Abstract
The transcription factor NF-κB regulates gene expression involved in cell growth and survival and has been implicated in progression of hormone-independent breast cancer. By expressing a dominant-active form of mitogen-activated protein kinase kinase kinase 1, by exposure to tumor necrosis factor alpha, or by overexpression of p50/p65, we show that NF-κB activates a transcription regulatory element of the prostate-specific antigen (PSA)-encoding gene, a marker for prostate cancer development, treatment, and progression. By DNase I footprinting, we identified four NF-κB binding sites in the PSA core enhancer. We also demonstrate that androgen-independent prostate cancer xenografts have higher constitutive NF-κB binding activity than their androgen-dependent counterparts. These results suggest a role of NF-κB in prostate cancer progression.
Prostate cancer begins as an androgen-dependent (AD) tumor and regresses in response to androgen ablation therapy. This therapy eventually fails, and the tumor progresses despite castrate levels of androgen. This stage of the disease is referred to as androgen-independent (AI) or hormone-refractory prostate cancer. The prostate-specific antigen (PSA) level in serum is a clinical marker for prostate cancer progression and effectiveness of treatment. Rising PSA levels typically signal progression from androgen dependence to androgen independence. Biochemical and genetic analyses have identified two regulatory regions responsible for androgen-regulated, tissue-specific PSA expression--the proximal promoter containing a core TATA box and an enhancer region located approximately 4.2 kb upstream of the transcription start site (7, 19, 33). We have used these regions as tools with which to identify molecular mechanisms responsible for PSA expression at castrate levels of androgen to gain insight into mechanisms of prostate cancer progression (2, 9).
The NF-κB/Rel genes encode a family of heterodimeric transcription factors that share a 300-amino-acid Rel homology domain (RHD) (16, 26). Classical NF-κB is a heterodimer composed of a 50-kDa (p50) subunit and a 65-kDa (p65 or RelA) subunit and was discovered as a κ-immunoglobulin enhancer DNA binding protein (38). c-Rel, another member of this family, was originally identified as a proto-oncogene. The activity of these proteins is regulated primarily through posttranslational modification. In unstimulated cells, the NF-κB/Rel proteins are sequestered in the cytoplasm by association with inhibitory proteins, termed inhibitor of NF-κB (IκBα and IκBβ). Upon phosphorylation by IκB kinases (IKKs), IκB is modified by ubiquitination and degraded, allowing NF-κB to translocate into the nucleus and activate target gene transcription (16).
Breast cancer studies have proposed a role for NF-κB in the progression of hormone-dependent cancers to hormone independence. Constitutive activation of NF-κB was found in estrogen receptor (ER)-negative breast cancer cell lines and poorly differentiated primary tumors (29). Progression of the rat mammary carcinoma cell line RM22-F5 from an ER-positive, nonmalignant phenotype to an ER-negative, malignant phenotype was accompanied by constitutive activation of NF-κB (29). The current data on prostate cancer are less certain. In the AI prostate cell lines PC-3 and DU-145, the DNA binding activity of NF-κB is constitutively activated whereas the androgen-sensitive prostate cancer cell line LNCaP has a low level of NF-κB binding activity (31). However, PC-3 and DU-145 do not express androgen receptor (AR) and PSA; therefore, the relevance of this finding to clinical AI prostate cancer progression is not clear. In a different set of studies, the RelA subunit of NF-κB was shown to inhibit AR function by competing for the coactivator CREB binding protein (CBP) (1, 32). This result suggests that NF-κB may play a negative role in AI progression since AR activation (not suppression) is associated with prostate cancer progression.
We have examined this issue in two xenograft models of AD-to-AI prostate cancer progression in which AR expression and PSA expression are retained (8, 23). We showed previously that mitogen-activated protein kinase kinase kinase 1 (MEKK1) activates PSA expression in prostate cancer cells (2). This activation is only partially abolished by the AR inhibitor bicalutamide (Casodex), suggesting that pathways other than AR may be involved in this activation. MEKK1 can activate Jun N-terminal kinase (JNK) or IκB kinase (IKK), resulting in activation of the AP-1 or NF-κB transcription factor complex, respectively. In this report, we demonstrate that NF-κB, but not AP-1, is required for MEKK1 to fully activate PSA transcription. We also identify previously unrecognized NF-κB binding sites in the PSA enhancer and show that progression to AI is associated with increased constitutive NF-κB binding activity in both xenograft models.
MATERIALS AND METHODS
Cell culture and xenograft.
The human prostate cancer cell lines LNCaP and DU145 were obtained from the American Type Culture Collection and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). For phorbol 12-myristate 13-acetate (PMA) and tumor necrosis factor alpha (TNF-α) treatment, LNCaP cells were grown in the presence of 10% FBS overnight and subsequently in the absence of serum for 2 days. The serum-starved LNCaP cells were challenged with 5% charcoal-stripped FBS with or without PMA (10 ng/ml) or TNF-α (50 ng/ml) overnight, and the media were subjected to enzyme-linked immunosorbent assay (ELISA; American Qualex) to determine PSA expression. The LAPC-4 and LAPC-9 xenograft models were established as previously described (9, 23) and maintained subcutaneously in mice. Progression to AI was modeled by castration of mice bearing AD tumors.
Plasmids.
The cDNA of MEKK1-DA (MEKK1 dominant active) was subcloned into pcDNA3 as previously described (2). MEKK1-DA is a truncated form of MEKK1 in which the N-terminal 351 amino acids were deleted. The PSA promoter enhancer luciferase reporter (PSA-P/E-Luc) was constructed by inserting a 600-bp fragment of the PSA promoter and a 2.4-kb enhancer sequence upstream of luciferase (pSE) as previously described (2, 9, 33). Constructs containing mutations in the NF-κB binding sites on PSA-P/E-Luc were kindly provided by Michael Carey (University of California at Los Angeles) and were prepared as previously described (19). The IκBα mutant contains amino acid substitutions of serines 32 and 36 to alanine. Plasmids encoding the IκBα mutant, p50, p65, and c-Rel were kindly provided by Genhong Cheng (University of California at Los Angeles).
Transfection.
LNCaP or DU145 cells were plated at 40 to 50% confluency and transfected 1 day later in accordance with the manufacturer's instructions using TFX-50 (Promega) for LNCaP and Lipofectamine plus (Promega) for DU145. To minimize interference from androgen, transfected cells were maintained in RPMI 1640 medium supplemented with 5% charcoal-stripped serum. Two days after transfection, cells were harvested and the luciferase activity of the reporter constructs was measured with a luciferase assay kit or a dual luciferase assay kit (Promega). Luciferase activity was normalized to the percentage of green fluorescent protein (GFP)-positive cells in transfections that included pcDNA3-enhanced GFP or to Renilla luciferase when the pRL-TK plasmid was used as the normalization control.
DNase I footprinting.
Probes were created by PCR with the reporter plasmid PSA-P/E-Luc, which contains the PSA core enhancer, as a template. The 3′ primers were ACCAGCTCAATCAGTCAC, CATGTTCACATTAGTACACC, AACCTGAGATTAGGAATCC, and TGAGAGAGATATCATCTTGC. The common 5′ primer was CGTTGAGACTGTCCTGCAG. The 3′ primers were end labeled by T4 polymerase kinase with [γ-32P]ATP, and each 3′ primer was combined with the common 5′ primer to PCR amplify DNA probe for footprinting. The binding reactions for DNase footprinting were as previously described (13). The DNA was precipitated and resolved on a 6 to 8% polyacrylamide-7 M urea sequencing gel. Recombinant human p50 was purchased from Promega.
EMSAs.
Concentrated nuclear extracts for electrophoretic mobility shift assays (EMSAs) were prepared as previously described (6). Nuclear extracts were diluted in buffer containing 2 mM EDTA, 25 mM HEPES (pH 7.5), 150 mM NaCl, 1% Triton X-100, and 10% glycerol. The wild-type and mutant double-strand probes were purchased from Operon. They are GCCATGGGGGGATCCCCGAAGTCC and GCCATGGGCCGATCCCCGAAGTCC. The probes were end labeled by T4 polymerase kinase with [γ-32P]ATP. Diluted nuclear extracts were incubated with the indicated probe in the same buffer as for DNase I footprinting (13), except that glycerol was added to a final concentration of 1%. Antibodies against NF-κB p50 (SC-114 X), p65 (SC-372-G), and AR (SC-816) were also incubated in supershift experiments. After 1 h of incubation, the reaction mixture was resolved in a 6% native polyacrylamide electrophoresis gel in buffer containing 0.5× Tris-borate-EDTA and 1% glycerol.
Western blot analysis.
Nuclear extracts and cytoplasmic fractions of xenograft tumors were prepared as previously described (6). Western blot analysis was performed in accordance with standard procedures (35). The antibodies used in this study were SC-1643 against IκBα, SC-945 against IκBβ, SC-372-G against NF-κB p65, SC-1190 against NF-κB p50 (Santa Cruz Biotechnology), and AC-15 against β-actin (Sigma).
RESULTS
NF-κB, but not AP-1, is required for MEKK1-mediated activation of the PSA-P/E.
Previously, we demonstrated that a constitutively active form of MEKK1 (MEKK1-DA) induces AR-independent activation of the PSA promoter-enhancer (PSA-P/E) (2). To identify mechanisms responsible for this activation, we used dominant-negative mutant forms to examine downstream pathways of MEKK1.
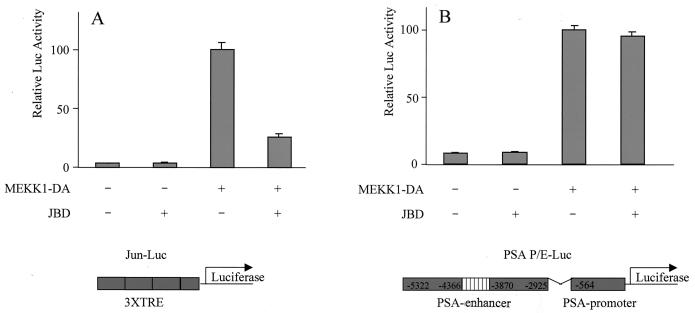
An AP-1 binding site was previously identified in the PSA enhancer by sequence inspection (36). Because MEKK1 activates AP-1 through JNK, we asked if JNK is required for MEKK1-mediated induction of PSA. LNCaP cells were cotransfected with MEKK1-DA and JBD, a truncated form of JIP-1 that selectively inhibits JNK (11). In a titration experiment, the minimal dose of plasmid MEKK1-DA required to fully activate the PSA-P/E was defined (data not shown). Transfected cells were maintained in 5% charcoal-stripped serum to minimize interference from androgen. As expected, MEKK1 stimulated transcription of a c-Jun-responsive element and the activation was inhibited by JBD (Fig. 1A). MEKK1-DA activated the PSA-P/E, but this activation could not be blocked by JBD (Fig. 1B). This result is consistent with our previous finding that JBD does not impair the effect of MEKK1 on prostate cancer cell survival (2) and suggests that AP-1 is not involved in MEKK1-mediated activation of PSA.
FIG. 1.
JBD does not inhibit MEKK1-mediated activation of PSA. LNCaP cells were transfected with empty vector pcDNA3, JBD (500 ng), MEKK1-DA (400 ng), or JBD with MEKK1-DA, together with the reporter construct driven either by a Jun-responsive element (Jun-Luc; 200 ng) (A) or by PSA-P/E-Luc (200 ng) (B). Luciferase activity was measured 2 days after transfection and normalized by transfection efficiency, which was determined by GFP cotransfection. The data shown represent three experiments.
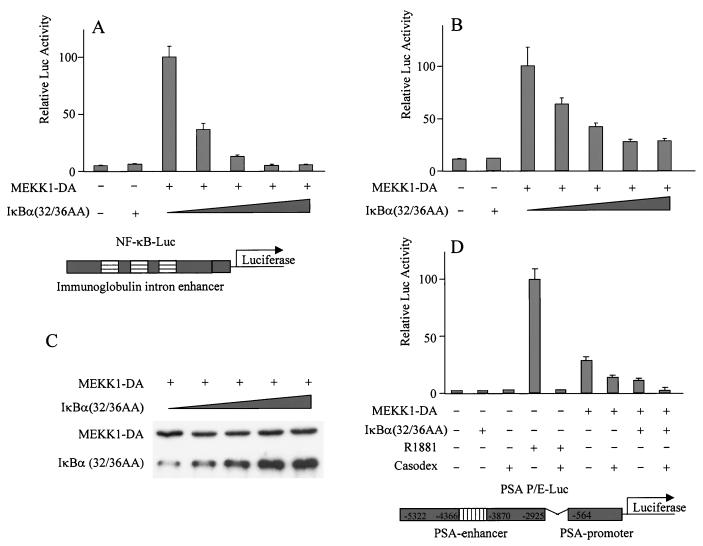
Another target of MEKK1 is IKK. MEKK1 activates IKK, which phosphorylates IκB, leading to NF-κB activation. To determine if NF-κB is involved in MEKK1-mediated activation of PSA, LNCaP cells were transfected with MEKK1-DA and a dominant-active form of IκBα containing alanine substitutions at serines 32 and 36. These mutations prevent phosphorylation by IKK, thereby preventing NF-κB translocation to the nucleus to activate transcription. Cotransfection of the mutant IκBα blocked the effect of MEKK-DA on an NF-κB-responsive element (Fig. 2A) and on the PSA-P/E (Fig. 2B) in a dose-dependent manner. The expression level of MEKK-DA did not change when the mutant IκBα was cotransfected (Fig. 2C). While large doses of the mutant IκBα completely abolished the effect of MEKK1-DA on the NF-κB-responsive element (Fig. 2A), the maximal effect on the PSA-P/E was a reduction by 65% (Fig. 2B). However, PSA activation was completely abolished when both bicalutamide and the mutant IκBα were used (Fig. 2D), consistent with our previous observation that MEKK1-DA also activates AR (2).
FIG. 2.
Dominant-active IκBα mutant inhibits MEKK1-mediated activation of PSA. LNCaP cells were transfected with empty vector pcDNA3, the mutant form IκBα(32/36AA) (800 ng), MEKK1-DA (300 ng), or MEKK1-DA with increasing amounts (100, 200, 400, and 800 ng) of the mutant form IκBα(32/36AA), together with the reporter construct driven either by an NF-κB-responsive element (NF-κB-Luc; 200 ng) (A) or by PSA-P/E-Luc (200 ng) (B and D). (C) Lysates from the transfected cells were examined for MEKK1-DA expression. Bicalutamide was used as indicated in the experiment whose results are shown in panel D. Luciferase activity was measured 2 days after transfection and normalized by transfection efficiency, which was determined by GFP cotransfection. The data shown represent three experiments.
NF-κB activates the PSA-P/E.
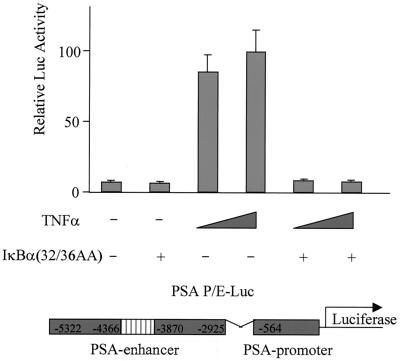
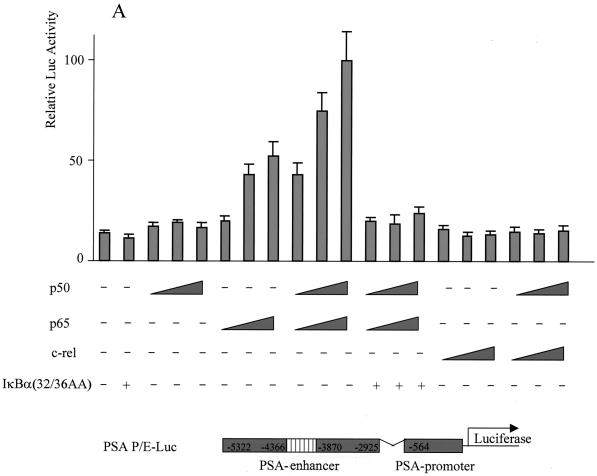
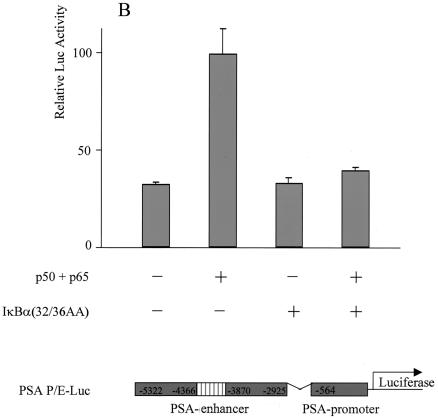
If NF-κB is required for transcriptional activation of PSA by MEKK1-DA, we reasoned that other signals that lead to activation of NF-κB may also activate the PSA-P/E. Indeed, TNF-α, a cytokine that activates NF-κB, was sufficient to activate PSA-P/E transcription in LNCaP cells, and this activation was completely blocked by the mutant IκBα (Fig. 3). To provide more direct evidence that NF-κB plays a regulatory role in PSA transcription, we asked if NF-κB is sufficient to increase PSA-P/E transcriptional activity. Overexpression of p50, the NF-κB subunit without a transcriptional activation domain, did not activate the PSA-P/E (Fig. 4A). However, overexpression of p65, or the combination of p50 and p65, did activate the PSA-P/E and this activation was abolished by cotransfection of the mutant IκBα (Fig. 4A). It is noteworthy that c-Rel, another member of the p65 family, did not activate the PSA-P/E (Fig. 4A). These results indicate that NF-κB is sufficient to activate the PSA-P/E in the absence of androgen and suggests that this activation may be specific to the classical p50/p65 NF-κB complex. We performed a similar experiment with DU145 cells, a prostate cancer cell line that does not express AR. Cotransfection of p50/p65 activated the PSA-P/E reporter in the absence of AR, and this activation was blocked by the mutant IκBα (Fig. 4B). While the levels of NF-κB-induced PSA reporter induction are lower than those achieved by transfection of AR and addition of androgen (data not shown), these data demonstrate that NF-κB is sufficient to activate PSA expression independently of AR.
FIG. 3.
TNF-α activates PSA expression through the NF-κB pathway. LNCaP cells were transfected with the reporter construct PSA-P/E-Luc (200 ng) with or without the mutant form IκBα(32/36AA) (800 ng) as indicated. After transfection, cells were treated with or without TNF-α (10 or 50 ng/ml) as indicated. Luciferase activity was measured 2 days after transfection and normalized to Renilla luciferase. The data shown represent two experiments.
FIG. 4.
Overexpression of NF-κB activated PSA expression, and the activation was abolished by mutant IκBα. (A) LNCaP cells were transfected with the reporter construct PSA-P/E-Luc (200 ng) with increasing amounts of human p50 (p50) (25, 50, and 100 ng), human p65 (p65) (25, 50, and 100 ng), a combination of p50 and p65, c-Rel (25, 50, and 100 ng), or a combination of p50 and c-Rel and with or without the mutant form IκBα(32/36AA) (800 ng) as indicated. (B) DU145 cells were cotransfected with the reporter construct PSA-P/C-Luc (200 ng) and p50 (50 ng) and p65 (50 ng) and with or without the mutant form IκBα(32/36AA) (800 ng) as indicated. Luciferase activity was measured 2 days after transfection and normalized to Renilla luciferase. The data shown represent two experiments.
TNF-α and PMA stimulate endogenous PSA expression.
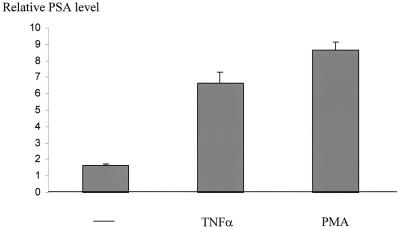
To determine whether NF-κB activates endogenous PSA expression, LNCaP cells were challenged with two commonly used NF-κB-inducing agents, TNF-α and PMA, both of which are known to activate NF-κB in LNCaP cells (20, 28). This approach, rather than cotransfection of p50/p65 plasmids, was necessary to ensure NF-κB activation in a large population of cells (because of low transfection efficiency). Cells were maintained in RPMI 1640 medium without serum for 2 days to deplete PSA and then challenged with TNF-α or PMA in 5% charcoal-stripped FBS. PSA expression was determined by ELISA (26, 37). Both TNF-α and PMA stimulated PSA production (Fig. 5), suggesting that NF-κB activation alone is sufficient to stimulate PSA expression.
FIG. 5.
TNF-α and PMA induce endogenous PSA expression. Serum-starved LNCaP cells were grown in 5% charcoal-stripped FBS with or without TNF-α (50 nM) or PMA (10 ng/ml) overnight, and the media were subjected to ELISA to determine PSA expression.
NF-κB directly binds the PSA enhancer.
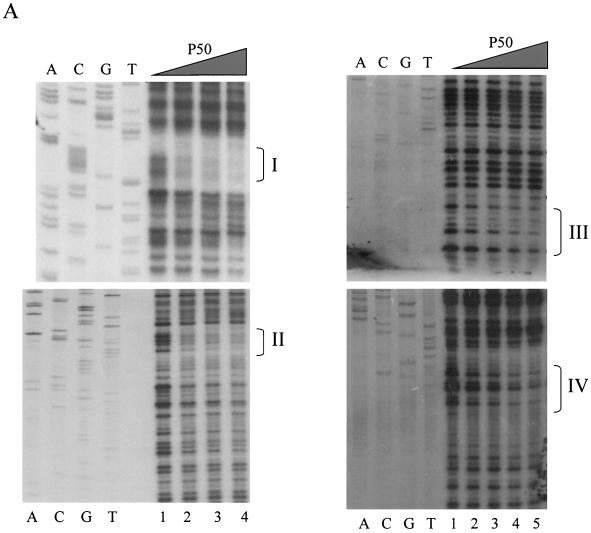
To determine whether activation of PSA transcription by NF-κB was a direct or indirect effect, DNase footprinting analysis was performed. We analyzed a core enhancer between −4366 and −3824 because this region was previously shown to be important for androgen-induced PSA expression (7, 19, 33). The 32P-labeled PSA core enhancer was incubated with recombinant p50 protein, and the reaction mixture was subjected to DNase I digestion. p50 protected four regions in the PSA core enhancer with the following order of binding affinity: I > II > IV > III (Fig. 6A). One-half of a gel shift unit (defined as the amount of p50 required to shift 0.38 pmol of the NF-κB oligonucleotide) completely protected site I from DNase digestion, and one gel shift unit greatly reduced DNase digestion of site II. The four protected sequences (Fig. 6B) in the PSA core enhancer resemble a κB consensus sequence (GGGRNNYYCC) (26), and the order of NF-κB binding affinity is consistent with the prediction from a comparative analysis (42). This result indicates that NF-κB can directly bind to the PSA enhancer.
FIG. 6.
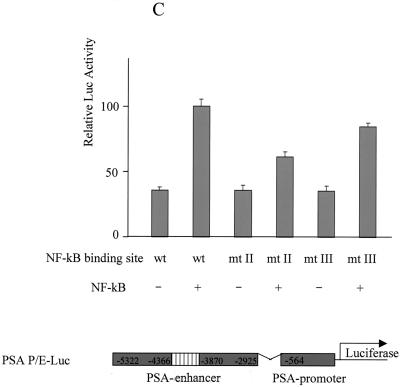
Identification of NF-κB binding sites in PSA core enhancer. (A) Increasing amounts of recombinant human p50 protein (0, 0.5, 1.0, 2.0, and 4.0 gel shift units in lanes 1, 2, 3, 4, and 5, respectively) were incubated with a 32P-labeled enhancer fragment, from −4366 to −3824, to detect footprints. The sites identified are designated I, II, III, and IV in sequence order. Sequencing ladders are included alongside the footprints to localize the protected sites. The order of binding affinity was I > II > III > IV. (B) Sequence of the PSA core enhancer. Footprinted regions are underlined and compiled below the consensus. The sequence of site I is reversed. (C) Wild-type (wt) or mutant PSA-P/E-Luc was cotransfected with p50 and p65 into LNCaP cells. Luciferase activity was measured 2 days after transfection and normalized by transfection efficiency, which was determined by GFP cotransfection. The data shown represent three experiments. Sites II and III were mutated to CCGGTTTGTG and ACGGAGTACT, respectively.
To determine the functional role of these sites, we examined the effect of NF-κB on promoters containing mutations in sites II and III. We chose mutations in these two sites because they have intermediate DNA binding affinity (Fig. 6B). Both mutations abolished the footprinting of p50, as expected (data not shown). In addition, both mutations showed reduced activation by NF-κB (Fig. 6C).
Increased NF-κB binding activity in AI prostate cancer.
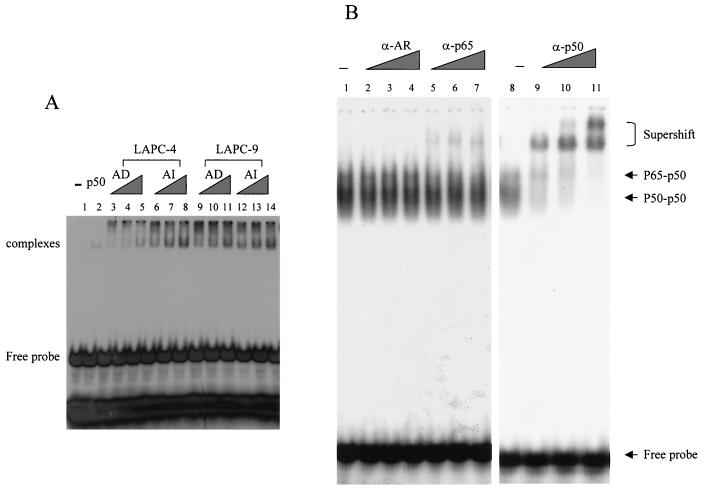
To determine if NF-κB activity correlates with ligand-independent PSA expression during AI prostate cancer progression, we measured NF-κB binding activity in the LAPC-4 and LAPC-9 prostate cancer xenograft models by EMSA. A strong band shift was detected when 2 μg of nuclear extract from LAPC-4 AI tumor tissue was used (Fig. 7A, lane 8). The band shift was also seen when smaller quantities of nuclear extracts from LAPC-4 AI tumor tissue were used (Fig. 7A, lanes 6 and 7). However, nuclear extracts from LAPC-4 AD tumor tissue formed barely detectable shifted complexes, even at high concentrations (Fig. 7A, lanes 3 to 5). This result suggests that AI tumor tissue has a higher constitutive NF-κB binding activity than its AD counterpart. Similar results were observed in the LAPC-9 xenograft model, although the basal level of NF-κB activation in LAPC-9 AD is already elevated (Fig. 7A, lanes 9 to 14).
FIG. 7.
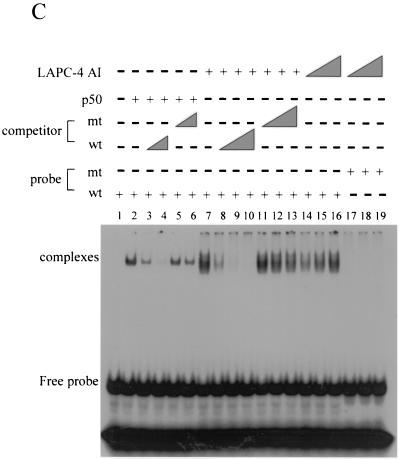
AI tumors have a higher level of constitutive NF-κB binding activity than do AD tumors. (A) Increasing amounts (0.5, 1.0, and 2.0 μg) of nuclear extracts from both AD and AI LAPC-4 and LAPC-9 tumors were incubated with a 32P-labeled NF-κB binding sequence to detect NF-κB binding by gel shift analysis. The shifted complex and free probe are indicated. (B) Identity of the shifted complexes. Two micrograms of LAPC-4 AI tumor nuclear extract was incubated with a 32P-labeled NF-κB binding sequence with or without increasing amounts of antibodies against AR, p65, or p50. (C) Specificity of the shifted complexes. Shifted complexes (lane 2) formed by the recombinant p50 protein were competed away by increasing amounts of the unlabeled wild-type (wt) probe (lanes 3 and 4) but not by the unlabeled mutant (mt) probe (lanes 5 and 6). LAPC-4 AI tumor nuclear extracts (0.5, 1.0, and 2.0 μg) formed a band shift with the wild-type probe (lanes 14 to 16) and but not with the mutant probe (lanes 17 to 19). The band shift was competed away by increasing amounts of the unlabeled wild-type probe (lanes 8 to 10) but not by the unlabeled mutant probe (lanes 11 to 13).
The band shift pattern was similar to that of previous reports describing the p65/p50 heterodimer and p50/p50 homodimer complexes (10, 29, 41). To confirm the identities of the shifted complexes, we performed antibody supershift experiments with nuclear extracts from LAPC-4 AI tumor tissue. Addition of p65 antibody caused the upper band to shift (Fig. 7B, lanes 5 to 7), and addition of p50 antibody shifted both complexes to higher positions in a dose-dependent fashion (Fig. 7B, lanes 9 to 11). The supershift was specific because antibody against an unrelated protein (AR) did not alter the mobility of the complexes (Fig. 7B, lanes 2 to 4). These results indicated that the upper complex contains the p65/p50 heterodimer and the lower band contains p50.
Binding specificity was determined by using nuclear extracts from LAPC-4 AI tumor tissue. One-half to two micrograms of nuclear extracts produced the band shift with the wild-type probe (Fig. 7C, lanes 14 to 17) but not with a mutant probe (Fig. 7C, lanes 17 to 19). The shifted complex was competed away by a 5- to 80-fold excess of unlabeled wild-type probe (Fig. 7B, lanes 8 to 10) but not by unlabeled mutant probe (Fig. 7C, lanes 11 to 13). The wild-type, but not the mutant, competitor also competed away the shifted complex from recombinant p50 (Fig. 7C, lanes 2 to 6) with comparable efficiency. These results demonstrate an increase in constitutive NF-κB binding activity during progression to androgen independence.
Nuclear accumulation of NF-κB correlates with its binding activity.
To examine the mechanism for the elevated NF-κB binding activity in the AI xenografts, we measured the levels of p50, p65, and IκB in nuclear and cytoplasmic extracts of LAPC-4 AD and AI tumors. p50 and p65 protein levels were higher in nuclear extracts and lower in cytoplasmic extracts from AI tumors (Fig. 8A and B). Therefore, the higher NF-κB binding activity in LAPC-4 AI tumor tissue is associated with an increased concentration of NF-κB proteins in the nucleus. We also noted that the level of the IκBβ protein, but not that of the IκBα protein, was lower in cytoplasmic extracts from AI tumor (Fig. 8C), raising the possibility that an increased level of NF-κB in the AI tumor nucleus results from a decreased level of IκBβ in the cytoplasm. A full understanding of the mechanism for increased NF-κB activity in these xenografts requires further study.
FIG. 8.
Western blot analysis of NF-κB and IκB protein levels in LAPC-4 xenograft tumors. (A) Nuclear extracts were subjected to Western blot analysis with antibodies against p50 and p65. (B) Cytoplasmic fractions were subjected to Western blot analysis with antibodies against p50 and p65. (C) Cytoplasmic fractions were subjected to Western blot analysis with antibodies against IκBα and IκBβ.
DISCUSSION
Our data from analysis of prostate cancer cell lines and xenografts support a role for NF-κB in prostate cancer progression. We demonstrate that NF-κB regulates the expression of PSA, an important clinical marker of prostate cancer progression. We also show that NF-κB directly binds to the PSA core enhancer. By using matched AD and AI prostate cancers derived from two xenograft models, we demonstrated that NF-κB binding activity is upregulated in AI tumors.
Previous work has shown that NF-κB negatively regulates AR function. This conclusion is based on cotransfection studies showing that NF-κB and AR are mutually inhibitory due to competition for a common transcriptional coactivator, CBP (1, 32). Because AR is activated in AI prostate cancer (9, 17, 25), these findings imply an inhibitory role for NF-κB in prostate cancer progression. However, NF-κB inhibition of AR was observed when artificial promoters containing either consensus AR response element (ARE) or NF-κB binding sites were used. Natural promoters are more complex and typically contain cis-acting elements for different transcription factors. The PSA core enhancer studied here contains multiple low-affinity AREs that act synergistically (19). Among the four NF-κB binding sites we identified (Fig. 5B), three (I, II, and IV) are adjacent to AREs and NF-κB binding site III overlaps an ARE (19). These observations raise the possibility that cooperative NF-κB and AR binding contributes to PSA transcriptional regulation. Cooperation between nuclear receptors and NF-κB has been demonstrated in other systems (1, 5, 21, 32). For example, retinoid receptors and NF-κB synergistically induce ICAM-1 expression, which promotes metastasis potential (5). NF-κB and the aryl hydrocarbon receptor cooperate to activate c-myc expression in mammary cells (21).
The transition from AD to AI prostate cancer is a multistep process (8). AI cells must first survive androgen deprivation and then grow to become AI tumors. NF-κB may participate in both processes. NF-κB is known to be a critical regulator of survival (3). p65/RelA knockout mice die from extensive liver apoptosis during embryogenesis (4). NF-κB also functions as a survival factor in neurons in response to cell injury through the upregulation of antiapoptotic genes (27). Accumulating evidence indicates that NF-κB also promotes proliferation. Inhibiting NF-κB activation by dominant-negative p65 blocks cell cycle progression in human glioma cells (30). Lymphocytes lacking p50, p65, or c-Rel show defective responses to mitogenic stimulation (12, 24, 39, 40). Inhibiting NF-κB activation by IκBα expression, or by phenylarsine oxide, blocks focus formation in NIH 3T3 cells (15) and colony-forming cell proliferation of acute myelogenous leukemia cells (14). The proliferation-promoting effect of NF-κB may result from its ability to activate c-myc expression (22) and/or cyclin D1 expression (18, 34).
The link between NF-κB activation and prostate cancer androgen independence may provide an opportunity for drug development. Therapeutic interventions might target upstream pathways that lead to activation of NF-κB, NF-κB itself, or downstream effectors that participate in cancer progression. Because NF-κB is involved in multiple signal transduction pathways, identification of the pathway(s) responsible for increased activity of NF-κB in AI progression will be of great interest.
Acknowledgments
We thank Genhong Cheng for expression vectors of mutant IκBα, p50, p65, and c-Rel, Michael Carey for wild-type and mutant PSA-P/E-Luc reporters, and Katherine Ellwood for assistant in DNase I footprinting and EMSA. We also thank Ke Shuai, Mitch Gross, and Ingo Mellinghoff for critical reading of the manuscript.
This work was supported by a postdoctoral award from the DOD (C.D.C.) and by grants from the DOD, the NCI, and CaPCure (C.L.S.)
REFERENCES
- 1.Aarnisalo, P., H. Santti, H. Poukka, J. J. Palvimo, and O. A. Janne. 1999. Transcription activating and repressing functions of the androgen receptor are differentially influenced by mutations in the deoxyribonucleic acid binding domain. Endocrinology 140:3097-3105. [DOI] [PubMed] [Google Scholar]
- 2.Abreu-Martin, M. T., A. Chari, A. A. Palladino, N. A. Craft, and C. L. Sawyers. 1999. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol. 19:5143-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 2001. Series introduction: the transcription factor NF-κB and human disease. J. Clin. Investig. 107:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 5.Chadwick, C. C., L. J. Shaw, and R. C. Winneker. 1998. TNF-α and 9-cis-retinoic acid synergistically induce ICAM-1 expression: evidence for interaction of retinoid receptors with NF-κB. Exp. Cell Res. 239:423-429. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. D., and D. M. Helfman. 1999. Donor site competition is involved in the regulation of alternative splicing of the rat β-tropomyosin pre-mRNA. RNA 5:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleutjens, K. B., H. A. van der Korput, C. C. Ehren-van Eekelen, R. A. Sikes, C. Fasciana, L. W. Chung, and J. Trapman. 1997. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol. Endocrinol. 11:1256-1265. [DOI] [PubMed] [Google Scholar]
- 8.Craft, N., C. Chhor, C. Tran, A. Belldegrun, J. DeKernion, O. N. Witte, J. Said, R. E. Reiter, and C. L. Sawyers. 1999. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 59:5030-5036. [PubMed] [Google Scholar]
- 9.Craft, N., Y. Shostak, M. Carey, and C. L. Sawyers. 1999. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 5:280-285. [DOI] [PubMed] [Google Scholar]
- 10.Davis, J. N., O. Kucuk, and F. H. Sarkar. 1999. Genistein inhibits NF-κB activation in prostate cancer cells. Nutr. Cancer 35:167-174. [DOI] [PubMed] [Google Scholar]
- 11.Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693-696. [DOI] [PubMed] [Google Scholar]
- 12.Doi, T. S., T. Takahashi, O. Taguchi, T. Azuma, and Y. Obata. 1997. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med. 185:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellwood, K., W. Huang, R. Johnson, and M. Carey. 1999. Multiple layers of cooperativity regulate enhanceosome-responsive RNA polymerase II transcription complex assembly. Mol. Cell. Biol. 19:2613-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrov, Z., S. K. Manna, D. Harris, Q. Van, E. H. Estey, H. M. Kantarjian, M. Talpaz, and B. B. Aggarwal. 1999. Phenylarsine oxide blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, and induces apoptosis of acute myelogenous leukemia cells. Blood 94:2844-2853. [PubMed] [Google Scholar]
- 15.Finco, T. S., J. K. Westwick, J. L. Norris, A. A. Beg, C. J. Der, and A. S. Baldwin, Jr. 1997. Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J. Biol. Chem. 272:24113-24116. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 17.Gregory, C. W., R. T. Johnson, Jr., J. L. Mohler, F. S. French, and E. M. Wilson. 2001. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61:2892-2898. [PubMed] [Google Scholar]
- 18.Guttridge, D. C., C. Albanese, J. Y. Reuther, R. G. Pestell, and A. S. Baldwin, Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19:5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, W., Y. Shostak, P. Tarr, C. Sawyers, and M. Carey. 1999. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J. Biol. Chem. 274:25756-25768. [DOI] [PubMed] [Google Scholar]
- 20.Keller, E. T., C. Chang, and W. B. Ershler. 1996. Inhibition of NF-κB activity through maintenance of IκBα levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J. Biol. Chem. 271:26267-26275. [DOI] [PubMed] [Google Scholar]
- 21.Kim, D. W., L. Gazourian, S. A. Quadri, R. Romieu-Mourez, D. H. Sherr, and G. E. Sonenshein. 2000. The RelA NF-κB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19:5498-5506. [DOI] [PubMed] [Google Scholar]
- 22.Kim, D. W., M. A. Sovak, G. Zanieski, G. Nonet, R. Romieu-Mourez, A. W. Lau, L. J. Hafer, P. Yaswen, M. Stampfer, A. E. Rogers, J. Russo, and G. E. Sonenshein. 2000. Activation of NF-κB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis 21:871-879. [DOI] [PubMed] [Google Scholar]
- 23.Klein, K. A., R. E. Reiter, J. Redula, H. Moradi, X. L. Zhu, A. R. Brothman, D. J. Lamb, M. Marcelli, A. Belldegrun, O. N. Witte, and C. L. Sawyers. 1997. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 3:402-408. [DOI] [PubMed] [Google Scholar]
- 24.Kontgen, F., R. J. Grumont, A. Strasser, D. Metcalf, R. Li, D. Tarlinton, and S. Gerondakis. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965-1977. [DOI] [PubMed] [Google Scholar]
- 25.Linja, M. J., K. J. Savinainen, O. R. Saramaki, T. L. Tammela, R. L. Vessella, and T. Visakorpi. 2001. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 61:3550-3555. [PubMed] [Google Scholar]
- 26.Miyamoto, S., and I. M. Verma. 1995. Rel/NF-κB/I κB story. Adv. Cancer Res. 66:255-292. [PubMed] [Google Scholar]
- 27.Moerman, A. M., X. Mao, M. M. Lucas, and S. W. Barger. 1999. Characterization of a neuronal κB binding factor distinct from NF-κB. Brain Res. Mol. Brain Res. 67:303-315. [DOI] [PubMed] [Google Scholar]
- 28.Muenchen, H. J., D. L. Lin, M. A. Walsh, E. T. Keller, and K. J. Pienta. 2000. Tumor necrosis factor-α-induced apoptosis in prostate cancer cells through inhibition of nuclear factor-κB by an IκBα “super-repressor.” Clin. Cancer Res. 6:1969-1977. [PubMed] [Google Scholar]
- 29.Nakshatri, H., P. Bhat-Nakshatri, D. A. Martin, R. J. Goulet, Jr., and G. W. Sledge, Jr. 1997. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 17:3629-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otsuka, G., T. Nagaya, K. Saito, M. Mizuno, J. Yoshida, and H. Seo. 1999. Inhibition of nuclear factor-κB activation confers sensitivity to tumor necrosis factor-α by impairment of cell cycle progression in human glioma cells. Cancer Res. 59:4446-4452. [PubMed] [Google Scholar]
- 31.Palayoor, S. T., M. Y. Youmell, S. K. Calderwood, C. N. Coleman, and B. D. Price. 1999. Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene 18:7389-7394. [DOI] [PubMed] [Google Scholar]
- 32.Palvimo, J. J., P. Reinikainen, T. Ikonen, P. J. Kallio, A. Moilanen, and O. A. Janne. 1996. Mutual transcriptional interference between RelA and androgen receptor. J. Biol. Chem. 271:24151-24156. [DOI] [PubMed] [Google Scholar]
- 33.Pang, S., J. Dannull, R. Kaboo, Y. Xie, C. L. Tso, K. Michel, J. B. deKernion, and A. S. Belldegrun. 1997. Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res. 57:495-499. [PubMed] [Google Scholar]
- 34.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schuur, E. R., G. A. Henderson, L. A. Kmetec, J. D. Miller, H. G. Lamparski, and D. R. Henderson. 1996. Prostate-specific antigen expression is regulated by an upstream enhancer. J. Biol. Chem. 271:7043-7051. [DOI] [PubMed] [Google Scholar]
- 37.Sen, R., and D. Baltimore. 1986. Inducibility of κ immunoglobulin enhancer binding protein NF-κB by a posttranslational mechanism. Cell 47:921-928. [DOI] [PubMed] [Google Scholar]
- 38.Sen, R., and D. Baltimore. 1986. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705-716. [DOI] [PubMed] [Google Scholar]
- 39.Sha, W. C., H. C. Liou, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80:321-330. [DOI] [PubMed] [Google Scholar]
- 40.Snapper, C. M., P. Zelazowski, F. R. Rosas, M. R. Kehry, M. Tian, D. Baltimore, and W. C. Sha. 1996. B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol 156:183-191. [PubMed] [Google Scholar]
- 41.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 42.Zabel, U., R. Schreck, and P. A. Baeuerle. 1991. DNA binding of purified transcription factor NF-κB. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J. Biol. Chem. 266:252-260. [PubMed] [Google Scholar]