Abstract
Self-aggregation of tumor necrosis factor receptor type 1 (TNFR1) induces spontaneous downstream signaling and results in cell death. It has been suggested that silencer of death domain (SODD) binds TNFR1 monomers to prevent self-aggregation. We found that SODD binds through its BAG domain to the ATPase domain of Hsp70. We also determined that SODD binds through its BAG domain to TNFR1. ATP, but not nonhydrolyzable ATP-γS, regulates the SODD binding by Hsp70 or TNFR1. ATP binding by TNFR1 was abolished when a point mutation was introduced into a phosphate-binding loop motif characteristic of ATP-binding proteins, suggesting that TNFR1 functions as an ATPase. Furthermore, TNFR1 was present in aggregates in ATP-depleted cells and SODD disassembled aggregates in vitro only in the presence of ATP. These data suggest that SODD functions as a cofactor analogous to the nucleotide exchange factor BAG-1, which modulates the ATPase cycle of Hsp70 proteins. We propose a new model in which a nucleotide-dependent conformational change in TNFR1 has a key role in regulating TNF signaling.
Cell surface receptors use a variety of mechanisms to initiate signal transduction. In most cases, receptors are phosphorylated or undergo other modifications to fix the active state and amplify the signal. However, death receptors belonging to the tumor necrosis factor receptor (TNFR) superfamily trigger apoptosis by a different process. This is exemplified by TNFR type 1 (TNFR1), one of the best-characterized death receptors. According to the accepted model, TNFR1 forms oligomers upon TNF binding (1), the TNFR-associated death domain (TRADD) adaptor protein then binds to the oligomers through the death domain in the C-terminal region of TNFR1, and TRADD thereupon recruits signaling proteins that activate the apoptosis cascade (12, 13, 41). Activation is also induced by receptor-aggregating antibodies (7, 43) or by the overexpression and self-aggregation of receptors (3, 37). However, recent evidence shows that TNFR1, TNFR2, and Fas receptor chains preassemble into complexes on the cell surface prior to ligand binding (5, 33). Two models have been proposed to explain these data. One holds that ligand trimers induce clustering of preassembled receptor trimers, thereby initiating downstream signaling (8). The other holds that trimerization of the receptor chains does not initiate death signaling but rather that signaling involves rearrangement of the preassembled chains in response to the binding of ligand trimers (22).
Silencer of death domain (SODD) was isolated during a search for proteins interacting with the cytoplasmic portion of death receptor 3 (DR3), a member of the TNFR family (17). SODD also binds to TNFR1 and is released upon TNF binding, and overexpression of SODD suppresses the ability of TNF to induce cell death. It has been suggested that the interaction between SODD and the death domain of TNFR1 inhibits intrinsic receptor self-aggregation and prevents spontaneous signaling (17). SODD has a protein-binding domain characteristic of BAG family proteins (38). Six human protein family members containing the BAG domain are known currently, and BAG-1 is the best characterized of these (39). BAG-1 binds to the ATPase domain of Hsp70 and Hsc70 through its BAG domain, a three-helix bundle near the carboxy-terminal end (35, 40). While Hsp40 facilitates the hydrolysis of ATP bound by Hsp70 or Hsc70, BAG-1 binding induces a conformational change in the ATPase domain, thereby causing an exchange of ADP for ATP (35). This allows BAG-1 to regulate the chaperone activities of Hsp70 and Hsc70, because their binding affinities for an unfolded substrate are strongly influenced by the nucleotide-binding state (2, 11, 23, 40). These observations led to the suggestion that SODD acts as an adaptor protein that targets Hsp70 or Hsc70 to the cytoplasmic domain of TNFR1 to maintain the receptor in a silent state, thereby negatively regulating downstream signaling (44). It has been surmised that the BAG domain is not responsible for binding SODD to the death domain of TNFR1 (44).
We isolated SODD in a yeast two-hybrid screen for proteins that interact with Hsp70 family members and found that the BAG domain of SODD binds competitively to TNFR1 and the Hsp70 ATPase domain. We propose a new model based on these and other observations, wherein TNFR1 is an ATPase whose oligomeric state and function are modulated by SODD binding to the ATPase domain.
MATERIALS AND METHODS
Isolation of cDNA and construction of expression plasmids.
A cDNA encoding the full-length testis-specific HSC70t mouse protein (24, 25) was cloned into the yeast expression vector pAS2-1 (Clontech) and used for screening a mouse testis cDNA library (Clontech) as described previously (26). A cDNA encoding the full-length SODD protein was isolated. The cDNAs for full-length TNFR1 and HSC70 were synthesized from mouse testis total RNA by reverse transcriptase PCR, verified by sequencing, and used as templates for PCR. The cDNA encoding TNFR1 residues 211 to 425 (TNFR1211-425) was ligated into the pFLAG-CMV-2 (Sigma) and pGST-4T-1 (Amersham Pharmacia Biotech) expression vectors. The cDNA encoding SODD residues 2 to 457 was ligated into the pCMV-Tag3 (Stratagene) expression vector that contained the myc-tag sequence. The cDNA for full-length HSC70 was ligated into the pET28a (Novagen) vector for the production of His6-tagged fusion protein. Various HSC70 and SODD deletion mutants were produced by PCR and cloned into pFLAG-CMV-2 and pGEX-4T-1, respectively. GEX and FLAG plasmids encoding SODD312-457 and TNFR1211-425, respectively, were used as templates for site-directed mutagenesis as described previously (27).
In vitro binding assay.
SODD, TNFR1, or HSC70 expression plasmids constructed in pFLAG-CMV-2 were transfected into COS-7 cells by using Lipofectamine reagent (Life Technologies). The COS-7 cells were maintained in Dulbecco modified Eagle medium (DMEM) containing 4.5 g of glucose per liter supplemented with 10% (vol/vol) fetal calf serum (FCS). Two days after transfection, cells were harvested in lysis buffer (140 mM NaCl, 0.1% Triton X-100, 0.1 mM dithiothreitol, protease inhibitor cocktail [Complete; Boehringer Mannheim], 20 mM HEPES buffer [pH 7.4]). Lysates were centrifuged at 18,000 × g for 10 min, and the supernatant was stored at −70°C until use. Glutathione S-transferase (GST)-SODD fusion protein (∼1 μg) was immobilized on glutathione-Sepharose resin (Amersham Pharmacia Biotech) and incubated for 1 h at 4°C in 0.5 ml of lysis buffer with ∼50 μg of COS-7 cell lysate containing FLAG-TNFR1 protein. His6-tagged HSC70 was purified on Ni-nitrilotriacetic acid agarose (Qiagen) and used as a competitor for TNFR1 and SODD interaction. Resins were washed four times with lysis buffer, and proteins binding to GST-SODD were released by treatment with sodium dodecyl sulfate (SDS)-sample buffer. Proteins were analyzed by Western blotting with the M2 antibody to FLAG (Sigma) or monoclonal antibody BB70 (StressGen) to HSP70/HSC70.
For the ATP binding assay, ATP-Sepharose (Upstate Biotechnology) with a capacity of 150 pmol of ATP was incubated with 5 μg of COS-7 cell lysates containing truncated FLAG-TNFR1211-425 in 0.5 ml of lysis buffer for 1 h at 4°C. The resin was washed four times with the same buffer, and proteins were released by treatment with SDS-sample buffer and analyzed by Western blotting as described above.
Analysis of TNFR1 complexes by gel filtration chromatography.
The FLAG-TNFR1211-425 expression plasmid was transfected into COS-7 cells with or without the SODD expression vector constructed in pCMV-Tag3. Ten micrograms of GST or GST-SODD was immobilized on glutathione-Sepharose resin and incubated for 60 min at 4°C with 500 μg of COS-7 cell lysates containing FLAG-TNFR1211-425 and 1 mM ATP in 0.5 ml of lysis buffer. GST or GST-SODD bound to the resin was removed by centrifugation, and the supernatant was fractionated over a 1- by 35-cm Sephacryl S-200 column (Amersham Pharmacia Biotech) equilibrated with HBS buffer (140 nM NaCl, 20 mM HEPES buffer [pH 7.4]). For the experiment with GST-SODD alone (see Fig. 5), lysates were incubated with 10 μg of nonimmobilized GST-SODD because FLAG-TNFR1211-425 binds GST-SODD immobilized to glutathione-Sepharose resin in the absence of ATP. Eluted proteins were detected by Western blotting with anti-FLAG antibody. The column was calibrated with protein standards (Amersham Pharmacia Biotech), including blue dextran, catalase, aldolase, bovine serum albumin, ovalbumin, and chymotrypsinogen A.
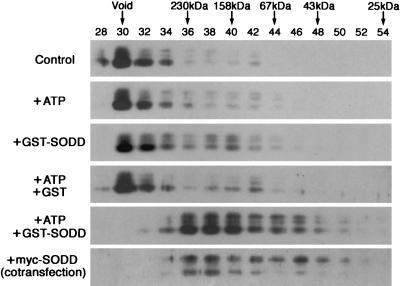
FIG. 5.
Disassembly of TNFR1 aggregates by ATP and SODD. Lysates of COS-7 cells expressing FLAG-TNFR1211-425 were incubated for 60 min at 4°C without ATP (Control), with 1 mM ATP alone, with GST-SODD alone, with GST and ATP, or with GST-SODD and ATP. Following incubation, cell lysates were fractionated over Sephacryl S-200 columns. The fraction numbers are indicated at the top of the gels. Arrows indicate positions where molecular mass marker proteins eluted. Eluted proteins were detected by Western blotting with anti-FLAG antibody. In the bottom gel, COS-7 cells were cotransfected with FLAG-TNFR1211-425 and myc-SODD2-457.
Cross-linking of cell surface proteins.
HeLa cells were cultured with DMEM containing 4.5 g of glucose per liter supplemented with 10% FCS. H9 cells (American Type Culture Collection), a clonal derivative of the Hut 78 cell line, were cultured in RPMI 1640 medium with 2 mM glutamine, 4.5 g of glucose per liter, 1 mM sodium pyruvate, and 10 mM HEPES (pH 7.4) supplemented with 20% (vol/vol) FCS. Confluent HeLa cells on a 100-mm-diameter dish or 107 H9 cells were washed and incubated with phosphate-buffered saline (PBS) containing 100 ng of TNF-α (R&D Systems) per ml for 15 min at 37°C. For ATP depletion, cells were washed with PBS and cultured for 4 h with glucose-free DMEM (Life Technologies) containing 10 μM oligomycin (Sigma) supplemented with 10% (vol/vol) dialyzed FCS (Life Technologies). Intracellular ATP levels were restored by replacing the ATP depletion medium with DMEM containing 4.5 g of glucose per liter supplemented with 10% (vol/vol) FCS.
Cells were washed twice with PBS, and cell surface proteins were cross-linked by incubation with a 2 mM concentration of the thiol-cleavable cross-linker 3,3′-dithiobis(sulfosuccinimidylpropionate) (DTSSP) (Pierce) in PBS for 30 min at 4°C. The reaction was terminated by adding 20 mM Tris-Cl, pH 7.4, followed by additional incubation for 15 min at 4°C. Cells were washed with PBS and harvested in sonication buffer (HBS containing protease inhibitor cocktail). Cell lysates were prepared by brief sonication to disrupt the plasma membrane. After centrifugation at 18,000 × g for 20 min, the pellets were resuspended in sonication buffer containing 1% Triton X-100 and put on ice for 30 min. A soluble fraction containing membrane proteins was obtained by centrifuging lysates at 18,000 × g for 5 min.
Detection of TNFR complexes by SDS-PAGE.
Proteins from HeLa (30 μg) and H9 (20 μg) cells were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with 4 to 15% gradient polyacrylamide gel (Bio-Rad) under reducing or nonreducing (with or without 1% [vol/vol] β-mercaptoethanol, respectively) conditions. For two-dimensional SDS-PAGE, 40 μg of protein was separated by using a 7% polyacrylamide gel under nonreducing conditions. After electrophoresis, gel slices were boiled for 5 min in SDS-sample buffer containing β-mercaptoethanol and loaded onto an 8% polyacrylamide gel. TNFR1 and TNFR2 were detected by Western blotting with anti-TNFR1 antibody (sc-8436; Santa Cruz) or anti-TNFR2 antibody (sc-7862; Santa Cruz). Prestained standard proteins (Bio-Rad) and laminin α subunit (400 kDa) from Engelbreth-Holm-Swarm tumor cells (Life Technologies) were used to estimate protein mass.
Nucleotide sequence accession number.
The sequence of the mouse SODD cDNA reported in this paper has been deposited in GenBank under accession number AF332863.
RESULTS
The BAG domain of SODD is a dual binding domain for Hsc70 and TNFR1.
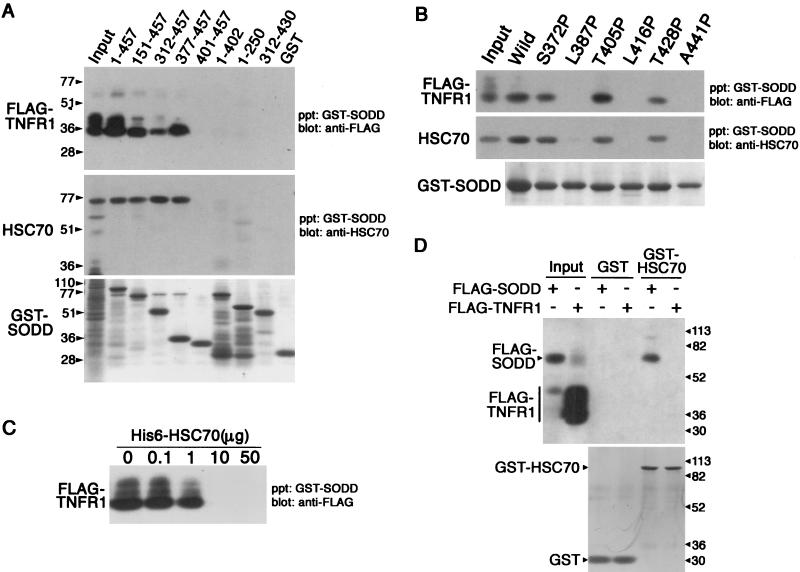
We identified SODD as an Hsc70-binding protein and found that it also had been reported to bind to TNFR1 (17). To determine how SODD binds to Hsc70 and TNFR1, we mapped the binding domains on SODD for these two proteins (Fig. 1A). Wild-type or deletion mutant GST-SODD proteins were immobilized on glutathione-Sepharose and incubated with lysates of COS-7 cells expressing transfected FLAG-TNFR1211-425 and endogenous HSC70. The C-terminal region of SODD containing the BAG domain bound HSC70 as expected. However, an unanticipated finding was that the same region of SODD also bound FLAG-TNFR1211-425. Site-directed mutagenesis was used to determine if these interactions were specific and involved the BAG domain. Chou-Fasman analysis predicts that the BAG domain contains three α-helices, corresponding to amino acid residues 381 to 403, 408 to 426, and 435 to 453. When any of the putative α-helices were disturbed by a proline substitution, HSC70 and FLAG-TNFR1211-425 binding was abolished, while a proline substitution in the vicinity of the helices did not affect binding (Fig. 1B). This implies that α-helices in the BAG domain form a signature motif essential for Hsc70 and FLAG-TNFR1211-425 binding. It also is consistent with structural analyses indicating that the BAG domain of BAG-1 forms a three-helix bundle that contacts the ATPase domain of Hsc70 (35). In addition, His6-HSC70 competed with FLAG-TNFR1211-425 for binding to SODD (Fig. 1C). These results provide strong evidence that SODD binds to HSC70 and FLAG-TNFR1211-425 by its BAG domain. Because SODD binds TNFR1 in the yeast two-hybrid system (17) and GST-HSC70 binds FLAG-SODD but not FLAG-TNFR1211-425 (Fig. 1D), we conclude that HSC70 is unlikely to be an adaptor protein that targets SODD to TNFR1 or specifies the TNFR1 binding site on SODD.
FIG. 1.
BAG domain is responsible for SODD binding to TNFR1 and HSC70. Binding assays were performed with GST-SODD deletion mutants (A) or GST-SODD312-457 point mutants (B) immobilized on glutathione-Sepharose and incubated with lysates of COS-7 cells expressing endogenous HSC70 and transfected FLAG-TNFR1211-425. Proteins associating with SODD were eluted from the glutathione-Sepharose precipitate (ppt), separated by SDS-PAGE, and detected by Western blotting with either anti-FLAG (top gels) or anti-HSP70/HSC70 (middle gels) antibodies. Minor bands with slower mobility probably correspond to phosphorylated species (45). The gels also were analyzed by Coomassie blue staining to visualize GST-SODD mutant proteins (bottom gels). Input lanes shown in the top and middle gels received 10 and 20% of the total cell lysates, respectively. (C) GST-SODD1-457 was used for binding assays as described above, except that cell lysates containing the indicated amounts of His6-HSC70 were used. (D) GST or GST-HSC70 was used for binding to FLAG-SODD2-457 or FLAG-TNFR1211-425 as described above. Input lanes received 10% of the total cell lysates. The bottom gel shows the results of Coomassie blue staining to visualize GST and GST-HSC70 in eluants. Protein standards (in kilodaltons) are shown to the left of the gels in panel A and to the right of the gels in panel D.
TNFR1 is an ATPase.
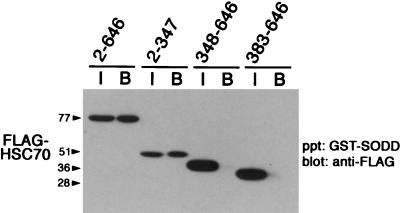
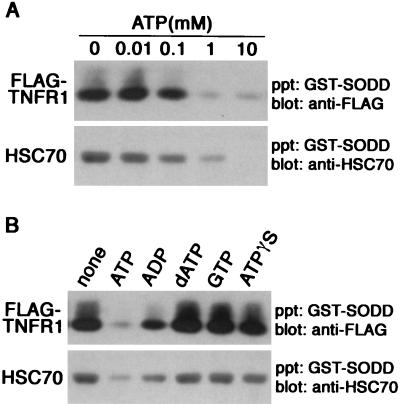
Upon finding that SODD binding to HSC70 and FLAG-TNFR1211-425 is abolished by disruption of the secondary structure of the BAG domain (Fig. 1B), we next examined whether the BAG domain of SODD binds a motif common to HSC70 and FLAG-TNFR1211-425. We found that GST-SODD binds FLAG-HSC702-347 containing the ATPase domain but not FLAG-HSC70348-646 or FLAG-HSC70383-646 lacking this domain (Fig. 2). This suggested that SODD binds to an ATPase domain or a related structure in TNFR1. Because binding of ATP causes a change in conformation of HSC70 and alters its association with other proteins (2, 29, 40), we determined whether ATP affected binding of SODD to FLAG-TNFR1211-425 or HSC70. The addition of 0.1 mM ATP to lysates of transfected COS-7 cells reduced the association of SODD with both (Fig. 3A). A greater reduction occurred when ATP was added than when ADP, dATP, or GTP was added. Nonhydrolyzable ATP-γS failed to substitute for ATP, indicating that ATP hydrolysis rather than simple ATP binding is required for the regulation of TNFR1 binding by SODD (Fig. 3B). FLAG-TNFR1211-425 expressed in COS-7 cells also bound to ATP-Sepharose (Fig. 4A, gel labeled Wild), and the binding was inhibited by the addition of ATP. These results suggested that the BAG domain of SODD associates with an ATPase domain in TNFR1.
FIG. 2.
SODD binding domain on HSC70. GST-SODD1-457 was immobilized on glutathione-Sepharose and incubated with lysates of COS-7 cell expressing mutant FLAG-HSC70 proteins (lanes B [bound]). Proteins that bound were analyzed by Western blotting with anti-FLAG antibody. Input lanes (I) received 20% of the total cell lysates. Protein standards (in kilodaltons) are shown to the left. ppt, precipitate.
FIG. 3.
ATP regulates interaction between SODD and TNFR1. GST-SODD1-457 was immobilized on glutathione-Sepharose and incubated with lysates of COS-7 cells expressing endogenous HSC70 and transfected FLAG-TNFR1211-425 in the presence of ATP at the indicated concentrations (A) or of no nucleotide or 1 mM concentrations of ATP or other nucleotides (B). Binding proteins were analyzed as described in the legend to Fig. 1. precipitate.
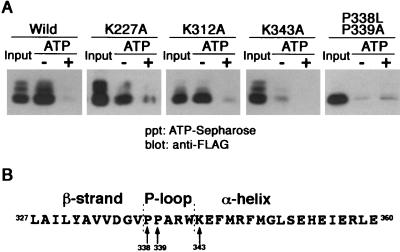
FIG. 4.
TNFR1 possesses ATP-binding activities. (A) ATP-Sepharose was incubated with lysates of COS-7 cells expressing wild-type or mutant FLAG-TNFR1211-425 proteins in the absence (−) or presence (+) of 10 mM ATP. Proteins that bound in the presence or absence of exogenous ATP were analyzed by Western blotting with anti-FLAG antibody. Input lanes received 50% of the total cell lysates. (B) The secondary structure of mouse TNFR1 was analyzed using Chou-Fasman methods. The predicted secondary structure is shown above the amino acid sequence. Arrows indicate amino acid residues analyzed by mutagenesis. The numbers correspond to amino acid residues of mouse TNFR1 (21). ppt, precipitate.
ATP- and GTP-binding proteins usually contain a phosphate-binding loop (P-loop) with the consensus sequence [G/A](X)4GK[S/T] (46). Although TNFR1 lacks a typical P-loop consensus sequence, this motif is not apparent in some ATP- and GTP-binding proteins. To determine if TNFR1 contains an atypical ATP-binding domain, we focused on the consensus lysine residue of the P-loop that is directed towards and interacts with the phosphate of ATP (32, 34). Comparison of the amino acid sequences of mouse and human TNFR1 revealed that three conserved lysine residues are present in the cytoplasmic region of the protein (21). Their possible role in ATP binding was examined by site-directed mutagenesis. Although K227A or K312A substitutions did not affect ATP binding, the K343A substitution dramatically reduced ATP binding to FLAG-TNFR1211-425 (Fig. 4A). Structural analysis of ATP- and GTP-binding proteins indicated that a β strand always precedes the P-loop and that a lysine residue begins the α-helix following the P-loop (46). Chou-Fasman analysis predicts that the secondary structure of TNFR1 surrounding K343 has this consensus secondary structure (Fig. 4B). An additional feature frequently present in the P-loop is one or more proline residues that are believed to provide flexibility. ATP binding to FLAG-TNFR1211-425 was reduced by the double substitution of P338L and P339A (Fig. 4A). The P-loop motif of TNFR1 is located near the N terminus of the death domain, and a similar sequence is present in DR3 but is not apparent in other death receptors. It remains to be determined if DR3 or other death receptors bind ATP.
SODD is a cofactor for TNFR1 disassembly.
The observation that HSC70 competes with FLAG-TNFR1211-425 for binding to SODD (Fig. 1C) argues against the proposal that SODD is an adaptor protein that targets Hsp70 and Hsc70 to the cytoplasmic domain of TNFR1 and inhibits self-aggregation until TNF binding occurs (44). To further examine the interactions between these three proteins, we analyzed the FLAG-TNFR1211-425 complex present in COS-7 cell lysates by gel filtration chromatography over a Sephacryl S-200 column. The majority of FLAG-TNFR1211-425 eluted in the void volume (Fig. 5). This was determined by Superose 6 column chromatography to be a complex of ∼2 MDa (data not shown), which is consistent with data indicating that TNFR1 forms aggregates by interacting through the death domain (3, 37). FLAG-TNFR1211-425 remained in the ∼2-MDa complex upon addition of ATP or GST-SODD (immobilized on glutathione-Sepharose resin) alone or ATP plus GST but was found in ∼200-kDa complexes when cell lysates were incubated with both ATP and GST-SODD (Fig. 5). This may be an intermediate complex that is incompletely disassembled or a complex of TNFR1211-425 with unknown proteins. However, this complex was free of GST-SODD, which remained immobilized on the resin (see Materials and Methods). Furthermore, GST-SODD bound TNFR1 in the ∼2-MDa complex but not in the ∼200-kDa complex after fractionation (data not shown). These results argue against the proposal that SODD associates with the death domain of monomeric TNFR1 to inhibit intrinsic self-aggregation (17). They suggest instead that SODD associates with TNFR1 aggregates and serves as a cofactor in the disassembly process upon binding or hydrolysis of ATP.
ATP depletion causes aggregation of TNFR1 in the plasma membrane.
The oligomeric status of TNFR1 in the plasma membrane was analyzed under physiological and ATP-depleted conditions. HeLa cells were treated with DTSSP to cross-link cell surface proteins, and TNFR1 complexes were then analyzed by SDS-PAGE under reducing or nonreducing conditions (Fig. 6A, TNFR1 gels). Although much of the TNFR1 resides in the Golgi apparatus and is inaccessible to the cross-linker (18), substantial amounts of TNFR1 aggregates that exhibit a molecular size approximately three times the unit size were found, which is consistent with a previous report of ligand-independent receptor trimer assembly (5). As expected, these aggregates increased in amount upon treatment with TNF-α for 15 min.
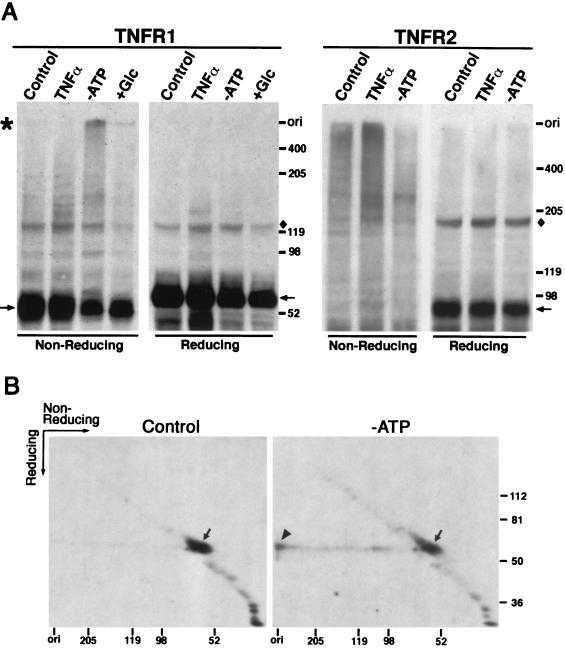
FIG. 6.
TNFR1 aggregates form in ATP-depleted cells. HeLa and H9 cells were treated with 100 ng of TNF-α per ml for 15 min (TNFα) or with glucose-free DMEM containing 10 μM oligomycin for 4 h (−ATP). The ATP depletion medium for HeLa cells was then replaced with DMEM containing 4.5 g of glucose per liter and incubated for an additional 2 h (+Glc). Cell surface proteins were cross-linked by DTSSP and solubilized with 1% Triton X-100. (A) Cell lysates were separated by SDS-PAGE under reducing or nonreducing conditions. TNFR1 and TNFR2 from HeLa and H9 cells, respectively, were detected by Western blotting. Shown are the positions of protein monomers (arrows), TNFR1 aggregates (asterisk), an unidentified protein recognized by the rabbit antiserum (diamonds), and the origin of the separating gel (ori). (B) HeLa cell lysates were separated by SDS-PAGE under nonreducing conditions for the first dimension. The gel was then boiled in SDS-sample buffer containing β-mercaptoethanol, and the proteins were separated in the second dimension. TNFR1 was detected by Western blotting. The arrowhead indicates the spot of TNFR1 aggregates that stacked on top of the gel in the first dimension, while arrows indicate the locations of the TNFR1 monomers. The protein standards (in kilodaltons) are shown on the right and bottom of the gels.
We next cultured HeLa cells in glucose-free medium containing oligomycin, an inhibitor of mitochondrial F0F1-ATPase, to reduce intracellular ATP levels. This treatment blocks the production of ATP by both glycolysis and oxidative phosphorylation, and intracellular ATP becomes undetectable within 60 min (6, 19). When cells were cultured under these conditions for 4 h, TNFR1 aggregates that migrated more slowly than a 400-kDa marker protein were present and the relative amount of TNFR1 monomers was reduced (Fig. 6A). TNFR1 aggregates were not seen when cell lysates were analyzed under conditions that cleaved the cross-linker. Proteins in the complex were confirmed to be TNFR1 by two-dimensional SDS-PAGE under nonreducing conditions in the first dimension and then under reducing conditions in the second. Monomers of TNFR1 (Fig. 6B) appeared in a diagonal line, while cross-linked TNFR1 that was cleaved under reducing conditions migrated with a mass of 60 kDa and formed a horizontal line (Fig. 6B, right gel). The analysis of ATP-depleted HeLa cells cultured for 4 h without ATP showed that TNFR1 derived from the complexes that had remained at the top of the nonreducing gel migrated into the gel under reducing conditions (Fig. 6B, right gel). TNFR2 also transduces the TNF signal but lacks a death domain on its cytoplasmic portion. TNFR2 aggregates were observed in H9 cells (Fig. 6A, TNFR2 gels), and TNF-α treatment for 15 min facilitated aggregate formation. However, ATP depletion for 4 h did not induce the accumulation of TNFR2 aggregates but rather reduced them. This indicates that aggregate formation in response to ATP depletion is not a general characteristic of all members of the TNFR superfamily but is specific to TNFR1.
Cells with reduced ATP levels show an increase in ATP to a new steady-state level within 15 min after addition of glucose (19). TNFR1 aggregates were reduced and the relative amount of monomers was increased when cells depleted of ATP for 4 h were treated for 2 h with a medium containing glucose (Fig. 6A, lanes +Glc), which suggests that TNFR1 aggregation is reversible and dependent on intracellular ATP levels. TNFR1 aggregation occurs spontaneously in the plasma membrane (Fig. 6A) (5), but we suggest that under physiological conditions this is a dynamic process and the aggregates are disassembled immediately after their formation. In support of this suggestion, we note that disassembled FLAG-TNFR1211-425 was observed in vivo when COS-7 cells were cotransfected with myc-SODD2-457 (Fig. 5). This suggests that the intracellular ATP level usually is sufficient for SODD to facilitate disassembly of TNFR1 aggregates. From these results, we conclude that in the presence of normal ATP levels SODD serves as a cofactor for disassembly of self-aggregated TNFR1.
DISCUSSION
The ATPase domain of TNFR1 is a novel cell surface receptor feature.
The identification of an ATPase domain in TNFR1 arose from the unexpected finding that the BAG domain of SODD binds to both Hsc70 and TNFR1. The BAG domain recently was reported to form a three-helix bundle that binds BAG-1 to the Hsc70 ATPase domain, thereby inducing a conformation incompatible with nucleotide binding (35). Because the α-helix triplet of the BAG domain binds SODD to Hsc70 and TNFR1, we hypothesized that TNFR1 contains an ATPase domain. This hypothesis was supported by the following findings. (i) Hsc70 and TNFR1 compete for binding to SODD. (ii) TNFR1 interaction with SODD is inhibited by ATP. (iii) FLAG-TNFR1211-425 binds ATP. (iv) TNFR1 contains a P-loop motif. (v) Mutations in the P-loop motif abolish ATP binding by TNFR1.
Nuclear magnetic resonance studies showed that the death domains of the Fas receptor and the Fas-associated death domain (FADD) adaptor protein are folded similarly and contain six antiparallel α-helices (14, 16). Because of the conserved structure of death domains, it is surprising that a P-loop sequence is not apparent in the Fas receptor. However, the P-loop of TNFR1 is located at the N terminus of the death domain, and Chou-Fasman analysis predicts that TNFR1 assumes a β-strand structure (Fig. 4C) instead of the α1-helix structure present in the Fas receptor. The sequences in this area are diverse among the different death receptors, except for TNFR1 and DR3. In addition, Lys (K343 of TNFR1) is a key amino acid in the P-loop because it interacts by its positive charge with the phosphate of ATP, but DR4 and DR5 have Asp and Glu, respectively, in this position (32, 34). These sequence differences may explain why SODD binds TNFR1 and DR3 but not other death receptors (17). Point mutagenesis has been used to search for the amino acids required for death signaling, and some of these are located in TNFR1 between K343 and the C terminus (42). The corresponding region of Fas includes the amino acids required for binding FADD (14), and it is the region where monomers bind by their death domains to form trimers. We hypothesize that the N-terminal region of the death domain, together with the adjacent sequence, serves as a receptor-specific regulatory motif that modulates the oligomeric states of death receptors. However, structural analysis will be necessary to test this hypothesis.
The TNFR1 ATPase cycle is regulated by SODD.
It has been shown that SODD binds to the death domain of monomeric TNFR1 and prevents self-aggregation (17). However, we found that TNFR1 interaction with SODD is inhibited by ATP but not by nonhydrolyzable ATP-γS, indicating that ATP hydrolysis is required for the regulation of TNFR1 binding by SODD. We also learned that self-aggregated TNFR1 binds SODD and disassembles into oligomers upon addition of ATP, suggesting that ATP has an important role in the silencing of TNFR1. It appeared that the interaction between SODD and TNFR1 was similar to the one between BAG-1 and Hsc70, in which BAG-1 serves as a nucleotide exchange factor for the Hsc70 ATPase cycle (35). In this cycle, conformational changes in Hsc70 in response to binding of ATP or ADP modulate its peptide binding affinity (10). The conversion of the ATP-bound form of Hsc70 into the ADP-bound state occurs upon Hsp40-stimulated ATP hydrolysis, resulting in a stable association of Hsc70 with an unfolded peptide. ADP dissociation from Hsc70 destabilizes the complex, and the peptide dissociates upon ATP binding to Hsc70. Although BAG-1 does not participate in protein refolding (2, 28), it acts as the nucleotide exchange factor in this cycle (11, 35). Because SODD is a member of the BAG family, we hypothesized that SODD is a nucleotide exchange factor associated with the TNFR1 disassembly process and that ATP binding causes a conformational change in TNFR1, leading to dissociation of TNFR1 complexes and silencing of the death signal. A corollary of the hypothesis is that a change in TNFR1 conformation upon ATP hydrolysis allows spontaneous aggregation to occur. However, this is probably the rate-limiting step in the ATPase cycle of TNFR1, with physiological levels of ATP causing SODD to quickly disassemble self-aggregated TNFR1, shifting the equilibration towards the monomeric state. To confirm this model, the enzymatic characteristics of TNFR1 and the stoichiometry of this reaction will need to be determined.
BAG proteins as nucleotide exchange factors in cellular events.
A conformational change upon ATP binding is common in protein-protein interactions, assembly and disassembly of protein complexes, and other dynamic processes. For example, N-ethylmaleimide-sensitive fusion protein oligomerizes into hexamers in the presence of ATP and changes shape upon ATP hydrolysis (9, 20). Similarly, structural differences in p97 hexamers were found between ATP- and ADP-bound states (30). Because members of the AAA (ATPase associated with various cellular activities) protein family have the conserved ATPase motifs found in N-ethylmaleimide-sensitive fusion protein and p97, many other proteins apparently are capable of conformational change that is dependent on their nucleotide-bound state. It is noteworthy that Apaf-1, which is not an AAA protein, oligomerizes into biologically active complexes in the presence of dATP and cytochrome c (4, 31). Our data suggest that TNFR1 undergoes such a conformational change during its assembly-disassembly cycle. Because ATP regulates the oligomeric state of TNFR1, this receptor fits the definition of an oligomeric ATPase. In such proteins, ATP hydrolysis causes oligomer formation and/or modification of interactions with other proteins. To maintain the cycle, an ATPase needs to release ADP and bind ATP to return to the ready state. However, the mechanisms and factors involved in this step are well studied only for Hsc70/Hsp70/DnaK. We anticipate that other BAG proteins will be found to serve as nucleotide exchange factors that regulate ATPase cycles associated with various cellular activities.
Possible mode of action of Hsc70.
Coimmunoprecipitation experiments demonstrated that SODD binds TNFR1 (17) or Hsc70 (our unpublished results). We showed in this study that the BAG domain of SODD binds to both Hsc70 and TNFR1 and that Hsc70 competed with TNFR1 for binding to SODD. These results suggest that SODD forms a binary complex with TNFR1 rather than a ternary complex with TNFR1 and Hsc70. If Hsc70/Hsp70 binding to SODD leads to conformational changes that allow it to bind to TNFR1, overexpressed Hsp70 could diminish TNF signaling by acceleration of receptor disassembly and thereby support cell survival (15). This is consistent with the general chaperone function of Hsc70, the binding and delivery of a partner protein to target proteins. Alternatively, Hsc70/Hsp70 and TNFR1 may compete for SODD in vivo. BAG-1 binds the catalytic domain of the protein kinase Raf-1 to stimulate its activity (47). Interestingly, Hsp70 and Raf-1 compete for binding to the BAG domain of BAG-1 even though the binding sites do not completely overlap (36). Under stressful conditions that induce Hsp70, BAG-1 bound to Raf-1 is replaced by Hsp70. This results in inhibition of DNA synthesis and cell growth because of diminished Raf-1 signaling (36). SODD may function in the stress response to determine if cell death or survival occurs, while Hsc70/Hsp70 may modulate SODD interaction with TNFR1 by functioning like a sensor, as in Raf-1 signaling.
TNF signaling.
The knowledge that TNFR1 has ATPase activity changes our understanding of TNF-triggered signaling and spontaneous signaling by this receptor. The general concept has been that ligand binding causes receptor monomers to form trimeric complexes that recruit signal transduction proteins to associate with the cytoplasmic death domain of the receptor (1). However, it has been shown recently that TNF receptors constitutively form ligand-independent receptor trimers through conserved domains in the extracellular region (5). This suggests that signaling is not due to receptor trimerization but involves rearrangement of the preassembled chains. We propose that TNF binding changes the nucleotide-bound state of preassembled TNFR1, inducing a conformational change in the cytoplasmic portion of the receptor, and thereby causes the death domain to recruit adaptors for downstream signaling. An alternative proposal is that ligand binding causes formation of clusters from preassembled receptor trimers, inducing the signal transduction cascade that leads to cell death (8). In either case, the role of SODD is to promote disassembly of randomly formed TNFR1 aggregates and prevent spontaneous signaling.
Acknowledgments
We thank Masuo Goto for helpful technical advice and valuable discussions.
REFERENCES
- 1.Banner, D. W., A. D'Arcy, W. Janes, R. Gentz, H. J. Schoenfeld, C. Broger, H. Loetscher, and W. Lesslauer. 1993. Crystal structure of the soluble human 55 kd TNF receptor-human TNFβ complex: implications for TNF receptor activation. Cell 73:431-445. [DOI] [PubMed] [Google Scholar]
- 2.Bimston, D., J. Song, D. Winchester, S. Takayama, J. C. Reed, and R. I. Morimoto. 1998. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 17:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldin, M. P., I. L. Mett, E. E. Varfolomeev, I. Chumakov, Y. Shemer-Avni, J. H. Camonis, and D. Wallach. 1995. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J. Biol. Chem. 270:387-391. [DOI] [PubMed] [Google Scholar]
- 4.Cain, K., D. G. Brown, C. Langlais, and G. M. Cohen. 1999. Caspase activation involves the formation of the aposome, a large (∼700 kDa) caspase-activating complex. J. Biol. Chem. 274:22686-22692. [DOI] [PubMed] [Google Scholar]
- 5.Chan, F. K., H. J. Chun, L. Zheng, R. M. Siegel, K. L. Bui, and M. J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288:2351-2354. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi, Y., S. Shimizu, and Y. Tsujimoto. 1997. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57:1835-1840. [PubMed] [Google Scholar]
- 7.Engelmann, H., H. Holtmann, C. Brakebusch, Y. S. Avni, I. Sarov, Y. Nophar, E. Hadas, O. Leitner, and D. Wallach. 1990. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J. Biol. Chem. 265:14497-14504. [PubMed] [Google Scholar]
- 8.Golstein, P. 2000. FasL binds preassembled Fas. Science 288:2328-2329. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, P. I., R. Roth, H. Morisaki, R. Jahn, and J. E. Heuser. 1997. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90:523-535. [DOI] [PubMed] [Google Scholar]
- 10.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 11.Höhfeld, J., and S. Jentsch. 1997. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 16:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 13.Hsu, H., H. Shu, M. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 14.Huang, B., M. Eberstadt, E. T. Olejniczak, R. P. Meadows, and S. W. Fesik. 1996. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384:638-641. [DOI] [PubMed] [Google Scholar]
- 15.Jäättelä, M., D. Wissing, P. A. Bauer, and G. C. Li. 1992. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 11:3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong, E. J., S. Bang, T. H. Lee, Y. I. Park, W. S. Sim, and K. S. Kim. 1999. The solution structure of FADD death domain. Structural basis of death domain interactions of Fas and FADD. J. Biol. Chem. 274:16337-16342. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Y., J. D. Woronicz, W. Liu, and D. V. Goeddel. 1999. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 283:543-546. [DOI] [PubMed] [Google Scholar]
- 18.Jones, S. J., E. C. Ledgerwood, J. B. Prins, J. Galbraith, D. R. Johnson, J. S. Pober, and J. R. Bradley. 1999. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 162:1042-1048. [PubMed] [Google Scholar]
- 19.Leist, M., B. Single, A. F. Castoldi, S. Kühnle, and P. Nicotera. 1997. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenzen, C. U., D. Steinmann, S. W. Whiteheart, and W. I. Weis. 1998. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell 94:525-536. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, M., L. A. Tartaglia, A. Lee, G. L. Bennett, G. C. Rice, G. H. Wong, E. Y. Chen, and D. V. Goeddel. 1991. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. USA 88:2830-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 23.Lüders, J., J. Demand, O. Papp, and J. Höhfeld. 2000. Distinct isoforms of the cofactor BAG-1 differentially affect Hsc70 chaperone function. J. Biol. Chem. 275:14817-14823. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa, M., D. A. O'Brien, R. L. Allen, and E. M. Eddy. 1989. Heat-shock cognate protein (hsc71) and related proteins in mouse spermatogenic cells. Biol. Reprod. 40:843-852. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto, M., and H. Fujimoto. 1990. Cloning of a hsp70-related gene expressed in mouse spermatids. Biochem. Biophys. Res. Commun. 166:43-49. [DOI] [PubMed] [Google Scholar]
- 26.Miki, K., and E. M. Eddy. 1998. Identification of tethering domains for protein kinase A type Iα regulatory subunits on sperm fibrous sheath protein FSC1. J. Biol. Chem. 273:34384-34390. [DOI] [PubMed] [Google Scholar]
- 27.Miki, K., and E. M. Eddy. 1999. Single amino acids determine specificity of binding of protein kinase A regulatory subunits by protein kinase A anchoring proteins. J. Biol. Chem. 274:29057-29062. [DOI] [PubMed] [Google Scholar]
- 28.Nollen, E. A., J. F. Brunsting, J. Song, H. H. Kampinga, and R. I. Morimoto. 2000. Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol. Cell. Biol. 20:1083-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palleros, D. R., W. L. Welch, and A. L. Fink. 1991. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc. Natl. Acad. Sci. USA 88:5719-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouiller, I., V. M. Butel, M. Latterich, R. A. Milligan, and E. M. Wilson-Kubalek. 2000. A major conformational change in p97 AAA ATPase upon ATP binding. Mol. Cell 6:1485-1490. [DOI] [PubMed] [Google Scholar]
- 31.Saleh, A., S. M. Srinivasula, S. Acharya, R. Fishel, and E. S. Alnemri. 1999. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 274:17941-17945. [DOI] [PubMed] [Google Scholar]
- 32.Saraste, M., P. R. Sibbald, and A. Wittinghofer. 1990. The P-loop: a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15:430-434. [DOI] [PubMed] [Google Scholar]
- 33.Siegel, R. M., J. K. Frederiksen, D. A. Zacharias, F. K. Chan, M. Johnson, D. Lynch, R. Y. Tsien, and M. J. Lenardo. 2000. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 288:2354-2357. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova, I. N., V. N. Kasho, and L. D. Faller. 1998. Inferences about the catalytic domain of P-type ATPases from the tertiary structures of enzymes that catalyze the same elementary reaction. FEBS Lett. 431:309-314. [DOI] [PubMed] [Google Scholar]
- 35.Sondermann, H., C. Scheufler, C. Schneider, J. Höhfeld, F. U. Hartl, and I. Moarefi. 2001. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291:1553-1557. [DOI] [PubMed] [Google Scholar]
- 36.Song, J., M. Takeda, and R. I. Morimoto. 2001. Bag1-Hsp70 mediates a physiological stress signaling pathway that regulates Raf-1/ERK and cell growth. Nat. Cell Biol. 3:276-282. [DOI] [PubMed] [Google Scholar]
- 37.Song, H. Y., J. D. Dunbar, and D. B. Donner. 1994. Aggregation of the intracellular domain of the type 1 tumor necrosis factor receptor defined by the two-hybrid system. J. Biol. Chem. 269:22492-22495. [PubMed] [Google Scholar]
- 38.Takayama, S., Z. Xie, and J. C. Reed. 1999. Evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J. Biol. Chem. 274:781-786. [DOI] [PubMed] [Google Scholar]
- 39.Takayama, S., and J. C. Reed. 2001. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 3:E237-E241. [DOI] [PubMed] [Google Scholar]
- 40.Takayama, S., D. M. Bimston, S. Matsuzawa, B. C. Freeman, C. Aime-Sempe, Z. Xie, R. I. Morimoto, and J. C. Reed. 1997. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16:4887-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tartaglia, L. A., and D. V. Goeddel. 1992. Two TNF receptors. Immunol. Today 13:151-153. [DOI] [PubMed] [Google Scholar]
- 42.Tartaglia, L. A., T. M. Ayres, G. H. Wong, and D. V. Goeddel. 1993. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845-853. [DOI] [PubMed] [Google Scholar]
- 43.Trauth, B. C., C. Klas, A. M. Peters, S. Matzku, P. Moller, W. Falk, K. M. Debatin, and P. H. Krammer. 1989. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245:301-305. [DOI] [PubMed] [Google Scholar]
- 44.Tschopp, J., F. Martinon, and K. Hofmann. 1999. Apoptosis: silencing the death receptors. Curr. Biol. 9:R381-R384. [DOI] [PubMed] [Google Scholar]
- 45.Van Linden, A. A., V. Cottin, C. Leu, and D. W. Riches. 2000. Phosphorylation of the membrane proximal region of tumor necrosis factor receptor CD120a (p55) at ERK consensus sites. J. Biol. Chem. 275:6996-7003. [DOI] [PubMed] [Google Scholar]
- 46.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, H. G., S. Takayama, U. R. Rapp, and J. C. Reed. 1996. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc. Natl. Acad. Sci. USA 93:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]