Abstract
Hypoxia (low-oxygen tension) is an important physiological stress that influences responses to a wide range of pathologies, including stroke, infarction, and tumorigenesis. Prolonged or chronic hypoxia stimulates expression of the stress-inducible transcription factor gene c-jun and transient activation of protein kinase and phosphatase activities that regulate c-Jun/AP-1 activity. Here we describe evidence obtained by using wild-type and HIF-1α nullizygous mouse embryonic fibroblasts (mEFs) that the induction of c-jun mRNA expression and c-Jun phosphorylation by prolonged hypoxia are completely dependent on the presence of the oxygen-regulated transcription factor hypoxia-inducible factor 1α (HIF-1α). In contrast, transient hypoxia induced c-jun expression in both types of mEFs, showing that the early or rapid induction of this gene is independent of HIF-1α. These findings indicate that the c-jun gene has a biphasic response to hypoxia consisting of inductions that depend on the degree or duration of exposure. To more completely define the relationship between prolonged hypoxia and c-Jun phosphorylation, we used mEFs from mice containing inactivating mutations of critical phosphorylation sites in the c-Jun N-terminal region (serines 63 and 73 or threonines 91 and 93). Exposure of these mEFs to prolonged hypoxia demonstrated an absolute requirement for N-terminal sites for HIF-1α-dependent phosphorylation of c-Jun. Taken together, these findings suggest that c-Jun/AP-1 and HIF-1 cooperate to regulate gene expression in pathophysiological microenvironments.
The proto-oncogene c-jun encodes a major component of AP-1 transcription factors, which are important regulators of immediate-early signals directing cellular proliferation, survival, differentiation, and environmental stress responses (reviewed in references 31, 39, and 56). AP-1 transcription factors are dimers of basic-region leucine zipper (bZIP) proteins and consist of members of the Jun, Fos, ATF, and Maf families as well as the Nrl protein (20, 31). Regulation of AP-1 activity is complex but depends critically on mechanisms controlling the abundance and biochemical modifications of its subunits (14, 31). At a higher level of organization, AP-1 activity also depends on interactions with other transcription factors and transcriptional coregulators associated with target genes (reviewed in references 23, 65, and 72). Presumably, multiple levels of AP-1 regulation are necessary to ensure that its activation by diverse signals generates specific cellular responses. Biochemical modifications of c-Jun include phosphorylation, reduction, ubiquitination, and sumoylation (48, 49, 56). Of these modifications, the phosphorylation state of c-Jun is a primary determinant of the activity of c-Jun/AP-1.
We have been investigating the response of c-Jun/AP-1 to hypoxia, particularly pathophysiological or tumor-like hypoxia (5, 35, 36). Activation of c-Jun/AP-1, defined mainly in terms of DNA binding and reporter gene assays, has been described for both normal and transformed cells exposed to various low-oxygen conditions (5, 8, 46, 59, 69, 74, 76). However, while these studies have demonstrated that c-Jun/AP-1 is poised to respond to hypoxia, they have not established the pathways responsible for its activation by hypoxic signals. Among the protein kinases that target c-Jun/AP-1 in vivo, the mitogen-activated protein kinase (MAPK) family members stress-activated protein kinases (SAPKs)/c-Jun N-terminal kinases (JNKs) and extracellular signal-regulated kinases 1 and 2 (ERK1/2) are activated by hypoxia (36, 47). Certain p38 MAPKs (p38 MAPKα and -γ) are also hypoxia inducible (18), but these enzymes have not been found to phosphorylate c-Jun. Nevertheless, because p38 MAPKs can phosphorylate ATF and MEF2 transcription factors (52, 57), in principle they could activate AP-1/ATF and/or MEF2 complexes in the c-jun-proximal promoter region and thus induce c-jun expression in hypoxic cells. Recently the ERK1/2 pathway has also been reported to activate the hypoxia-responsive transcription factors hypoxia-inducible factor 1α and 2α (HIF-1α and -2α) (17, 58). HIF-1α is the hypoxia-responsive subunit of HIF-1, a ubiquitous regulator of hypoxia-responsive gene expression (reviewed in references 44, 63, and 70). Under physiologically relevant low-oxygen conditions (e.g., partial O2 pressure [pO2] ≤ 2% of atmospheric O2 [29]), HIF-1α protein is stabilized, leading to modulation of specific gene expression through binding of HIF-1 to hypoxic response element (HRE) sites in chromatin (63, 70). Stabilization of HIF-1α protein is dependent on escape from targeted proteolysis mediated by the von Hippel-Lindau tumor suppressor protein (pVHL) in normoxic cells (27, 28).
The findings that hypoxia-inducible MAPK pathways have both c-jun or c-Jun/AP-1 and HIF-1 as targets suggested that there could be a physiological relationship between these two stress-responsive transcription factors. Thus, c-Jun/AP-1 and HIF-1 could be part of a transcriptional network underlying the adaptation of cells to hypoxia or anoxia. To investigate the potential relationship between c-Jun/AP-1 and HIF-1 in hypoxic or anoxic cells, we used the Cre/loxP system to generate mouse embryonic fibroblasts (mEFs) conditionally nullizygous for HIF-1α and then compared c-jun expression in aerobic and hypoxic cultures of wild-type and HIF-1α null mEFs produced by Cre recombinase expression. Here we present findings demonstrating that the induction of c-jun mRNA accumulation and c-Jun phosphorylation (e.g., N-terminal phosphorylation) by hypoxia has HIF-1α-independent and -dependent components. We demonstrated the involvement of c-Jun N-terminal phosphorylation using mEFs from mice that we had generated lacking either the SAPK/JNK phosphorylation sites at serines 63 and 73 or other sites at threonines 91 and 93. In general, we found that there is an early or rapid response of the c-jun gene to hypoxia that is independent of HIF-1α but dependent on serum and a late or delayed response that is dependent on HIF-1α but not on serum. We propose that the HIF-1α-dependent component constitutes a secondary cascade of transcriptional stress responses to hypoxia or anoxia.
MATERIALS AND METHODS
Construction of a conditional HIF-1α gene targeting vector and generation of immortalized HIF-1α null mEFs.
HIF-1α null mEFs were generated by conditional targeting of the HIF-1α gene using the Cre/loxP system (25). Exon 2 of the HIF-1α gene was chosen for targeting because it encodes the helix-loop-helix domain, which is essential for the dimerization of HIF-1-α with HIF-1β/ARNT and thus for the formation of HIF-1 (68). Genomic DNA for the HIF-1α targeting vector was obtained from a 129/SvJ BAC clone from Genome Systems, Inc., as described elsewhere (60). The targeting vector was constructed by engineering a loxP site in the first intron, just 5′ of exon 2, and a loxP-flanked neo resistance gene in the second intron (25, 61). The targeting vector was linearized and electroporated into R1 embryonic stem (ES) cells (50). After selection with G418 (150 μg/ml), targeted clones were identified by Southern blotting and hybridization. In order to excise the neo gene, an expression vector encoding Cre recombinase was transiently expressed in the targeted ES cells by electroporation (61). ES cells from which the neo gene had been excised were identified by Southern blotting, and those containing 5′ and 3′ loxP sites surrounding exon 2 of the HIF-1α locus were identified by PCR analysis.
Chimeric mice were generated by injection of these cells into C57Bl/6 blastocysts (53). Mice containing loxP-flanked HIF-1α alleles were crossed with mice heterozygous for HIF-1α (+/−) (60), and mEFs were later harvested from E13.5 embryos that were heterozygous (+f/−;f, floxed) for the conditionally targeted allele. These mEFs were immortalized by stable transfection with TAg expressed from the vector pOT (26), and HIF-1α null cells (−f/−) were subsequently generated by transient expression of Cre recombinase from an adenovirus expression vector to excise the loxP-flanked exon 2. The T-antigen immortalized +f/− and −f/− mEFs are designated below as wild-type and HIF-1α null cells. In addition, mEFs homozygous for the conditionally targeted HIF-1α gene were generated by crossing mice containing loxP-flanked alleles of HIF-1α and then immortalizing the resultant (+f/+f) mEFs either with TAg or using the classic “3T3 protocol” (66).
Generation of fibroblast cell lines containing a c-jun allele with serines 63 and 73 or threonines 91 and 93 mutated to alanine.
The generation of these mice will be described in detail elsewhere (C. Gustafson-Brown et al., unpublished data). Briefly, knock-in mutations were created in which the c-Jun phosphorylation sites at Ser63 and Ser73 or Thr91 and Thr93 were converted to alanine by PCR-based mutagenesis, and the resulting mice were crossed to obtain litters homozygous for each mutation (c-jun S63A S73A or c-jun T91A T93A). Knock-in mEFs isolated from these litters at E13.5 were immortalized with TAg expressed from pOT to create cell lines for the studies described here.
Cell culture and hypoxia or anoxia protocols.
Wild-type and HIF-1α null cells were cultured for no more than 20 passages before being replaced from frozen early passage cultures. Cells were plated in 60- or 100-mm-diameter plastic culture dishes (500 cells/mm2) in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (Sigma, St. Louis, Mo.) and 25 mM HEPES buffer (pH 7.4). In vitro hypoxia experiments were performed according to a standard protocol described in detail elsewhere (36). Briefly, cells were incubated in a 5% CO2 air atmosphere at 37°C overnight and then placed in aluminum gas-exchange chambers maintained at 37°C. The chambers containing the cells were placed in a 37°C circulating water bath, and the original atmosphere was repeatedly exchanged with 5% CO2--95% N2 using a manifold equipped with a vacuum pump and a gas cylinder. Atmospheric oxygen levels in this apparatus were calibrated by using a polarographic oxygen electrode (Oxygen Sensors, Inc., Norristown, Pa.) in an attached test chamber. Using this system, defined atmospheric pO2 values are achieved within the range of approximately 1 to ≤0.01% (relative to air at pO2 ≈ 21%). For example, atmospheric pO2 values are 1 and 0.01% at 30 min and 2 h, respectively, after the initiation of a hypoxic exposure in this apparatus. Severely hypoxic or anoxic conditions simulate those detectable in regions of solid tumors and in solid tumor models (e.g., see references 21 and 67). Following various hypoxic exposures, the chambers were opened under anoxic conditions in a glove box (Bactron X; Sheldon Manufacturing Inc., Cornelius, Ore.) maintained at 5% CO2-95% N2 to prepare cell lysates without significant reoxygenation.
To prepare serum-starved cultures, cells were plated in 60-mm-diameter plastic culture dishes (106 cells/dish) in DMEM containing 10% FBS (Sigma) and 25 mM HEPES buffer (pH 7.4) and incubated in a 5% CO2 air atmosphere at 37°C overnight. The cells were rinsed with serum-free DMEM and then incubated in 4 ml of this medium overnight. Cells were exposed to hypoxia (pO2 ≤ 1%) as described above and then harvested for total RNA for reverse transcription (RT)-PCR analysis.
Immunoblotting analysis.
The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.): a monoclonal anti-phospho-c-Jun antibody (KM-1; immunizing antigen amino acids 56 to 69 of human c-Jun; KM-1 recognizes c-Jun phosphorylated on Ser63) and a rabbit polyclonal anti-c-Jun antibody (H79; immunizing antigen amino acids 1 to 79 of human c-Jun). In addition, the following anti-phospho-c-Jun antibodies were purchased from New England BioLabs, Inc. (Beverly, Mass.): a rabbit polyclonal anti-phospho-Ser63 antibody (9261S; immunizing antigen a phospho-Ser63 peptide corresponding to human c-Jun) and a rabbit polyclonal anti-phospho-Ser73 antibody (9164S; immunizing antigen a phospho-Ser73 peptide corresponding to human c-Jun). To detect HIF-1α protein, a monoclonal anti-HIF-1α antibody was purchased from Novus Biologicals, Inc. (Littleton, Colo.; immunizing antigen amino acids 432 to 528 of human HIF-1α).
Following a hypoxic exposure, cells were immediately placed on ice in air or on Super Ice cold packs in the anoxic glove box, and the medium was removed. Cells were washed with 2 ml of ice-cold phosphate-buffered saline (PBS) before addition of 200 μl of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 250 mM NaCl, 0.5% NP-40, 50 mM NaF, 15 mM sodium pyrophosphate, 1 mM glycerophosphate, 1 mM Na3VO4, 500 nM okadaic acid or 1 μM microcystin, 20 μg of aprotinin/ml, 5 μg of leupeptin/ml, 0.7 μg of pepstatin/ml, 1 mM phenylmethylsulfonyl fluoride). These solutions were degassed before total protein was harvested from hypoxic cells. After centrifugation at 12,000 × g for 20 min at 4°C, portions of the supernatants were diluted with equal volumes of 2× sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min. The protein concentrations of the supernatants were determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.). Equal total protein samples (typically 10 to 15 μg) were resolved in SDS-polyacrylamide gels (8 to 11% polyacrylamide) and electroblotted in a buffer containing 25 mM Tris-HCl (pH 8.3), 192 mM glycine, 0.1% SDS, and 15% methanol onto Immobilon P membranes (Millipore, Marlborough, Mass.) by using a TE 22 Mighty Small Transphor Tank Transfer Unit (Hoefer Pharmacia Biotech Inc., San Francisco, Calif.). To blot a single protein sample for simultaneous analysis by different antibodies in a slot blotting apparatus, the PR 150 Mini Dec-Probe system (Hoefer Pharmacia Biotech, Inc.) was used according to the manufacturer's protocol. Blots were blocked in 5% nonfat dried milk in PBS containing 0.1% Tween 20 at 4°C overnight. For protein detection, blots were washed once in PBS-0.1% Tween 20 and then incubated with rocking at room temperature for 2 h with a primary antibody typically diluted 1:1,000 in PBS-0.1% Tween 20 containing 5% nonfat dried milk. A secondary anti-mouse or anti-rabbit immunoglobulin G antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) diluted 1:10,000 or 1:5,000, respectively, in PBS-0.1% Tween 20 was added, and blots were gently rocked at room temperature for 1 h. After three washes in PBS-0.1% Tween 20, primary antibody binding was detected and visualized by using the ECL Plus Western blotting Detection System (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.), according to the supplier's instructions.
RNA analysis.
Purification of total cellular RNA for Northern analysis was performed by the RNeasy method (Qiagen Inc., Santa Clarita, Calif.) according to the supplier's instructions. RNA was resolved in 1% denaturing agarose gels and blotted onto nylon membranes (37). A 1.8-kb c-jun cDNA probe was prepared by digestion of the plasmid pcD10 with EcoRI and PstI (5), and the probe was labeled with [α-32P]dCTP (Amersham Pharmacia Biotech, Arlington Heights, Ill.) by the random primer method. To provide a normalization standard for RNA loading and transfer, blots were stripped and probed with a DNA oligomer corresponding to a human 28S rRNA sequence (BD Biosciences Clontech, Palo Alto, Calif.) end labeled with [γ-32P]ATP (Amersham).
Total RNA was harvested for quantitative RT-PCR analysis of c-jun expression by using a LightCycler System instrument (Roche Diagnostics) according to the manufacturer's protocols to detect signals generated by the SYBER Green method (sense primer, 5′-CATGGAGTCTCAGGAGCGGATCA-3′; antisense primer, 5-′TGAGTACGATTGCGTCGTCAACG-3′).
RESULTS
Early induction of c-jun mRNA expression by hypoxia is independent of the presence of HIF-1α.
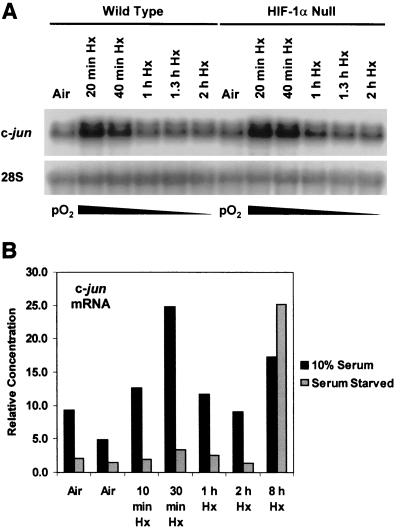
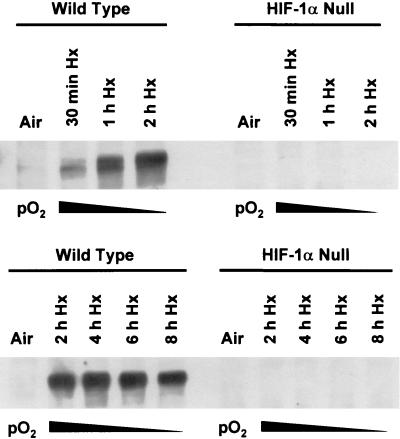
The c-jun gene is a classical member of the immediate-early group, genes that are characterized by rapid and transient induction by diverse biological and physical stimuli in the absence of new protein synthesis (e.g., see references 22, 38, and 62). Because hypoxia, like many other physical stimuli, induces c-jun mRNA expression (5), we first determined whether the deregulation of hypoxia-responsive gene expression in HIF-1α null cells could affect this induction shortly after the onset of hypoxic stress. Figure 1A shows that c-jun mRNA accumulated rapidly and transiently in both wild-type and HIF-1α null cells exposed to hypoxia, similar to the immediate-early response of c-jun mRNA which occurs on serum stimulation (62). This finding demonstrates that the early induction of c-jun mRNA expression by hypoxia is independent of the presence of HIF-1α and thus of HIF-1. In the absence of serum, this induction of c-jun mRNA expression was not detectable (Fig. 1B), indicating that the early response of hypoxia-inducible c-jun expression is mediated by serum-responsive signaling pathways. Figure 2 shows that this early response occurred under low-oxygen conditions (pO2 ≤ 1%) that simultaneously caused the accumulation of the HIF-1α protein. These findings indicate that physiological hypoxia simultaneously transmits serum-dependent signals to c-jun that may or may not overlap with those necessary for HIF-1α protein stabilization (27, 28).
FIG. 1.
(A) Hypoxia Hx) induces the immediate-early accumulation of c-jun mRNA independently of the presence of HIF-1α. Autoradiograph of a Northern blot of total RNA from wild-type and HIF-1α null mEFs incubated under aerobic conditions (air [5% CO2]) or exposed to hypoxia (pO2 ≤ 1%) for the indicated times. The blot was probed sequentially for c-jun and 28S rRNA transcripts. This finding is representative of three independent experiments. (B) The early hypoxia-inducible accumulation of c-jun mRNA is dependent on serum (10% FBS). Histogram of relative amounts of c-jun mRNA detected by quantitative RT-PCR amplification of total RNA harvested at the indicated times. For details, see Materials and Methods.
FIG. 2.
Hypoxia (Hx) rapidly induces the accumulation of HIF-1α protein in wild-type cells. Autoradiographs of immunoblots of total protein from wild type and HIF-1α null mEFs showing the accumulation of HIF-1α in wild-type cells exposed to hypoxia (pO2 ≤ 1%) for the indicated times. In these studies and in all others, hypoxic cells were harvested for protein assays under anoxic conditions. For details, see Materials and Methods.
Late induction of c-jun mRNA expression by hypoxia is dependent on the presence of HIF-1α.
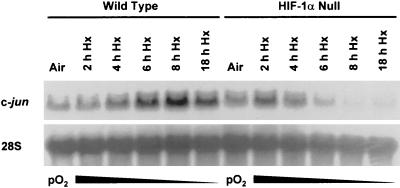
In vitro studies of c-Jun/AP-1 activation by low-oxygen conditions have generally involved exposing cell lines to prolonged hypoxia or anoxia (e.g., see references 5, 59, and 76). Moreover, HIF-1α protein accumulation has been reported to increase exponentially with decreasing oxygen levels (29), suggesting that its maximal transcriptional effect could occur beyond the time scale of immediate-early gene activation. Therefore, we investigated the induction of c-jun mRNA expression in wild-type and HIF-1α null cells exposed to prolonged hypoxia (pO2 ≤ 0.01%, up to 8 h). Figure 3 shows that while c-jun mRNA accumulated in the wild-type cells with increasing time under this hypoxic stress, it declined in the HIF-1α null cells exposed in parallel to identical low-oxygen conditions. This finding contrasts with that shown in Fig. 1A, where c-jun mRNA accumulated in both wild-type and HIF-1α null cells exposed to hypoxia for shorter times. Therefore, the overall response of c-jun mRNA expression to hypoxia or anoxia has both HIF-1α-independent and -dependent components, where the early response (transient hypoxia) is independent and the late response (prolonged hypoxia) is dependent. Because the HIF-1α-dependent, late response suggested that HIF-1-dependent genes could regulate c-jun expression and therefore c-Jun/AP-1 activity in cells exposed to tumor-like hypoxia, we focused on investigating potential mechanisms of this response. In this context, the negative effect of a HIF-1α null mutation on hypoxia-inducible c-jun mRNA expression (Fig. 3) suggested that the transmission of hypoxic signals was impaired in HIF-1α null cells exposed to prolonged hypoxia. Based on this possibility and the positive feedback model for the activation of c-jun expression involving c-Jun phosphorylation (positive autoregulation), particularly in the N-terminal region (3, 31), we hypothesized that diminished c-Jun phosphorylation could in part explain the late response of c-jun mRNA expression in HIF-1α null cells exposed to prolonged hypoxia.
FIG. 3.
The delayed accumulation of c-jun mRNA induced by prolonged hypoxia (Hx) is dependent on the presence of HIF-1α. Autoradiograph of a Northern blot of total RNA from wild-type and HIF-1α null mEFs incubated under aerobic conditions (air [5% CO2]) or exposed to hypoxia (pO2 ≤ 0.01%) for the indicated times. The blot was probed sequentially for the mRNAs for c-jun and 28S rRNA. This finding is representative of three independent experiments.
Phosphorylation of c-Jun in response to prolonged hypoxia is strongly attenuated in the absence of HIF-1α.
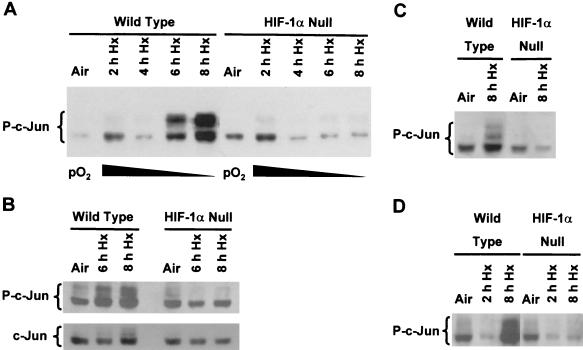
We used anti-c-Jun antibodies specific for phosphorylation sites within the N-terminal region (e.g., KM-1; see Materials and Methods) to further investigate the relationship between c-Jun/AP-1 and HIF-1 in wild-type and HIF-1α null cells exposed to prolonged hypoxia. Figure 4A shows that exposure of the wild-type cells to hypoxia (pO2 ≤ 0.01%) for at least 6 to 8 h significantly increased phosphorylation of c-Jun, whereas this increase did not occur in the HIF-1α null cells. The enhanced phosphorylation of c-Jun was made evident by the appearance of a ladder of electrophoretic bands (indicated as P-c-Jun in Fig. 4) as well as by an overall increase in the intensity of the c-Jun phospho-isoform signals. Ladders of electrophoretic bands very similar to those shown in Fig. 4 have been reported in studies of c-Jun phosphorylation by MAPKs (e.g., see references 6, 49, and 54). Importantly, no difference in the relative levels of total cellular c-Jun was found between the wild-type and HIF-1α null cells exposed to hypoxia for up to 8 h, as determined by immunoblotting analysis with antibodies generated against a nonphosphorylated c-Jun peptide or the full-length protein (Fig. 4B and data not shown). Thus, the attenuation of hypoxia-inducible c-Jun phosphorylation in the HIF-1α null cells compared with the wild-type cells cannot be attributed to a stress-associated decline in total cellular c-Jun levels. To determine whether the presence of TAg influenced hypoxia-inducible c-Jun phosphorylation, we exposed spontaneously immortalized, wild-type and HIF-1α null cells to prolonged hypoxia and harvested total protein under aerobic or anoxic conditions for immunoblotting analysis with the KM-1 antibody. Figure 4C shows that hypoxia-inducible c-Jun phosphorylation in spontaneously immortalized cells was also dependent on HIF-1α, demonstrating that immortalization by TAg did not influence c-Jun phosphorylation under these conditions. The attenuated response of hypoxia-inducible c-Jun phosphorylation in HIF-1α null cells (Fig. 4) parallels the inhibition of c-jun mRNA accumulation found on exposure of these cells to identical low-oxygen conditions (Fig. 3), supporting a role for positive autoregulation of c-jun expression by hypoxia. This possibility is discussed further below. Finally, because protein kinase activities known to stimulate c-jun expression are commonly associated with serum growth factor receptors (e.g., see reference 15), we investigated the serum dependence of hypoxia-inducible c-Jun phosphorylation in response to prolonged stress. The results in Fig. 4D demonstrate that HIF-1α-dependent phosphorylation of c-Jun was independent of serum. Together, the findings shown in Fig. 4 suggest that HIF-1-dependent gene expression is important for mediating the phosphorylation of c-Jun in cells exposed to prolonged hypoxia and that this response does not depend on the addition of serum growth factors.
FIG. 4.
Phosphorylation of c-Jun in response to prolonged hypoxia (Hx). (A) Immunoblot of total protein from wild-type and HIF-1α null mEFs harvested under aerobic conditions or following exposure to hypoxia (pO2 ≤ 0.01%) for the indicated times. The blot was probed with an anti-phospho-c-Jun antibody (KM-1). This immunoblot is representative of multiple independent experiments. (B) Immunoblots of total protein from wild-type mEFs harvested under aerobic conditions or following exposure to hypoxia (pO2 ≤ 0.01%) for the indicated times. Replicate blots were probed with the KM-1 antibody (top panel) or with an antibody that recognizes nonphosphorylated c-Jun (bottom panel; antibody H79). (C) Immunoblot of total protein from spontaneously immortalized wild-type or HIF-1α null mEFs exposed to hypoxia (pO2 ≤ 0.01%) for 8 h. The blot was probed with the KM-1 antibody. (D) Immunoblots of protein from serum-starved wild-type and HIF-1α null mEFs incubated under aerobic conditions (air [5% CO2]) or exposed to hypoxia (pO2 ≤ 0.01%) for the indicated times. Aerobic cells and cells to be exposed to hypoxia were incubated in medium containing no serum for 18 h before the study was performed. The blot was probed with the KM-1 antibody.
Phosphorylation of c-Jun in response to prolonged hypoxia involves c-Jun N-terminal sites.
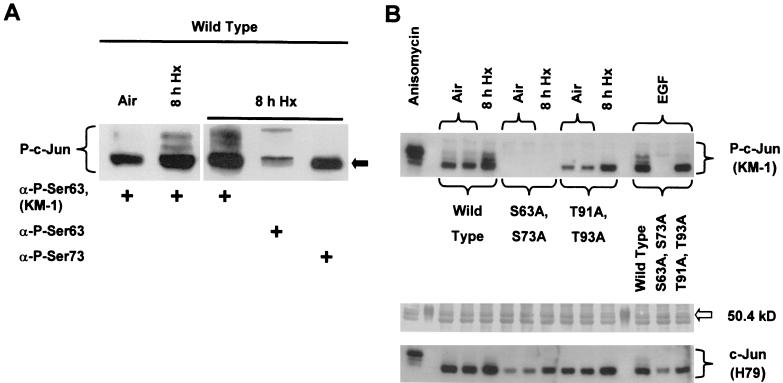
The specific antibody used to detect hypoxia-inducible c-Jun phosphorylation shown in Fig. 4 recognizes at least phospho-Ser63 in the c-Jun N-terminal activation region (see Materials and Methods). Phosphorylation of Ser63 and Ser73 within the activation region is critical for increasing the transcriptional activity of c-Jun and for stabilizing it against ubiquitin-mediated degradation (45, 49). To investigate the contribution of Ser63/Ser73 to hypoxia-inducible c-Jun phosphorylation, we harvested total protein from wild-type cells exposed to prolonged hypoxia and loaded the sample in a single well extending across the width of an SDS-polyacrylamide slab gel. As described in Materials and Methods, following SDS-PAGE the fractionated sample was transferred to an immobilizing membrane, and the membrane was simultaneously probed with different anti-c-Jun antibodies in adjacent lanes of a slot blotting apparatus. Figure 5A shows that the electrophoretic band having the greatest mobility (designated by an arrow) in the cluster of hypoxia-inducible bands contained c-Jun phosphorylated on Ser63/Ser73. Because this band is the major signal for phosphorylated c-Jun present in aerobic control cells (Fig. 5A, left panel), the presence of less mobile bands in hypoxic cells indicates that hypoxia-inducible c-Jun phosphorylation involves sites in addition to Ser63 and/or Ser73. Interestingly, Fig. 5A (right panel) suggests that c-Jun phosphoisoforms containing phospho-Ser63 but not phospho-Ser73 were further modified by hypoxia-inducible phosphorylation. An intramolecular mechanism has been described in which N-terminal phosphorylation of c-Jun influences the phosphorylation state of the C-terminal region (54). Further research is necessary to identify the c-Jun phosphoisoforms responsive to hypoxic signals.
FIG. 5.
(A) Immunoblot of total protein from wild-type mEFs harvested under aerobic conditions (left panel) or following exposure to hypoxia (Hx) (pO2 ≤ 0.01%; right panel) for 8 h. The same total protein sample from hypoxic cells was probed with different anti-c-Jun antibodies on one membrane. For details, see Materials and Methods. (B) Top panel: immunoblot of total protein from aerobic (two identical cultures) and hypoxic (pO2 ≤ 0.01%; 8 h) wild-type mEFs and mEFs containing the knock-in alleles c-jun (S63A S73A) or c-jun (T91A T93A). Control cells from each genotype were exposed to EGF (50 ng/ml, 15 min). Wild-type cells were also exposed to anisomycin (10 μg/ml, 1 h). Middle panel: Ponceau red staining of total protein on the immunoblot shown above. The position of a marker having an electrophoretic mobility within the range appropriate for c-Jun phospho-isoforms (50.4 kDa; lanes 2 and 12 from the left; Bio-Rad Laboratories, Hercules, Calif.) is indicated by an arrow. Bottom panel: replicate immunoblot of total protein from aerobic and hypoxic wild-type mEFs and mEFs containing the knock-in alleles c-jun (S63A S73A) or c-jun (T91A T93A). The blot was probed with the H79 antibody that recognizes nonphosphorylated c-Jun.
To directly investigate the contribution of N-terminal sites to hypoxia-inducible c-Jun phosphorylation, we exposed wild-type and c-jun knock-in cells having the genotypes S63A S73A or T91A T93A to prolonged hypoxia. Exposure to epidermal growth factor (EGF) or anisomycin was used as a positive control for c-Jun phosphorylation (11, 40). Figure 5B shows two important findings relevant for hypoxia-inducible c-Jun phosphorylation. First, no c-Jun phospho-isoform signals were detected in immunoblotted total protein harvested from control or treated cells containing only the S63A S73A knock-in allele and probed with the KM-1 antibody. The failure to detect the most electrophoretically mobile c-Jun phospho-isoform(s) in immunoblotted total protein from the S63A S73A knock-in cells demonstrates that this band contained phospho-Ser63 and/or phospho-Ser73, in agreement with the conclusion drawn from the immunoblotting analysis shown in Fig. 5A. Second, only one band having an electrophoretic mobility corresponding to that of phospho-Ser63 and/or phospho-Ser73 c-Jun was detected in immunoblotted total protein from control or treated cells containing only the T91A T93A knock-in allele. This finding shows that additional phosphorylation involving at least Thr91 and/or Thr93 occurred in cells exposed to hypoxia or EGF. In addition, compared with the signal from control cell protein (air/untreated), the corresponding signals from treated T91A T93A knock-in cell protein were substantially more intense, suggesting that they contained unresolved c-Jun phospho-isoforms in addition to phospho-Ser63 and/or phospho-Ser73 c-Jun (Fig. 5B). These findings obtained using cells containing c-jun knock-in alleles with specific c-Jun N-terminal phosphorylation site mutations indicate that known sites within the N-terminal region are susceptible to hypoxia-inducible phosphorylation. However, it will be necessary to determine which sites or combinations of these sites (Ser63, Ser73, Thr91, and/or Thr93) are responsive to hypoxic or anoxic signaling pathways.
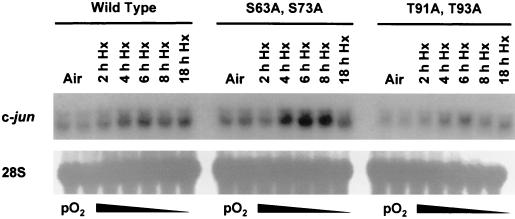
Finally, to further investigate the hypothesis that hypoxia-inducible c-Jun phosphorylation could positively autoregulate c-jun expression, we compared the effect of prolonged hypoxia on the induction of c-jun mRNA in the wild-type and knock-in cells. Figure 6 shows that hypoxia-inducible c-jun mRNA expression occurred in both the wild-type and knock-in cells, indicating that the c-jun gene was not positively autoregulated in these cells by changes in N-terminal phosphorylation in response to prolonged hypoxia. This finding indicates that the effects of prolonged hypoxia on c-jun mRNA expression and c-Jun phosphorylation are functionally separate.
FIG. 6.
Autoradiograph of a Northern blot of total RNA from wild-type mEFs and mEFs containing the knock-in alleles c-jun (S63A S73A) or c-jun (T91A T93A) incubated under aerobic conditions (air [5% CO2]) or exposed to hypoxia (Hx) (pO2 ≤ 0.01%) for the indicated times. The blot was probed sequentially for c-jun and 28S rRNA transcripts.
DISCUSSION
The major conclusion of this study is that the response of c-jun mRNA expression and c-Jun phosphorylation to prolonged or chronic hypoxia is dependent on the presence of HIF-1α and therefore on HIF-1. HIF-1 is an essential regulator of gene expression under both physiological and pathophysiological low-oxygen conditions, such as those present in the tumor microenvironment. This conclusion is based in part on findings that experimental tumors prepared from cells in which HIF-1α expression or activity has been eliminated are significantly smaller than control tumors (24, 33, 43, 60, 61). For example, when teratocarcinomas were produced from wild-type and HIF-1α null embryonic stem cells (a HIF-1α−/− genotype), the wild-type tumors were approximately 75% larger than the HIF-1α null tumors after 3 weeks of growth (60). A similar effect on average tumor mass was found using fibrosarcoma xenografts prepared from wild-type and HIF-1α null mEFs transformed with oncogenic ras (61). The impaired growth of the HIF-1α null fibrosarcomas was not simply the result of inadequate vascularization caused by diminished expression of hypoxia-inducible pro-angiogenic factors (24), as no significant differences in vascular density were found between the wild-type and HIF-1α null tumors (61). These findings indicate that the role of HIF-1 in the growth of rodent tumor xenografts is pleiotropic, affecting tumor development through various processes (reviewed in reference 44).
The c-jun proto-oncogene also has pleiotropic effects on tumor development (e.g., see references 4, 30, 56, and 72). The ability of c-jun to contribute to tumorigenesis requires cooperation with a strong oncogenic signal (1, 13, 30, 34) and involves persistent N-terminal phosphorylation, particularly but not exclusively at Ser63 and/or Ser73 (7, 10, 12, 64). In general, N-terminal phosphorylation of c-Jun is necessary for its transcriptional activity (56). Presumably, the importance of c-Jun N-terminal phosphorylation for oncogenesis arises from the aberrant expression of c-Jun/AP-1 modulated genes that could accompany sustained c-Jun/AP-1 activation (72). Given the sensitivity of c-Jun N-terminal phosphorylation to environmental stimuli, the oncogenic effects of c-Jun/AP-1 could involve activation by pathophysiological stresses within the tumor microenvironment, such as hypoxia or anoxia. In support of this hypothesis, it was reported that the growth of fibrosarcomas prepared from transformed knock-in cells expressing c-Jun (S63A S73A) was significantly less than that of control tumors (10). Interestingly, fibrosarcomas prepared from H-ras-transformed mEFs completely lacking c-jun expression (c-jun−/−) either did not grow or grew extremely poorly (30), suggesting that sites other than Ser63 and/or Ser73 could contribute to the fibrosarcoma growth defect.
The similar growth defects reported for c-jun or HIF-1α null compared with wild-type fibrosarcomas suggest a model in which c-Jun/AP-1 and HIF-1 cooperate to regulate gene expression in the microenvironment of solid tumors. In the model, both transcription factors control a common subset of genes necessary both for adaptation to hypoxic and glucose stress and for optimal tumor growth. This control could involve both direct and indirect cooperation. Evidence for direct cooperation between AP-1 and HIF-1 can be found in studies of the hypoxic response of the genes for vascular endothelial growth factor and tyrosine hydroxylase (19, 32, 51), which contain functional AP-1 and HRE sites. Recently it was reported that ectopically overexpressed c-Jun cooperates with HIF-1 to regulate HRE-dependent reporter gene expression, although without direct binding to AP-1 sites (2). We have observed that basal expression of transiently transfected AP-1-dependent reporter genes is constitutively high in the cells used for our studies, even in the presence of low serum, and thus, we have not been able to demonstrate reliable hypoxia-inducible expression of these constructs except under extreme stress (e.g., 18 to 24 h, pO2 ≤ 0.01%). A similar negative result was described for transient reporter gene studies of the proximal promoter region of MKP-1 (41), another stress- and hypoxia-responsive immediate-early gene (36). It was suggested that chromatin remodeling or distant genetic regulatory elements is critical for transcriptional activation of MKP-1 by stress (41), constraints that are also important for the transcriptional response of c-jun (16). The proximal promoter region of c-jun does not seem to bind HIF-1 (unpublished results), indicating that its response to prolonged hypoxia is an indirect or secondary effect of HIF-1-dependent gene expression.
The present study addresses potential mechanisms of cooperation between c-Jun/AP-1 and HIF-1 in terms of the effect of prolonged hypoxia on c-jun mRNA expression and c-Jun phosphorylation. The finding that hypoxia-inducible c-jun mRNA expression was not greatly perturbed in cells containing knock-in alleles for S63A S73A or T91A T93A c-jun (Fig. 6) indicates that at least part of this response is posttranscriptional rather than the result of an autoregulatory positive feedback loop involving c-Jun phosphorylation. Indeed, in earlier work members of our group demonstrated that the accumulation of c-jun mRNA in cells exposed to prolonged hypoxia involves mRNA stabilization (5). It will be important to further evaluate the contribution of mRNA stabilization to HIF-1α-dependent c-jun mRNA expression.
The dependence of hypoxia-inducible c-Jun phosphorylation on HIF-1 could influence the expression of multiple target genes in hypoxic or anoxic cells through changes in c-Jun/AP-1 activity. Nine c-Jun phosphorylation sites have been described, including four sites in the N-terminal region (Ser63, Ser73, Thr91, and Thr93), one site in the middle of the protein (Tyr170), and four sites just before the C-terminal DNA-binding region (Thr231, Thr 239, Ser243, and Ser249 in human c-Jun; Thr234, Thr 242, Ser246, and Ser252 in mouse c-Jun) (9, 56). Unlike N-terminal phosphorylation, phosphorylation of the C-terminal sites inhibits c-Jun/AP-1 DNA-binding (54), and therefore its transcriptional activity requires dephosphorylation of at least one of these sites. A model has been proposed in which N-terminal phosphorylation facilitates dephosphorylation at the C-terminal region (54). The findings reported here that Ser63 and/or Ser73 and Thr91 and/or Thr93 in the N-terminal region are sensitive to phosphorylation in mEFs exposed to prolonged hypoxia demonstrate that there is at least one c-Jun kinase pathway that is both hypoxia responsive and HIF-1 dependent. For example, the SAPK/JNK pathway phosphorylates Ser63 and Ser73 of c-Jun (reviewed in reference 45), and thus, it is possible that the expression of components of this pathway is HIF-1 dependent. However, other MAPKs target these sites: Ser63 and/or Ser73 are phosphorylated by ERK1/2 in PC12 cells exposed to nerve growth factor (40). The kinase (or kinases) that phosphorylates Thr91 and Thr93 in the N-terminal region is unknown. Although these sites are present in a motif that could be an ERK1/2 target (54), direct phosphorylation of Thr91 and/or Thr93 by these MAPKs has not been demonstrated. ERK1/2 is generally believed to activate c-Jun/AP-1 indirectly, by phosphorylating glycogen synthase kinase 3β (GSK3β) and thus decreasing its ability to phosphorylate the C-terminal inhibitory sites in c-Jun (71). In this connection, we found that neither GSK3β nor casein kinase II, both of which phosphorylate the C-terminal region (42, 55), is activated in hypoxic mEFs (unpublished results). Therefore, the enzymes responsible for altering c-Jun phosphorylation in response to prolonged hypoxia remain to be identified.
In summary, using genetically manipulated cells that are wild type or null for the hypoxia-responsive transcription factor HIF-1α, we have demonstrated that hypoxia-inducible c-jun mRNA expression has an early response that is independent of HIF-1 and a late response that requires its presence. We have also demonstrated that c-Jun phosphorylation in response to prolonged or chronic hypoxia is dependent on HIF-1. Using c-jun knock-in alleles for N-terminal phosphorylation sites, we demonstrated that N-terminal phosphorylation is an important contribution to the late response of hypoxia-inducible c-Jun phosphorylation. However, the present results indicate that the effect of prolonged hypoxia on c-jun mRNA expression does not depend on N-terminal phosphorylation and positive autoregulation. It is worth stating that a biphasic activation of AP-1 has been reported for hypoxic HeLa cells, although this phenomenon was attributed to the synthesis of new components of AP-1 complexes rather than stress-dependent c-Jun/AP-1 phosphorylation (59). We hypothesize that c-jun, like other hypoxia-inducible immediate-early genes, such as c-fos, Egr-1, DEC1/Sta13, and MKP-1 (36, 47, 73, 75), are activated by hypoxic signals to participate in a program of gene expression controlled by HIF-1, the principal transcriptional regulator of the mammalian hypoxic response. In the context of the tumor microenvironment, this hypothesis suggests that gene expression modulated by hypoxia involves cooperation between c-Jun/AP-1 and HIF-1, as well as other transcription factors that influence tumor development. Identifying genes regulated by both c-Jun/AP-1 and HIF-1 is an important area for further research that will increase our understanding of how tumor hypoxia contributes to malignant progression.
Acknowledgments
This work was supported by grants CA73807, CA74100, and CA82515 from the NCI.
REFERENCES
- 1.Alani, R., P. Brown, B. Binetruy, H. Dosaka, R. K. Rosenberg, P. Angel, M. Karin, and M. J. Birrer. 1991. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol. Cell. Biol. 11:6286-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfranca, A., M. Gutierrez, A. Vara, J. Aragones, F. Vidal, and M. Landazuri. 2002. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol. Biol. Cell. 22:12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel, P., K. Hattori, T. Smeal, and M. Karin. 1988. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 55:875-885. [DOI] [PubMed] [Google Scholar]
- 4.Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 5.Ausserer, W. A., B. Bourrat-Floeck, C. J. Green, K. R. Laderoute, and R. M. Sutherland. 1994. Regulation of c-jun expression during hypoxic and low-glucose stress. Mol. Cell. Biol. 14:5032-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, S. J., T. K. Kerppola, D. Luk, M. T. Vandenberg, D. R. Marshak, T. Curran, and C. Abate. 1992. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Mol. Cell. Biol. 12:4694-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakiri, L., D. Lallemand, E. Bossy-Wetzel, and M. Yaniv. 2000. Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J. 19:2056-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandyopadhyay, R. S., M. Phelan, and D. V. Faller. 1995. Hypoxia induces AP-1-regulated genes and AP-1 transcription factor binding in human endothelial and other cell types. Biochim. Biophys. Acta 1264:72-78. [DOI] [PubMed] [Google Scholar]
- 9.Barila, D., R. Mangano, S. Gonfloni, J. Kretzschmar, M. Moro, D. Bohmann, and G. Superti-Furga. 2000. A nuclear tyrosine phosphorylation circuit: c-Jun as an activator and substrate of c-Abl and JNK. EMBO J. 19:273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens, A., W. Jochum, M. Sibilia, and E. F. Wagner. 2000. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene 19:2657-2663. [DOI] [PubMed] [Google Scholar]
- 11.Bost, F., R. McKay, N. Dean, and D. Mercola. 1997. The JUN kinase/stress-activated protein kinase pathway is required for epidermal growth factor stimulation of growth of human A549 lung carcinoma cells. J. Biol. Chem. 272:33422-33429. [DOI] [PubMed] [Google Scholar]
- 12.Brown, P. H., R. Alani, L. H. Preis, E. Szabo, and M. J. Birrer. 1993. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877-886. [PubMed] [Google Scholar]
- 13.Chiariello, M., M. J. Marinissen, and J. S. Gutkind. 2000. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol. Cell. Biol. 20:1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claret, F. X., M. Hibi, S. Dhut, T. Toda, and M. Karin. 1996. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature 383:453-457. [DOI] [PubMed] [Google Scholar]
- 15.Clarke, N., N. Arenzana, T. Hai, A. Minden, and R. Prywes. 1998. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol. Cell. Biol. 18:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton, A. L., S. Rose, M. J. Barratt, and L. C. Mahadevan. 2000. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J. 19:3714-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad, P. W., T. L. Freeman, D. Beitner-Johnson, and D. E. Millhorn. 1999. EPAS1 trans-activation during hypoxia requires p42/p44 MAPK. J. Biol. Chem. 274:33709-33713. [DOI] [PubMed] [Google Scholar]
- 18.Conrad, P. W., R. T. Rust, J. Han, D. E. Millhorn, and D. Beitner-Johnson. 1999. Selective activation of p38alpha and p38gamma by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J. Biol. Chem. 274:23570-23576. [DOI] [PubMed] [Google Scholar]
- 19.Damert, A., E. Ikeda, and W. Risau. 1997. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 327:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsey, M. J., H. J. Tae, K. G. Sollenberger, N. T. Mascarenhas, L. M. Johansen, and E. J. Taparowsky. 1995. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene 11:2255-2265. [PubMed] [Google Scholar]
- 21.Evans, S. M., S. Hahn, D. R. Pook, W. T. Jenkins, A. A. Chalian, P. Zhang, C. Stevens, R. Weber, G. Weinstein, I. Benjamin, N. Mirza, M. Morgan, S. Rubin, W. G. McKenna, E. M. Lord, and C. J. Koch. 2000. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 60:2018-2024. [PubMed] [Google Scholar]
- 22.Fambrough, D., K. McClure, A. Kazlauskas, and E. S. Lander. 1999. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97:727-741. [DOI] [PubMed] [Google Scholar]
- 23.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 24.Grunstein, J., W. G. Roberts, O. Mathieu-Costello, D. Hanahan, and R. S. Johnson. 1999. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 59:1592-1598. [PubMed] [Google Scholar]
- 25.Hadjantonakis, A. K., M. Pirity, and A. Nagy. 1999. Cre recombinase mediated alterations of the mouse genome using embryonic stem cells. Methods Mol. Biol. 97:101-122. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan, D., D. Lane, L. Lipsich, M. Wigler, and M. Botchan. 1980. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell 21:127-139. [DOI] [PubMed] [Google Scholar]
- 27.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, B. H., G. L. Semenza, C. Bauer, and H. H. Marti. 1996. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271:C1172-C1180. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, R., B. Spiegelman, D. Hanahan, and R. Wisdom. 1996. Cellular transformation and malignancy induced by ras require c-jun. Mol. Cell. Biol. 16:4504-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin, M., Z. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 32.Kimura, H., A. Weisz, Y. Kurashima, K. Hashimoto, T. Ogura, F. D'Acquisto, R. Addeo, M. Makuuchi, and H. Esumi. 2000. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 95:189-197. [PubMed] [Google Scholar]
- 33.Kung, A. L., S. Wang, J. M. Klco, W. G. Kaelin, and D. M. Livingston. 2000. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat. Med. 6:1335-1340. [DOI] [PubMed] [Google Scholar]
- 34.Laderoute, K. R., J. M. Calaoagan, A. M. Knapp, H. L. Mendonca, and R. S. Johnson. 2001. c-jun cooperates with SV40 T-antigen to sustain MMP-2 expression in immortalized cells. Biochem. Biophys. Res. Commun. 284:1134-1139. [DOI] [PubMed] [Google Scholar]
- 35.Laderoute, K. R., J. M. Calaoagan, H. L. Mendonca, W. A. Ausserer, E. Y. Chen, A. J. Giaccia, and R. M. Sutherland. 1996. Early responses of SiHa human squamous carcinoma cells to hypoxic signals: evidence of parallel activation of NF-kB and AP-1 transcriptional complexes. Int. J. Oncol. 8:875-882. [DOI] [PubMed] [Google Scholar]
- 36.Laderoute, K. R., H. L. Mendonca, J. M. Calaoagan, A. M. Knapp, A. J. Giaccia, and P. J. Stork. 1999. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem. 274:12890-12897. [DOI] [PubMed] [Google Scholar]
- 37.Laderoute, K. R., B. J. Murphy, S. M. Short, T. D. Grant, A. M. Knapp, and R. M. Sutherland. 1992. Enhancement of transforming growth factor-alpha synthesis in multicellular tumor spheroids of A431 squamous carcinoma cells. Br. J. Cancer 65:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau, L. F., and D. Nathans. 1987. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc. Natl. Acad. Sci. USA 84:1182-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leppa, S., and D. Bohmann. 1999. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18:6158-6162. [DOI] [PubMed] [Google Scholar]
- 40.Leppa, S., R. Saffrich, W. Ansorge, and D. Bohmann. 1998. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 17:4404-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, J., M. Gorospe, D. Hutter, J. Barnes, S. M. Keyse, and Y. Liu. 2001. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol. Cell. Biol. 21:8213-8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin, A., J. Frost, T. Deng, T. Smeal, N. al-Alawi, U. Kikkawa, T. Hunter, D. Brenner, and M. Karin. 1992. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 71:777-789. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell, P. H., G. U. Dachs, J. M. Gleadle, L. G. Nicholls, A. L. Harris, I. J. Stratford, O. Hankinson, C. W. Pugh, and P. J. Ratcliffe. 1997. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 94:8104-8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell, P. H., C. W. Pugh, and P. J. Ratcliffe. 2001. Activation of the HIF pathway in cancer. Curr. Opin. Genet. Dev. 11:293-299. [DOI] [PubMed] [Google Scholar]
- 45.Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta 1333:F85-F104. [DOI] [PubMed] [Google Scholar]
- 46.Minet, E., G. Michel, D. Mottet, J. P. Piret, A. Barbieux, M. Raes, and C. Michiels. 2001. c-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp. Cell Res. 265:114-124. [DOI] [PubMed] [Google Scholar]
- 47.Muller, J. M., B. Krauss, C. Kaltschmidt, P. A. Baeuerle, and R. A. Rupec. 1997. Hypoxia induces c-fos transcription via a mitogen-activated protein kinase-dependent pathway. J. Biol. Chem. 272:23435-23439. [DOI] [PubMed] [Google Scholar]
- 48.Muller, S., M. Berger, F. Lehembre, J. S. Seeler, Y. Haupt, and A. Dejean. 2000. c-Jun and p53 activity is modulated by SUMO-1 modification. J. Biol. Chem. 275:13321-13329. [DOI] [PubMed] [Google Scholar]
- 49.Musti, A. M., M. Treier, and D. Bohmann. 1997. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science 275:400-402. [DOI] [PubMed] [Google Scholar]
- 50.Nagy, A., and J. Rossant. 1993. Production of completely ES-cell derived fetuses, p. 147-179. In A. L. Joyner (ed.), Gene targeting: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 51.Norris, M. L., and D. E. Millhorn. 1995. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J. Biol. Chem. 270:23774-23779. [DOI] [PubMed] [Google Scholar]
- 52.Ornatsky, O. I., D. M. Cox, P. Tangirala, J. J. Andreucci, Z. A. Quinn, J. L. Wrana, R. Prywes, Y. T. Yu, and J. C. McDermott. 1999. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 27:2646-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papaioannou, V., and R. Johnson. 1993. Production of chimeras and genetically defined offspring from targeted ES cells, p. 107-146. In A. L. Joyner (ed.), Gene targeting: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 54.Papavassiliou, A. G., M. Treier, and D. Bohmann. 1995. Intramolecular signal transduction in c-Jun. EMBO J. 14:2014-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plyte, S. E., K. Hughes, E. Nikolakaki, B. J. Pulverer, and J. R. Woodgett. 1992. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim. Biophys. Acta 1114:147-162. [DOI] [PubMed] [Google Scholar]
- 56.Rahmsdorf, H. J. 1996. Jun: transcription factor and oncoprotein. J. Mol. Med. 74:725-747. [DOI] [PubMed] [Google Scholar]
- 57.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 58.Richard, D. E., E. Berra, E. Gothie, D. Roux, and J. Pouyssegur. 1999. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 274:32631-32637. [DOI] [PubMed] [Google Scholar]
- 59.Rupec, R. A., and P. A. Baeuerle. 1995. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-κB. Eur. J. Biochem. 234:632-640. [DOI] [PubMed] [Google Scholar]
- 60.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan, H. E., M. Poloni, W. McNulty, D. Elson, M. Gassmann, J. M. Arbeit, and R. S. Johnson. 2000. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 60:4010-4015. [PubMed] [Google Scholar]
- 62.Ryder, K., and D. Nathans. 1988. Induction of protooncogene c-jun by serum growth factors. Proc. Natl. Acad. Sci. USA 85:8464-8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semenza, G. L. 2001. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 13:167-171. [DOI] [PubMed] [Google Scholar]
- 64.Smeal, T., B. Binetruy, D. A. Mercola, M. Birrer, and M. Karin. 1991. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature 354:494-496. [DOI] [PubMed] [Google Scholar]
- 65.St-Arnaud, R., and I. Quelo. 1998. Transcriptional coactivators potentiating AP-1 function in bone. Front. Biosci. 3:D838-D848. [DOI] [PubMed] [Google Scholar]
- 66.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaupel, P., and M. Hoeckel. 1999. Predictive power of the tumor oxygenation status. Adv. Exp. Med. Biol. 471:533-539. [DOI] [PubMed] [Google Scholar]
- 68.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Webster, K. A., D. J. Discher, and N. H. Bishopric. 1993. Induction and nuclear accumulation of Fos and Jun proto-oncogenes in hypoxic cardiac myocytes. J. Biol. Chem. 268:16852-16858. [PubMed] [Google Scholar]
- 70.Wenger, R. H. 2000. Mammalian oxygen sensing, signalling and gene regulation. J. Exp. Biol. 203:1253-1263. [DOI] [PubMed] [Google Scholar]
- 71.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 72.Wisdom, R. 1999. AP-1: one switch for many signals. Exp. Cell Res. 253:180-185. [DOI] [PubMed] [Google Scholar]
- 73.Wykoff, C. C., C. W. Pugh, P. H. Maxwell, A. L. Harris, and P. J. Ratcliffe. 2000. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene 19:6297-6305. [DOI] [PubMed] [Google Scholar]
- 74.Xu, L., K. Xie, N. Mukaida, K. Matsushima, and I. J. Fidler. 1999. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 59:5822-5829. [PubMed] [Google Scholar]
- 75.Yan, S. F., J. Lu, Y. S. Zou, J. Soh-Won, D. M. Cohen, P. M. Buttrick, D. R. Cooper, S. F. Steinberg, N. Mackman, D. J. Pinsky, and D. M. Stern. 1999. Hypoxia-associated induction of early growth response-1 gene expression. J. Biol. Chem. 274:15030-15040. [DOI] [PubMed] [Google Scholar]
- 76.Yao, K. S., S. Xanthoudakis, T. Curran, and P. J. O'Dwyer. 1994. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol. Cell. Biol. 14:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]