Abstract
Following B-cell antigen receptor (BCR) ligation, the cytoplasmic domains of immunoglobulin α (Igα) and Igβ recruit Syk to initiate signaling cascades. The coupling of Syk to several distal substrates requires linker protein BLNK. However, the mechanism by which BLNK is recruited to the BCR is unknown. Using chimeric receptors with wild-type and mutant Igα cytoplasmic tails we show that the non-immunoreceptor tyrosine-based activation motif (ITAM) tyrosines, Y176 and Y204, are required to activate BLNK-dependent pathways. Subsequent analysis demonstrated that BLNK bound directly to phospho-Y204 and that fusing BLNK to mutated Igα reconstituted downstream signaling events. Moreover, ligation of the endogenous BCR induced Y204 phosphorylation and BLNK recruitment. These data demonstrate that the non-ITAM tyrosines of Igα couple Syk activation to BLNK-dependent pathways.
Specific immune responses to foreign antigens require one or more of a family of multimeric receptors including the B-cell antigen receptor (BCR), the T-cell antigen receptor, and the Fc receptors (2, 9). Each consists of an antigen-specific subunit noncovalently associated with signal-transducing subunits (35). Within the BCR complex, membrane-bound immunoglobulin (Ig) recognizes antigen and the Igα/Igβ heterodimer activates key signaling molecules including phospholipase C-γ (PLC-γ), Ras, Rac, extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK) (7, 11, 13, 27, 32). Coordinated activation of these effectors controls cell differentiation, proliferation, and development.
BCR aggregation induces phosphorylation of conserved tyrosines in the immunoreceptor tyrosine-based activation motifs (ITAMs) (8, 48) present in the cytoplasmic tails of Igα and Igβ (20, 25). ITAM phosphorylation is mediated by Src family tyrosine kinases assembled with the resting BCR (6, 16). Although Igα and Igβ both contain ITAMs, Igβ may serve to regulate Igα phosphorylation rather than initiate primary signaling (15, 36, 42, 51). Once phosphorylated, the Igα ITAM recruits and activates the tyrosine kinase Syk (50), which is both necessary (38, 49) and sufficient (37) to initiate many BCR-mediated signaling pathways. While the processes regulating Syk activation are well defined, the mechanisms linking Syk to downstream effectors are unclear.
The linker protein BLNK (21), also known as SLP-65 (63) and BASH (22), is preferentially expressed in B cells. BLNK phosphorylation is dependent on Syk, and cotransfection studies indicate that BLNK is a direct substrate (21, 22, 28, 63). Deletion of BLNK in DT40 cells blocks BCR-induced JNK and PLC-γ2 activation (30). Expression of BLNK is required for normal B-cell development in mice (33, 34, 47, 64) and has been implicated in human immunodeficiency (43).
BLNK contains a carboxy-terminal Src homology 2 (SH2) domain, a proline-rich region, and 13 potential tyrosine phosphorylation sites (21). Six of these tyrosines are part of YXXP motifs, predicted to bind the SH2 domains of PLC-γ, Vav, and Nck (55, 56). In addition, the SH2 domain of Btk binds to phosphorylated BLNK in vitro (28), and the proline-rich region is predicted to bind the SH3 domain of Grb2 (3). Although these molecules can be coimmunoprecipitated with BLNK following receptor ligation, specific binding sites on BLNK have not been definitively identified.
BLNK and related T-cell adapter protein SLP-76 act as scaffolds to integrate the activation of multiple signaling cascades (17, 21, 31). For example, the coassembly of Vav and Nck on SLP-76 directly couples the guanine nucleotide exchange factor activity of Vav to Nck-associated serine/threonine kinase Pak to facilitate JNK activation and actin polymerization (61). A possible integration point coordinated by BLNK is at PLC-γ2, which requires both Syk and Btk for full activation (57, 58).
A simple way in which BLNK could be brought into proximity to Syk is through direct recruitment to the BCR. In addition to the ITAM tyrosines, Igα contains two additional tyrosines, Y176 and Y204, which flank the ITAM (9) and which, we now report, function to recruit BLNK. Mutation of these tyrosines uncoupled a chimeric receptor from BLNK-dependent pathways. Subsequent analysis demonstrated that Y204 was phosphorylated following receptor ligation and bound directly to the SH2 domain of BLNK. In addition, fusion of BLNK to the carboxy-terminal tail of mutant Igα rescued distal signaling. These data provide a model in which the recruitment of BLNK to Igα links Syk activation to downstream pathways.
MATERIALS AND METHODS
Mutagenesis and expression of cDNAs.
Construction of the platelet-derived growth factor receptor β (PDGFRβ)/Igα chimera has been previously described (42). Single tyrosine-to-phenylalanine mutations at residue 176 or 204 of Igα were generated by site-directed mutagenesis with the Altered Sites system (Promega). The double tyrosine-to-phenylalanine mutations at residues 182 and 193 or residues 176 and 204 were generated by complementary-primer PCR (54). To fuse BLNK to the carboxy terminus of PDGFRβ/Igα176,204, DNA containing the open reading frame of BLNK was first amplified by PCR from murine cDNA. The 5′ primer contained an EcoRI site, and the 3′ primer contained an XhoI site and an in-frame stop codon. These sites were used to clone the BLNK DNA 3′ to cDNA encoding PDGFRβ/Igα176,204. All constructs were cloned into a modified pcDNA3 expression vector (Invitrogen) in which the cytomegalovirus promoter had been replaced with the thymidine kinase promoter and μ heavy chain enhancer (54). Plasmids were transfected into A20IIA1.6, an Fc receptor-γRII-negative B-cell lymphoma, by electroporation and grown by plating at limiting dilution in medium containing 1.2 mg of G-418 (Gibco-BRL)/ml. For transfection of full-length murine Igα into J558Lμm cells (J. Cambier, National Jewish Center, Denver, Colo.), murine Igα cDNA was obtained from A20IIA1.6 cells by first isolating total RNA with the RNeasy kit (Qiagen), followed by reverse transcription-PCR utilizing random hexamer primers (First Strand cDNA kit; Pharmacia). The entire open reading frame of Igα was amplified with primers containing an EcoRI site at the 5′ end and an XhoI site and an in-frame stop codon at the 3′ end. After the Igα open reading frame was cloned into pGEM (Promega) and sequenced, the Y204F mutant was created by PCR with the following 3′ primer, with the mutated base underlined (TAC [tyrosine]) to TTC ([phenylalanine]): GGGGCTCGAGTCATGGCTTTTCCAGCTGGGCATCTCCAATGTGGAGGTTGCCCACATCCTGGAAGGTGCCCTGGAG. After ligation into pGEM and sequencing, both the wild type and Y204F mutant were cloned into the green fluorescent protein (GFP) containing retrovirus vector MIGR1 (H. Singh, University of Chicago), modified to include an altered multiple cloning site between the EcoRI and XhoI sites. J558Lμm cells were transfected with these clones by using retrovirus collected from packaging cell line GP-293 (Clontech), and productive infection was confirmed by both flow cytometry (for GFP) and protein blotting (for Igα).
Cell culture and generation of clones.
Cells were grown in Iscove's modified Dulbecco's medium (IMDM) (Gibco-BRL) supplemented with 10% fetal calf serum (FCS; HyClone), 2 mM glutamine, 100 U of penicillin/ml, and 10 μg of streptomycin/ml (Gibco-BRL) at 37°C in 5% CO2. Clones were screened by fluorescence-activated cell sorter with an antibody that recognizes the extracellular domain of PDGFRβ (R&D Systems), followed by a fluorescein isothiocyanate (FITC)-coupled anti-mouse IgG1 antibody (Zymed). Cells were tested for BCR expression by staining with a FITC-labeled rabbit anti-mouse IgG antibody (Zymed).
Cell stimulations.
Cells (1.5 × 107) were resuspended in 0.4 ml of serum-free media, the ligand and antibodies were added on ice, and the cells were then transferred to 37°C for various lengths of time. To stimulate the PDGFRβ/Igα chimera, platelet-derived growth factor BB (PDGF-BB; Sigma) was first added to a final concentration of 1 μg/ml and the cells were incubated for 5 min. The cells were then sequentially incubated with an anti-PDGFRβ antibody (final concentration, 10 μg/ml) for 3 min and with rabbit anti-mouse IgG1 antibodies (20 μg/ml) for 5 min. To stimulate the BCR, rabbit anti-mouse IgG (Jackson) was added for 5 min at a final concentration of 20 μg/ml. In experiments assaying the association of BLNK with endogenous Igα/Igβ, cells were first warmed to 37°C and then stimulated with rabbit anti-mouse IgG (A20IIA1.6 cells) or goat anti-mouse IgM (WEHI-231 cells) (Jackson Laboratories) for 2 min. In all cases, reactions were terminated by the addition of ice-cold phosphate-buffered saline (PBS). For the J558Lμm experiment, cells (4 × 106) were stimulated with 20 μg of goat anti-mouse IgM/ml for 3 min at 37°C.
Immunoprecipitations and immunoblotting.
Stimulated cells were washed with ice-cold PBS and lysed in 1 ml of modified radioimmunoprecipitation assay buffer (1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.5% deoxycholate, 150 mM NaCl, 10 mM Tris [pH 7.3], 10 mM Na4P2O7, 0.4 mM Na3VO4, 0.4 mM EDTA, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 1 μg each of aprotinin, leupeptin, and α-1-antitrypsin/ml) for 20 min on ice. Lysates were clarified by centrifugation, precleared with protein A- and G-Sepharose, and then subjected to immunoprecipitation with protein A- and G-Sepharose-coupled antibodies at 4°C. The beads were boiled in Laemmli SDS sample buffer, subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene difluoride membranes (Millipore), which were then blocked in 3% bovine serum albumin-Tris-buffered saline with 1% Triton X-100 (TBST). Membranes were then incubated with the antibodies listed in the figure legends, washed with TBST, incubated with horseradish peroxidase-labeled secondary antibodies (Amersham), and then washed with TBST. ECL (Amersham) was used to visualize immunoreactive proteins. To strip blots, membranes were incubated in TBST-0.4% SDS, pH 2.5, for 1 h at room temperature before being washed and reblotted as described above. Antibodies utilized included antiphosphotyrosine antibody 4G10 (Upstate Biotechnology), anti-ERK (Zymed), anti-phospho-ERK (Promega), anti-PLC-γ2 (Santa Cruz Biotechnology), and anti-Igβ extracellular domain (Pharmingen). Anti-Syk and anti-BLNK antisera were generated by immunizing rabbits (Strategic Biosolutions) with glutathione S-transferase (GST) fusion proteins containing the hinge region of Syk (gift from J. Cambier, National Jewish Center) and the BLNK SH2 domain, respectively. For some experiments, anti-BLNK antiserum purified against GST-BLNK-SH2 bound to Sepharose was used. The anti-Igα antisera have been described previously (42).
Syk kinase assay.
Cells (5 × 106) were stimulated and lysed in lysis buffer (50 mM Tris-HCl [pH 7.4], 1% Brij 96, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1 μg of leupeptin and aprotinin/ml). After clarification by centrifugation, lysates were incubated for 2 h at 4°C with anti-Syk antibodies bound to protein A-Sepharose. Immunoprecipitates were washed three times in 700 μl of wash buffer (25 mM HEPES [pH 7.4], 0.1% Brij 96, 150 mM NaCl, 1 mM Na3VO4) and twice in wash buffer without Brij 96 (5). Washed immunoprecipitates were then incubated with 4 μg of GST-Igα cytoplasmic tail (1) in kinase buffer (25 mM HEPES [pH 7.5], 10 mM MnCl2, 20 mM p-nitrophenylphosphate, 10 μCi of [γ-32P]ATP) for 3 min at 30°C. Samples were immediately boiled in 2× Laemmli sample buffer and separated by SDS-PAGE. Phosphorylation of GST-Igα was detected by autoradiography.
JNK kinase assay.
Cells were stimulated and lysed in lysis buffer (25 mM HEPES [pH 7.7], 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 0.5 mM dithiothreitol [DTT], 20 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 μg of leupeptin/ml, 100 μg of PMSF/ml) on ice for 30 min. The cell lysates were clarified by centrifugation and incubated with GST-c-Jun coupled to glutathione-Sepharose beads for 3 h at 4°C. The beads were washed four times with HEPES binding buffer (20 mM HEPES [pH 7.7], 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100, 0.1 mM Na3VO4, 2 μg of leupeptin/ml, 100 μg of PMSF/ml), resuspended in 30 μl of kinase buffer (20 mM HEPES [pH 7.6], 20 mM MgCl2, 20 mM β-glycerolphosphate, 20 mM p-nitrophenylphosphate, 0.1 mM Na3VO4, 2 mM DTT, 20 μM ATP, 5 μCi of [γ-32P]ATP), and incubated at 30°C for 15 min. The beads were washed with HEPES binding buffer, resuspended in 50 μl of Laemmli SDS sample buffer, boiled, and subjected to SDS-PAGE. Dried gels were exposed to X-ray film for autoradiography.
Intracellular calcium mobilization.
Cells (107) were incubated in IMDM with 10% FCS in the presence of 4 μM fluo-3 and 10 μM Fura red (Molecular Probes) for 45 min at 37°C (45). Cells expressing the PDGFRβ/Igα chimeric proteins were washed, resuspended in 300 μl of IMDM with 10% FCS, warmed to 37°C in the presence of PDGF-BB, and stimulated by addition of anti-PDGFRβ and rabbit anti-mouse IgG1 antibodies. BCR signaling was initiated with rabbit anti-mouse IgG antibodies. Data were collected as histograms of fluorescence over time on a BD LSR flow cytometer. The fluo-3/Fura red ratio versus time was plotted with Multitime software (Phoenix Flow Systems, San Diego, Calif.).
Peptides.
Peptides were synthesized at the University of Chicago Cancer Research Center Peptide Core Facility. The sequence flanking tyrosine 176 is MPDDYEDENLY, and the sequence flanking tyrosine 204 is LQGTYQDVGNL. The peptides were synthesized with or without a phosphate group on the tyrosine corresponding to the fifth residue of each peptide. One microgram of peptide was covalently coupled to 1 ml of a 50% slurry of N-hydroxy-succinimide-activated Sepharose 4 Fast Flow (Pharmacia) for precipitation studies in accordance with the manufacturer's directions.
GST fusion proteins.
The coding sequence for the SH2 domain of BLNK (amino acid residues 324 to 456) was cloned into pGEX3X (Pharmacia). The plasmid encoding GST-c-Jun was generously provided by Marsha Rosner (University of Chicago). Bacteria containing the GST fusion protein constructs were grown in Luria-Bertani medium containing ampicillin. Expression of the fusion proteins was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.1 mM for 3 h at 37°C. The cells were harvested and resuspended in ice-cold PBS with 1% Triton X-100 and 1 mM PMSF. Cells were then frozen at −80°C, thawed at 37°C, and sonicated for maximum lysis. Insoluble debris was pelleted by centrifugation. Glutathione-Sepharose beads were added to the supernatant and incubated at 4°C for 2 h. The beads were then washed in PBS. Fusion proteins were eluted with 10 mM reduced glutathione in 10 mM Tris-HCl (pH 8), dialyzed against PBS, and quantitated by SDS-PAGE and Coomassie staining.
GST fusion protein binding assays.
For far-Western blotting, the eluted fusion protein was used to probe transferred membranes at a concentration of 4 μg/ml in 5% milk-TBST at 4°C overnight. Blots were washed extensively and then blotted sequentially with rabbit anti-GST (Zymed) and horseradish peroxidase-labeled anti-rabbit IgG (Amersham). For pull-down assays, 10 μg of fusion protein bound to glutathione-Sepharose was used for each precipitation.
In vivo phosphopeptide mapping.
For in vivo labeling, 107 WEHI-231 cells were incubated with 1.5 mCi of 32PO4 (Amersham) in phosphate-free medium for 1.5 h at 37°C (23). The cells were stimulated with 50 μg of goat anti-mouse IgM antibodies/ml for 5 min and then lysed in a solution containing 20 mM Tris-HCl (pH 8), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1% Triton X-100, 1 mM Na3VO4, 1 mM PMSF, 10 μg of leupeptin/ml, and 1 μg of aprotinin/ml. After insoluble debris was removed by centrifugation, SDS was added to a final concentration of 0.3% and sodium deoxycholate was added to a final concentration of 0.4%. Igα/Igβ was immunoprecipitated with rabbit polyclonal antibodies directed against the cytoplasmic tail of Igα. Immunoprecipitations were resolved by two-dimensional (2D) nonreducing and reducing SDS-PAGE, and Igα was identified by autoradiography. Gel slices containing Igα were excised and digested with V8 protease. Recovered peptides were resolved by thin-layer electrophoresis (TLE) in the first dimension and ascending thin-layer chromatography in the second dimension as described previously (23). Plates were then subjected to autoradiography. To identify each phosphorylated peptide, a synthetic peptide corresponding to the entire cytoplasmic domain of Igα was labeled in vitro with baculovirus-produced Lck, digested with V8 protease in solution, and then subjected to 2D phosphopeptide mapping. Radiolabeled spots were eluted from the thin-layer chromatography (TLC) plate and sequenced by tandem mass spectrometry.
RESULTS
Construction and expression of chimeras.
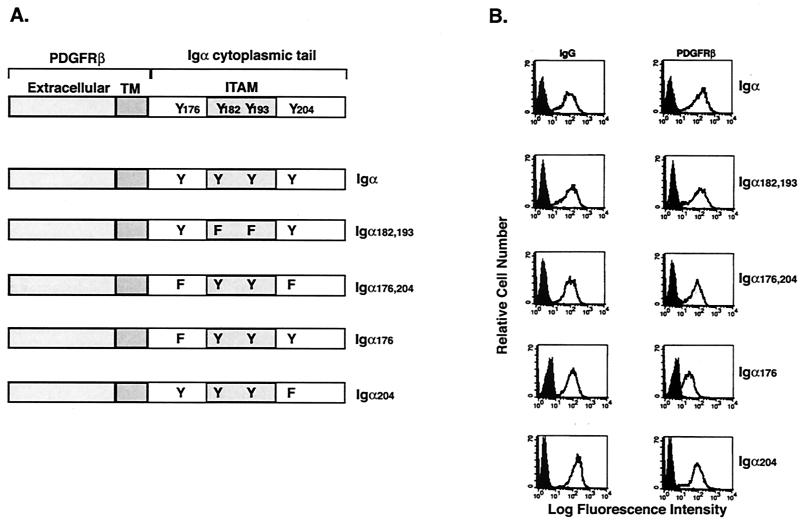
The cytoplasmic tail of Igα contains four tyrosines, two within the ITAM (Y182 and Y193) and two flanking the ITAM (Y176 and Y204). While the importance of the ITAM tyrosines has been demonstrated (20, 46), we postulated that phosphorylation of Y176 and/or Y204 might contribute to the activation of receptor-dependent signaling pathways. Therefore, we constructed cDNAs encoding chimeric proteins in which the extracellular and transmembrane domains of the PDGFRβ chain were fused to wild-type or mutant Igα cytoplasmic tails (Fig. 1A). The mutant tails contained tyrosine-to-phenylalanine mutations at both ITAM tyrosines (Igα182,193) or at one or both non-ITAM tyrosines (Igα176, Igα204, or Igα176,204). Stable clones of murine B-cell lymphoma A20IIA1.6 cells expressing each chimera were derived. Flow cytometry was used to select clones expressing similar levels of PDGFRβ and the endogenous BCR for further analysis (Fig. 1B).
FIG. 1.
Expression of the PDGFRβ/Igα chimeric proteins. (A) Chimeric proteins were constructed by fusing the extracellular and transmembrane (TM) domains of PDGFRβ to the cytoplasmic domain of either wild-type or mutated Igα. The mutants contain tyrosine-to-phenylalanine mutations at both ITAM tyrosines (Igα182,193), at both non-ITAM tyrosines (Igα176,204), or at either non-ITAM tyrosine individually (Igα176 and Igα204). (B) B-cell surface expression of BCR (left) and PDGFRβ (right) on transfected cells as measured by flow cytometry. Cells stained with the secondary antibody only were used as a negative control (shaded peaks).
Y176 and Y204 are necessary for activating BLNK-dependent pathways.
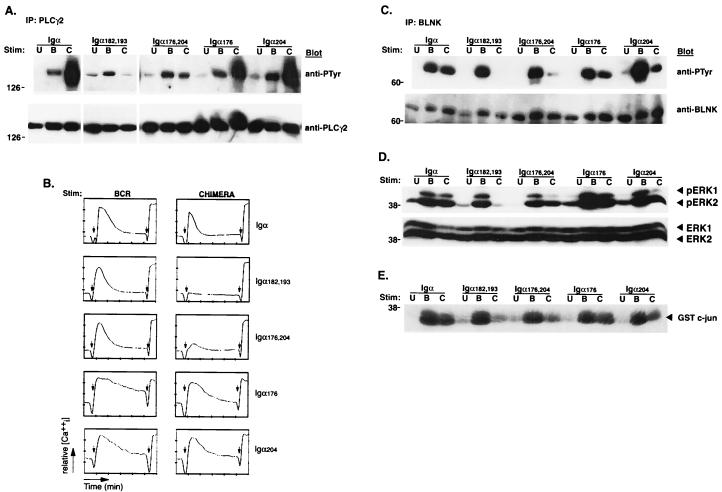
Cross-linking of the BCR induces the rapid tyrosine phosphorylation of intracellular substrates (12, 24). To investigate the ability of the different PDGFRβ/Igα chimeras to induce tyrosine phosphorylation, cells from each line were stimulated through the BCR or through each chimera for 2 min and analyzed for inductive protein tyrosine phosphorylation. Cross-linking PDGFRβ/Ιgα induced a pattern of tyrosine phosphorylation similar to that for the corresponding endogenous BCR (Fig. 2A). As expected, stimulation of PDGFRβ/Igα182,193, in which both ITAM tyrosines were mutated, induced no detectable cytosolic phosphorylation. Interestingly, mutation of Y204 and, to a lesser degree, Y176 resulted in a selective defect in the phosphorylation of at least four proteins with relative molecular masses of approximately 42, 52, 65, and 75 kDa. Similar results were observed when cells were stimulated for 1 or 5 min (data not shown).
FIG. 2.
Igα176 and Igα204 are required for tyrosine phosphorylation of selected substrates. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated PDGFRβ/Igα chimeras (C) for 2 min. Lysates were analyzed by immunoblotting with antiphosphotyrosine antibodies. Arrows, proteins with decreased tyrosine phosphorylation induced through the mutant chimeras compared to that induced through BCR. (B) Cells were left unstimulated (U) or were stimulated through various PDGFRβ/Igα chimeras (C) for 2 min. Lysates were immunoprecipitated (IP) with anti-PDGFRβ antibodies to precipitate the chimera and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PDGFRβ antibodies (bottom). (C) Cells were stimulated as for panel B. Lysates were immunoprecipitated with anti-Syk antibodies and immunoblotted with antiphosphotyrosine antibodies (top). The blot was then stripped and reprobed with anti-Syk antibodies (bottom). (D) Cells were stimulated as for panel B. Brij lysates (1%) were immunoprecipitated with anti-Syk antibodies and subjected to a kinase assay using a GST-Igα cytoplasmic tail as an exogenous substrate. After SDS-PAGE, the gel was dried and phosphorylation of GST-Igα was detected by autoradiography.
Although phosphorylation of the Igα ITAM tyrosines is required to recruit and activate Syk (38, 50), it was possible that mutating Y176 and/or Y204 compromised Syk activation. Therefore, cells from each line were stimulated through their respective chimeras, lysed, and immunoprecipitated with anti-PDGFRβ (Fig. 2B) or anti-Syk antibodies (Fig. 2C and D). Immunoblotting with antiphosphotyrosine antibodies revealed that stimulation through the wild-type chimera and chimeras bearing Y176 and/or Y204 mutations induced similar levels of both chimera and Syk phosphorylation (Fig. 2B and C). In addition, Syk kinase activity, as measured by phosphorylation of the exogenous substrate GST-Igα cytoplasmic tail, paralleled the phosphorylation status of Syk (Fig. 2D).
Downstream of Syk, the activation of PLC-γ2 generates inositol-1,4,5-triphosphate, which, through specific receptors on the endoplasmic reticulum, mobilizes intracellular calcium stores (53, 60). Therefore, we next investigated whether the different chimeric receptors could induce PLC-γ2 phosphorylation. Cells from each line were stimulated through the BCR or their respective chimeric receptors, and PLC-γ2 immunoprecipitates were analyzed for tyrosine phosphorylation (Fig. 3A). Cross-linking either PDGFRβ/Igα, PDGFRβ/Igα176, or PDGFRβ/Igα204 induced robust phosphorylation of PLC-γ2. In contrast, mutation of both non-ITAM tyrosines reduced PDGFRβ/Igα-mediated PLC-γ2 phosphorylation. We next assayed the ability of each chimeric complex to increase intracellular calcium. As seen in Fig. 3B, the requirements for calcium mobilization paralleled those for PLC-γ2 phosphorylation. Mutation of either Y176 or Y204 had minimal effects on the ability of PDGFRβ/Igα to mobilize intracellular calcium. However, mutation of both Y176 and Y204 severely inhibited calcium mobilization. Similar results were obtained with independently derived clones (data not shown). These data indicate that the non-ITAM residues are redundant for PLC-γ2 activation.
FIG. 3.
Igα176 and Igα204 are required for the phosphorylation of BLNK and for the activation of distal pathways. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated PDGFRβ/Igα chimeras (C) for 3 min. Lysates were immunoprecipitated (IP) with anti-PLC-γ2 antibodies and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PLC-γ2 antibodies (bottom). (B) Cells were loaded with Fura red and fluo-3 and stimulated through either the BCR (left) or the indicated chimera (right). The relative mean increase in intracellular calcium is plotted as a function of time. Left arrows, addition of stimulating antibody; right arrows, addition of ionomycin. (C) Cells were stimulated as for panel A. Lysates were immunoprecipitated with anti-BLNK antibodies and immunoblotted with antiphosphotyrosine antibodies (top). The blot was then stripped and reprobed with anti-BLNK antibodies (bottom). (D) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 5 min. Lysates were immunoblotted with anti-phospho-ERK1 and -ERK2 antibodies (top). The blot was then stripped and reprobed with anti-ERK1 and -ERK2 antibodies (bottom). (E) Cells were stimulated as for panel D. Lysates were precipitated with GST-c-Jun and subjected to a kinase assay and SDS-PAGE, followed by detection of phosphorylated c-Jun by autoradiography.
Genetic and biochemical evidence indicate that the linker protein BLNK couples Syk to PLC-γ2 activation (30, 33, 47). Therefore, we next examined whether Igα-mediated BLNK phosphorylation was dependent on the Igα non-ITAM tyrosines. BLNK immunoprecipitates from the lysates of chimera- or BCR-stimulated cells were analyzed for tyrosine phosphorylation. As seen in Fig. 3C, BLNK was phosphorylated following stimulation by either BCR or PDGFRβ/Igα. In contrast, mutation of Y204 and, to a lesser degree, Y176, inhibited receptor-induced BLNK phosphorylation. Mutation of both tyrosines almost completely eliminated BLNK phosphorylation. Therefore, as opposed to their redundant contributions to PLC-γ2 activation, both Y176 and Y204 are required for optimal receptor-induced BLNK phosphorylation.
If Y176 and Y204 contribute to PLC-γ2 activation by mediating the phosphorylation of BLNK, then mutation of these tyrosines should inhibit the activation of other effectors distal to BLNK such as the mitogen-activated protein kinases JNK and ERK (30). To assay for ERK activation, whole-cell lysates of cells stimulated through the BCR, or the chimeras, were immunoblotted with anti-phospho-ERK antibodies. As seen in Fig. 3D, the activation of ERK1 and, to some degree ERK2, was diminished in cells stimulated through chimeras bearing mutations of Y204. Mutation of Y176 had a modest effect on ERK activation. The partial dependency of receptor-induced ERK activation on Y176 and Y204 may reflect the calcium-dependent and -independent pathways through which ERK can be activated in B lymphocytes (26). In contrast, BCR-induced JNK activation is fully dependent on BLNK (30). Therefore, in vitro kinase assays with GST-c-Jun, the JNK substrate, were performed. Mutation of Y204 significantly diminished PDGFRβ/Igα-mediated JNK activation. In parallel with BLNK phosphorylation and ERK activation, Y204 was the primary determinant of JNK activation (Fig. 3E). Together, these data indicate that phospho-Y176 and -Y204 make differential contributions to the coupling of BCR-initiated kinase activation to BLNK phosphorylation and BLNK-dependent signaling pathways. To explore how Y176 and Y204 mediate receptor-induced BLNK phosphorylation, we first sought to identify what proteins, if any, they bound.
BLNK is recruited directly to phospho-Y204.
When phosphorylated, Y204, in the context of the three amino acids C-terminal to it, is predicted to bind group I SH2 domains (55). In contrast, the sequence flanking Y176 does not contain a consensus motif for binding either the SH2 (18) or phosphotyrosine binding (PTB) (4) domain. To determine if proteins could bind to either Y176 or Y204, we synthesized phosphorylated and nonphosphorylated peptides containing the 11 amino acids flanking these tyrosines. The peptides were covalently coupled to Sepharose beads and assayed for their ability to bind tyrosine-phosphorylated proteins from BCR-stimulated cells. As seen in Fig. 4A, a single tyrosine-phosphorylated band with a relative molecular mass of 65 kDa, suggestive of BLNK, was precipitated specifically by the phospho-Y204 peptide from the lysates of stimulated cells. When the blot was stripped and reprobed, strong BLNK immunoreactivity was detected in phospho-Y204 peptide precipitations from both unstimulated and stimulated lysates (bottom). Less BLNK was recovered from the lysates of stimulated cells than from those of unstimulated cells, possibly because of competition for the BLNK SH2 domain by other tyrosine-phosphorylated proteins in the cell lysate.
FIG. 4.
BLNK binds directly to phosphorylated Y204 in vivo and in vitro. (A) Eleven-amino-acid peptides spanning Y176 (MPDDYEDENLY) or Y204 (LQGTYQDVGNL) were synthesized with or without a phosphate on the middle tyrosine and covalently coupled to N-hydroxy-succinimide-activated Sepharose. Cells were either left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with peptide-coupled beads and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blot was then stripped and reprobed with anti-BLNK antibodies (bottom) (54a). (B) Cells were left unstimulated (U) or were stimulated through the indicated chimeras (C) for 2 min. Lysates were precipitated with GST-BLNK-SH2. The samples were immunoblotted with antiphosphotyrosine antibodies (top). The blot was stripped and reprobed with anti-Igα antibodies (bottom). (C) Cells were stimulated as for panel B. Lysates were immunoprecipitated (IP) with anti-PDGFRβ antibodies, divided into three equal aliquots, and far-Western blotted with GST-BLNK-SH2 (top) or GST (middle) followed by anti-GST antibodies or Western blotted with antiphosphotyrosine antibodies (bottom). (D) Cells were stimulated as for panel B for the times indicated. Lysates were immunoprecipitated with anti-PDGFRβ antibodies, divided into two equal aliquots, and Western blotted with antiphosphotyrosine antibodies (top) and far-Western blotted with the GST-BLNK-SH2 fusion protein followed by anti-GST antibodies (bottom).
BLNK contains a single SH2 domain at its carboxy terminus (21, 63). To investigate whether this domain mediated binding to phospho-Y204, we constructed a GST fusion protein containing the BLNK SH2 domain. Lysates from chimera-stimulated cells were precipitated with GST-BLNK-SH2 coupled to glutathione-Sepharose and analyzed for tyrosine phosphorylation (Fig. 4B). GST-BLNK-SH2 only precipitated chimeras which had both intact ITAM and Y204 tyrosines (top). Stripping and reprobing with anti-Igα antibodies confirmed that the precipitated bands were the PDGFRβ/Igα chimeras (bottom). We conclude that the BLNK SH2 domain can bind to phospho-Y204, but not Y176, following receptor stimulation.
We next examined if the BLNK SH2 domain interacted directly with phospho-Y204 or required an intermediate molecule by far-Western blotting (Fig. 4C). PDGFRβ immunoprecipitates from chimera-stimulated cells were blotted with either GST-BLNK-SH2 (top), GST alone (middle), or antiphosphotyrosine antibodies (bottom). Aggregation induced the tyrosine phosphorylation of all the PDGFRβ/Igα chimeras containing an intact ITAM. This suggested that Syk recruitment was required for the phosphorylation of the non-ITAM tyrosines. In contrast, GST-BLNK-SH2 bound only the PDGFRβ/Igα chimeras containing an intact ITAM as well as Y204. These data indicate that aggregation of the PDGFRβ/Igα chimera induces the phosphorylation of Y204, which then directly and specifically binds the SH2 domain of BLNK.
Utilizing the high specificity and sensitivity of the BLNK SH2 domain, we also examined the kinetics of Y204 phosphorylation (Fig. 4D). Cells expressing the PDGFRβ/Igα chimera were stimulated for the times indicated, lysed, and subjected to anti-PDGFRβ immunoprecipitation. Total chimera phosphorylation, as visualized by antiphosphotyrosine blotting (top), illustrated that the phosphorylation of the chimera was transient and peaked at 2 min following stimulation. The far-Western data illustrated that Y204 was more transiently phosphorylated than the entire chimera: phosphorylation peaked at 1 min and rapidly decreased thereafter.
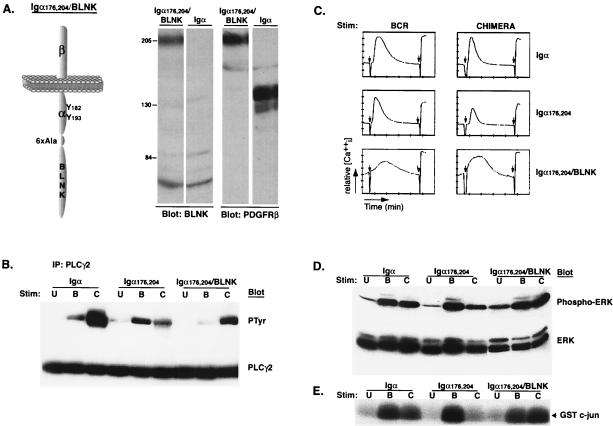
BLNK reconstitutes mutant receptor signaling.
Membrane recruitment of BLNK is necessary for the proper activation and assembly of downstream signaling molecules. Therefore, we next examined if constitutive membrane localization of BLNK would restore PLC-γ2 phosphorylation, calcium mobilization, and JNK activation. A chimeric protein was constructed by fusing full-length BLNK to the carboxy terminus of the PDGFRβ/Igα176,204 chimera (Fig. 5A, left). A clone expressing levels of chimera and BCR similar to those expressed by the previously analyzed clones was selected and analyzed. Expression of the PDGFRβ/Igα176,204/BLNK protein was confirmed by Western blotting using both anti-BLNK and anti-PDGFRβ antibodies (Fig. 5A, right). As shown previously, cross-linking of PDGFRβ/Igα induces more-robust tyrosine phosphorylation of PLC-γ2 than does cross-linking of the BCR, while PDGFRβ/Igα176,204 induces minimal tyrosine phosphorylation. However, BLNK fused to PDGFRβ/Igα176,204 induced PLC-γ2 phosphorylation that was significantly enhanced over that induced by PDGFRβ/Igα176,204 or the BCR (Fig. 5B). Moreover, fusing BLNK to Igα176,204 did not merely rescue, but augmented, the magnitude of initial calcium responses (Fig. 5C). JNK and ERK activation, which is deficient when Y176 and Y204 are mutated, was also reconstituted (Fig. 5D and E). Therefore, constitutive receptor localization of BLNK reconstitutes BLNK-dependent signaling pathways.
FIG. 5.
Fusion of BLNK to Igα176,204 rescues signaling. (A) Diagram and expression of PDGFRβ/Igα176,204/BLNK. Full-length BLNK was fused to the Igα176,204 construct with a six-alanine linker inserted between Igα and BLNK. Whole-cell lysates were prepared from 2 × 106 cells expressing the Igα176,204/BLNK or wild-type Igα chimeras and immunoblotted with anti-BLNK (left) and anti-PDGFRβ (right) antisera. PDGFRβ/Igα migrates at approximately 130 kDa, and PDGFRβ/Igα176,204/BLNK migrates at 205 kDa. (B) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 1 min. Lysates were immunoprecipitated with anti-PLC-γ2 antibodies and immunoblotted with antiphosphotyrosine (PTyr) antibodies (top). The blot was then stripped and reprobed with anti-PLC-γ2 antibodies (bottom). (C) Cells were loaded with Fura red and fluo-3 and stimulated either through the BCR (left) or the indicated chimeras (right). The relative mean increase in intracellular calcium is plotted as a function of time. Left arrows, addition of stimulating antibody; right arrows addition of ionomycin. The PDGF-BB ligand was added at the same time as the stimulating antibodies. (D) Cells were left unstimulated (U) or were stimulated through the BCR (B) or the indicated chimeras (C) for 5 min. Lysates were immunoblotted with anti-phospho-ERK1 and -ERK2 antibodies (top). The blot was then stripped and reprobed with anti-ERK1 and -ERK2 antibodies (bottom). (E) Cells were stimulated as for panel D. Lysates were precipitated with GST-c-Jun and subjected to a kinase assay and SDS-PAGE, followed by detection of phosphorylated c-Jun by autoradiography.
BLNK associates with endogenous phospho-Igα through its SH2 domain.
Our results demonstrate that phosphorylation of Y204 can functionally and structurally couple PDGFRβ/Igα to BLNK. However, it is not clear if Y204 has a similar function in the intact BCR. Therefore, we examined whether the BLNK SH2 domain bound to endogenous Igα. Lysates from unstimulated and BCR-stimulated A20IIA1.6 cells were precipitated with either anti-Igβ antibodies to precipitate the Igα/Igβ heterodimer, GST-BLNK-SH2, or GST and then analyzed for tyrosine phosphorylation (Fig. 6A). Although no bands appeared in the GST precipitation, a tyrosine-phosphorylated band the size of endogenous Igα was present in both the Igβ and GST-BLNK-SH2 precipitations. Stripping and reprobing confirmed that the band was indeed Igα. These data demonstrate that the BLNK SH2 domain can precipitate endogenous Igα from BCR-stimulated cells.
FIG. 6.
BLNK binds to endogenous phospho-Igα. (A) Cells were left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with anti-Igβ antibodies, GST, or GST-BLNK-SH2 and immunoblotted with antiphosphotyrosine (anti-PTyr) antibodies (top). The blots were then stripped and reprobed with anti-Igα antibodies (bottom). ∗, position of the BLNK-SH2 fusion protein; ∗∗, position of Igα. (B) Samples were stimulated as for panel A. Lysates were immunoprecipitated (IP) with control Ig (cIg) antibodies, antiphosphotyrosine antibodies, or anti-Igβ antibodies, and each was then divided into three equal aliquots. Immunoprecipitations were blotted with antiphosphotyrosine antibodies (left) or the GST-BLNK-SH2 fusion protein (middle) or GST (right) followed by anti-GST antibodies. (C) Samples were stimulated as for panel A for the times indicated. Lysates were immunoprecipitated with anti-Igβ antibodies and divided into two equal aliquots. Immunoprecipitations were blotted with antiphosphotyrosine antibodies (top) or the GST-BLNK-SH2 fusion protein followed by anti-GST antibodies (bottom). (D) A20IIA1.6 cells were left unstimulated (U) or were stimulated through the BCR (B) for 2 min. Lysates were precipitated with cIg or anti-Igβ antibodies and analyzed by immunoblotting with antiphosphotyrosine antibodies (left). In a parallel experiment, lysates were immunoprecipitated with anti-Igβ antibodies and immunoblotted with anti-BLNK antibodies (right). Longer exposures of the same blots illustrating the interaction of BLNK with the unstimulated BCR are also presented (bottom). Arrows, migratory position of BLNK. (E) (Left) J558Lμm cells were left untransfected (J558Lμm) or transfected by means of a retrovirus with either wild-type, full-length Igα (J558Lμm/wt Igα) or full-length Igα containing a Y204F mutation (J558Lμm/IgαY204F) and stimulated through the BCR with anti-IgM antibodies (5 × 106 cells/lane). Lysates were precipitated with GST-BLNK-SH2, and associated phosphorylated proteins were detected by antiphosphotyrosine blotting. (Right) Lysates of each cell type (5 × 106 cells) were subjected to anti-Igα immunoprecipitations followed by anti-Igα Western blotting to confirm equal Igα expression in the two transfectants. (F) WEHI-231 cells were labeled with 32PO4 and then stimulated through the BCR for 5 min. Anti-Igα immunoprecipitates were resolved by reducing or nonreducing 2D SDS-PAGE. Excised bands were digested with V8 protease, and eluted peptides were resolved by TLE and then TLC (left). To identify each peptide, a synthetic peptide corresponding to the entire cytoplasmic tail of Igα was phosphorylated with baculovirus-produced Lck and digested with V8 protease and subjected to TLE and TLC (right). Elution of each spot and sequencing using tandem mass spectrometry revealed the listed sequences. O, origin; Y*, phosphotyrosine.
We next examined if, as demonstrated for the PDGFRβ chimeras, the interaction between Igα and the BLNK-SH2 domain was direct. Lysates from unstimulated and BCR-stimulated A20IIA1.6 cells were precipitated with control Ig, antiphosphotyrosine, or anti-Igβ antibodies and immunoblotted with either antiphosphotyrosine (left), GST-BLNK-SH2 (middle), or GST (right) (Fig. 6B). Short exposures revealed a large number of phosphoproteins in the antiphosphotyrosine immunoprecipitates of stimulated cells, and a single phosphorylated band at 33 kDa was detected in the Igβ immunoprecipitation. Subsequent immunoblotting confirmed that this phosphoprotein was Igα (data not shown). When immunoprecipitations were far-Western blotted with GST-BLNK-SH2, Igα was detected in the antiphosphotyrosine and anti-Igβ immunoprecipitations from BCR-stimulated cells (middle). These results demonstrate that the BLNK SH2 domain binds directly to the Igα chain of the endogenous BCR following receptor stimulation.
We next examined the kinetics of Y204 phosphorylation in the endogenous receptor by a time course assay (Fig. 6C). A20IIA1.6 cells were stimulated through the BCR for the times shown, lysed, and subjected to immunoprecipitation with anti-Igβ antibodies. Total Igα phosphorylation was detected by antiphosphotyrosine blotting (top), and Y204-specific phosphorylation was visualized by far-Western blotting with the GST-BLNK-SH2 fusion protein (bottom). The phosphorylation patterns of endogenous Igα and Y204 were strikingly similar to those of Igα and Y204 in the chimeric system (Fig. 4D). Total Igα phosphorylation was transient and peaked at 2 min, whereas Y204 phosphorylation peaked at 1 min and rapidly decreased thereafter.
We next sought to determine if BLNK was recruited to the BCR following ligation. Stimulated A20IIA1.6 lysates were immunoprecipitated with antibodies to the extracellular domain of Igβ and immunoblotted with either antiphosphotyrosine (left) or anti-BLNK antibodies (right). Although short exposures of antiphosphotyrosine blots of Igβ immunoprecipitates detected only phosphorylated Igα (Fig. 6B), longer exposures revealed several coprecipitating bands (Fig. 6A and D). Some of these bands, such as the p40/42 complex, have been characterized previously (41). In the Igβ immunoprecipitates, a phosphoprotein with a relative molecular mass of 65 kDa was detected (Fig. 6D). When similar immunoprecipitations were blotted with anti-BLNK antibodies, only one band was preferentially and reproducibly detected in BCR-stimulated samples. Another band of weaker intensity at 60 kDa was observed in some, but not all, experiments. These data indicate that, upon BCR stimulation, BLNK is recruited to the Igα/Igβ heterodimer. Interestingly, longer exposures revealed a faint anti-BLNK immunoreactive band in precipitations from unstimulated A20IIA1.6 cells (Fig. 6D, bottom). This suggests that the BCR may be preassembled with receptor-coupled substrates as well as their kinases (16, 62).
We next examined whether phosphorylation of Y204 in the endogenous BCR mediated BLNK recruitment. To address this, we used a retrovirus to transfect Igα-negative murine plasmacytoma J558Lμm with constructs containing wild-type and Y204F Igα. Cell populations that expressed similar levels of GFP (by flow cytometry) and Igα (by Western blotting; Fig. 6E, right) were established. After anti-IgM stimulation, cell samples were lysed and subjected to precipitation with the GST-BLNK-SH2 fusion protein. Blotting with antiphosphotyrosine antibodies indicated that the BLNK-SH2 domain precipitated transfected, phosphorylated Igα molecules in an IgM-stimulated, Y204-dependent manner (Fig. 6E, left).
Finally, we sought to determine if Igα Y204 in the endogenous BCR was phosphorylated in vivo following receptor ligation. The demonstrated binding specificity of the BLNK SH2 domain for phospho-Y204, and its direct binding to Igα, made this likely. For these experiments, we used the B-cell line WEHI-231 because BCR stimulation in this cell line induces a very robust tyrosine phosphorylation of cellular substrates including Igα and BLNK is recruited directly to the BCR (data not shown). WEHI-231 cells labeled with 32PO4 were stimulated through the BCR, Igα immunoprecipitates were digested with V8 protease, and Igα peptides were resolved by 2D TLE and TLC (23). Three prominent phosphopeptides, labeled 1, 2, and 3, were observed (Fig. 6F, left). To identify the peptide in each spot, a synthetic peptide corresponding to the entire cytoplasmic tail of Igα was phosphorylated by baculovirus-produced Lck and then digested with V8 protease. After TLE and TLC the same three spots were identified (Fig. 6F, right). Elution of each spot and sequencing using tandem mass spectrometry revealed the indicated sequences (Fig. 6F). A similar pattern of three prominent spots was obtained when in vivo- and in vitro-labeled and V8-digested materials were mixed and resolved by TLE and TLC (data not shown). From these data, we conclude that Y182 and Y204 are phosphorylated following receptor stimulation. Consistent with previous reports (44, 46), we did not detect the phosphorylation of Igα Y193. There was also no detectable phosphorylation of Igα Y176. These results and the results presented in Fig. 4 indicate that, while Y204 phosphorylation is necessary to recruit BLNK, Y176 may contribute to functional coupling of the receptor to BLNK through a phosphorylation-independent mechanism.
DISCUSSION
Within seconds of ligation, the BCR initiates complex signaling pathways which determine cell fate (9, 10). One of the first demonstrable signaling events following BCR ligation is the phosphorylation of the Igα ITAM and the recruitment of Syk (25, 29, 52). Genetic and biochemical evidence indicates that Syk activation is linked to several distal pathways through BLNK, which acts to assemble effectors of these pathways at the plasma membrane (21, 30). However, it was unknown how activated Syk coupled to BLNK. Herein, we demonstrate that BLNK is recruited by its SH2 domain directly to phospho-Y204, which lies carboxy-terminal to the Igα ITAM tyrosines. Using these chimeras, we showed that mutation of Y204 and another functionally important tyrosine, Y176, uncoupled Igα from BLNK-dependent pathways leading to PLC-γ2 and JNK activation. The activation of these pathways was reconstituted when BLNK was added back to the receptor complex. In addition, BLNK and Igα coprecipitate in a Y204-dependent manner in the endogenous BCR. Our data indicate that the close juxtaposition of kinase and substrate on the Igα cytoplasmic tail facilitates the rapid assembly and initiation of several signaling pathways.
Both Igα Y176 and Y204 contribute to coupling the BCR to PLC-γ2 and JNK activation. Mutation of either tyrosine alone resulted in a partial decrease in BLNK phosphorylation, although it did not significantly affect PLC-γ2 phosphorylation or calcium mobilization in response to maximal stimulation through the chimeric receptor. However, mutation of Y204 did attenuate calcium mobilization in response to suboptimal cross-linking (data not shown). Therefore, while both tyrosines function to mediate the phosphorylation of BLNK, Y176 cannot fully compensate for mutation of Y204. However, it is likely that there are other mechanisms that couple the BCR to BLNK activation, as demonstrated by the ability of chimeric Igα molecules which contain only the ITAM and not Y204 to mobilize intracellular calcium (14, 40). Our chimeric Igα receptors with a mutation of Y204 were not completely deficient at inducing BLNK phosphorylation or calcium mobilization.
Although functionally redundant, Y176 and Y204 probably link the BCR to BLNK through different mechanisms. As demonstrated, BLNK is directly recruited through its SH2 domain to phospho-Y204 following receptor ligation. This interaction was also suggested in a recent preliminary report (19). The binding of the BLNK SH2 domain appears highly specific in that only the pYQDV motif in Igα was selected from the array of cytosolic phosphotyrosines available following receptor stimulation (Fig. 4A and 6A and B). Closely related motif YDDV in serine/threonine kinase HPK-1 has also been shown to bind the BLNK SH2 domain (59). These sequences suggest a possible consensus motif of Y-hydrophilic amino acid-D-V. A BLAST search of this motif did not reveal additional potential BLNK binding partners, indicating a very limited array of potential ligands for the BLNK SH2 domain. Furthermore, similar unpaired tyrosines could not be detected within the cytosolic tails of other ITAM-containing receptors. Therefore, the non-ITAM tyrosines of Igα appear to represent a unique signaling mechanism.
In contrast to the role of phospho-Y204 in the activation of BLNK, the mechanism by which Y176 contributes to signaling is unclear. We were unable to detect any protein that specifically bound to either unphosphorylated or phosphorylated Y176. This Y176 motif (YEDE) is not part of any recognizable SH2 or PTB domain consensus sequence (4, 55). Furthermore, there was no detectable phosphorylation of Igα Y176 following receptor stimulation (Fig. 6F). Y176 is only six amino acids from Y182, the binding site for Syk, making it less likely that Y176 independently binds a distinct protein. Rather, the hydroxyl moiety of Y176 might provide a secondary binding site for BLNK or a BLNK-associated protein. This would be consistent with the relatively mild reduction in BLNK phosphorylation observed when only Y176 was mutated.
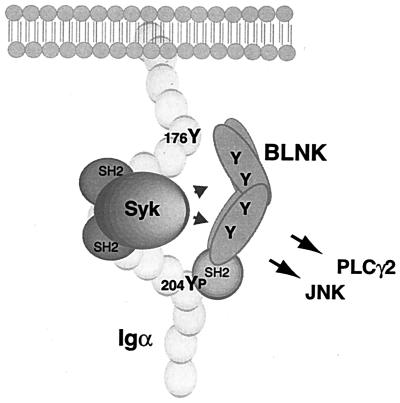
We did not determine if BLNK and Syk bound to the same or to different Igα molecules. If BLNK and Syk were bound in cis, the amino terminus of BLNK, which contains several potential tyrosine phosphorylation sites, might be in close proximity to the catalytic domain of Syk (14, 40) (Fig. 7). Furthermore, numerous proteins in an Igα-based complex, including Syk, BLNK, and other BLNK-associated molecules, could mediate cooperative binding events favoring the assembly of multimeric signalsomes. Alternatively, it might be difficult to accommodate both Syk and BLNK on the same Igα chain, which raises the possibility that they might be recruited to different Igα molecules. The cross-linking of the receptor forms large caps, and proteins associated with adjacent Igα molecules would be brought into close apposition, facilitating the phosphorylation of BLNK in trans. Igβ, which has no non-ITAM tyrosines or obvious recruitment sites for BLNK, cannot compensate for mutation of Y176 and Y204 (54a). Therefore, it is likely that only Igα recruits BLNK to the BCR.
FIG. 7.
Model of Syk and BLNK cooperation in mediating PLC-γ2 and JNK activation. Upon BCR stimulation Syk is recruited to, and activated by, the phosphorylated ITAM tyrosines in the Igα cytoplasmic tail. Subsequent or concurrent recruitment of BLNK to phospho-Y204, through its SH2 domain, brings it into proximity to Syk. We postulate that the secondary, phosphotyrosine-independent association site at Y176 orients the tyrosines of BLNK to the kinase domain of Syk, facilitating BLNK phosphorylation and linkage to downstream pathways.
Both Igα and Igβ contain ITAMs, which, if phosphorylated, should recruit Syk (50). However, this potential does not always translate into competency to signal. Igβ alone induces weak tyrosine phosphorylation (36, 51) and chaotic intracellular calcium mobilizations (15). It is likely that Igβ functions to regulate Igα phosphorylation and lower the activation threshold (42).
Immunoprecipitations from resting B cells demonstrate that the BCR is constitutively associated with Src family kinases and with Syk. Our observation that BLNK coimmunoprecipitates with the unstimulated BCR indicates that a proximal substrate is present as well. The preassembly of these signaling molecules at the BCR may prime the receptor for rapid activation in response to ligation. Alternatively, receptor-facilitated assembly of both kinases and substrate may be required for resting BCR function. Indeed, surface expression of membrane Ig has been demonstrated to be required for maintenance of mature B lymphocytes in the periphery (39). The presence of BLNK may link the receptor to pathways required for survival.
In summary, our observations indicate that coupling of kinase to substrate is accomplished by the direct recruitment of BLNK to Igα. This simple and efficient solution provides an explanation for the rapidity with which the BCR can initiate signals. Furthermore, it provides a model for how receptor assembly, in the absence of ligation, could transmit signals sufficient for cell survival.
Acknowledgments
S. Kabak and B. J. Skaggs contributed equally to this work.
We thank Lee Ann Garrett-Sinha and Leo D. Wang for critical reading of the manuscript and helpful discussions.
This work was supported by National Institutes of Health grants HL07065 (S.K.), AI42787 (A.C.C.), and GM52736 and GM56187 (M.R.C.).
REFERENCES
- 1.Adachi, T., J. Wienands, C. Wakabayashi, H. Yakura, M. Reth, and T. Tsubata. 2001. SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates. J. Biol. Chem. 276:26648-26655. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila, J., S. Takaki, J. D. Kerner, and R. M. Perlmutter. 1997. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 15:125-154. [DOI] [PubMed] [Google Scholar]
- 3.Alexandropoulos, K., G. Cheng, and D. Baltimore. 1995. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc. Natl. Acad. Sci. USA 92:3110-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bork, P., and B. Margolis. 1995. A phosphotyrosine interaction domain. Cell 80:693-694. [DOI] [PubMed] [Google Scholar]
- 5.Burg, D. L., M. T. Furlong, M. L. Harrison, and R. L. Geahlen. 1994. Interactions of Lyn with the antigen receptor during B cell activation. J. Biol. Chem. 269:28136-28142. [PubMed] [Google Scholar]
- 6.Burkhardt, A. L., M. Brunswick, J. B. Bolen, and J. J. Mond. 1991. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc. Natl. Acad. Sci. USA 88:7410-7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustelo, X. R., and M. Barbacid. 1992. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science 256:1196-1199. [DOI] [PubMed] [Google Scholar]
- 8.Cambier, J. C. 1995. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL). Immunol. Today 16:110.. [DOI] [PubMed] [Google Scholar]
- 9.Cambier, J. C., C. M. Pleiman, and M. R. Clark. 1994. Signal transduction by the B cell antigen receptor and its coreceptors. Annu. Rev. Immunol. 12:457-486. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, K. S. 1999. Signal transduction from the B cell antigen-receptor. Curr. Opin. Immunol. 11:256-264. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, K. S., and J. C. Cambier. 1990. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide-linked, inducibly phosphorylated glycoprotein complex. EMBO J. 9:441-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell, M. A., and B. M. Sefton. 1990. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 9:2125-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter, R. H., D. J. Park, S. G. Rhee, and D. T. Fearon. 1991. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc. Natl. Acad. Sci. USA 88:2745-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassard, S., D. Choquet, W. H. Fridman, and C. Bonnerot. 1996. Regulation of ITAM signaling by specific sequences in Ig-β B cell antigen receptor subunit. J. Biol. Chem. 271:23786-23791. [DOI] [PubMed] [Google Scholar]
- 15.Choquet, D., G. Ku, S. Cassard, B. Malissen, H. Korn, W. H. Fridman, and C. Bonnerot. 1994. Different patterns of calcium signaling triggered through two components of the B lymphocyte antigen receptor. J. Biol. Chem. 269:6491-6497. [PubMed] [Google Scholar]
- 16.Clark, M. R., S. A. Johnson, and J. C. Cambier. 1994. Analysis of Ig-α tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-α stimulation of Fyn activity. EMBO J. 13:1911-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements, J. L., S. E. Ross-Barta, L. T. Tygrett, T. J. Waldschmidt, and G. A. Koretzky. 1998. SLP-76 expression is restricted to hematopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J. Immunol. 161:3880-3889. [PubMed] [Google Scholar]
- 18.Cohen, G. B., R. Ren, and D. Baltimore. 1995. Modular binding domains in signal transduction proteins. Cell 80:237-248. [DOI] [PubMed] [Google Scholar]
- 19.Engels, N., B. Wollscheid, and J. Wienands. 2001. Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-α. Eur. J. Immunol. 31:2126-2134. [DOI] [PubMed] [Google Scholar]
- 20.Flaswinkel, H., and M. Reth. 1994. Dual role of the tyrosine activation motif of the Ig-alpha protein during signal transduction via the B cell antigen receptor. EMBO J. 13:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu, C., C. W. Turck, T. Kurosaki, and A. C. Chan. 1998. BLNK: a central linker protein in B cell activation. Immunity 9:93-103. [DOI] [PubMed] [Google Scholar]
- 22.Goitsuka, R., Y.-I. Fujimura, H. Mamada, A. Umeda, K. Morimura, K. Uetsuka, S. Doi, S. Tsuji, and D. Kitamura. 1998. BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J. Immunol. 161:5804-5808. [PubMed] [Google Scholar]
- 23.Gold, M. R., R. Chiu, R. J. Ingham, T. M. Saxton, I. V. Oostveen, J. D. Watts, M. Affolter, and R. Aebersold. 1994. Activation and serine phosphorylation of the p56 lck protein tyrosine kinase in response to antigen receptor cross-linking in B lymphocytes. J. Immunol. 153:2369-2380. [PubMed] [Google Scholar]
- 24.Gold, M. R., D. A. Law, and A. L. DeFranco. 1990. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature 345:810-813. [DOI] [PubMed] [Google Scholar]
- 25.Gold, M. R., L. Matsuuchi, R. B. Kelly, and A. L. DeFranco. 1991. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc. Natl. Acad. Sci. USA 88:3436-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harwood, A. E., and J. C. Cambier. 1993. B cell antigen receptor cross-linking triggers rapid protein kinase C independent activation of p21ras. J. Immunol. 151:4513-4522. [PubMed] [Google Scholar]
- 27.Hashimoto, A., H. Okada, A. Jiang, M. Kurosaki, S. Greenberg, and E. A. Clark. 1998. Involvement of guanoside triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 188:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto, S., A. Iwamatsu, M. Ishiai, K. Okawa, T. Yamadori, M. Matsushita, Y. Baba, T. Kishimoto, T. Kurosaki, and S. Tsukada. 1999. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood 94:2357-2364. [PubMed] [Google Scholar]
- 29.Hutchcroft, J. E., M. L. Harrison, and R. L. Geahlen. 1991. B lymphocyte activation is accompanied by phosphorylation of a 72kDa protein-tyrosine kinase. J. Biol. Chem. 266:14846.. [PubMed] [Google Scholar]
- 30.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 31.Jackman, J. K., D. G. Motto, Q. Sun, M. Tanemoto, C. W. Turck, G. A. Peltz, G. A. Koretzky, and P. R. Findell. 1995. Molecular cloning of SLP-76, a 76 kDa tyrosine phosphoprotein associated with Grb2 in T cells. J. Biol. Chem. 270:7029-7032. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, A., A. Craxton, T. Kurosaki, and E. A. Clark. 1998. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J. Exp. Med. 188:1297-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jumaa, H., B. Wollscheid, M. Mitterer, J. Wienands, M. Reth, and P. J. Nielsen. 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11:547-554. [DOI] [PubMed] [Google Scholar]
- 34.Katsuhiko, H., R. Nittono, N. Okamoto, T. Sachiyo, Y. Hara, R. Goitsuka, and D. Kitamura. 2000. The B cell-restricted adaptor BASH is required for normal development and antigen receptor mediated activation of B cells. Proc. Natl. Acad. Sci. USA 97:2755-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keegan, A. D., and W. E. Paul. 1992. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol. Today 13:63-68. [DOI] [PubMed] [Google Scholar]
- 36.Kim, K. M., G. Alber, P. Weiser, and M. Reth. 1993. Differential signaling through the Ig-alpha and Ig-beta components of the B-cell antigen receptor. Eur. J. Immunol. 23:911-916. [DOI] [PubMed] [Google Scholar]
- 37.Kolanus, W., C. Romeo, and B. Seed. 1993. T cell activation by clustered tyrosine kinases. Cell 74:171-183. [DOI] [PubMed] [Google Scholar]
- 38.Kurosaki, T., S. A. Johnson, L. Pao, K. Sada, H. Yamamura, and J. C. Cambier. 1995. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 182:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam, K.-P., R. Kuhn, and K. Rajewsky. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90:1073-1083. [DOI] [PubMed] [Google Scholar]
- 40.Law, D. A., V. W. F. Chan, S. K. Datta, and A. L. DeFranco. 1993. B-cell antigen receptor motifs have redundant signaling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr. Biol. 3:645-657. [DOI] [PubMed] [Google Scholar]
- 41.Lee, J., P. Luisiri, and M. R. Clark. 1996. A novel complex, p40/42, is constitutively associated with the B cell antigen receptor and phosphorylated upon receptor stimulation. J. Immunol. 157:3828-3837. [PubMed] [Google Scholar]
- 42.Luisiri, P., Y. J. Lee, B. J. Eisfelder, and M. R. Clark. 1996. Cooperativity and segregation of function within the Igα/β heterodimer of the B cell antigen receptor complex. J. Biol. Chem. 271:5158-5163. [DOI] [PubMed] [Google Scholar]
- 43.Minegishi, Y., J. Rohrer, E. Coustan-Smith, H. M. Lederman, R. Pappu, D. Campana, A. C. Chan, and M. E. Conley. 1999. An essential role for BLNK in human B cell development. Science 286:1954-1957. [DOI] [PubMed] [Google Scholar]
- 44.Muller, R., J. Wienands, and M. Reth. 2000. The serine and threonine residues in the Ig-α cytoplasmic tail negatively regulate immunoreceptor tyrosine based activation motif-mediated signal transduction. Proc. Natl. Acad. Sci. USA 97:8451-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novak, E. J., and P. S. Rabinovitch. 1994. Improved sensitivity in flow cytometric intracellular ionized calcium measurement using fluo-3/Fura red fluorescence ratios. Cytometry 17:135-141. [DOI] [PubMed] [Google Scholar]
- 46.Pao, L. I., S. J. Famiglietti, and J. C. Cambier. 1998. Asymmetrical phosphorylation and function of immunoreceptor tyrosine-based activation motif tyrosines in B cell antigen receptor signal transduction. J. Immunol. 160:3305-3314. [PubMed] [Google Scholar]
- 47.Pappu, R., A. M. Cheng, B. Li, Q. Gong, C. Chiu, N. Griffin, M. White, B. P. Sleckman, and A. C. Chan. 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286:1949-1954. [DOI] [PubMed] [Google Scholar]
- 48.Reth, M. 1989. Antigen receptor tail clue. Nature 338:383-384. [PubMed] [Google Scholar]
- 49.Richards, J. D., M. R. Gold, S. L. Hourihane, A. L. DeFranco, and L. Matsuuchi. 1996. Reconstitution of B cell antigen receptor-induced signaling events in a nonlymphoid cell line by expressing the Syk protein-tyrosine kinase. J. Biol. Chem. 271:6458-6466. [DOI] [PubMed] [Google Scholar]
- 50.Rowley, R. B., A. L. Burkhardt, H. G. Chao, G. R. Matsueda, and J. B. Bolen. 1995. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270:11590-11594. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez, M., Z. Misulovin, A. L. Burkhardt, S. Mahajan, T. Costa, R. Franke, J. B. Bolen, and M. Nussenzweig. 1993. Signal transduction by immunoglobulin is mediated through Ig-alpha and Ig-beta. J. Exp. Med. 178:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saouaf, S. J., S. Mahajan, R. B. Rowley, S. A. Kut, J. Fargnoli, A. L. Burkhardt, S. Tsukada, O. N. Witte, and J. B. Bolen. 1994. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc. Natl. Acad. Sci. USA 91:9524-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scharenberg, A. M., O. El-Hillal, D. A. Fruman, L. O. Beitz, Z. Li, S. Lin, I. Gout, L. C. Cantley, D. J. Rawlings, and J.-P. Kinet. 1998. Phosphatidylinositol-3,4,5,-triphosphate (PtdIns-3,4,5P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 17:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siemasko, K., B. J. Eisfelder, C. Stebbins, S. Kabak, A. J. Sant, W. Song, and M. R. Clark. 1999. Igα and Igβ are required for efficient trafficking to late endosomes and to enhance antigen presentation. J. Immunol. 162:6518-6525. [PubMed] [Google Scholar]
- 54a.Siemasko, K., B. Skaggs, S. Kabak, E. Williamson, B. Brown, W. Song, and M. R. Clark. 2002. Receptor-facilitated antigen presentation requires the recruitment of B-cell linker protein to Igα. J. Immunol. 168:2127-2138. [DOI] [PubMed] [Google Scholar]
- 55.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajando, M. M. Chou, H. Hanafusa, B. Schafthausen, and L. C. Cartley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 56.Songyang, Z., S. E. Shoelson, J. McGlade, P. Olivier, T. Pawson, X. R. Bustelo, M. Barbacid, H. Sabe, H. Hanafusa, T. Yi, R. Ren, D. Baltimore, R. A. Feldman, and L. C. Cartley. 1994. Specific motifs recognized by the SH2 Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takata, M., and T. Kurosaki. 1996. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma2. J. Exp. Med. 184:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takata, M., H. Sabe, A. Hata, T. Inazu, Y. Homma, T. Nukada, H. Yamamura, and T. Kurosaki. 1994. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuji, S., M. Okamoto, K. Yamada, N. Okamoto, R. Goitsuka, R. Arnold, F. Kiefer, and D. Kitamura. 2001. B cell adapter containing Src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1. J. Exp. Med. 194:529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, D., J. Feng, R. Wen, J.-C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 61.Wardenburg, J. B., P. Rajita, J.-Y. Bu, B. Mayer, J. Chernoff, D. Straus, and A. C. Chan. 1998. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9:607-616. [DOI] [PubMed] [Google Scholar]
- 62.Wienands, J., O. Larbolette, and M. Reth. 1996. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc. Natl. Acad. Sci. USA 93:7865-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wienands, J., J. Schweikert, B. Wollscheid, H. Jumaa, P. J. Nielsen, and M. Reth. 1998. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 188:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, S., E.-I. Tan, P.-Y. Wong, A. Manickam, S. Ponniah, and K.-P. Lam. 2000. B cell development and activation defects resulting in xid-like immunodeficiency in BLNK/SLP-65 deficient mice. Int. Immunol. 12:397-404. [DOI] [PubMed] [Google Scholar]