Abstract
The fps/fes proto-oncogene encodes a cytoplasmic protein tyrosine kinase implicated in growth factor and cytokine receptor signaling and thought to be essential for the survival and terminal differentiation of myeloid progenitors. Fps/Fes-null mice were healthy and fertile, displayed slightly reduced numbers of bone marrow myeloid progenitors and circulating mature myeloid cells, and were more sensitive to lipopolysaccharide (LPS). These phenotypes were rescued using a fps/fes transgene. This confirmed that Fps/Fes is involved in, but not required for, myelopoiesis and that it plays a role in regulating the innate immune response. Bone marrow-derived Fps/Fes-null macrophages showed no defects in granulocyte-macrophage colony-stimulating factor-, interleukin 6 (IL-6)-, or IL-3-induced activation of signal transducer and activator of transcription 3 (Stat3) and Stat5A or LPS-induced degradation of IκB or activation of p38, Jnk, Erk, or Akt.
Fps/Fes (hereafter referred to as Fps) and Fer comprise the only two members of subgroup IV of the nonreceptor protein tyrosine kinase family (68). They share a similar structure, comprised of a unique N-terminal Fps/Fes/Fer/CIP4 homology (FCH) domain (6), two regions of predicted coiled coils (CC), a central Src homology 2 (SH2) domain, and a C-terminal tyrosine kinase domain (4, 89). The FCH domain was first described as a region of homology between Fps/Fes/Fer protein tyrosine kinases and a Cdc42-interacting protein, CIP4 (6). It is conserved in proteins implicated in the regulation of cytoskeletal rearrangements, vesicular trafficking, and endocytosis (20, 31, 49, 57, 67, 82, 83, 92), and the CIP4 FCH domain has been shown to bind microtubules (82). The CC domains of Fps and Fer protein tyrosine kinases direct homotypic oligomerization (10, 12, 64). The SH2 domain, by virtue of its high affinity for specific phosphotyrosine-containing peptides, is able to specify substrate binding, localization, and regulation of the kinase (14, 43, 44, 72, 74). Fps and Fer kinases participate in their own regulation through autophosphorylation both in cis and in trans (12, 73). The kinase activity of Fps is required for proper subcellular localization to the Golgi apparatus and other vesicular compartments of the cell (96). The substrates of cellular Fps, or oncogenic v-Fps/Fes forms, include signal transducer and activator of transcription 3 (Stat3) (21, 63), Ras GTPase-activating protein (p120Ras-Gap) (32), the Ras-Gap-associated p190Rho-Gap and p62Dok proteins (59), Bcr (53), Shc (55), connexin 43 (45), platelet-derived growth factor β (5), and p130Cas and Fyb/SLAP130 (36).
Fps expression was initially thought to be restricted to hematopoietic cells of the monocytic and granulocytic lineages (52, 76). However, more recent studies have shown that fps is expressed in all three germ layers during development and in neuronal, epithelial, and endothelial, as well as hematopoietic, cells in the adult (9, 26). In contrast to the tissue-specific expression pattern of Fps, the closely related Fer kinase is ubiquitously expressed (17, 30, 65). Another distinct feature of fer is the expression of a shorter 51-kDa Fer T isoform which lacks the N-terminal FCH and CC domains and is produced from an internal testis-specific promoter (41).
Fps has been shown to promote myeloid differentiation of the K562 leukemia cell line (95). Furthermore, antisense oligonucleotides to fps blocked the differentiation of the promyelocytic leukemia cell line HL60 (18, 93, 94) and leukemic blast cells from acute promyelocytic leukemia with the concomitant induction of apoptosis in these cells (19). From these studies it was concluded that Fps plays an important role in the terminal differentiation of the monocyte/granulocyte lineages and that this may involve a role in regulation of cell-survival signaling pathways. The Fps kinase has also be described as activated by or associated with the receptors for granulocyte-macrophage colony-stimulating factor (GM-CSF) (8, 28, 50), interleukin 3 (IL-3) (28), IL-4 (34, 35), cytokines using the common gp130 subunit (including IL-6, leukemia-stimulating factor, oncostatin M, IL-11, and ciliary neurotrophic factor) (2, 54), and erythropoietin (29). These interactions are consistent with Fps playing a role in hematopoiesis, because all of these receptors are expressed in hematopoietic cells. Fps also plays roles in fibroblast growth factor 2-induced chemotaxis in endothelial cells (38) and IL-4-mediated insulin receptor substrate 2 activation in B cells (35). Fps may signal via the Ras pathway to the mitogen-activated protein kinases Erk and Jnk (48, 62), and Fps may modulate additional growth regulatory pathways through its interactions with and phosphorylation of Bcr (47, 51, 66).
Fps was initially identified as a transforming protein encoded by avian (fps) (24) or feline (fes) retroviral oncogenes (27). Mice expressing a v-fps transgene driven by a minimal β-actin promoter developed lymphoid and mesenchymal tumors as well as enlargement of the heart and trigeminal nerves (90, 91). In contrast, tissue-specific overexpression of a wild-type human fps transgene did not perturb mouse development or lead to any hematological or other physiological defects (23). However, when Fps was targeted to lipid membranes within cells by providing a Src-like myristylation sequence at its N terminus, the myristylated Fps protein was able to transform cultured NIH 3T3 cells and transgenic mice expressing this myristylated form of Fps developed widespread hypervascularity, which progressed to multiple hemangiomas (22). The relative ease of generating endothelial cell lines from the yolk sacs of these mice also suggested that myristylated Fps imparted a growth advantage to the endothelial lineage (87). While these approaches have suggested potential pathophysiological consequences of deregulated fps expression or mutations affecting subcellular localization, they have not yet clarified the function of the Fps kinase.
We have used a gene-targeting approach in mice to further understand the function of the Fps kinase. We first explored the requirement of Fps kinase activity by generating mice in which the endogenous fps gene was targeted with a kinase-inactivating missense mutation (80). Mice homozygous for this mutation produced a full-length, catalytically inactive protein. In spite of the many functions ascribed to Fps, these mice were viable and fertile and had no gross abnormalities in their hematopoietic system (80). Subtle biochemical defects observed included reduced Stat3 and Stat5 phosphorylation after GM-CSF stimulation of bone marrow-derived macrophages (BMDM), as well as reduced activation of Erk after lipopolysaccharide (LPS) treatment (80).
Recently Hackenmiller and coworkers reported the characterization of Fps-null mice that were generated by substituting a PGKneo selection cassette for sequences encompassing the fps promoter region and up to exon 3 (25). These Fps-null mice were described as having hematopoietic homeostasis defects, including decreased B lymphocytes and increased monocytes, neutrophils, and granulocytes. They also showed enhanced Stat3 and Stat5 activation in macrophages after GM-CSF or IL-6 stimulation and suggested a model whereby Fps might compete with Jak2 for binding to and phosphorylation of Stat proteins. These authors also described several low-penetrance phenotypes in their Fps-null mice, including embryonic lethality, runting, skin lesions, conjunctivitis, and cardiovascular defects. They also reported an increased sensitivity of Fps-null mice to infection with Borrelia burgdorferi, although these data were not shown (25). The severer phenotypes described in these Fps-null mice than in the previously reported mice which express kinase-inactive Fps have suggested the possibility that Fps may have some kinase-independent functions.
We describe here the generation and characterization of a distinct line of Fps-null mice that show subtle phenotypes which are substantially different from the Fps-null mice described recently by Hackenmiller and coworkers. In our targeting strategy we deleted sequences encoding the kinase domain in the 3′ region of the gene. We chose this approach to avoid effects on expression of the furin gene, which is located immediately upstream from fps. The 3′ end of furin lies 1,350 bp upstream from the beginning of the fps transcription start site (R. A. Zirngibl and P. A. Greer, unpublished data), and it is transcribed in the same direction as fps (70, 71). By avoiding deletions in the fps promoter or insertion of a drug-selectable PGKneo cassette in this region of the gene, we excluded potential effects on expression of the furin gene. furin encodes a proprotein convertase that is involved in the processing of a number of cytokines and growth factors (61, 79). Deletion of the mouse furin gene resulted in heart development defects and embryonic death (69).
The Fps-null mice described here generated no detectable Fps protein and have no Fps kinase activity. These Fps-null mice are viable and fertile and show no evidence for low-penetrance embryonic lethality, runting, or skin lesions. They do have minor defects in hematopoietic homeostasis which are different from those described by Hackenmiller and coworkers. Slightly reduced numbers of bone marrow myeloid progenitors and circulating mature myeloid cells were seen. They also displayed an increase in circulating erythrocytes and some evidence for defective B-lymphocyte maturation. BMDM from Fps-null mice showed no defects in GM-CSF-, IL-3-, or IL-6-induced activation of Stat3 and Stat5A. The most striking defect was revealed when the innate immune system of these mice was challenged with LPS to mimic a gram-negative bacterial infection. This resulted in a pronounced reduction in survival of the Fps-null mice compared to that of wild-type mice. All of these phenotypes of the Fps-null mice were rescued when the fps-targeted mice were crossed with a strain that carried a human fps transgene. This genetic rescue experiment demonstrated that the observed defects in the fps-targeted mice were indeed specific for loss of Fps expression.
MATERIALS AND METHODS
Construction of the fps-null targeting vector.
A 15-kb genomic fragment containing the complete c-fps locus was cloned from a 129SvJ mouse genomic library in λDASH2 and sequenced (unpublished data). Since the NotI and XhoI sites in the drug selection vector pPNT partially overlapped (85), we modified pPNT by digestion with XhoI, annealing, and ligating two complementary oligonucleotides, PNT-NHS1 (5′-TCGAAGCTAGCATGTTAACATGTCGACT-3′) and PNT-NHS2 (5′-TCGAAGTCGACATGTTAACATGCTAGCT-3′) into the XhoI site. These oligonucleotides destroyed the XhoI site and introduced three unique sites, SalI, HpaI, and NheI, at the 5′ end of the PGKneo positive selection cassette. This vector was designated pPNT-NHS14. A short 3′ arm of fps homology consisting of exon 19 within a 2.5-kb KpnI-EcoRI fragment was cloned into the corresponding sites in pPNT-NHS14 downstream from the PGKneo cassette. A long 5′ arm of fps homology consisting of exons 1 through 14 within a 8.2-kb NotI-to-XhoI fragment was then cloned upstream of the PGKneo cassette between the NotI and SalI sites, giving the final targeting vector pPNT-NXKpR. Homologous recombination resulted in the deletion of exons 15 through 18, which codes for most of the kinase domain, and insertion of the PGKneo cassette in its place.
ES cell culture and chimeric mouse production.
Mouse embryonic stem (ES) cells (RI; passage 8) were kindly provided by Andras Nagy (60). Propagation, electroporation, and selection of recombinant RI clones were carried out essentially as described previously (80). RI ES cells were electroporated with the NotI linearized targeting vector and were selected by using 200 μg of active Geneticin (G418; Gibco BRL)/ml and 2 μM ganciclovir (Syntex, Inc.). Drug-selected clones were picked, replica plated, and expanded on gelatin-coated tissue culture plates. DNA from individual clones was digested with BglI restriction enzyme and analyzed by Southern blot hybridization using a 1.6-kb EcoRI fragment (probe A) isolated from the 3′ end of the isolated genomic clone as a probe. Positive clones were expanded on gelatin-coated tissue culture plates and used to make chimeric mice using the darning needle aggregation method (60). Chimeric males were crossed to albino CD1 outbred females, and the genotypes of agouti pups were determined as described above using DNA prepared from tail biopsy. A chimeric male from clone NXKpR144 transmitted the mutation through the germ line. Subsequent routine genotyping of the fps-null mutation was performed using a SacI digest of genomic DNA with an internal SacI-XhoI fragment (probe B) as a probe. This probe B was also capable of cross-hybridizing to the human fps locus and allowed for the simultaneous detection of the human fps transgene in compound fps-targeted/fps transgenic mice.
Immune complex kinase assays and immunoblotting analysis.
Bone marrow was isolated from dissected femurs as previously described (84). Immune complex kinase assays were performed as previously described (80) using αFpsQE serum, which detects both Fps and Fer equally well, or αFerLA serum, which is cross-reactive toward Fps and Fer but is far more sensitive toward Fer (26). A human genomic PstI-PstI fragment containing part of the last exon of fur, as described in reference 70, was cloned into pGEM-1 and then subcloned as a BamHI-to-HindIII fragment into bacterial expression vector pATH-3. A TrpE-furin fusion protein containing the last 59 amino acids of furin was then produced and used to generate rabbit polyclonal antisera. Antisera from three rabbits (Larry, Curly, and Moe) recognized both the TrpE-furin fusion protein and a 100-kDa protein in lysates from mouse bone marrow. Antisera specific for p120Ras-Gap were prepared against a TrpE fusion protein as previously described (16). Proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were either dried and exposed to film or transferred to polyvinyl difluoride membrane (Immobilon) using a semidry apparatus (Bio-Rad) following the manufacturer's instructions. Membranes were probed with primary antisera at 1:1,000 dilutions in 5% BLOTTO and were visualized by enhanced chemiluminescence (NEN Life Science Products) using peroxidase-conjugated anti-rabbit secondary antibody (Boehringer Mannheim Biochemical; 1:10,000).
Peripheral blood cell counts.
Mice between the ages of 5 and 18 weeks were deeply anesthetized with chloroform, and blood was collected via cardiac puncture. Blood was mixed with EDTA as the anticoagulant at a final concentration of 1.5 ± 0.25 mg/ml. Blood was collected at the same time of day, to minimize circadian rhythm variation between animals (1). The blood was analyzed on a Cell-Dyn 3500 multiparameter hematology analyzer (Abbott Diagnostics, Santa Clara, Calif.) using the veterinary package.
Flow cytometry of bone marrow, spleen, and thymus.
Mice between the ages of 8 and 23 weeks were euthanatized by cervical dislocation, and their spleens and thymi were placed in 1× phosphate-buffered saline (PBS), pH 7.4, on ice. Both femurs were cleared of muscle tissue and were flushed with 5 ml of 1× PBS, pH 7.4, containing 1.5 mg of EDTA/ml on ice. The spleen and thymi were gently teased apart using dissection needles in 5 ml of 1× PBS-0.5% bovine serum albumin-0.1% sodium azide (pH 7.2) (PAB), and the cell suspension was transferred to a 15-ml tube. Large cell aggregates were allowed to settle, and the top 4 ml was transferred into a fresh tube. Cells were spun at 300 × g for 5 min and were resuspended at 20 × 106 cells/ml in PAB. Before staining with the individual combinations of cell surface markers, the Fc receptors were blocked with anti-CD16/32 (Pharmingen) for 5 min at 4°C. The cells were incubated for 15 min at room temperature with the following combinations of rat anti-mouse antibodies, conjugated with phycoerythrin (PE) or fluorescein isothiocyanate (FITC) in 10 μl of PAB: PE-Ly6-G (RB6-8C5) and FITC-CD11b (M1/70); PE-TER119 (TER-119) and FITC-CD44 (IM7); PE-CD45R/B220 (RA3-6B2) and FITC-immunoglobulin M (IgM) (LO-MM-9); PE-CD8 (53-6.72) and FITC-CD4 (GK1.5); FITC-CD11b and PE-Pan NK (DX5); FITC-IgM and PE-Pan NK; PE-CD45R/B220 and FITC-CD23 (B3B5); and PE-CD45R/B220 and FITC-IgE (23G2). All antibodies were from Pharmingen, except the IgM (Serotec), CD4/CD8 (Leinco Technologies, Inc.), and IgE (Southern Biotechnology Associates, Inc.) antibodies. Erythrocytes were lysed by the addition of 3 ml of ice-cold ACK buffer (4.13 g of NH4Cl, 0.5 g of KHCO3, and 15.3 mg of EDTA in 500 ml of double-distilled water), and cells were mixed at 4°C for 5 min. The cells were pelleted at 300 × g for 5 min, washed once in 3 ml of PAB, and pelleted again. Cells were suspended in 400 μl of PAB containing 2 μg of propidium iodide/ml to gate out dead cells.
LPS stimulation of mice.
Mice between the ages of 10 and 17 weeks were weighed the night before receiving an intraperitoneal bolus of the indicated amounts of LPS (serotype 055:B5; Sigma). LPS was diluted in a total volume of 200 μl of 0.9% saline. Control mice were injected with saline alone. Initial experiments were carried out to determine the optimal dose to be used in our outbred mice. Mice were monitored three times daily over the 6-day observation period, and mortality was charted.
All experimental procedures were approved by the Queen's University Animal Care Committee and conform to the guidelines established by the Canadian Council for Animal Care.
Isolation, culture, and stimulation of BMDM.
Femurs from the various genotypes were isolated as described above. Culturing of BMDM was essentially as described by Tushinski et al. (84) with minor modifications. The femurs were flushed with Iscove’s modified Dulbecco’s medium (IMDM; Gibco BRL) and 2% fetal bovine serum (FBS) (HYCLONE). The bone marrow cells were plated in 100-mm-diameter tissue culture plates (Sarstedt) at a concentration of 106 cells/ml and at a density of 2.9 × 105 cells/cm2 in complete macrophage medium consisting of IMDM plus 15% FBS, 15% G-CSF-conditioned medium, 5% IL-3-conditioned medium, 2 mM glutamine, 1% antibiotic-antimycotic, and 50 μM α-monothioglycerol. For the conditioned media we used NIH 3T3 cells that express murine G-CSF or IL-3 from retroviral vectors. After 24 h in culture, the nonadherent cells were centrifuged at 700 × g and were resuspended in half the original volume of complete macrophage media and replated on 35-mm-diameter tissue culture plates. Following a further 48 h, the nonadherent cells were harvested and spun at 700 × g. The cells were then plated at a concentration of 0.5 × 106 to 1.0 × 106 cells/ml and at a density of 1.9 × 104 cells/cm2 on 35-mm-diameter tissue culture plates in complete macrophage media. The media were changed every 2 or 3 days until the plates had reached 90% confluence.
The BMDM were starved in macrophage starvation medium (IMDM, 0.5% FBS, 1% antibiotic-antimycotic, and 50 μM α-monothioglycerol) for 48 h. Prior to stimulation the cells were rinsed with prewarmed PBS, and the medium was changed to prewarmed IMDM for 2 h. The IMDM was replaced with prewarmed IMDM that contained either 30 ng of recombinant murine GM-CSF, IL-3, or IL-6 (all from R&D Systems)/ml for 15 min. For the stimulation with LPS, cells were incubated with 1 μg of LPS serotype 055:B5/ml in prewarmed IMDM for the times indicated. Plates were immediately placed on ice after the incubation period, the medium was aspirated, and ice-cold PBS containing 100 μM sodium orthovanadate was placed on the cells. The PBS was aspirated, and the cells were directly lysed in 500 μl of boiling 1× SDS-sample buffer with a rubber policeman. The samples were heated to 100°C for 10 min, and the lysates were then passed through a pipette tip several times to shear the high-molecular-weight DNA and then stored at −20°C before being resolved on SDS-PAGE gels. The proteins were transferred as described above. The blots were probed following the manufacturer's suggestions with the following antibodies: mouse anti-human pStat3 (clone9E12; Upstate Biotechnology), rabbit anti-mouse Stat3 (New England Biolabs), mouse anti-human pStat5A and -B (clone 8-52; Upstate Biotechnology), rabbit anti-human Stat5A (Upstate Biotechnology), mouse anti-human pErk (clone E-4; Santa Cruz), rabbit anti-rat Erk (clone K-23; Santa Cruz), mouse monoclonal anti-pTyr (clone PY99; Santa Cruz), mouse anti-mouse pAkt (clone 4E2; New England Biolabs), rabbit anti-mouse Akt (New England Biolabs), rabbit anti-IκBα (New England Biolabs), rabbit anti-human pp38 (New England Biolabs), rabbit anti-mouse p38 (clone C-20; Santa Cruz), mouse anti-human pJnk (clone G-7; Santa Cruz), and rabbit anti-human Jnk (clone FL, Santa Cruz). The secondary antibody used was peroxidase-conjugated goat anti-rabbit (Boehringer Mannheim) or sheep anti-mouse (Amersham Life Sciences), depending on the primary antibody used. The blots were exposed to enhanced chemiluminescence reagent (NEN Life Science Products) and then to film.
RESULTS
Generation of targeted fps-null mice.
In order to explore the role of Fps in murine development, we designed a gene-targeting strategy to eliminate Fps expression. This targeting resulted in the replacement of exons 15 through 18 with the positive selection marker PGKneo (Fig. 1A). This deletion eliminated coding sequences for kinase subdomains IV to X, which was predicted to result in the production of an unstable truncated Fps protein. Out of the 165 G418+ TK− clones isolated by Southern blotting, seven were found to contain the correctly targeted fps allele (data not shown). One of these clones, NXKpR144, transmitted the mutation through the germ line, as shown by a shift in the 4.5-kb endogenous BglI restriction fragment to 6.7 kb by the insertion of the PGKneo gene (Fig. 1B). Since Fps has been implicated in signaling downstream from several cytokine and growth factor receptors, we expected Fps-null mice to have defects in development or hematopoiesis. In order to rescue any defects which might be observed, we crossed the heterozygous (fps+/−) animals with a transgenic mouse line generated by zygote microinjection of a 13-kb EcoRI fragment containing the entire human fps locus (Tg). We have previously shown that this fps transgenic mouse line recapitulated the tissue-specific and temporal expression pattern of the endogenous murine fps gene (22, 23). A second screening strategy was devised for routine genotyping of the resulting compound transgenic animals. Using a SacI digestion and an internal SacI-to-XhoI fragment as a probe (probe B), a 1.7-kb wild-type fragment and the 3.5-kb fragment for the targeted fps allele were detected. Because probe B cross-hybridized to the corresponding 0.85-kb human SacI fragment, this strategy also allowed for the simultaneous detection of the human fps transgene. A Southern blot hybridization analysis of mice generated from a fps+/− mouse crossed with a heterozygous mouse carrying the human fps transgene (fps+/−:Tg) is shown in Fig. 1C.
FIG. 1.
Organization of the fps locus and targeting strategy employed. (A) The top line indicates the spatial arrangement of the fps locus with the solid black boxes indicating coding regions and the gray boxes indicating noncoding regions. The letters X, Y, and Z indicate the last three exons of the furin gene. The middle line indicates the targeting vector with the positive selection marker PGKneo replacing fps exons 15 to 18. Probe A indicates the 3′ external probe to the targeting vector used to identify correctly targeted ES cell clones and mice using a BglI digest. Probe A also detects an additional 2-kb fragment. Probe B is used for routine genotyping of mouse DNA digested with SacI, and this probe also cross-reacts with the human fps transgene (Tg). Restriction enzyme sites for BglI (B), KpnI (K), NotI (N), EcoRI (R), SacI (S), and XhoI (X) are indicated. Restriction sites within brackets (N) and (R) define the ends of the cloned genomic DNA and were derived from the cloning vector. (B) Southern blot of BglI-digested DNA from the offspring of a heterozygous founder animal derived from the correctly targeted NXKpR144 ES cell line. The wild-type 4.5-kb band was shifted to 6.7 kb by the insertion of the PGKneo cassette. (C) Southern blot of SacI-digested DNA from a heterozygous intercross, where one of the animals also had the human fps transgene (Tg). A 1.7-kb wild-type fragment was shifted to 3.5 kb by the inserted PGKneo cassette. The appearance of the 0.85-kb band indicates animals that also carried the human fps transgene (Tg).
Fps is not required for mouse development.
In order to determine if Fps was required for development, we assessed the genotypes of offspring produced from breeding pairs of mice in various combinations of genotypes as listed in Table 1. We observed no significant deviation from the expected Mendelian ratio in fps+/− intercrosses (180 of 700 [25.7%] for fps+/+, 358 of 700 [51.1%] for fps+/−, and 162 of 700 [23.1%] for fps−/−; χ2 = 0.524). In breeding pairs where one parent was homozygous and the other was heterozygous for the fps mutation, there was no skewing in the predicted genotype of the offspring. The different combinations of intercrosses did not reveal any differences in the male-to-female ratio (data not shown) and are thus presented as combined numbers of animals in Table 1. While runting, skin lesions, or infections were occasionally seen in the offspring from this breeding colony, we did not observe an increase of runted or distressed animals that segregated with any particular genotype in fps+/− × fps+/− intercrosses. Furthermore, in the fps−/− × fps−/− intercrosses, we did not observe animals that appeared runted or distressed at a higher frequency. We actually observed slightly larger litter sizes in breeding pairs that had only one or no wild-type fps allele between the parents (Table 1). Finally, from measuring the weights of newborn mice from days 4 through 40, it was apparent that all possible genotypes produced in fps+/− × fps+/− intercrosses gained weight at the same rate. This breeding data clearly indicated that Fps was dispensable for murine embryonic, fetal, and postnatal development.
TABLE 1.
Breeding analysis of fps-targeted mice
| Breeding paira genotypes | No. (%) of indicated genotype
|
No. of breeding pairs | Total no. of litters born | No. of pups per litter (mean ± SEM) | χ2 value | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Wild type | Heterozygous | Homozygous | ||||
| Wild type | Wild type | 333 (100) | 5 | 35 | 9.51 ± 0.56 | |||
| Wild type | Homozygous | 131 (100) | 2 | 12 | 11.17 ± 0.74 | |||
| Homozygous | Wild type | 70 (100) | 2 | 6 | 11.67 ± 1.05 | |||
| Heterozygous | Heterozygous | 180 (25.71) | 358 (51.14) | 162 (23.14) | 10 | 76 | 10.08 ± 0.36 | 0.524 |
| Heterozygous | Homozygous | 65 (58.04) | 47 (41.96) | 3 | 11 | 12.27 ± 1.33b,c | 0.089 | |
| Homozygous | Heterozygous | 75 (46.30) | 87 (53.70) | 2 | 13 | 12.69 ± 0.80b,d | 0.346 | |
| Homozygous | Homozygous | 407 (100) | 6 | 39 | 11.41 ± 0.59b,d | |||
Breeding pairs are on a mixed 129SvJ and CD1 background.
These breeding pairs have statistically significantly different litter sizes compared to wild-type × wild-type or heterozygous × heterozygous crosses.
P < 0.045 using the Student t test.
P < 0.0063 using the Student t test.
Mice homozygous for the fps mutation have no detectable Fps protein or kinase activity, both of which can be rescued by a human fps transgene.
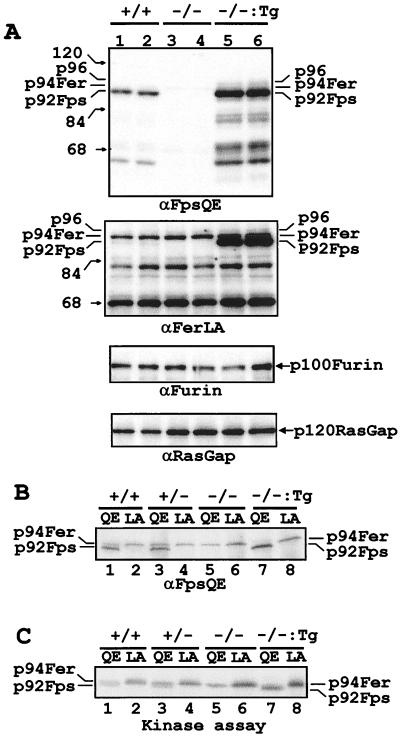
To verify that the targeted fps mutation did indeed abolish expression of the Fps kinase, we performed Western blotting on whole-cell lysates from bone marrow. The αFpsQE serum used in this analysis was raised against a peptide consisting of glutamine-381 through to glutamate-563 of Fps, which includes part of the second CC domain, the complete SH2 domain, and part of the kinase domain. The αFpsQE serum has been shown previously to recognize both Fps and Fer equally well (26). Since the predicted 70-kDa truncated Fps protein encoded by the targeted fps allele would retain all of the sequences against which the αFpsQE serum was raised, it was expected that this antiserum would detect any residual, stable, truncated Fps protein that might accumulate. Bands corresponding to p92Fps and a small amount of p94Fer were detected in lysates from fps+/+ mice (Fig. 2A, upper panel). In contrast, p92Fps was completely absent in lysates from the fps−/− mice, while p94Fer levels remained constant. In lysates from fps−/−:Tg cells, the overexpressed p92Fps protein was the most prominent band, and this signal largely masked the fainter p94Fer band. In these Fps-overexpressing cells, there was also a 96-kDa species which may be a hyperphosphorylated or otherwise modified form of Fps. Other lower-molecular-weight peptides detected by the αFpsQE serum included putative breakdown products of Fps and Fer, as well as cross-reactive, unrelated peptides. The identity of some of these as Fps breakdown products is substantiated by their increased amounts in lysates from the Fps-overexpressing fps−/−:Tg cells. The predicted size of a truncated Fps protein from the targeted fps allele would be 70 kDa. When a comparison of fps−/− and fps+/+ lysates was done, a unique band of this molecular weight was not detected in lysates from the fps−/− bone marrow cells. The band comigrating with the 68-kDa marker is present in equivalent amounts in all genotypes and most likely represents a breakdown product of p94Fer, because a prominent band of this molecular weight is also recognized with the αFerLA serum (Fig. 2A, second panel). This antiserum is cross-reactive for Fps and Fer, but it is much more sensitive toward Fer, because it was raised against a portion of the N-terminal domain which is less conserved between Fps and Fer (26). In more extensive Western blotting analysis on those tissues which are known to express substantial Fps levels (bone marrow, spleen, lung, and liver), we have failed to identify a truncated Fps protein in the targeted animals (data not shown).
FIG. 2.
(A) Immunoblotting analysis of Fps, Fer, furin, and RasGap proteins in whole-cell lysates from bone marrow. Lysates from the indicated genotypes were resolved on four identical SDS-PAGE gels, blotted onto membranes, and immunoblotted with the indicated polyclonal antisera. αFpsQE detected both p92Fps and p94Fer with equal sensitivity. αFerLA is relatively specific for p94Fer but is also partially cross-reactive to p92Fps. The αFurin sera are specific for p100Furin, and αRasGap is specific for p120Ras-Gap. The position of putative higher-molecular-weight forms of Fps and Fer is indicated as p96. The migration positions of 120-, 84-, and 68-kDa molecular weight standards are also indicated. (B) Analysis of Fps and Fer protein expression in bone marrow by immunoprecipitation followed by immunoblotting. (C) Analysis of Fps and Fer kinase activity in spleen lysates by immune complex kinase assay. For panels B and C, proteins were first immunoprecipitated using either αFpsQE or αFerLA antisera as indicated above each lane (QE or LA) and either were immunoblotted with αFpsQE (B) or subjected to immune complex kinase assay (C). Samples were from wild-type (+/+), homozygous mutant (−/−), heterozygous (+/−), or homozygous mutant:fps transgenic (−/−:Tg) animals, and the positions of full-length p92Fps and p94Fer proteins are indicated.
The 3′ end of the fur locus, which encodes the proprotein convertase furin, is located only 1,350 nucleotides upstream of the fps transcriptional start site (70, 71) (Zirngibl and Greer, unpublished). We were therefore concerned that targeting of the fps locus might effect expression of furin, which is important for the processing of a large number of growth factors, cytokines, and receptors (69). Antibodies were generated against p100Furin and were used to compare furin levels in fps−/− tissues with those in fps+/+ or fps−/−:Tg tissues. Furin levels in bone marrow (Fig. 2A, third panel) or platelets (data not shown) were unaffected by disruption of the fps locus.
We next looked for evidence of a truncated protein or any Fps kinase activity in fps−/− mice by using immunoprecipitation and Western blotting or immune complex kinase assays. Samples from the different genotypes were immunoprecipitated with either the αFpsQE serum or αFerLA, resolved on SDS-PAGE gels, and immunoblotted with the FpsQE antiserum. In bone marrow from fps+/+ and fps+/− mice, an αFpsQE immunoreactive p92Fps band was apparent, which migrated slightly faster than the p94Fer band (Fig. 2B, lanes 1 and 3). In contrast, only the p94Fer band was immunoprecipitated with αFerLA antiserum (Fig. 2B, lanes 2 and 4). As expected, the p92Fps band was absent in the fps−/− bone marrow (Fig. 2B, lane 5), and no additional lower-molecular-weight Fps-specific peptides were detected in either fps+/− or fps−/− bone marrow (data not shown). This suggested that, if made, the truncated Fps peptide was highly unstable and was therefore rapidly degraded. The loss of Fps expression in the fps−/− background was rescued by the human fps transgene (Fig. 2B, lanes 7 and 8), and in αFpsQE immunoprecipitates there appears to be substantially more p92Fps and p94Fer (Fig. 2B, lane 7). Similar results were obtained using spleen lysates (data not shown). To evaluate Fps kinase activity, we next performed immunoprecipitation kinase assays on bone marrow (data not shown) and spleen lysates (Fig. 2C). The band corresponding to Fer kinase alone seen in the αFerLA immunoprecipitates was constant between different genotypes (Fig. 2C, lanes 2, 4, 6, and 8). In contrast, using αFpsQE, in fps+/+ and fps+/− mice we observed kinase activities in bands corresponding to both Fps and Fer (Fig. 2C, lanes 1 and 3), but in fps−/− mice, only the upper band corresponding to Fer kinase was seen (Fig. 2C, lanes 5 and 6). As expected, the faster-migrating Fps kinase activity was restored in the presence of the human fps transgene (Fig. 2C, lane 7). These experiments demonstrated that deletion of sequences encoding part of the Fps kinase domain abolished Fps protein expression and kinase activity. Therefore, the targeted fps mutation effectively resulted in a fps-null allele.
Hematological analysis of peripheral blood.
High Fps expression levels in monocytes and granulocytes have suggested a biological function in cells of these lineages (26, 52, 76). Antisense blocking experiments have also implicated Fps in the regulation of differentiation and apoptosis in cultured myeloid cell lines (18, 19). If Fps played an important role in these processes, we expected to see a defect in the peripheral circulating levels of granulocytes and monocytes. Peripheral blood was collected from fps+/+, fps+/−, fps−/−, and fps−/−:Tg mice, and 14 different blood parameters were analyzed (Table 2). The overall numbers of white blood cells, neutrophils, lymphocytes, monocytes, and eosinophils were unaffected by the loss of Fps function. However, there was a 30% decrease in the percentage of basophils in the fps−/− mice. Interestingly, the opposite effect was seen in the erythroid lineage, where there was a 5% increase in the number of red blood cells, hemoglobin concentration, and hematocrit in the fps−/− mice. These statistically significant differences in basophils and erythrocytes were both corrected by the human fps transgene, which strongly supports the conclusion that the observed biological alterations were indeed due to loss of Fps expression. Interestingly, mean corpuscular hemoglobin was elevated in fps−/−:Tg mice relative to that in fps+/+ mice (P = 0.046) and fps−/− mice (P = 0.0009), indicating that simple tissue-specific overexpression of wild-type Fps may impact on the differentiation of erythroid cells. We also checked the spleen for any abnormalities because we had previously shown that mice expressing kinase-inactive Fps showed a tendency toward splenomegaly (80). In the Fps-null mice described here, there was a statistically significant decrease in spleen weight with fps+/+ > fps+/− > fps−/− > fps−/−:Tg. However, when the spleen weights were expressed as a percentage of body weight, no statistically significant differences were noted except for the fps−/−:Tg mice (P = 0.000086 versus fps+/+; P = 0.013 versus fps−/−).
TABLE 2.
Peripheral blood analysisa
| Hematological parameter | Value for mice with genotype:
|
|||
|---|---|---|---|---|
| fps+/+ | fps+/− | fps−/− | fps−/−:Tg | |
| White blood cells (103/μl) | 8.69 ± 3.42 (89) | 7.91 ± 2.39 (94) | 8.62 ± 2.67 (53) | 9.47 ± 2.98 (29) |
| Neutrophils (%) | 23 ± 14.6 (89) | 21 ± 14.5 (94) | 19.5 ± 13.6 (53) | 25.5 ± 14.6 (29) |
| Lymphocytes (%) | 76.2 ± 18.2 (89) | 70.4 ± 17 (94) | 72.6 ± 17.2 (53) | 65.6 ± 18.3 (29) |
| Monocytes (%) | 5.82 ± 5.55 (89) | 5.14 ± 4.75 (94) | 4.77 ± 4.99 (53) | 5.22 ± 4.73 (29) |
| Eosinophils (%) | 1.68 ± 1.57 (89) | 1.6 ± 1.69 (94) | 1.4 ± 1.39 (53) | 1.82 ± 1.95 (29) |
| Basophils (%) | 2.3 ± 2.47 (89) | 1.91 ± 2.18 (94) | 1.64 ± 1.36+ (53) | 1.87 ± 1.17 (29) |
| Erythrocytes (106/μl) | 9.20 ± 1.15 (89) | 9.20 ± 0.97 (94) | 9.72 ± 0.55* (53) | 8.87 ± 0.87 (29) |
| Hemoglobin (g/liter) | 147.2 ± 12.98 (89) | 147.6 ± 10.99 (94) | 154 ± 7.28# (53) | 145.9 ± 9.58 (29) |
| Hematocrit (liter/liter) | 0.460 ± 0.038 (89) | 0.459 ± 0.035 (94) | 0.480 ± 0.022# (53) | 0.456 ± 0.031 (29) |
| Mean corpuscular vol (fl) | 50.30 ± 3.51 (89) | 50.25 ± 2.91 (94) | 49.47 ± 2.04 (53) | 51.61 ± 2.89 (29) |
| Mean corpuscular hemoglobin (pg) | 16.1 ± 1.01 (89) | 16.16 ± 0.94 (94) | 15.85 ± 0.65 (53) | 16.52 ± 0.89 (29) |
| Mean corpuscular hemoglobin concn (g/liter) | 319.82 ± 6.49 (89) | 321.35 ± 5.30 (93) | 320.57 ± 5.44 (53) | 320.13 ± 4.05 (29) |
| Platelets (103/μl) | 966.6 ± 304.7 (89) | 1,043 ± 282.1 (94) | 926.1 ± 267 (53) | 1013 ± 320 (29) |
| Mean platelet vol (fl) | 6.83 ± 0.64 (89) | 6.91 ± 0.58 (94) | 6.74 ± 0.56 (53) | 7.14 ± 1.2 (29) |
| Body wt (g) | 31.63 ± 6.82 (75) | 28.17 ± 6.24 (71) | 29.56 ± 7.72 (38) | 30.29 ± 4.08 (27) |
| Spleen wt (mg) | 139.25 ± 65.91 (75) | 117.62 ± 34.88 (71) | 112.51 ± 32.74# (38) | 94.86 ± 38.24 (27) |
| % Spleen wt | 0.46 ± 0.22 (75) | 0.44 ± 0.16 (71) | 0.41 ± 0.16 (38) | 0.32 ± 0.12 (27) |
| Age of mice (days) | 65.12 ± 19.13 (89) | 63.43 ± 24.15 (94) | 67.66 ± 19.26 (53) | 81.55 ± 17.42 (29) |
Values represent the mean ± standard deviation (sample size) for each parameter. Statistically significant differences between wild-type and homozygous animals are indicated in boldface. P values were calculated using the Student t test: +, P < 0.04; *, P ≤ 0.0022; and #, P ≤ 0.0005.
Loss of Fps affects B lymphoid and myeloid compartments in bone marrow.
We next wanted to determine if the erythroid, lymphoid, or myeloid lineages in bone marrow were affected by the loss of Fps protein. The erythroid lineage was sorted with the TER119 and CD44 markers as shown in Fig. 3A. There was no significant difference in any of the erythroid populations, suggesting that erythropoiesis was normal in fps−/− mice. This is intriguing since erythrocytes in the peripheral blood of fps−/− are increased relative to those in wild-type animals. We then assessed the B-cell compartment for surface expression of B220 (CD45R) and IgM. A statistically significant increase in the B220+ IgM− pre- and pro-B-cell subpopulation was observed in fps−/− (17.8%) mice relative to that in fps+/+ (13.9%) mice (Fig. 3B, P = 0.0224). This increase was not observed in the numbers of mature B cells (B220+ IgM+). The effect on pre- and pro-B cells in fps−/− bone marrow was rescued in the compound fps−/−:Tg animals, suggesting that the defect was truly caused by the absence of Fps. The granulocyte/myeloid lineage was next examined using the Ly6-G (Gr-1) and CD11b (Mac-1) markers. There was a statistically significant decrease in the Ly6-G+ CD11b+ population in the fps−/− mice (63.0%) compared to that in the fps+/+ mice (68.2%) (Fig. 3C, P = 0.0322). Again this difference was restored to wild-type levels in the compound fps transgenic, fps−/−:Tg animals. Further experiments will be required to look at subsets of myeloid cells to determine if Fps is required for their production. Finally, there was no difference in the overall cellularity of the bone marrow between the genotypes (data not shown).
FIG. 3.
Flow cytometry analysis of bone marrow hematopoietic precursors. Analysis of bone marrow from wild-type (fps+/+, n = 22), Fps-null (fps−/−, n = 22), and Fps-null:fps transgenic (fps−/−:Tg, n = 22) animals. (A) Erythroid precursors were sorted with the Ter119 and CD44 markers. (B) B- cell precursors were sorted with the B220 and IgM markers. (C) Myeloid precursors were sorted with the Ly6-G and CD11b markers. The percentage of positive staining cells in each of the quadrants as well as the standard deviation is given below each scattergram. Twenty thousand live cells were counted by propidium iodide exclusion for each pair of cell markers used. There was no difference between the 11 males and 11 females used for each genotype, and percentages shown are combined for the analysis presented. ∗, P < 0.034 by Student's t test.
Flow cytometry analysis of spleen and thymus.
We next examined the secondary lymphoid organs to see if there were any defects in the maturation of T and B cells. T cells in both the thymus (Fig. 4A) and spleen (Fig. 4B) were normal in Fps-null mice. Interestingly, the spleen of fps−/−:Tg mice displayed a slight decrease in the CD4 and CD8 single-positive populations, with a concomitant increase in the double-negative population that was significantly different from that in both the fps+/+ and the fps−/− animals (Fig. 4B). When we examined the B cells in the spleen (Fig. 4C), we noted an increase in the B220+ IgM− population and a decrease in the B220+ IgM+ population in the fps−/− mice that was significantly different from what was found in the fps+/+ animals. The B220+ IgM+ levels were partially rescued in the fps−/−:Tg mice, but the increase in the B220+ IgM− population was not. Since the B220+ IgM− population could represent immature B cells or natural killer (NK) cells, we examined this population using the pan-NK cell marker DX5. However, there were no differences in the NK cell population in either the spleen or the thymus using this marker (data not shown). The reduction in B220+ IgM+ cells in the spleens of fps−/− mice suggested some difference in the kinetics of maturation or class switching. This was consistent with the idea that class switching in B cells might be compromised in the Fps -null mice and that the differences were not due to infiltration of NK cells into the spleen and thymus. To further assess the maturation status of B cells in the spleen, we looked at the expression of CD23. CD23 is a marker for resting mature B cells that are IgMlo IgDhi and thus represent cells that have undergone class switching (40, 86). This analysis showed a slightly increased number of mature resting B cells in the fps−/− mice that was restored to wild-type levels in the fps−/−:Tg mice. However, these changes using the CD23 marker did not achieve statistical significance. Although the slight differences observed in the expression of B-cell markers are not likely to result in a biologically significant phenotype, they are consistent with some minor, perhaps indirect, role for Fps in B-cell maturation.
FIG. 4.
Flow cytometry analysis of secondary lymphoid organs. Analysis of the T-cell populations in thymus (A) or spleen (B) from fps+/+, fps−/−, and fps−/−:Tg animals sorted using CD4 and CD8. (C) The B-cell compartment of the spleen is perturbed in the fps−/− animals as assayed by the B220 IgM markers. (D) Analysis of class switching in B cells from the spleen using B220 and CD23 markers. CD23-positive cells represent resting B cells that have undergone class switching. The percentage of positive staining cells in each of the quadrants, as well as the standard deviation, is given below each representative scattergram. Ten thousand live cells were counted by propidium iodide exclusion for each pair of cell markers used. P values were calculated using the Student t test. +, P < 0.03; ∗, P < 0.014; and #, P < 0.008, compared to values for wild-type mice.
Fps-null macrophages display normal Stat3 and Stat5 activation in response to GM-CSF and IL-3.
We had previously found that, in fps-targeted mice which express normal levels of kinase-dead Fps, there was a slight reduction in the activation of Stat3 and Stat5A upon GM-CSF stimulation (80). Surprisingly, Hackenmiller and colleagues reported that, in their Fps-null mice, they observed increased Stat3 and Stat5 activation when macrophages were stimulated with GM-CSF (25). In order to address this apparent paradox, we have now examined cytokine-induced Stat activation in BMDM from our Fps-null mice and compared it with wild-type or compound fps−/−:Tg mice. BMDM were starved and then stimulated for 15 min with vehicle alone or with the indicated cytokines. Successful cytokine stimulation was initially confirmed by immunoblotting with phosphospecific Erk antibody, which detected activated Erk after stimulation with IL-6, GM-CSF, and IL-3 (Fig. 5, last row). The apparent differences in the degree of Erk activation were attributable to differences in loading as determined by reprobing with a control Erk antibody (data not shown). Activation of Stat proteins was then examined by immunoblotting with activation-specific pStat3 antibody and control Stat3 antibody (Fig. 5). Stimulation with IL-6, GM-CSF, and IL-3 induced the activation of Stat3 with apparently equal effectiveness in wild-type, fps−/−, and fps−/−:Tg BMDM. Apparent differences in the amount of phospho-Stat3 were simply due to differences in loading, as determined by reprobing the same blots with the Stat3 control antibody (Fig. 5).
FIG. 5.
Fps-null macrophages display normal, cytokine-induced Stat activation. BMDM were starved or stimulated with IL-6, GM-CSF, or IL-3, and Stat3, Stat5, and Erk activities were determined as outlined in Materials and Methods. The top panel shows activation of Stat3 by immunoblotting with a phosphospecific (α-pStat3) antibody. Protein loading was determined by stripping and reblotting the same membrane with the control (α-Stat3) antibody. The middle panels show activation of p94Stat5A by GM-CSF and IL-3 only (α-pStat5A/B). The faster-migrating p92Stat5B displayed some basal activation that was not enhanced by cytokine stimulation. Equal loading of Stat5A was confirmed by stripping and reprobing with a control (α-Stat5A) antibody. The bottom panel shows cytokine-induced activation of Erk by immunoblotting for phosphorylated Erk proteins (α-pErk).
The same analysis was also performed on Stat5. In this case we used a phosphospecific antibody which detects the two distinct Stat5 proteins, p94Stat5A and p92Stat5B. In the starved BMDM, the faster-migrating Stat5B was already activated to a basal level, and this did not change upon IL-6, GM-CSF, or IL-3 treatment (Fig. 5). In contrast, when BMDM were stimulated with GM-CSF or IL-3, we observed a pronounced increase in Stat5A activation. However, there were no differences in the degree of activation between wild-type, fps−/−, or fps−/−:Tg BMDM. Control blots using an antibody that detected only Stat5A confirmed that a similar level of the Stat5A protein was present in each lane. These experiments were performed three different times and were quantitated by densitometry. Stat3 and Stat5A were consistently activated by these cytokines to the same degree whether Fps was present or absent or overexpressed as it was in the fps−/−:Tg BMDM.
Enhanced LPS sensitivity in Fps-null mice.
Since fps−/− mice displayed only slight differences in hematopoietic homeostasis, we next sought to determine if they would reveal a more dramatic phenotype when their immune system was challenged with LPS. We had previously shown that cultured BMDM from mice expressing only the kinase-inactive Fps displayed an attenuated Erk activation in response to LPS (80). We therefore examined the in vivo response of Fps-null mice to LPS challenge. Male fps−/− mice were significantly more susceptible than fps+/+ controls to intraperitoneal injections of LPS at 7 mg/kg of body weight, as measured by survival over 6 days (Fig. 6A). Female mice were more susceptible to this dose of LPS, and there was no difference in survival rates for the Fps-null and control animals (Fig. 6B). Others have shown that female mice are more susceptible to LPS than males, because estrogen augments, while progesterone has a protective effect, on LPS administration (33, 46, 78). At the reduced LPS dose of 5 mg/kg, the fps−/− and fps+/+ male mice showed no statistically significant difference in survival (Fig. 6C). However, overexpression of human Fps in the compound fps−/−:Tg male mice protected them against LPS-mediated toxicity. At this lower LPS dose, the fps−/− females also showed a clear increase in the susceptibility to LPS relative to wild-type mice (Fig. 6D), and this was partially rescued in the compound fps−/−:Tg female mice.
FIG. 6.
Survival of mice challenged with LPS. Mice were administered LPS at 7 mg/kg (A and B) or 5 mg/kg (C and D), and survival was monitored over 6 days. (A) fps−/− males (n = 36) are more sensitive to the effects of LPS at the higher dose than their fps+/+ cohorts (n = 37). (B) Females from fps−/− (n = 43) and fps+/+ (n = 36) genotypes display similar survival at the higher dose of LPS. (C) fps−/−:Tg male mice (n = 18) are protected against the effects of LPS compared to their fps−/− (n = 28) and fps+/+ (n = 27) controls. (D) fps−/− females (n = 27) show decreased survival at lower doses of LPS than do their fps+/+ (n = 22) controls, and the fps transgene partially rescued these fps−/−:Tg females (n = 15). The number of animals (n) used represents the combined total from three to five separate experiments. Calculated P values using Fisher's exact test were as follows: +, P < 0.032; and ++, P < 0.007, for fps−/− versus fps+/+; and ∗, P < 0.033; and ∗∗, P < 0.008, for fps+/+ and fps−/− compared to fps−/−:Tg.
Fps-null macrophages display normal signaling responses to LPS.
Stimulation of macrophages with LPS leads to the activation of tyrosine kinases, the mitogen-activated protein kinase cascades, Akt, and NF-κB (reviewed in references 3, 15, and 88). Since we found that Fps plays a role in survival of mice treated with LPS, we looked at several of the downstream signaling pathways known to be activated. Because we had confirmed that female mice were more susceptible to LPS, we performed these experiments with BMDM from both males and female mice. Cultures were stimulated with 1 μg of LPS/ml for various times, and immunoblotting analysis was performed on whole-cell lysates using a panel of activation-specific and control antibodies to key signaling proteins. There was a time-dependent increase in tyrosine phosphorylation of a number of proteins in the 90- to 150-kDa molecular weight range in the male BMDM over the 2-h time course that was not apparent in female BMDM (data not shown). Thus, LPS-induced activation of tyrosine kinases appeared to be delayed in BMDM from females compared to those from males. However, there was no consistent difference in the phosphotyrosine profile between fps−/− and fps+/+ male BMDMs, suggesting that none of the observed major tyrosine-phosphorylated proteins are distinct Fps substrates.
It has been shown that Akt is activated in macrophages stimulated with LPS (58, 75) and that in neutrophils activated Akt can delay the onset of apoptosis (42). We therefore examined the activation of Akt using an antibody that recognizes activated or Ser473-phosphorylated Akt protein. There appeared to be no difference in the activation state of Akt between the different genotypes or sexes (data not shown). Degradation of IκB is diagnostic for activation of the transcription factor NF-κB, which is involved in the transcription of a number of proinflammatory cytokines (39). The degradation of IκB was delayed in the male BMDM compared to the female BMDM, indicating that NF-κB is activated earlier in the female macrophages. However, there was no apparent difference in the kinetics of IκB degradation between wild-type and Fps-null cells (data not shown).
We next checked the mitogen-activated protein kinase pathways for the activation of p38, Jnk, and Erk, again using phosphospecific and control antibodies. There was no difference between male and female or wild-type and Fps-null cells in the kinetics of activation of p38 or Erk. While Jnk activation was similar between wild-type and Fps-null cells, there was reduced and delayed activation of both the p46 and p54 forms of Jnk in the female BMDM compared to the male BMDM (data not shown). As Jnk is capable of phosphorylating the transcription factor Jun, this could result in a delayed transcriptional response from AP-1 target genes in female cells. However, it should be stressed that these apparent differences in cultured BMDM may not necessarily reflect differences in the intact animal. While this initial survey of signaling pathways downstream from the LPS receptor system did not reveal differences between wild-type and Fps-null BMDM, the enhanced sensitivity in Fps-null mice and the rescue from this sensitivity using the human fps transgene strongly implicate Fps in regulating the innate immune response.
DISCUSSION
The Fps protein tyrosine kinase has been proposed to play an important role in regulating the differentiation and maturation of macrophages and granulocytes and in the survival of myeloid cells (18, 19, 93-95). Using gene targeting, we showed that Fps kinase activity was not required for in vivo myelopoiesis by targeting the fps locus with a kinase-inactivating mutation (80). These results cast some doubts on the proposed roles of Fps in myelopoiesis. However, Hackenmiller and coworkers recently reported that mice targeted with a null mutation in fps displayed defects in both lymphopoiesis and myelopoiesis (25). Those observations suggested that Fps may have kinase-independent functions which were not revealed in mice targeted with a kinase-inactivating mutation. We have now characterized and independently generated a Fps-null line of mice, and interestingly these mice show distinctly different phenotypes from those described by Hackenmiller and coworkers. In particular, we saw no evidence for embryonic lethality, runting, or skin lesions in our Fps-null mice. On the contrary, our Fps-null animals showed normal viability and fertility and appeared overtly healthy.
The targeting strategy that we employed deleted fps exons 15 to 18, which encode most of the Fps catalytic domain. Immune complex kinase assays showed that there was no residual Fps kinase activity in cells or tissues homozygous for the mutation. Theoretically, a truncated Fps peptide consisting of the N-terminal half, the SH2 domain, and part of the small lobe of the catalytic domain could have been produced from this targeted fps allele. Using a polyclonal antibody raised against sequences that would all remain in this theoretical peptide, we were unable to detect a truncated Fps protein in whole-cell lysates from bone marrow or other tissues. Furthermore, a truncated Fps peptide was not seen in immunoprecipitates from bone marrow or spleen lysates. It is highly unlikely that the hypothetical, truncated Fps peptide, if present in amounts below our level of detection, could be present in sufficient amounts to act as a dominant negative. It is likelier that the truncated Fps protein was not folded properly and was therefore rapidly degraded. Therefore, it appears that this mutation is essentially Fps null. Interestingly, there was no evidence for up-regulation of the highly homologous Fer kinase in Fps-null tissues. It is possible that Fer provides some redundant biochemical activity to Fps. However, the lack of an observable Fer up-regulation in Fps-null tissues argues against the existence of feedback regulatory mechanisms that would promote the ability of Fer to compensate for a loss of Fps.
In designing our targeting strategy, we chose to avoid deletions in the fps promoter region because of its close proximity to the gene encoding the proprotein convertase furin (70, 71). furin is transcribed in the same direction as fps, and its 3′ end is only 1,350 bp upstream of the fps transcription start site in the mouse genome (Zirngibl and Greer, unpublished). This is very similar to the organization of the human locus, where furin is a mere 1,108 bp upstream of fps (70, 71). We reasoned that deletions or insertion of a PGKneo selection cassette into the fps-furin intergenic region might affect furin expression. Furin is capable of processing a large variety of growth factors and growth factor receptors, so it is conceivable that a number of biological processes, including hematopoiesis, could be affected by even subtle alterations in its expression (61, 79). By confining our modification to the 3′ end of the fps locus, we have avoided any effects on expression of furin, and this was confirmed by immunoblotting analysis in bone marrow and platelet lysates.
Although Hackenmiller and coworkers did not give a detailed description of the generation of their fps-targeting vector, three potentially problematic features could be determined from the limited information provided (25). First, the direction of transcription of the PGKneo selection cassette appears to be into the furin locus rather than away from it. Second, the 2.4-kb deletion was large enough to place the neo poly(A) signal within or very close to the 3′ untranslated region of furin. Third, the short arm of homology must have contained part of the furin locus itself, potentially part of the coding region of the last exon. This targeting strategy could therefore have affected furin expression in two ways. First, there was the potential for generating furin antisense RNA from the PGKneo cassette, which could have interfered with transcription of furin or destabilized the furin mRNA. Second, mutations could have been introduced into furin that might have destabilized the mRNA or led to alternations in the furin polypeptide itself. Interestingly, some phenotypes described by Hackenmiller and coworkers that we did not observe in our Fps-null mice were similar to phenotypes seen in furin-null mice. Mice lacking Furin die at midgestation and fail to fuse their heart tube and undergo looping morphogenesis (69). Hackenmiller and coworkers reported a partial embryonic lethality in their Fps-null mice and described cardiovascular defects in affected embryos. They also observed runting, unhealthy appearance, and ulcerated skin lesions. It is noteworthy that perturbed expression of bone morphogenic proteins (BMPs), members of the transforming growth factor β superfamily, and known substrates of furin (11, 13), leads to skin lesions resembling psoriasis (7, 37, 81).
Although bone marrow hematopoiesis and peripheral blood parameters showed no substantial abnormalities in our Fps-null mice, there were several subtle defects. A statistically significant 5% reduction of Ly6-G+ CD11b+ cells was seen in the bone marrow of fps−/− mice, and this correlated with reduced numbers of circulating neutrophils, monocytes, eosinophils, and basophils in the fps−/− mice. While only the circulating basophils showed a statistically significant reduction in the fps−/− mice, all of the reductions in circulating myeloid cells were rescued by the human fps transgene in the fps−/−:Tg animals. This genetic rescue provided significantly greater confidence that the observed changes truly reflected a biological role for Fps in myelopoiesis. While these small changes were consistent with a role for Fps in myeloid differentiation and maturation, the modest effect clearly demonstrated that Fps was not essential. A slight but statistically significant increase in erythrocyte numbers was also seen in Fps-null mice, and the hemoglobin content and hematocrit were also elevated. Again, these erythroid phenotypes were rescued by the human fps transgene in the fps−/−:Tg animals, indicating that the loss of Fps was responsible for the observed increases. Given the observed trend of decreased myeloid and increased erythroid cells in the Fps-null mice, it is interesting to speculate that Fps promotes the differentiation of committed multipotential progenitors down the myeloid lineage at the expense of the erythroid lineage. Fps was shown to be activated by erythropoietin in the erythroleukemia cell line TF-1 (29). Given the increased numbers of circulating erythroid cells in Fps-null mice in the absence of any effects on erythroid progenitor numbers in the bone marrow, an intriguing possibility would be that Fps plays a role in down-regulating signaling from the erythropoietin receptor. Loss of that function could therefore result in increased proliferation or survival of erythroid cells.
We also observed statistically significant increases in the B220+ IgM− immature B-cell fraction in both the bone marrow and the spleen of Fps-null animals. In the bone marrow, this difference was rescued to normal levels by the fps transgene. There was also a statistically significant decrease in the B220+ IgM+ fraction in the spleens of Fps-null animals that was rescued by the fps transgene. At first, these observations suggested that there might be a defect in class switching. However, the numbers of B220+ CD23+ mature resting B cells were actually slightly elevated in Fps-null mice, and this was again rescued by the fps transgene. These observations argue against a defect in class switching, but they are consistent with Fps playing some subtle, perhaps indirect, role in regulating the B-cell maturation process. Interestingly, as was seen in the case of erythropoiesis, our observations would be consistent with an inhibitory role of Fps in the B-cell maturation process. On the other hand, T-cell development did not appear to be affected by the loss of Fps, because there were no differences in the subpopulations of T cells in either the spleen or the thymus of Fps-null animals.
The small perturbations in the hematopoietic system seen in our Fps-null mice are more substantial than those seen in mice targeted with a kinase-inactivating missense mutation in the fps gene (80). Those mice did not display statistically significant differences in the hematopoietic lineages. This could be explained if an inactive Fps protein were able to provide a scaffolding function that was independent of kinase activity. This has been demonstrated for the Src kinase, where a kinase-deficient Src rescued the osteopetrotic phenotype of src-null mice (77). While we have observed differences in the hematopoietic lineages in Fps-null mice that were not apparent in kinase-inactive Fps mice, the defects were quite subtle and therefore do not strongly support the concept of a scaffolding function for Fps.
In contrast to the minor changes in hematopoietic homeostasis in Fps-null mice described here, Hackenmiller and coworkers reported more dramatic, and in some cases opposite, changes in their independently generated Fps-null strain (25). They described rather large increases in the number of monocytes (twofold in spleen and bone marrow and sevenfold in peripheral blood) and granulocytes (20% in bone marrow and fourfold in spleen and peripheral blood). These differences in the myeloid component are quite distinct from what we described for our Fps-null strain, and generally they are opposite to what we observed. They also reported that their Fps-null mice had a reduction of B220+ IgM+ cells of 54% in the spleen and 63% in peripheral blood, while the B220+ IgM− cells of the bone marrow and peripheral blood were reduced 30 and 75%, respectively (25). While we did see a reduction in the B220+ IgM+ cells in the spleen, it was not of the same magnitude (13 versus 54%), and we observed the opposite effect on the B220+ IgM− population in the bone marrow (28% increase versus 30% decrease).
Fps has previously been implicated in signaling downstream from several cytokine receptors (2, 8, 28, 29, 34, 50, 54). Using mice targeted with the kinase-inactivating mutation, we previously showed only a slightly reduced GM-CSF-induced activation of Stat3 and Stat5 in BMDM (80). This suggested that Fps kinase was not essential for this process; however, given the potential for redundancy in the cytokine signaling pathways regulating myeloid differentiation, we could not rule out a redundant nonessential role for the Fps kinase in this process. Interestingly, Hackenmiller and coworkers showed that there was greater Stat3 and Stat5 activation in their Fps-null macrophages stimulated with GM-CSF or IL-6. They proposed an intriguing model whereby Fps would compete with Jak kinase for access to Stat proteins but whereby Fps would not phosphorylate Stat as efficiently as activated Jak. Thus, by removing Fps, more Stat protein could be phosphorylated by activated Jak (25). This model was consistent with data generated using our kinase-inactive Fps mice, where we showed a decrease of Stat3 and Stat5 phosphorylation upon GM-CSF stimulation (80). In this case, a kinase-inactive Fps might still compete with Jak for access to Stat but would not phosphorylate Stat at all. However, in the present study we did not see increased phosphorylation of Stat proteins in our Fps-null mice using GM-CSF, IL-3, or IL-6. This also argues against a model where Fps and Jak competed for Stat proteins and where Fps was simply less efficient at Stat phosphorylation than was Jak.
The different phenotypes in Fps-null mice described here relative to those reported previously could involve strain differences. The mice used in our study were a 129SvJ × CD1 hybrid rather than the 129SvJ × C57BL/6 hybrid used by Hackenmiller and coworkers. We have started to backcross our mice into the 129SvJ strain, and after six generations of backcrossing, we see no evidence for embryonic lethality, runting, or skin lesions in the fps−/− mice. If strain differences are responsible for the different phenotypes, we would speculate that this is something intrinsic to the C57BL/6 genotype. It will be important to backcross our Fps-null mutation onto C57BL/6 to investigate this possibility. However, we cannot exclude other possibilities, such as an effect on the expression of the closely linked furin gene. A compelling argument that the changes we saw were truly due to loss of Fps function and did not involve effects on furin or any other gene came from the genetic rescue that we included in our experimental design. Essentially all the reported phenotypes in our fps−/− mice were rescued when we crossed them into a transgenic strain expressing a human fps transgene.
The most dramatic phenotype that we observed in our Fps-null mice suggests a role for Fps in regulation of innate immunity. Fps-null mice were more sensitive to LPS challenge than wild-type cohorts, and this difference was rescued by the human fps transgene. These data provide the first documented genetic evidence for a role of the Fps kinase in regulation of the innate immune system. This observation could place Fps in a signaling pathway downstream from the recently discovered family of Toll-like receptors (TLRs) (3, 88). Tyrosine kinases have been implicated in signaling from TLRs (15), but the important ones have so far eluded discovery. The Src family of kinases were the first tyrosine kinases thought to play a role in signaling downstream from TLRs, as inhibition of Src kinases with the specific inhibitor PP1 abrogated responsiveness in cultured macrophage lines. Since Hck, Fgr, and Lyn are the three Src kinases most highly expressed in macrophages, it was somewhat surprising that macrophages isolated from a triple-null mouse for these kinases were still responsive to LPS challenge (56). This suggested that the inhibitor PP1 was not specific for Src family kinases or that other tyrosine kinases may be involved in Toll signaling.
We examined some of the signaling pathways known to be activated downstream of TLR 4 (TLR4), including Akt and IκB and the Erk, Jnk, and Erk pathways (3, 15, 58, 75). We did not observe differences between wild-type and fps−/− BMDM in the LPS-induced activation of Akt, p38, Jnk, and Erk or in the degradation of IκB. There were more pronounced differences between males and females in the whole-cell tyrosine phosphorylation profiles and in the kinetics of IκB degradation and Jnk activation. It is interesting to speculate that these differences between the sexes might be related to the observed differences in LPS sensitivity. While we have been unable to definitively detect an LPS signaling defect in cultured macrophages in our Fps-null mice, this could also be due to the short initial window of stimulation that we studied. We intend to do a more exhaustive analysis of LPS-induced signaling in cultured macrophages in an attempt to gain further evidence for a role for Fps in some aspect of signaling downstream from TLR4. However, the model that we presently favor is that Fps is not involved in the early part of TLR4 signaling but that it may play a role further downstream, perhaps in regulating the production or release of inflammatory cytokines, anti-inflammatory cytokines or in signaling mediated by them. It has recently been shown that Fps localizes to vesicles in the secretory pathway of cells and that this localization is kinase dependent (96). If a lack of Fps were to upset the balance in the secretion or expression of inflammatory versus anti-inflammatory cytokines, then we would expect to see an increased mortality due to LPS. Preliminary gene microarray analysis on liver samples isolated from LPS-treated mice has revealed provocative differences in the induction of a number of known Stat3 and Stat5 target genes.
In conclusion, we have demonstrated that fps−/− mice are healthy and fertile and have only minor defects in hematopoietic homeostasis. The slight differences seen were consistent with a role for Fps in promoting differentiation of hematopoietic stem cell progenitors along the myeloid lineages, perhaps even at the expense of the lymphoid and erythroid lineages. However, this effect was not significant enough to result in any substantial disruption in hematopoiesis. Of more significance, we also showed that Fps-null mice have a clear defect in their response to challenge with LPS. It is tempting to speculate that Fps may play an important role in regulating signaling pathways involved in the production of or signaling downstream of inflammatory or anti-inflammatory cytokines in macrophages and other cells. This provides the first documented evidence of a role for Fps in regulating the innate immune response.
Acknowledgments
We thank Karen Williams and Kari Newcombe for technical assistance, Derek Schulze for flow cytometry, and staff members of the Kingston General Hospital Hematology Laboratories for access to the hematology analyzer. We also thank Andrew Craig, Donna May, Waheed Sangrar, and Peter Truesdell for comments on the manuscript.
This work was supported by the Canadian Cancer Society with a grant from the National Cancer Institute of Canada, and R.A.Z. was the recipient of an Ontario Graduate Scholarship award.
REFERENCES
- 1.Aardal, N. P., and O. D. Laerum. 1983. Circadian variations in mouse bone marrow. Exp. Hematol. 11:792-801. [PubMed] [Google Scholar]
- 2.Adunyah, S. E., G. C. Spencer, R. S. Cooper, J. A. Rivero, and K. Ceesay. 1995. Interleukin-11 induces tyrosine phosphorylation, and c-jun and c-fos mRNA expression in human K562 and U937 cells. Ann. N. Y. Acad. Sci. 766:296-299. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Alcalay, M., F. Antolini, W. J. Van de Ven, L. Lanfrancone, F. Grignani, and P. G. Pelicci. 1990. Characterization of human and mouse c-fes cDNA clones and identification of the 5′ end of the gene. Oncogene 5:267-275. [PubMed] [Google Scholar]
- 5.Anderson, D. H., and P. M. Ismail. 1998. v-Fps causes transformation of inducing tyrosine phosphorylation and activation of the PDGFβ receptor. Oncogene 16:2321-2331. [DOI] [PubMed] [Google Scholar]
- 6.Aspenström, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. [DOI] [PubMed] [Google Scholar]
- 7.Blessing, M., P. Schirmacher, and S. Kaiser. 1996. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J. Cell Biol. 135:227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brizzi, M. F., M. G. Aronica, A. Rosso, G. P. Bagnara, Y. Yarden, and L. Pegoraro. 1996. Granulocyte-macrophage colony-stimulating factor stimulates JAK2 signaling pathway and rapidly activates p93fes, STAT1 p91, and STAT3 p92 in polymorphonuclear leukocytes. J. Biol. Chem. 271:3562-3567. [DOI] [PubMed] [Google Scholar]
- 9.Care, A., G. Mattia, E. Montesoro, I. Parolini, G. Russo, M. P. Colombo, and C. Peschle. 1994. c-fes expression in ontogenetic development and hematopoietic differentiation. Oncogene 9:739-747. [PubMed] [Google Scholar]
- 10.Cheng, H., J. A. Rogers, N. A. Dunham, and T. E. Smithgall. 1999. Regulation of c-Fes tyrosine kinase and biological activities by N-terminal coiled-coil oligomerization domains. Mol. Cell. Biol. 19:8335-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constam, D. B., and E. J. Robertson. 1999. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J. Cell Biol. 144:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig, A. W., R. Zirngibl, and P. Greer. 1999. Disruption of coiled-coil domains in Fer protein-tyrosine kinase abolishes trimerization but not kinase activation. J. Biol. Chem. 274:19934-19942. [DOI] [PubMed] [Google Scholar]
- 13.Cui, Y., F. Jean, G. Thomas, and J. L. Christian. 1998. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 17:4735-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeClue, J. E., I. Sadowski, G. S. Martin, and T. Pawson. 1987. A conserved domain regulates interactions of the v-fps protein-tyrosine kinase with the host cell. Proc. Natl. Acad. Sci. USA 84:9064-9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFranco, A. L., M. T. Crowley, A. Finn, J. Hambleton, and S. L. Weinstein. 1998. The role of tyrosine kinases and map kinases in LPS-induced signaling. Prog. Clin. Biol. Res. 397:119-136. [PubMed] [Google Scholar]
- 16.Ellis, C., M. Moran, F. McCormick, and T. Pawson. 1990. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature 343:377-381. [DOI] [PubMed] [Google Scholar]
- 17.Feldman, R. A., J. P. Tam, and H. Hanafusa. 1986. Antipeptide antiserum identifies a widely distributed cellular tyrosine kinase related to but distinct from the c-fps/fes-encoded protein. Mol. Cell. Biol. 6:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari, S., A. Donelli, R. Manfredini, M. Sarti, R. Roncaglia, E. Tagliafico, E. Rossi, G. Torelli, and U. Torelli. 1990. Differential effects of c-myb and c-fes antisense oligonucleotides on granulocytic differentiation of human myeloid leukemia HL60 cells. Cell Growth Differ. 1:543-548. [PubMed] [Google Scholar]
- 19.Ferrari, S., R. Manfredini, E. Tagliafico, A. Grande, D. Barbieri, R. Balestri, M. Pizzanelli, P. Zucchini, G. Citro, G. Zupi, C. Franceschi, and U. Torelli. 1994. Antipoptotic effect of c-fes protooncogene during granulocytic differentiation. Leukemia 8(Suppl. 1):S91-S94. [PubMed]
- 20.Fuchs, U., G. Rehkamp, O. A. Haas, R. Slany, M. Konig, S. Bojesen, R. M. Bohle, C. Damm-Welk, W. D. Ludwig, J. Harbott, and A. Borkhardt. 2001. The human formin-binding protein 17 (FBP17) interacts with sorting nexin, SNX2, and is an MLL-fusion partner in acute myelogeneous leukemia. Proc. Natl. Acad. Sci. USA 98:8756-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia, R., C. L. Yu, A. Hudnall, R. Catlett, K. L. Nelson, T. Smithgall, D. J. Fujita, S. P. Ethier, and R. Jove. 1997. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 8:1267-1276. [PubMed] [Google Scholar]
- 22.Greer, P., J. Haigh, G. Mbamalu, W. Khoo, A. Bernstein, and T. Pawson. 1994. The Fps/Fes protein-tyrosine kinase promotes angiogenesis in transgenic mice. Mol. Cell. Biol. 14:6755-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greer, P., V. Maltby, J. Rossant, A. Bernstein, and T. Pawson. 1990. Myeloid expression of the human c-fps/fes proto-oncogene in transgenic mice. Mol. Cell. Biol. 10:2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groffen, J., N. Heisterkamp, M. Shibuya, H. Hanafusa, and J. R. Stephenson. 1983. Transforming genes of avian (v-fps) and mammalian (v-fes) retroviruses correspond to a common cellular locus. Virology 125:480-486. [DOI] [PubMed] [Google Scholar]
- 25.Hackenmiller, R., J. Kim, R. A. Feldman, and M. C. Simon. 2000. Abnormal Stat activation, hematopoietic homeostasis, and innate immunity in c-fes−/− mice. Immunity 13:397-407. [DOI] [PubMed] [Google Scholar]
- 26.Haigh, J., J. McVeigh, and P. Greer. 1996. The fps/fes tyrosine kinase is expressed in myeloid, vascular endothelial, epithelial, and neuronal cells and is localized in the trans-golgi network. Cell Growth Differ. 7:931-944. [PubMed] [Google Scholar]
- 27.Hampe, A., I. Laprevotte, F. Galibert, L. A. Fedele, and C. J. Sherr. 1982. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell 30:775-785. [DOI] [PubMed] [Google Scholar]
- 28.Hanazono, Y., S. Chiba, K. Sasaki, H. Mano, A. Miyajima, K. Arai, Y. Yazaki, and H. Hirai. 1993. c-fps/fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony stimulating factor and interleukin-3. EMBO J. 12:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanazono, Y., S. Chiba, K. Sasaki, H. Mano, Y. Yazaki, and H. Hirai. 1993. Erythropoietin induces tyrosine phosphorylation and kinase activity of the c-fps/fes proto-oncogene product in human erythropoietin-responsive cells. Blood 81:3193-3196. [PubMed] [Google Scholar]
- 30.Hao, Q.-L., N. Heisterkamp, and J. Groffen. 1989. Isolation and sequence analysis of a novel human tyrosine kinase. Mol. Cell. Biol. 9:1587-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilton, J. M., M. Plomann, B. Ritter, J. Modregger, H. N. Freeman, J. R. Falck, U. M. Krishna, and A. B. Tobin. 2001. Phosphorylation of a synaptic vesicle-associated protein by an inositol hexakisphosphate-regulated protein kinase. J. Biol. Chem. 276:16341-16347. [DOI] [PubMed] [Google Scholar]
- 32.Hjermstad, S. J., S. D. Briggs, and T. E. Smithgall. 1993. Phosphorylation of the ras GTPase-activating protein (GAP) by the p93c-fes protein-tyrosine kinase in vitro and formation of GAP-fes complexes via an SH2 domain-dependent mechanism. Biochemistry 32:10519-10525. [DOI] [PubMed] [Google Scholar]
- 33.Ikejima, K., N. Enomoto, Y. Iimuro, A. Ikejima, D. Fang, J. Xu, D. T. Forman, D. A. Brenner, and R. G. Thurman. 1998. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am. J. Physiol. 274:G669-G676. [DOI] [PubMed]
- 34.Izuhara, K., R. A. Feldman, P. Greer, and N. Harada. 1996. Interleukin-4 induces association of the c-fes proto-oncogene product with phosphatidylinositol-3 kinase. Blood 88:3910-3918. [PubMed] [Google Scholar]
- 35.Jiang, H., K. Foltenyi, M. Kashiwada, L. Donahue, B. Vuong, B. Hehn, and P. Rothman. 2001. Fes mediates the IL-4 activation of insulin receptor substrate-2 and cellular proliferation. J. Immunol. 166:2627-2634. [DOI] [PubMed] [Google Scholar]
- 36.Jucker, M., K. McKenna, A. J. da Silva, C. E. Rudd, and R. A. Feldman. 1997. The Fes protein-tyrosine kinase phosphorylates a subset of macrophage proteins that are involved in cell adhesion and cell-cell signaling. J. Biol. Chem. 272:2104-2109. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser, S., P. Schirmacher, A. Philipp, M. Protschka, I. Moll, K. Nicol, and M. Blessing. 1998. Induction of bone morphogenetic protein-6 in skin wounds. Delayed reepitheliazation and scar formation in BMP-6 overexpressing transgenic mice. J. Investig. Dermatol. 111:1145-1152. [DOI] [PubMed] [Google Scholar]
- 38.Kanda, S., E. C. Lerner, S. Tsuda, T. Shono, H. Kanetake, and T. E. Smithgall. 2000. The nonreceptor protein-tyrosine kinase c-Fes is involved in fibroblast growth factor-2-induced chemotaxis of murine brain capillary endothelial cells. J. Biol. Chem. 275:10105-10111. [DOI] [PubMed] [Google Scholar]
- 39.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 40.Kehry, M. R., and S. A. Hudak. 1989. Characterization of B-cell populations bearing Fc epsilon receptor II. Cell. Immunol. 118:504-515. [DOI] [PubMed] [Google Scholar]
- 41.Keshet, E., A. Itin, K. Fischman, and U. Nir. 1990. The testis-specific transcript (ferT) of the tyrosine kinase FER is expressed during spermatogenesis in a stage-specific manner. Mol. Cell. Biol. 10:5021-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein, J. B., A. Buridi, P. Y. Coxon, M. J. Rane, T. Manning, R. Kettritz, and K. R. McLeish. 2001. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell. Signal. 13:335-343. [DOI] [PubMed] [Google Scholar]
- 43.Koch, C. A., D. Anderson, M. F. Moran, C. Ellis, and T. Pawson. 1991. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science 252:668-674. [DOI] [PubMed] [Google Scholar]
- 44.Koch, C. A., M. Moran, I. Sadowski, and T. Pawson. 1989. The common src homology region 2 domain of cytoplasmic signaling proteins is a positive effector of v-fps tyrosine kinase function. Mol. Cell. Biol. 9:4131-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurata, W. E., and A. F. Lau. 1994. p130gag-fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v-src. Oncogene 9:329-335. [PubMed] [Google Scholar]
- 46.Laubach, V. E., P. L. Foley, K. S. Shockey, C. G. Tribble, and I. L. Kron. 1998. Protective roles of nitric oxide and testosterone in endotoxemia: evidence from NOS-2-deficient mice. Am. J. Physiol. 275:H2211-H2218. [DOI] [PubMed]
- 47.Li, J., and T. E. Smithgall. 1996. Co-expression with BCR induces activation of the FES tyrosine kinase and phosphorylation of specific N-terminal BCR tyrosine residues. J. Biol. Chem. 271:32930-32936. [DOI] [PubMed] [Google Scholar]
- 48.Li, J., and T. E. Smithgall. 1998. Fibroblast transformation by Fps/Fes tyrosine kinases requires Ras, Rac, and Cdc42 and induces extracellular signal-regulated and c-Jun N-terminal kinase activation. J. Biol. Chem. 273:13828-13834. [DOI] [PubMed] [Google Scholar]
- 49.Linder, S., K. Hufner, U. Wintergerst, and M. Aepfelbacher. 2000. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 113:4165-4176. [DOI] [PubMed] [Google Scholar]
- 50.Linnekin, D., S. M. Mou, P. Greer, D. L. Longo, and D. K. Ferris. 1995. Phosphorylation of a Fes-related protein in response to granulocyte-macrophage colony stimulating factor. J. Biol. Chem. 270:4950-4954. [DOI] [PubMed] [Google Scholar]
- 51.Lionberger, J. M., and T. E. Smithgall. 2000. The c-Fes protein-tyrosine kinase suppresses cytokine-independent outgrowth of myeloid leukemia cells induced by Bcr-Abl. Cancer Res. 60:1097-1103. [PubMed] [Google Scholar]
- 52.MacDonald, I., J. Levy, and T. Pawson. 1985. Expression of the mammalian c-fes protein in hematopoietic cells and identification of a distinct fes-related protein. Mol. Cell. Biol. 5:2543-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maru, Y., K. L. Peters, D. E. H. Afar, M. Shibuya, O. N. Witte, and T. E. Smithgall. 1995. Tyrosine phosphorylation of BCR by FPS/FES protein-tyrosine kinases induces association of BCR with GRB-2/SOS. Mol. Cell. Biol. 15:835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuda, T., T. Fukada, M. Takahashi-Tezuka, Y. Okuyama, Y. Fujitani, Y. Hanazono, H. Hirai, and T. Hirano. 1995. Activation of Fes tyrosine kinase by gp130, an interleukin-6 family cytokine signal transducer, and their association. J. Biol. Chem. 270:11037-11039. [DOI] [PubMed] [Google Scholar]
- 55.McGlade, J., A. Cheng, G. Pelicci, P. G. Pelicci, and T. Pawson. 1992. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc. Natl. Acad. Sci. USA 89:8869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng, F., and C. A. Lowell. 1997. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 185:1661-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modregger, J., B. Ritter, B. Witter, M. Paulsson, and M. Plomann. 2000. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J. Cell Sci. 113:4511-4521. [DOI] [PubMed] [Google Scholar]
- 58.Monick, M. M., A. B. Carter, P. K. Robeff, D. M. Flaherty, M. W. Peterson, and G. W. Hunninghake. 2001. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J. Immunol. 166:4713-4720. [DOI] [PubMed] [Google Scholar]
- 59.Moran, M. F., P. Polakis, F. McCormick, T. Pawson, and C. Ellis. 1991. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol. Cell. Biol. 11:1804-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakayama, K. 1997. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 327:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogiso, Y., T. Yokoyama, H. Watari, T. Y. Shih, and N. Kuzumaki. 1993. Resistance of NIH3T3 cells to v-fes transformation induced by a dominant negative H-ras mutant. Exp. Cell Res. 208:415-421. [DOI] [PubMed] [Google Scholar]
- 63.Park, W. Y., J. H. Ahn, R. A. Feldman, and J. S. Seo. 1998. c-Fes tyrosine kinase binds to and activates STAT3 after granulocyte-macrophage colony-stimulating factor stimulation. Cancer Lett. 129:29-37. [DOI] [PubMed] [Google Scholar]
- 64.Park, W. Y., and J. S. Seo. 1995. Leucine zipper-like domain regulates the autophosphorylation and the transforming activity of P130gag-fps. Biochem. Biophys. Res. Commun. 211:447-453. [DOI] [PubMed] [Google Scholar]
- 65.Pawson, T., K. Letwin, T. Lee, Q.-L. Hao, N. Heisterkamp, and J. Groffen. 1989. The FER gene is evolutionarily conserved and encodes a widely expressed member of the FPS/FES protein-tyrosine kinase family. Mol. Cell. Biol. 9:5722-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters, K. L., and T. E. Smithgall. 1999. Tyrosine phosphorylation enhances the SH2 domain-binding activity of Bcr and inhibits Bcr interaction with 14-3-3 proteins. Cell. Signal. 11:507-514. [DOI] [PubMed] [Google Scholar]
- 67.Qualmann, B., and R. B. Kelly. 2000. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148:1047-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson, D. R., Y. M. Wu, and S. F. Lin. 2000. The protein tyrosine kinase family of the human genome. Oncogene 19:5548-5557. [DOI] [PubMed] [Google Scholar]
- 69.Roebroek, A. J., L. Umans, I. G. Pauli, E. J. Robertson, F. van Leuven, W. J. Van de Ven, and D. B. Constam. 1998. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development 125:4863-4876. [DOI] [PubMed] [Google Scholar]
- 70.Roebroek, A. J. M., J. A. Schalken, M. J. G. Bussemakers, H. van Heerikhuizen, C. Onnekink, F. M. J. Debruyne, H. P. J. Bloemers, and W. J. M. Van de Ven. 1986. Characterization of human c-fps/fes reveals a new transcription unit (fur) in the immediately upstream region of the proto-oncogene. Mol. Biol. Rep. 11:117-125. [DOI] [PubMed] [Google Scholar]
- 71.Roebroek, A. J. M., J. A. Schalken, J. A. M. Leunissen, C. Onnekink, H. P. J. Bloemers, and W. J. M. Van de Ven. 1986. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 5:2197-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers, J. A., H. Y. Cheng, and T. E. Smithgall. 2000. Src homology 2 domain substitution modulates the kinase and transforming activities of the Fes protein-tyrosine kinase. Cell Growth Differ. 11:581-592. [PubMed] [Google Scholar]
- 73.Rogers, J. A., R. D. Read, J. Li, K. L. Peters, and T. E. Smithgall. 1996. Autophosphorylation of the Fes tyrosine kinase. Evidence for an intermolecular mechanism involving two kinase domain tyrosine residues. J. Biol. Chem. 271:17519-17525. [DOI] [PubMed] [Google Scholar]
- 74.Sadowski, I., J. C. Stone, and T. Pawson. 1986. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol. Cell. Biol. 6:4396-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salh, B., R. Wagey, A. Marotta, J. S. Tao, and S. Pelech. 1998. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J. Immunol. 161:6947-6954. [PubMed] [Google Scholar]
- 76.Samarut, J., B. Mathey-Prevot, and H. Hanafusa. 1985. Preferential expression of the c-fps protein in chicken macrophages and granulocytic cells. Mol. Cell. Biol. 5:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwartzberg, P. L., L. Xing, O. Hoffmann, C. A. Lowell, L. Garrett, B. F. Boyce, and H. E. Varmus. 1997. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 11:2835-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwarz, E., C. Schafer, J. C. Bode, and C. Bode. 2000. Influence of the menstrual cycle on the LPS-induced cytokine response of monocytes. Cytokine 12:413-416. [DOI] [PubMed] [Google Scholar]
- 79.Seidah, N. G., and M. Chretien. 1999. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 848:45-62. [DOI] [PubMed] [Google Scholar]
- 80.Senis, Y., R. Zirngibl, J. McVeigh, A. Haman, T. Hoang, and P. A. Greer. 1999. Targeted disruption of the murine fps/fes proto-oncogene reveals that Fps/Fes kinase activity is dispensable for hematopoiesis. Mol. Cell. Biol. 19:7436-7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stelnicki, E. J., M. T. Longaker, D. Holmes, K. Vanderwall, M. R. Harrison, C. Largman, and W. Y. Hoffman. 1998. Bone morphogenetic protein-2 induces scar formation and skin maturation in the second trimester fetus. Plast. Reconstr. Surg. 101:12-19. [DOI] [PubMed] [Google Scholar]
- 82.Tian, L., D. L. Nelson, and D. M. Stewart. 2000. Cdc42-interacting protein 4 mediates binding of the Wiskott-Aldrich syndrome protein to microtubules. J. Biol. Chem. 275:7854-7861. [DOI] [PubMed] [Google Scholar]
- 83.Tribioli, C., S. Droetto, S. Bione, G. Cesareni, M. R. Torrisi, L. V. Lotti, L. Lanfrancone, D. Toniolo, and P. Pelicci. 1996. An X chromosome-linked gene encoding a protein with characteristics of a rhoGAP predominantly expressed in hematopoietic cells. Proc. Natl. Acad. Sci. USA 93:695-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tushinski, R. J., I. T. Oliver, L. J. Guilbert, P. W. Tynan, J. R. Warner, and E. R. Stanley. 1982. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28:71-81. [DOI] [PubMed] [Google Scholar]
- 85.Tybulewicz, V. L. J., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 86.Waldschmidt, T., K. Snapp, T. Foy, L. Tygrett, and C. Carpenter. 1992. B-cell subsets defined by the Fc epsilon R. Ann. N. Y. Acad. Sci. 651:84-98. [DOI] [PubMed] [Google Scholar]
- 87.Wang, S.-J., P. Greer, and R. Auerbach. 1996. Isolation and propagation of yolk sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell. Dev. Biol. 32:292-299. [DOI] [PubMed] [Google Scholar]
- 88.Wasserman, S. A. 2000. Toll signaling: the enigma variations. Curr. Opin. Genet. Dev. 10:497-502. [DOI] [PubMed] [Google Scholar]
- 89.Wilks, A. F., and R. R. Kurban. 1988. Isolation and structural analysis of murine c-fes cDNA clones. Oncogene 3:289-294. [PubMed] [Google Scholar]
- 90.Yee, S.-P., D. Mock, P. Greer, V. Maltby, J. Rossant, A. Bernstein, and T. Pawson. 1989. Lymphoid and mesenchymal tumors in transgenic mice expressing the v-fps protein-tyrosine kinase. Mol. Cell. Biol. 9:5491-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yee, S. P., D. Mock, V. Maltby, M. Silver, J. Rossant, A. Bernstein, and T. Pawson. 1989. Cardiac and neurological abnormalities in v-fps transgenic mice. Proc. Natl. Acad. Sci. USA 86:5873-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeung, Y. G., S. Soldera, and E. R. Stanley. 1998. A novel macrophage actin-associated protein (MAYP) is tyrosine-phosphorylated following colony stimulating factor-1 stimulation. J. Biol. Chem. 273:30638-30642. [DOI] [PubMed] [Google Scholar]
- 93.Yu, G., and R. I. Glazer. 1987. Purification and characterization of p93fes- and p60src-related tyrosine protein kinase activities in differentiated HL-60 leukemia. J. Biol. Chem. 262:17543-17548. [PubMed] [Google Scholar]
- 94.Yu, G., S. Grant, and R. I. Glazer. 1988. Association of p93c-fes tyrosine protein kinase with granulocytic/monocytic differentiation and resistance to differentiating agents in HL-60 leukemia cells. Mol. Pharmacol. 33:384-388. [PubMed] [Google Scholar]
- 95.Yu, G., T. E. Smithgall, and R. I. Glazer. 1989. K562 leukemia cells transfected with the human c-fes gene acquire the ability to undergo myeloid differentiation. J. Biol. Chem. 264:10276-10281. [PubMed] [Google Scholar]
- 96.Zirngibl, R., D. Schulze, S. E. L. Mirski, S. P. C. Cole, and P. A. Greer. 2001. Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp. Cell Res. 266:87-94. [DOI] [PubMed] [Google Scholar]