50:255-265. [DOI] [PubMed] [Google Scholar]
- 39.Seno, M., M. De Santis, S. Kannan, C. Bianco, H. Tada, N. Kim, M. Kosaka, W. J. Gullick, H. Yamada, and D. S. Salomon. 1998. Purification and characterization of a recombinant human cripto-1 protein. Growth Factors 15:215-229. [DOI] [PubMed] [Google Scholar]
- 40.Shen, M. M., and A. F. Schier. 2000. The EGF-CFC gene family in vertebrate development. Trends Genet. 16:303-309. [DOI] [PubMed] [Google Scholar]
- 41.Shen, M. M., H. Wang, and P. Leder. 1997. A differential display strategy identifies cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development 124:429-442. [DOI] [PubMed] [Google Scholar]
- 42.Taylor-Papadimitriou, J., R. Wetzels, and F. Ramaekers. 1992. Intermediate filament protein expression in normal and malignant human mammary epithelial cells. Cancer Treat. Res. 61:355-378. [DOI] [PubMed] [Google Scholar]
- 43.ten Dijke, P., H. Ichijo, P. Franzen, P. Schulz, J. Saras, H. Toyoshima, C. H. Heldin, and K. Miyazono. 1993. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 8:2879-2887. [PubMed] [Google Scholar]
- 44.Wechselberger, C., A. D. Ebert, C. Bianco, N. I. Khan, Y. Sun, B. Wallace-Jones, R. Montesano, and D. S. Salomon. 2001. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp. Cell. Res. 266:95-105. [DOI] [PubMed] [Google Scholar]
- 45.Worley, D. S., J. M. Pisano, E. D. Choi, L. Walus, C. A. Hession, R. L. Cate, M. Sanicola, and S. J. Birren. 2000. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development 127:4383-4393. [DOI] [PubMed] [Google Scholar]
- 46.Xu, C., G. Liguori, M. G. Persico, and E. D. Adamson. 1999. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development 126:483-494. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto, M., C. Meno, Y. Sakai, H. Shiratori, K. Mochida, Y. Ikawa, Y. Saijoh, and H. Hamada. 2001. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 15:1242-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo, C. Y., and M. Whitman. 2001. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol. Cell 7:949-957. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J., W. S. Talbot, and A. F. Schier. 1998. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92:241-251. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, Y., H. Sun, D. C. Danila, S. R. Johnson, D. P. Sigai, X. Zhang, and A. Klibanski. 2000. Truncated activin type I receptor Alk4 isoforms are dominant negative receptors inhibiting activin signaling. Mol. Endocrinol. 14:2066-2075. [DOI] [PubMed] [Google Scholar]