Abstract
p27Kip1 is an important effector of G1 arrest by transforming growth factor β (TGF-β). Investigations in a human mammary epithelial cell (HMEC) model, including cells that are sensitive (184S) and resistant (184A1L5R) to G1 arrest by TGF-β, revealed aberrant p27 regulation in the resistant cells. Cyclin E1-cyclin-dependent kinase 2 (cdk2) and cyclin A-cdk2 activities were increased, and p27-associated kinase activity was detected in 184A1L5R cells. p27 from 184A1L5R cells was localized to both nucleus and cytoplasm, showed an altered profile of phosphoisoforms, and had a reduced ability to bind and inhibit cyclin E1-cdk2 in vitro when compared to p27 from the sensitive 184S cells. In proliferating 184A1L5R cells, more p27 was associated with cyclin D1-cdk4 complexes than in 184S. While TGF-β inhibited the formation of cyclin D1-cdk4-p27 complexes in 184S cells, it did not inhibit the assembly of cyclin D1-cdk4-p27 complexes in the resistant 184A1L5R cells. p27 phosphorylation changed during cell cycle progression, with cyclin E1-bound p27 in G0 showing a different phosphorylation pattern from that of cyclin D1-bound p27 in mid-G1. These data suggest a model in which TGF-β modulates p27 phosphorylation from its cyclin D1-bound assembly phosphoform to an alternate form that binds tightly to inhibit cyclin E1-cdk2. Altered phosphorylation of p27 in the resistant 184A1L5R cells may favor the binding of p27 to cyclin D1-cdk4 and prevent its accumulation in cyclin E1-cdk2 in response to TGF-β.
Transforming growth factor-beta (TGF-β) arrests or delays G1 progression in normal epithelial cells (22). In contrast, most cancer-derived cell lines show some resistance to G1 arrest by TGF-β, and in advanced cancers, TGF-β may stimulate proliferation and metastatic progression (11). In this study, we investigated cell cycle effects of TGF-β and how p27, a key effector of arrest by TGF-β, is regulated using finite-life-span, normal human mammary epithelial cell (HMEC) strain 184, which is sensitive to TGF-β (16), and an immortal derivative of 184, 184A1L5, which has undergone conversion to TGF-β resistance (46). For clarity, superscripts S (184S) and R (184A1L5R) indicate sensitivity and resistance to growth arrest by TGF-β.
Cell cycle progression through G1 phase is regulated by the sequential activation of D-type cyclin and then E-type cyclin-dependent kinases (cdk's) (37). Cyclin A-cdk2 is required for S-phase progression. cdk2 and cdk4 are both activated by cdk-activating kinase (CAK) (42), and cdk2 activation also requires dephosphorylation of inhibitory sites by Cdc25A (9). Both of these cdk-activating mechanisms are inhibited by TGF-β (17, 20, 41). G1 cdk's are also regulated by two families of cdk inhibitors (38, 39): the kinase inhibitor proteins (KIP) p21, p27, and p57 and the inhibitors of cdk4 (INK4) p15, p16, p18, and p19. Both p21 and p27 contribute to TGF-β arrest (reviewed in reference 7) and both p15 gene transcription (13, 55), and p15 protein stability (35) are increased by TGF-β.
p27 was first discovered as a heat-stable inhibitor of cyclin E-cdk2 in TGF-β-arrested cells (20, 33, 41). While p27 mRNA is constant across the cell cycle, p27 protein levels are regulated by translational controls (15, 28) and by ubiquitin-dependent proteolysis (32). p27 is maximal in G0, and phosphorylation at T187 in late G1 targets its proteasomal degradation (29, 36, 54). The cyclin E-cdk2-inhibitory activity of p27 is maximal in G0 and falls as cells move through G1 into S phase (14, 33, 41).
p27 plays two roles during G1-to-S phase progression. In addition to inhibition of cyclin E-cdk2, p27 also acts early in G1 to facilitate assembly, activation, and nuclear localization of cyclin D-cdk complexes (5, 21). Polyak et al. showed that p27's cyclin E-cdk2 inhibitory activity could be titrated out of TGF-β-arrested cell lysates by addition of recombinant cyclin D2-cdk4 (33). This seminal observation led to the hypothesis that the abundance of cyclin D1 may regulate p27 function, with p27 shifting out of cyclin E-cdk2 complexes into cyclin D-cdk4 complexes during G1 progression. It has been proposed that a key noncatalytic function of cyclin D-cdk’s is to titrate p21 and p27 away from cyclin E-cdk2, allowing the activation of the latter in late G1 (5, 33).
A number of observations suggest that p27 may not be passively regulated by the abundance of cyclin D1 alone. In most normal and many transformed cell types, cyclin D1-cdk4 activation precedes that of cyclin E-cdk2 by several hours and is not immediately or simultaneously accompanied by cyclin E1-cdk2 activation (2, 37). Moreover, despite its abundance in G0, p27 is unable to assemble exogenously overexpressed cyclin D1 into cdk4 complexes in serum-starved fibroblasts (26). However, overexpression of cyclin D1 together with constitutively activated MEK leads to sequestration of p27 away from cyclin E and into cyclin D1-cdk4-p27 complexes (6). These observations, together with data presented herein, suggest that p27 may be actively regulated by phosphorylation to function either as an inhibitor of cyclin E1-cdk2 or as an assembly factor for cyclin D1-cdk's. The shift of p27 out of cyclin E1-cdk2 and into cyclin D1-cdk complexes may be regulated by mitogenic kinases acting early in G1 and not merely passively regulated by the increasing abundance of cyclin D1 protein.
G1 arrest by TGF-β is brought about by a series of complementary and redundant mechanisms (7, 25). In epithelial cells, inhibition of G1 cyclin-cdk's by TGF-β involves the coordinate actions of p15 and p27 (34, 35). p15 induced by TGF-β was initially thought to dissociate p27 and cyclin D1 from cdk4 and promote the binding and inhibition of cyclin E-cdk2 by p27. Recent data suggest that, where p15 and p27 are both functional, they cooperate. However, cells lacking either p15 or p27 can still undergo TGF-β-mediated arrest (12, 17, 30). p15 is not required for binding and inhibition of cyclin E-cdk2 by p27, since cells bearing p15 deletions can still respond to TGF-β with accumulation of p27 in cyclin E-cdk2 and kinase inhibition (12, 17).
In HMECs, TGF-β mediates an increase in p15 stability and binding to cdk4, dissociation of p27 and cyclin D1 from cdk4, and binding and inhibition of cyclin E1-cdk2 by p27 in the sensitive 184S cells but not in resistant 184A1L5R cells (35). We showed that, while p15 from both cell types could dissociate in vitro cyclin D1-cdk4-p27 complexes isolated from 184S cells, cyclin D1-cdk4-p27 complexes from the 184A1L5R cells were resistant to dissociation by p15, suggesting an alteration in their conformation. The present investigation suggests that defective p27 regulation, with constitutive activation of p27 assembly function for cyclin D-cdk complexes, may underlie the TGF-β resistance in 184A1L5R. Our data suggest a model in which TGF-β regulates the affinity of p27 binding to its target cdk's by modulating p27 phosphorylation, shifting it from an assembly factor for D-type cyclin-cdk's to a form that binds and effectively inhibits cyclin E-cdk2.
MATERIALS AND METHODS
Culture of HMECs.
The derivation of normal finite-life-span HMECs from specimen 184 has been described (44). The immortal cell line 184A1 was derived following exposure of 184 HMECs to benzo(a)pyrene (45). Early-passage 184A1S is sensitive to growth inhibition by TGF-β. With progressive passage, cells undergo a gradual conversion process with outgrowth of a TGF-β-resistant population (46). Fully TGF-β-resistant cells can be readily isolated from late-passage 184A1 cells to give stable, growth-resistant sublines, such as 184A1L5R. The resistant subpopulation in 184A1S cannot arise as a result of a single genetic event, since all of four separate single-cell clones of 184A1S that were examined exhibited, when expanded, the presence of a TGF-β-resistant subpopulation. Thus, the switch from sensitive to resistant occurs at a population frequency that defies explanation on the basis of a single mutation. Hence, mutation of the cdk's, cyclins, or cdk inhibitors cannot underlie the conversion from TGF-β growth inhibited to growth resistant. Both 184S and 184A1L5R express normal TGF-β receptors and produce an extracellular matrix in response to TGF-β (16, 48).
HMECs were grown in MCDB 170 medium (Clonetics Corp., San Diego, Calif.). HMECs were G0 arrested by blocking epidermal growth factor (EGF) receptor signal transduction as described earlier (47). Asynchronously growing HMECs were treated with 2.5 ng of TGF-β1 (Genentech Inc., San Francisco, Calif.)/ml for 48 h.
Production of cyclin E1-cdk2 by baculovirus infection of Sf-9 cells.
Sf-9 cells and TNM-FH media were obtained from Invitrogen. Adherent Sf-9 cells were coinfected with baculoviruses expressing human cyclin E1 or human cdk2 genes, and cyclin E1 and cdk2 were prepared as described earlier (24). Sf-9 cell lysates containing cyclin E1 and cdk2 were used directly in p27 inhibitor assays and p27 binding assays.
Flow cytometry analysis.
Cells were pulse labeled with 10 μM bromodeoxyuridine and processed for flow cytometry as described (35).
Antibodies.
The anti-EGF receptor monoclonal antibody (MAb) 225 was provided by Steve Wiley (University of Utah Medical Center, Salt Lake City, Utah). Antibodies to cyclin A, cdk2, cdk4, cdk6, and p27 (C-19) were obtained from Santa Cruz Biotechnology; to cyclin D1 (DCS-6) and p27 (DCS-72) from Neomarkers; to PSTAIRE from S. Reed (The Scripps Research Institute, La Jolla, Calif.); and to cyclin E1 (MAbs E12 and E172) from E. Harlow (Massachusetts General Hospital, Boston, Mass.). These cyclin E1 antibodies are specific for cyclin E1 (24). Cyclin A MAb E67 was provided by J. Gannon and T. Hunt (Imperial Cancer Research Fund, London, England). p27 rabbit polyclonal serum (pAb5588) was provided by H. Toyoshima and T. Hunter (Salk Institute, La Jolla, Calif.). p27 and RCC1 antibodies were purchased from Transduction Laboratories.
Immunoblotting.
Cell lysis and immunoblotting were performed as described earlier (35). Equal protein loading was verified by blotting for β-actin. To assay cyclin E1-cdk-p27 complexes, cyclin E1 or p27 was immunoprecipitated from 200 μg of protein lysate. Immunoprecipitates were resolved, transferred, and blotted with cyclin E1, cdk2, and p27 antibodies. Proteins were detected by enhanced chemiluminescence (ECL). Antibody-alone controls were run along side all immunoprecipitates.
To compare the relative amounts of cyclin-bound p27, lysates from actively proliferating or G0-arrested cells were serially immunoprecipitated with cyclin D1 antibody three times. Cyclin E1 was then serially immunoprecipitated three times, followed by a final precipitation with anti-p27 antibody (C19). For G0 cells, cdk4 and cdk6 were immunoprecipitated prior to p27 precipitation. Precipitates were resolved and immunoblotted for associated proteins. The relative amounts of cyclin D1 and cyclin D1-bound p27 were measured by densitometric analysis of two different exposures on Western blots from each of two different biologic experiments. The relative amounts of cyclin D1 and of p27 bound to cyclin D1 were graphed as a percentage of the total in TGF-β-resistant 184A1L5R. For G0 lysates, cyclin D1-, E1-, cdk4- and cdk6-bound p27 and remaining p27 were quantitated from different ECL exposures from two different experiments.
cdk assays.
Cyclin E1, cyclin A, or p27 complexes were immunoprecipitated and reacted with [γ-32P]ATP and histone H1 as described earlier (10, 41). Radioactivity incorporated in histone substrate was quantitated using a Molecular Dynamics PhosphorImager and ImageQuant software. Radioactivity incorporated in control nonspecific mouse or rabbit polyclonal immunoglobulin G (IgG) immunoprecipitates was subtracted from test kinase values prior to quantitation of differences between activities in different cell types or different conditions.
Metabolic labeling.
Cells were labeled metabolically for 1 h with 500 μCi of [35S]methionine as described earlier (35). Lysates were precleared and incorporation of radioactivity was assayed by quantitation of trichloroacetic acid-insoluble counts. Volumes representing 108 cpm of trichloroacetic acid-insoluble radioactivity were precipitated with either cdk4 or cyclin D1 antibodies or nonimmune serum. Immune complexes were resolved, gels were dried, and proteins were visualized by autoradiography.
p27 immunocytochemistry.
HMECs were grown to 60% confluence on glass slides and were then depleted of EGF for 48 h. Cells were fixed and p27 immunoreactivity was detected using p27 MAb from Transduction Laboratories, as described earlier (4). Isotype-specific polyclonal mouse IgG was used in place of primary antibody for negative controls. The pattern of p27 immunostaining was confirmed using the polyclonal C-19 p27 antibody (Santa Cruz Biotechnology).
Nuclear cytoplasmic fractionation.
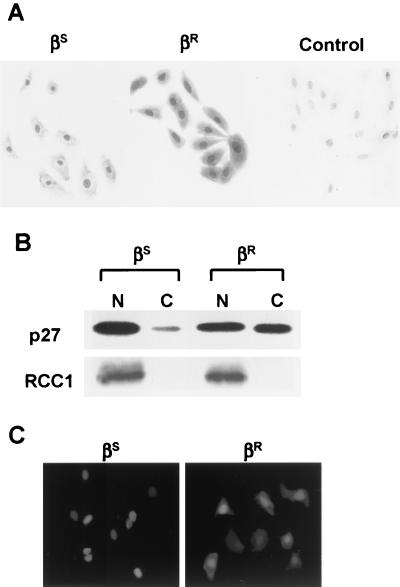
HMECs arrested by EGF depletion for 48 h were harvested and resuspended in ice-cold buffer (20 mM HEPES, pH 7.3, 110 mM KOAc, 5 mM NaOAc, 2 mM Mg[OAc]2, 1 mM EGTA, and 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin/ml, and 1 μg of aprotinin/ml). The cells were permeabilized at 4°C for 5 min by gradual addition of 2 to 3 μl of 0.1% digitonin until 95 to 98% permeabilization was achieved (detected by trypan blue staining). Nuclei were isolated by centrifugation, and the supernatant containing the cytosolic fraction was collected. The nuclei were washed, and ice-cold 0.1% NP-40 lysis buffer (see above for immunoblotting) was added to both fractions. To verify the adequacy of fractionation, immunoblots were probed for the nuclear protein RCC1.
Transfection of HMECs with fluorescent-tagged p27 and p27 localization.
A vector encoding wild-type human p27 cDNA fused to the yellow-green variant of the Aequorea victoria green fluorescent protein (YFPp27wt) was prepared in the pEYFP-C1 vector from Clontech Laboratories. HMECs were grown on glass slides to 60% confluence, depleted of EGF, and then transfected with the YFPp27wt vector using Lipofectamine PLUS Reagent (Life Technologies) according to the manufacturer's instructions. Fluorescent-tagged p27 was observed with a Zeiss Axiovert S100TV microscope with a C-Aprochromat 40× objective. Photographs were taken at 400× magnification, using an Empix digital camera and “CoolSnap” software.
Assays of p27 inhibitor function and binding to cyclin E1-cdk2.
184S or 184A1L5R cells were arrested in G0 by EGF depletion for 42 h following 48 h of contact inhibition. Fifty- to 300-μg cell lysates were precipitated with pAb5588 anti-p27 serum or control polyclonal rabbit IgG and protein A-Sepharose beads. For testing unboiled p27 inhibitory and binding activity, p27 immunoprecipitates were washed three times with 0.1% NP-40 lysis buffer and finally with reaction buffer (20 mM Tris, pH 7.5, 7.5 mM MgCl2, and 1 mM DTT). The immunoprecipitated p27 was incubated with a molar excess of recombinant cyclin E1-cdk2 at 30°C for 30 min. Complexes were then assayed either for H1 kinase activity or immunoblotted to detect associated proteins. The kinase activity of “uninhibited” recombinant cyclin E1-cdk2 was assayed following admixture with nonspecific IgG antibody control precipitates.
For testing heat-stable p27 inhibitor and cyclin E1-cdk2 binding activity, p27 immunoprecipitates were washed and then boiled for 5 min in 200 μl of reaction buffer, placed on ice, and then cleared by centrifugation. The p27 in the supernatant was recovered, recombinant cyclin E1-cdk2 and DTT (1 mM final concentration) were added, and the mixture was incubated at 30°C for 30 min, followed by immunoprecipitation with either anti-cyclin E1 (MAb E172) or control polyclonal mouse IgG (Sigma). Complexes were then either assayed for H1 kinase activity or immunoblotted to detect cyclin E1-bound cdk2 and p27.
2DIEF and phosphatase treatment.
Cells were lysed in ice-cold 0.1% Tween 20 lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, pH 8.0, 2.5 mM EGTA, pH 8.0, 10% glycerol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1% Tween 20, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM Na2VO4, 0.5 mM DTT, and 0.02 mg each of aprotinin, leupeptin, and pepstatin per ml). For two-dimensional isoelectric focusing (2DIEF), p27 immunoprecipitates were denatured in 8 M urea, loaded onto immobilized linear pH gradient (pH 3 to 10) or nonlinear (pH 3 to 10) IEF strips, and focused for 50,000 V·h, using the IPGphor apparatus from Amersham Pharmacia. The IEF strip was equilibrated in 50 mM Tris, pH 8.8, 6 M urea, 30% glycerol, and 2% sodium dodecyl sulfate (SDS) for 30 min before loading for SDS-polyacrylamide gel electrophoresis (PAGE). Gels were transferred to a polyvinyl difluoride membrane, and p27 isoforms were detected by immunoblotting using p27 antibody from Transduction Laboratories. For 2DIEF of cyclin D1-or cyclin E1-bound p27, 2 to 5 mg of protein lysate was precipitated with the appropriate antibody. For phosphatase treatment, p27 immunoprecipitates were washed twice with phosphatase buffer (50 mM Tris, pH 8.0, and 10% glycerol) and were then incubated at 37°C for 3 h with 10 U per 10-μl reaction of calf intestinal alkaline phosphatase (Boehringer Mannheim). For orthophosphate labeling, cells were transferred to phosphate-free medium for 1 h prior to labeling with 1 mCi of [32P]orthophosphate per p100 dish for 3 h. p27 was immunoprecipitated, and then complexes were resolved by 2DIEF. Immunoblotted p27 was detected by ECL and then exposed to film for autoradiography.
RESULTS
p27-cyclin-cdk2 complexes in TGF-β-resistant cells have increased kinase activity.
To investigate p27 effects on cdk2 complexes, lysates were prepared from asynchronous 184S (passage 12) and 184A1L5R populations with similar cell cycle distributions. 184S cells had 68% G1-, 25% S-, and 17% G2/M-phase cells, while the 184A1L5R cells had 65% G1-, 28% S-, and 17% G2/M-phase cells. The doubling times of the two cell types were similar at approximately 26 h. Levels of cyclin and cdk expression were quantitated by densitometry of different ECL exposures to ensure that the band densities were in the linear range of the film. Equal protein loading was verified by reprobing for β-actin. While the amounts of cyclin E1 and cyclin E1-bound cdk2 were similar, cyclin E1-cdk2 activity was higher in the resistant cells than in the sensitive cells (Fig. 1A). Similarly, cyclin A-dependent kinase activity was also elevated in 184A1L5R (Fig. 1A), despite similar levels of cyclin A and cyclin A-bound cdk2.
FIG. 1.
Cyclin-cdk2-p27 complexes and kinase activities. Cell lysates were prepared from asynchronously growing 184S (βS) and 184A1L5R (βR) cells that had similar cell cycle distributions (% S phase indicated). (A) Cyclin E1- and cyclin A-associated kinase activities. Cyclin E1 and cyclin A immunoprecipitates (IP) were assayed for associated kinase activities (left) or resolved by SDS-PAGE, transferred, and immunoblotted for cyclin and associated cdk2 (right) as described in Materials and Methods. For quantitation of the kinase reactions, radioactivity in nonspecific immune control (IgG) was subtracted and kinase activities were plotted as a percentage of the maximum (% max). Radioactivity in histone H1 bands is shown in the graph inset. (B) Cyclin E1 immunoprecipitated from asynchronously growing or TGF-β-treated 184S (βS) or 184A1L5R (βR). Complexes were resolved and proteins were detected by immunoblotting as indicated. Antibody-only control is shown on the right. (C) p27 immunoprecipitates in 184A1L5R cells contain active kinase. p27 immunoprecipitates from asynchronous HMECs with similar cell cycle distributions were assayed for cdk2 activity using histone H1 as substrate or resolved and immunoblotted for p27 and associated cdk2. Radioactivity in the nonspecific immune control (IgG) was subtracted prior to quantitation as for panel A above.
Cyclin E1-bound cdk2 in proliferating 184A1L5R cells showed an increase in the proportion of CAK-activated faster-mobility cdk2 isoform from that in 184S cells (Fig. 1A and B). Despite the higher cyclin E1-cdk2 activity, there was no loss of cyclin E1-bound p27 in proliferating 184A1L5R cells. Indeed, cyclin E1-bound p27 was somewhat increased in asynchronously growing resistant cells. These data raised the possibility that p27 from the 184A1L5R might bind cyclin E1-cdk2 with an altered conformation, possibly permitting cdk2 activity. To test this, equal amounts of p27 were precipitated from the two cell lines and were assayed for associated histone H1 kinase activity (Fig. 1C, left), and parallel p27 immunoprecipitations were immunoblotted to detect p27 and associated cdk2 (Fig. 1C, right). Asynchronously growing sensitive and resistant cells expressed p27 protein at similar levels. p27 immunoprecipitated from proliferating 184A1L5R showed significant histone H1 kinase activity, while that from 184S cells was only minimally above the nonspecific activity detected in nonimmune control precipitates. The amounts of cdk2 bound to p27 in asynchronous 184S and 184A1L5R cells were similar.
TGF-β inhibits cyclin D1-cdk4-p27 assembly in 184S but not in 184A1L5R cells.
The ability of newly synthesized cyclin D1 and cdk4 to assemble into cyclin D1-cdk4-p27 complexes was assayed by metabolic labeling in 184S and 184A1L5R cells. The HMECs were pulse labeled with [35S]methionine after 48 h of EGF depletion (G0 arrest) or at 12 h after release from G0 by readdition of EGF without (mid-G1) or with addition of TGF-β. Metabolically labeled cyclin D1 and cdk4 complexes are shown in Fig. 2. In G0, newly synthesized cyclin D1 precipitates did not bind cdk4, and cyclin D1 and p27 were barely detectable in cdk4 precipitates. In mid-G1, at a time when cyclin D1-cdk4 is active (35), cdk4 immunoprecipitates contained both cyclin D1 and p27. Although p27 is abundant in EGF-depleted, G0-arrested HMECs (see Fig. 5), it does not appear to facilitate the assembly of newly synthesized cyclin D1 with cdk4 (Fig. 2). Thus, the cyclin D1-cdk assembly function of p27 is lacking in G0, and p27 may require posttranslational modification to function as an assembly factor as cells move from G0 to mid-G1. When TGF-β was added to 184S cells at the same time that cells were stimulated to reenter cell cycle by readdition of EGF, the association of p27 and cyclin D1 with cdk4 was inhibited. While TGF-β caused a modest reduction in synthesis of cdk4 in 184S cells, it caused a more notable reduction in the relative amounts of cdk4-associated cyclin D1 and p27. In contrast, TGF-β did not prevent the assembly of p27-cyclin D1-cdk4 in resistant 184A1L5R cells.
FIG. 2.
p27-cyclin D1-cdk4 assembly detected by metabolic labeling. 184S (A) and 184A1L5R (B) were grown to 60% confluence and were then arrested by EGF deprivation (G0). Cells were then transferred to complete medium and cultured for 12 h without (mid-G1) or with addition of TGF-β. At the times indicated, cells were pulse labeled with [35S]methionine and were cyclin D1 or cdk4 immunoprecipitated. Nonimmune controls (IgG) were run alongside cyclin D1-cdk4 precipitates. Cyclin D1, PCNA, cdk4, and p27 are indicated with arrows on the right. Molecular weight markers in kilodaltons (kD) are indicated on the left.
FIG. 5.
p27 shows increased cytoplasmic localization in the 184A1L5R cells. (A) Immunocytochemistry shows strong nuclear p27 staining in G0-arrested 184S (βS, left). p27 is localized in both nucleus and cytoplasm in G0-arrested 184A1L5R cells (βR, center). The negative control in 184A1L5R cells is shown (right). (B) p27 content of nuclear (N) and cytoplasmic (C) fractions of quiescent βS and βR cells was assayed by Western blotting. (C) βS and βR cells were transfected with fluorescence-tagged p27 (YFPp27wt), and 24 h after transfection, p27 was detected by fluorescence microscopy. Cells in panels A and C were photographed at 400× magnification.
p27 from 184A1L5R cells is more cyclin D1 bound than p27 from 184S cells.
The steady-state levels of cyclin D1-bound p27 in asynchronous cells were compared by immunoprecipitation-Western blotting in the two lines. In addition to the differences in p27's functional association with cyclin E1-cdk2 complexes between the two lines (Fig. 1), proliferating 184A1L5R cells also showed an almost-twofold increase in steady-state binding of p27 to cyclin D1 from the level in 184S cells (Fig. 3A and B). Cyclin D1 immunoprecipitated from the resistant cells consistently showed, on repeat assays, a greater amount of associated p27 than did cyclin D1 from asynchronously proliferating 184S cells. p27 binding to cyclin D1 was corrected for the minor difference in total cyclin D1 immunoprecipitated from the two cell types and was graphed as a percentage of that observed in 184A1L5R in Fig. 3B. Moreover, the cyclin D1-bound p27 showed a slower mobility on SDS-PAGE than did cyclin E1-bound p27 (Fig. 3C). Cyclin E1-bound p27 from G0 cells had a mobility similar to that in asynchronous cells (not shown). The possibility that these different mobility forms of p27 may reflect altered p27 phosphorylation in cyclin D1 from that in cyclin E1 complexes was pursued further by 2DIEF (see below).
FIG. 3.
p27 binding to cyclin D1 and cyclin E1. (A) Asynchronously growing 184S (βS) or 184A1L5R (βR) cell lysates containing 200 μg of protein were serially immunodepleted of cyclin D1. Lanes 1 to 3 and 4 to 6 represent serial cyclin D1 immunoprecipitation (IP) from βS and βR cells, respectively. Immune complexes were resolved by SDS-PAGE and transferred, and blots were probed for cyclin D1 and p27. Lane C, control. (B) Cyclin D1 and p27 levels were quantitated by densitometry from different exposures of the blots in panel A, and the amounts of cyclin D1-bound p27 were corrected for the difference in total cyclin D1 between the two lines and graphed as a percentage of the maximum (% max) of that seen in the βR cells. (C) The mobilities of cyclin D1 (CycD1)- and cyclin E1 (CycE1)-bound p27 from asynchronous βS and βR cells are shown. Fifty micrograms of lysate was used for the cyclin D1 immunprecipitation, and 250 μg of lysate was used for cyclin E1 precipitation.
p27 from 184A1L5R cells has a reduced ability to bind and inhibit cyclin E1-cdk2.
To test p27 inhibitory function directly, p27 was immunoprecipitated and its ability to bind and inhibit recombinant cyclin E1 and glutathione transferase (GST)-tagged cdk2 complexes (cyclin E1-cdk2GST) was assayed. To ensure that differences in p27 function were not due to subtle differences in the cell cycle profiles between the two cell types, cells were G0 arrested by contact inhibition and EGF depletion (88 to 90% of cells with 2 N DNA content and <2% of cells in S phase). The amounts of p27 to be compared from the two cell types were titrated by immunoblotting and were arbitrarily designated 0.5×, 1×, and 2×. p27 immunoprecipitates from G0-arrested 184S cells contained more associated endogenous cellular cyclin E1 and cdk2 than did p27 from quiescent 184A1L5R cells (left side, Fig. 4A). On addition of recombinant cyclin E1-cdk2GST, approximately twice as much cyclin E1-cdk2GST bound to p27 from 184S cells than to p27 from 184A1L5R cells (right side, Fig. 4A).
FIG. 4.
In vitro assays of p27's ability to bind and inhibit cyclin E1-cdk2. (A) Titrated amounts of p27 were immunoprecipitated (IP) from G0-arrested 184S (βS) or 184A1L5R (βR) cells, resolved by SDS-PAGE, and associated cellular cyclin E1 and cdk2 (endogenous) detected by immunoblotting (left panels). On the right, the indicated amounts of immunoprecipitated p27 from βS and βR cells were mixed with recombinant (recomb) cyclin E1 and GST-tagged cdk2 and were then resolved by SDS-PAGE and p27-bound endogenous (endog) and recombinant proteins detected by immunoblotting. (B) The inhibitory activity of p27 is reduced in 184A1L5R. Recombinant cyclin E1-cdk2 (recomb E/k2) was mixed with the indicated amounts of p27 immunoprecipitated from the same 184S (βS) or 184A1L5R (βR) cell lysates as for panel A above, and histone H1 kinase activity was assayed. Inhibition of E/k2 activity by the addition of p27 is shown. Radioactivity incorporated into histone H1 is shown in the autoradiograph (inset, upper right) and graphed as a percentage of the maximum (% max) uninhibited E/k2 activity. (C) The reduced affinity for E/k2 is a heat-stable property of p27 from 184A1L5R cells. The indicated amounts of immunoprecipitated p27 from βS and βR cells were boiled (100°C for 5 min). The initial amount of p27 used is indicated in the immunoblot (top left), and p27 reprecipitated after boiling is shown (lower left). The free heat-stable p27, recovered after p27 complexes were boiled, was mixed with recombinant cyclin E1 and cdk2 proteins. Cyclin E1 was then immunoprecipitated, and the amount of cyclin E1-bound cellular p27 was detected by blotting. Results shown in panels A to C are a representative of at least three experiments.
The cyclin E1-cdk2 inhibitory function of p27 was assayed using the same G0-arrested 184S or 184A1L5R lysates as for Fig. 4A. Recombinant cyclin E1-cdk2GST was incubated with the indicated amounts of immunoprecipitated p27 from each cell type. Uninhibited cyclin E1-cdk2GST activity was quantitated and compared with the activity remaining after admixture of cellular p27. The cyclin E1-cdk2-inhibitory activity of p27 from 184S cells was approximately twice that of p27 from 184A1L5R cells (Fig. 4B). The data graphed are the mean of three kinase inhibition assays.
To test if this difference in inhibitory activities reflected different amounts of free, non-cyclin-bound p27, cyclins and cdk's were immunodepleted from G0 lysates and the amount of residual p27 was assayed by immunoblotting. Serial immunodepletion of cyclin D1, cyclin E1, and then of cdk4 and -6 followed by p27 precipitation and p27 immunoblotting showed a significant excess of p27 remaining in the final immunoprecipitation in both 184S and 184A1L5R G0 lysates (80 and 67% of total, respectively, as assayed by densitometry, data not shown). The non-cyclin D1- and E1-bound p27 in 184S cells was only 1.2-fold higher than that in 184A1L5R, and this difference in potentially unbound p27 could not fully account for the increased inhibitory activity in the 184S lysates. The cyclin E immunodepletion confirmed that G0 184S cells had more cyclin E1-bound p27 than did 184A1L5R cells. There was a modest amount of cyclin D1-bound p27 in the resistant cells, but cyclin D1-bound p27 was negligible in G0 184S cells. Thus, sequestration by cyclin D1 was not sufficient to account for the difference in inhibitory activities shown in Fig. 4B.
Reduced ability to bind and inhibit cyclin E1-cdk2 is a heat-stable property of p27 from 184A1L5R.
As a second measure to rule out p27 sequestration by associated heat-labile cyclins or novel proteins, we made use of the heat-stable property of p27. p27 immunoprecipitated from 184S or 184A1L5R cells was boiled (100°C for 5 min). Under these conditions, p27 is essentially monomeric (14, 41). The recovered heat-stable p27 was incubated with cyclin E1-cdk2GST. The binding of p27 to recombinant cyclin E1 was detected by immunoblotting after cyclin E1 immunoprecipitation. Less heat-stable p27 from 184A1L5R cells bound to the recombinant cyclin E1-cdk2GST than did p27 from the 184S cells (Fig. 4C). The difference in the kinase-inhibitory activities of heat-stable p27 from the two cell types was essentially the same as that observed for Fig. 4B. Approximately twice as much heat-stable p27 from the resistant cells was required to achieve the same inhibition of cyclin E1-cdk2GST as from the 184S cells (not shown). Thus, the reduced ability of the heat-stable p27 from 184A1L5R cells to bind and inhibit cyclin E1-cdk2 was not due to sequestration by cyclins nor likely due to the presence of a novel p27-associated protein but might rather reflect a heat-stable, posttranslational modification.
p27 shows altered intracellular localization in resistant cells.
Some advanced cancer-derived cell lines show mislocalization of p27 in the cytoplasm (31). To test whether the loss of p27 function in 184A1L5R cells was associated with altered cellular localization, the immunolocalization of p27 was assayed. p27 was predominantly nuclear in 184S cells, while quiescent 184A1L5R cells showed both nuclear and strong cytoplasmic p27 staining (Fig. 5A). Subcellular fractionation and immunoblotting confirmed a nuclear-to-cytoplasmic ratio of p27 in 184A1L5R cells of approximately 1.5:1, while that in the 184S cells was greater than 6:1 (representative blot, Fig. 5B). The nuclear protein RCC1 provided a control for fractionation (27). Similarly, TGF-β-arrested 184S cells showed nuclear p27 localization, while TGF-β-treated 184A1L5R cells showed both nuclear and cytoplasmic p27 staining (not shown). Moreover, when 184S and 184A1L5R cells were transiently transfected with a vector encoding YFPp27wt, the p27 fluorescence detected 24 h posttransfection was exclusively nuclear in 184S cells but both cytoplasmic and nuclear in 184A1L5R cells (Fig. 4C).
When nuclear and cytoplasmic fractions were compared from the two cell lines, equal amounts of p27 from both nuclear and cytoplasmic fractions from 184A1L5R showed a decreased ability to bind recombinant cyclin E1-cdk2GST, compared to nuclear p27 from the 184S cells (not shown). Thus, the reduced cyclin E1-cdk2 binding affinity of total cellular p27 from 184A1L5R cells could not be attributed solely to the presence of a dysfunctional p27 localized in the cytoplasm.
p27 phosphorylation is altered in TGF-β-resistant cells.
2DIEF showed that the differences in p27 function and localization between the 184S and 184A1L5R cells were associated with differences in p27 phosphorylation. In G0 cells, at least seven different p27 isoforms could be reproducibly distinguished by 2DIEF, using an amphotric carrier with a linear pH range of 3 to 10 (Fig. 6). The dominant isoform of p27 in G0 184S cells (form 1) was the least negatively charged isoform, migrating close to the predicted IEF point for p27 of 6.5, with others at pI's of 6.24 (form 2), 5.95 (form 3), 5.58 (form 6), and 5.48 (form 7). TGF-β-resistant 184A1L5R cells showed a different pattern of p27 isoforms in G0. Two major isoforms were seen at pI 6.5 and 5.95, with three minor forms at 6.24, 5.8 (form 4), and 5.65 (form 5). The isoforms at pI 5.54 and 5.48 observed in 184 were not detected in 184A1L5R cells. Forms 1 to 3 were common to both cell lines, as they comigrated when immunoprecipitates from each line were mixed prior to 2DIEF; however, their relative abundance differed significantly between the two cell types. p27 form 1 was the dominant isoform in 184S cells, while the relative abundance of isoform 3 (pI 5.95) was greater in 184A1L5R cells. With phosphatase treatment prior to 2DIEF, most p27 migrated as form 1, with a small amount migrating as form 2 (Fig. 6) (8). This small amount of form 2 remaining may result from an incomplete phosphatase reaction. However, 32P-orthophosphate labeling showed that forms 1 and 2 did not incorporate any radioactivity (data not shown). While form 1 probably represents unphosphorylated p27, form 2 may represent posttranslational modification (such as acetylation, myristylation, or glycosylation) other than phosphorylation. Taken together, these data suggest that isoforms 3 to 7 seen on 2DIEF are phosphoforms of p27.
FIG. 6.
p27 phosphorylation differs between 184S and 184A1L5R cells. p27 was immunoprecipitated (IP) using polyclonal p27 pAb5588 from G0-arrested 184S (βS) or 184A1L5R (βR) cells. For 2DIEF, complexes were resolved using the IEF strips with a linear gradient of pH 3 to 10 (3-10 L) followed by SDS-12% PAGE. Gels were transferred and p27 isoforms were detected by immunoblotting with the Transduction Laboratories p27 antibody. The IEF points (pI) of the p27 isoforms (forms 1 to 7) are indicated with arrows. In the third panel, the p27 immunprecipitates from βS and βR cells were mixed prior to 2DIEF. The bottom panel shows that, after phosphatase treatment, most of the p27 migrated at the highest IEF point, 6.5.
Phosphorylation of cyclin E1-bound p27 differs from that of cyclin D1-bound p27.
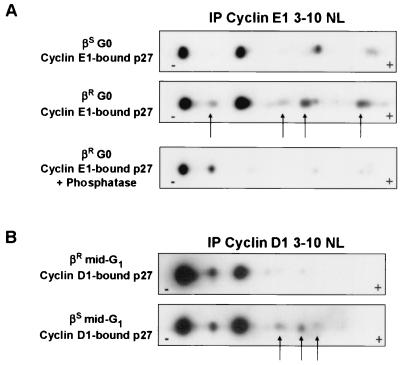
While the levels of cyclin D1 were similar in the sensitive and resistant cells, more p27 was bound to cyclin D1 complexes in proliferating 184A1L5R than in 184S cells (Fig. 3). Moreover, TGF-β did not inhibit the assembly of newly synthesized p27, cyclin D1, and cdk4 into complexes in 184A1L5R cells (Fig. 2). Since the mobilities of cyclin D1-bound and cyclin E1-bound p27 on SDS-PAGE gels differ (Fig. 3C), suggesting a different p27 phosphorylation state, we performed 2DIEF analysis on cyclin D1-bound p27 from mid-G1 cells and cyclin E1-bound p27 from G0-arrested cells using the nonlinear gradient pH 3 to 10 to ascertain whether the “assembly form” of p27 bound to cyclin D1 might differ in its phosphorylation from that bound to cyclin E1. The phosphorylation of cyclin E1-bound p27 (Fig. 7A) differed from that in cyclin D1 complexes (Fig. 7B). Phosphatase treatment confirmed that the different p27 forms were due to phosphorylation (Fig. 7A and data not shown).
FIG. 7.
The phosphorylation of cyclin E1-bound p27 differs from that of cyclin D1-bound p27. The 2DIEF profile of p27 in the 184S (βS) or 184A1L5R (βR) cells was assayed in cyclin E1 immune complexes from G0 (contact inhibition for 48 h, followed by 48 h of EGF deprivation) (A) and in cyclin D1 immune complexes from mid-G1 (B). Cyclin E1-bound p27 was treated with calf intestinal alkaline phosphatase prior to 2DIEF for the lower part of panel A. Complexes were resolved on pH 3 to 10 nonlinear gradient (3-10 NL) IEF strips prior to resolution on SDS-12% PAGE and immunoblotting for p27. The arrows (A and B) indicate cyclin-associated p27 isoforms in 184A1L5R that are not detected or less abundant in 184S cells. IP, immunoprecipitation.
Although there was only a minor difference in the phosphorylation patterns of cyclin D1-bound p27 between the two lines, Fig. 3 demonstrated a twofold increase in the total amount of cellular p27 bound to cyclin D1 in 184A1L5R cells compared to the amount in 184S cells. Taken together, these data suggest that there may be a constitutive alteration of p27 phosphorylation that shifts it toward the cyclin D1-cdk assembly phosphoform in the TGF-β-resistant cells. In addition, there was a subtle difference in phosphorylation patterns of cyclin-bound p27 between 184A1L5R and 184S cells. Certain cyclin E1-bound phosphoforms detected in 184A1L5R cells were not seen in cyclin E1 complexes in 184S cells (arrows, Fig. 7A). The most abundant cyclin D1-bound p27 isoform in 184S cells was the most hypophosphorylated, and the three most negatively charged (most strongly phosphorylated) p27 isoforms detected in 184A1L5R cells were not detected in cyclin D1 complexes in 184S cells (arrows, Fig. 7B). These data may reflect constitutive activation of certain p27 phosphorylation events in 184A1L5R cells.
DISCUSSION
Earlier investigation of TGF-β effects in these HMECs suggested a defect in p27 function (35). TGF-β increased p15 protein stability and its association with cdk4 and cdk6 and inhibited assembly of cyclin D1-p27-cdk4 and -cdk6 complexes, while p27 accumulated in cyclin E1-cdk2 in 184S cells but not in resistant 184A1L5R cells (35). Moreover, cdk4-cyclin D1-KIP complexes from 184A1L5R lysates were resistant to dissociation by p15 in vitro (35). These data led us to postulate that movement of p27 out of cyclin D1-cdk4 complexes might be necessary to allow p15 to displace cyclin D1 and inhibit cdk4 and that altered posttranslational modification of p27 may prevent its dissociation from cdk4 complexes and abrogate sensitivity to G1 arrest by TGF-β.
Several observations in TGF-β-resistant 184A1L5R cells suggest aberrant p27 regulation. The increased activities of cyclin E1- and cyclin A-dependent kinases and the increased CAK activation of cyclin E1-bound cdk2 are consistent with defective KIP function in 184A1L5R cells. There was more p27 bound to cyclin E1 in proliferating 184A1L5R cells, but p27 binding did not appear to have the same inhibitory consequences as in 184S cells. Indeed, p27 from proliferating 184A1L5R cells was associated with histone H1 kinase activity, while essentially none was detected in p27 complexes from proliferating 184S cells. p27 itself is not a kinase. The p27-immunoprecipitable kinase activity detected in 184A1L5R cells may arise through the dissociation of cyclin E1-cdk2 from p27 in vitro after immunoprecipitation. Alternatively, p27 from the resistant cells may bind cyclin E1-cdk2 with an altered conformation that allows both CAK access to cdk2 and cdk2 activity. The observation of p27-associated kinase activity is not without precedent (8, 21, 43). It was recently found that breast cancer cells resistant to antiestrogen-mediated G1 arrest showed altered p27 phosphorylation, p27-associated kinase activity, and reduced inhibitory function in vitro (8). p27 is a key mediator of G1 arrest by TGF-β and by antiestrogens, and, thus, it is not surprising that its function is altered in cells resistant to these different forms of G1 arrest.
In the resistant cells, altered p27 regulation was also manifested by the localization of p27 in both the nucleus and cytoplasm, in contrast to the largely nuclear localization of p27 in 184S cells. The expression of stable cytoplasmic p27 observed in some primary cancers (40) and in malignantly transformed cell lines (31) could reflect similar perturbations of the cell cycle-regulatory machinery. The strong stable cytoplasmic expression of p27 in 184A1L5R cells suggests either a dissociation of export and degradation (51) or impaired import of newly synthesized p27. It is not clear whether or how the increased cytoplasmic localization of p27 in the 184A1L5R cells is linked to its increased association with cyclin D1 or with its reduced affinity for cyclin E1-cdk2.
In vitro assays indicated a reduced p27 affinity for cyclin E1-cdk2 in G0-arrested 184A1L5R cells. p27 from 184A1L5R bound and inhibited less recombinant cyclin E1-cdk2 in vitro than did p27 from 184S. Phosphatase treatment of the p27 prior to cyclin E1-cdk2 inhibition assays abolished the inhibitory activity detected in both cell types (not shown), suggesting that certain p27 phosphorylation events are required for cyclin E1-cdk2 inhibition. Overexpression of c-myc has been shown to induce a heat-labile factor that binds p27 and inhibits its association with cyclin E-cdk2 (53). However, the differences in p27 inhibitory activity could not be attributed to sequestration by cyclin D1 or a heat-labile inhibitor of p27 in 184A1L5R cells, since they persisted after boiling and, thus, more likely reflect the differences observed in p27 phosphorylation. While a heat-stable protein could reassociate with p27 and prevent its binding and inhibition of cyclin E1-cdk2 in these assays, the relative rarity of heat-stable cellular proteins mitigates against this.
The reduced ability of p27 from G0 184A1L5R to bind and inhibit cyclin E1-cdk2 was associated with an altered p27 phosphorylation profile. 2DIEF identified several p27 isoforms. The dominant form 1 (70% of total) in 184S cells migrated with the predicted p27 IEF point of 6.5. This hypophosphorylated pI 6.5 isoform represented only 40% of the p27 from 184A1L5R cells. Whether the different p27 isoforms seen on 2DIEF reflect combinations of multiple phosphorylation events or specific changes in single phosphorylation sites is under investigation.
p27 function changes during G0-to-S-phase progression. p27 is a potent inhibitor of cyclin E1-cdk2 in G0- and in TGF-β-arrested cells. In quiescence or in TGF-β-arrested HMECs, newly synthesized cyclin D1 fails to bind cdk4. This is not likely attributable to the low abundance of cyclin D1 in quiescence, since cyclin D1 is clearly synthesized in G0 HMECs and since even ectopically overexpressed cyclin D1 fails to assemble with cdk4 in quiescent fibroblasts (26). The data of Matsushime et al. (26) suggested that a growth factor-stimulated event is required for cyclin D1-cdk4 assembly. Indeed, subsequent work showed that cyclin D1 overexpression mediates p27-cyclin D1-cdk4 complex formation only when coexpressed with an activated MEK, suggesting that MEK-dependent effects may facilitate p27-cyclin D1-cdk4 assembly (6). p27 may require posttranslational modification during G0-to-G1 progression in order to function in cyclin D-cdk assembly. We have shown that the pattern of p27 phosphorylation differs when it is bound to inactive cyclin E1-cdk2 in G0 from that present in cyclin D1-cdk complexes in mid-G1. Thus, as cells move from G0 into G1, p27 acquires the ability to function as a cyclin D1-cdk assembly factor in association with changes in its phosphorylation.
In the 184A1L5R cells, approximately twofold more cellular p27 was detected in cyclin D1-complexes than in 184S cells. This twofold increase in cyclin D1 binding could potentially be significant, since changes of this magnitude in the amount of p27 accessible for cyclin E-cdk2 binding can have significant effects on cyclin E-cdk2 activity. Reynisdottir et al. showed that a two- to threefold increase in p27 was sufficient to fully saturate cyclin E, leading to G1 arrest (34). However, there was no reduction in p27 binding to cyclin E1 in asynchronous 184A1L5R cells; indeed, it was modestly increased. Moreover, immunoprecipitated p27 from resistant cells contained associated histone H1 kinase activity. These observations raise the possibility that the changes in p27 phosphorylation that allow its cyclin D-cdk assembly function in mid-G1 may occur in association with a reduced ability to inhibit cyclin E complexes. The p27-associated kinase detected in 184A1L5R cells may reflect kinase-active p27-cyclin-cdk2 complexes or kinase complexes containing p27 that readily dissociate in vitro. There is some experimental evidence to suggest that p27 may exist transiently in a cyclin E-bound, noninhibitory conformation in vivo in G1 (8, 54). Unfortunately, the low abundance of cyclin E1-bound p27 in mid-G1 precluded analysis of its phosphorylation status by 2DIEF. Constitutive activation of pathways leading to increased assembly of p27 into cyclin D1-cdk4 complexes may also lead to a change in the inhibitory action of p27 toward cyclin E-cdk2 in the 184A1L5R cells. Alternatively, the pathways that regulate p27-cyclin D1-cdk4 assembly and p27's cyclin E-cdk2 inhibitory function may be independent of each other, and both may be altered in the TGF-β-resistant cells.
In the G0 184A1L5R cells, the reduced ability to bind stably to cyclin E1-cdk2 was associated with altered cellular p27 phosphorylation. T187 phosphorylation of p27 by cyclin E-cdk2 (36, 54) occurs near the G1-to-S-phase transition (3) and allows p27 recognition by the ubiquitin ligase SCFSkp2 complex (Skp2, Cul1, and Skp1) involved in its proteolysis (3, 49, 52). T187 phosphorylation is minimal in G0, and it does not reduce p27's affinity for cyclin E1-cdk2 in vitro (36, 54) (B. Amati, personal communication). Thus, phosphorylation of sites other than T187 must affect p27 function in 184A1L5R cells. Although serine 10 appears to be a major p27 phosphorylation site in cells arrested by p27 transfection, mutation of serine 10 did not detectably alter the inhibitory activity of p27 toward cyclin E-cdk2 in vitro (18). Thus, serine 10 phosphorylation may not be relevant to the poor cyclin-E-cdk2 inhibitory function of p27 in 184A1L5R cells.
Since the assembly and activation of cyclin D-cdk complexes (2, 5, 21) precede activation of cyclin E1-cdk2 during normal G1 progression, kinases other than cyclin E-cdk2 may phosphorylate p27 and condition it to function as a cyclin D-cdk assembly factor. The noncatalytic function of cyclin D-cdk complexes to titrate p27 may not be exclusively dependent on the abundance of D-type cyclins but is also actively regulated through p27 phosphorylation. As noted earlier, overexpression of activated MEK, together with cyclin D1, can lead to sequestration of p27 into cyclin D1-cdk4 and activate cyclin E-cdk2 though loss of p27 binding (6). Although mitogen-activated protein kinase activation occurs early and is required for G1-to-S-phase progression in HMECs, the increased assembly of p27 in cyclin D1 complexes in 184A1L5R cells could not be attributed to increased mitogen-activated protein kinase activation (J. Liang and J. M. Slingerland, unpublished data).
Constitutive ras activation has been shown to increase p27 phosphorylation, leading to both a reduced affinity of p27 binding to cdk2 in vitro and to p27 degradation. In human cancer-derived lines, oncogenic activation of receptor tyrosine kinase pathways (23) or of ras (1, 19, 50) may lead to TGF-β resistance through accelerated p27 proteolysis or reduced cdk-inhibitory function. Only the latter of these effects is observed in the TGF-β-resistant 184A1L5R line. In proliferating 184A1L5R cells, the equilibrium of p27 binding was shifted, with more p27 bound to cyclin D1 than in 184S cells. Moreover, p27 from the resistant cells showed reduced inhibitory activity against cyclin E1-cdk2. These data suggest a model in which TGF-β modulates the posttranslational regulation of p27, converting it from a factor with high affinity for cyclin D1-cdk4 to a form that binds with high affinity and inhibits cyclin E1-cdk2. In the resistant 184A1L5R cells, p27 phosphorylation was shifted, allowing increased p27 binding to and assembly of cyclin D1-cdk complexes and the presence of cdk2 bound p27 with reduced affinity for or inhibitory action on cyclin E-cdk2. In the TGF-β-resistant 184A1L5R cells, constitutive activation of one or more mitogenic pathways may alter p27 phosphorylation, causing failure of p27 to dissociate from cyclin D1-cdk4 and preventing p15 from displacing cyclin D1 and inhibiting cdk4 in response to TGF-β. These changes in p27 function that contribute to TGF-β resistance are likely secondary to alterations in mitogenic signaling pathways in the 184A1L5R cells. Moreover, the effects of TGF-β on the cell cycle are pleiotropic. While our data suggest a role for deregulated p27 function in the resistant cells, other changes in cell cycle effectors likely contribute to the resistance phenotype. Elucidation of the signal transduction pathways whose activation regulates these events in the 184A1L5R model may shed light not only on mechanisms of TGF-β resistance but may also reveal the pathways whose activation leads to p27 phosphorylation, acquisition of cyclin D1-cdk assembly function, and the subsequent degradation of p27 during G1-to-S-phase progression in normal cells.
Acknowledgments
Wesley Hung and Venkateswaran Subramaniam contributed equally to this work.
We thank Martha Stampfer, L. Hengst, M. Pagano, and T. Hunter for helpful discussions and M. Pagano and L. Hengst for critical reading of the manuscript. We thank T. Hunter and H. Toyoshima for the pAb5588 p27 antibody, M. Stampfer for 184S and 184A1L5R HMECs, B. Amati for reagents for cyclin E1 and cdk2 production by bacculovirus, L. Hengst for the YFPp27wt plasmid, and N. Bhattacharya and C. To for technical assistance.
This work was supported by a grant from the Canadian Breast Cancer Research Initiative to J.M.S. J.M.S. is supported by Cancer Care Ontario and by the Burroughs Wellcome Fund.
REFERENCES
- 1.Aktas, H., H. Cai, and G. M. Cooper. 1997. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 17:3850-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cariou, S., J. C. Donovan, W. M. Flanagan, A. Milic, N. Bhattacharya, and J. M. Slingerland. 2000. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc. Natl. Acad. Sci. USA 97:9042-9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 4.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3:227-230. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, M., V. Sexl, C. J. Sherr, and M. F. Roussel. 1998. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl. Acad. Sci. USA 95:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan, J., and J. Slingerland. 2000. Transforming growth factor-beta and breast cancer: cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. 2:116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan, J. C., A. Milic, and J. M. Slingerland. 2001. Constitutive MEK/MAPK activation leads to p27Kip1 deregulation and antiestrogen resistance in human breast cancer cells. J. Biol. Chem. 276:40888-40895. [DOI] [PubMed] [Google Scholar]
- 9.Draetta, G., and J. Eckstein. 1997. Cdc25 protein phosphatases in cell proliferation. Biochim. Biophys. Acta 1332:M53-M63. [DOI] [PubMed] [Google Scholar]
- 10.Dulic, V., E. Lees, and S. I. Reed. 1992. Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257:1958-1961. [DOI] [PubMed] [Google Scholar]
- 11.Dumont, N., and C. L. Arteaga. 2000. Transforming growth factor-beta and breast cancer tumor promoting effects of transforming growth factor-beta. Breast Cancer Res. 2:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florenes, V. A., N. Bhattacharya, M. R. Bani, Y. Ben-David, R. S. Kerbel, and J. M. Slingerland. 1996. TGF-beta mediated G1 arrest in a human melanoma cell line lacking p15INK4B: evidence for cooperation between p21Cip1/WAF1 and p27Kip1. Oncogene 13:2447-2457. [PubMed] [Google Scholar]
- 13.Hannon, G. J., and D. Beach. 1994. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371:257-261. [DOI] [PubMed] [Google Scholar]
- 14.Hengst, L., V. Dulic, J. M. Slingerland, E. Lees, and S. I. Reed. 1994. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 91:5291-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengst, L., and S. I. Reed. 1996. Translational control of p27Kip1 accumulation during the cell cycle. Science 271:1861-1864. [DOI] [PubMed] [Google Scholar]
- 16.Hosobuchi, M., and M. R. Stampfer. 1989. Effects of transforming growth factor beta on growth of human mammary epithelial cells in culture. In Vitro Cell Dev. Biol. 25:705-713. [DOI] [PubMed] [Google Scholar]
- 17.Iavarone, A., and J. Massague. 1997. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 387:417-422. [DOI] [PubMed] [Google Scholar]
- 18.Ishida, N., M. Kitagawa, S. Hatakeyama, and K. Nakayama. 2000. Phosphorylation at Serine 10, a major phosphorylation site of p27Kip1, increases its protein stability. J. Biol. Chem. 275:25146-25154. [DOI] [PubMed] [Google Scholar]
- 19.Kawada, M., S. Yamagoe, Y. Murakami, K. Suzuki, S. Mizuno, and Y. Uehara. 1997. Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene 15:629-637. [DOI] [PubMed] [Google Scholar]
- 20.Koff, A., M. Ohtsuki, K. Polyak, J. M. Roberts, and J. Massague. 1993. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science 260:536-539. [DOI] [PubMed] [Google Scholar]
- 21.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 22.Laiho, M., J. A. DeCaprio, J. W. Ludlow, D. M. Livingston, and J. Massague. 1990. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell 62:175-185. [DOI] [PubMed] [Google Scholar]
- 23.Lane, H. A., I. Beuvink, A. B. Motoyama, J. M. Daly, R. M. Neve, and N. E. Hynes. 2000. ErbB2 potentiates breast tumor proliferation through modulation of p27Kip1-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol. Cell. Biol. 20:3210-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauper, N., A. R. Beck, S. Cariou, L. Richman, K. Hofmann, W. Reith, M. M. Slingerland, and B. Amati. 1998. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene 17:2637-2643. [DOI] [PubMed] [Google Scholar]
- 25.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 26.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J.-Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melchior, F., and L. Gerace. 1998. Two-way trafficking with Ran. Trends Cell Biol. 8:175-179. [DOI] [PubMed] [Google Scholar]
- 28.Millard, S. S., J. S. Yan, H. Nguyen, M. Pagano, H. Kiyokawa, and A. Koff. 1997. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem. 272:7093-7098. [DOI] [PubMed] [Google Scholar]
- 29.Montagnoli, A., F. Fiore, E. Eytan, A. C. Carrano, G. F. Draetta, A. Hershko, and M. Pagano. 1999. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, and D. Y. Loh. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 31.Orend, G., T. Hunter, and E. Ruoslahti. 1998. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene 16:2575-2583. [DOI] [PubMed] [Google Scholar]
- 32.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. Del Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 33.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 34.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu, C., J. Garbe, N. Bhattacharya, J. Daksis, C.-H. Pan, P. Yaswen, J. Koh, J. M. Slingerland, and M. R. Stampfer. 1997. Transforming growth factor β stabilizes p15INK4B protein, increases p15INK4B-cdk4 complexes, and inhibits cyclin D1/cdk4 association in human mammary epithelial cells. Mol. Cell. Biol. 17:2458-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 37.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79:551-555. [DOI] [PubMed] [Google Scholar]
- 38.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 39.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 40.Singh, S. P., J. Lipman, H. Goldman, F. H. Ellis, L. Aizenman, M. G. Cangi, S. Signoretti, D. S. Chiaur, M. Pagano, and M. Loda. 1998. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 58:1730-1735. [PubMed] [Google Scholar]
- 41.Slingerland, J. M., L. Hengst, C.-H. Pan, D. Alexander, M. R. Stampfer, and S. I. Reed. 1994. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol. Cell. Biol. 14:3683-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon, M. J., and P. Kaldis. 1998. Regulation of CDKs by phosphorylation. Results Probl. Cell Differ. 22:79-109. [DOI] [PubMed] [Google Scholar]
- 43.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7:135-146. [PubMed] [Google Scholar]
- 44.Stampfer, M. 1985. Isolation and growth of human mammary epithelial cells. J. Tissue Cult. Methods 9:107-115. [Google Scholar]
- 45.Stampfer, M. R., and J. C. Bartley. 1985. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc. Natl. Acad. Sci. USA 82:2394-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stampfer, M. R., A. Bodnar, J. Garbe, M. Wong, A. Pan, B. Villeponteau, and P. Yaswen. 1997. Gradual phenotypic conversion associated with immortalization of cultured human mammary epithelial cells. Mol. Biol. Cell 8:2391-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stampfer, M. R., C.-H. Pan, J. Hosoda, J. Bartholomew, J. Mendelsohn, and P. Yaswen. 1993. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous re-entry into the cell cycle. Exp. Cell Res. 208:175-188. [DOI] [PubMed] [Google Scholar]
- 48.Stampfer, M. R., P. Yaswen, M. Alhadeff, and J. Hosoda. 1993. TGF beta induction of extracellular matrix associated proteins in normal and transformed human mammary epithelial cells in culture is independent of growth effects. J. Cell. Physiol. 155:210-221. [DOI] [PubMed] [Google Scholar]
- 49.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 50.Takuwa, N., and Y. Takuwa. 1997. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 17:5348-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomoda, K., Y. Kubota, and J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 52.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 53.Vlach, J., S. Hennecke, K. Alevizopoulos, D. Conti, and B. Amati. 1996. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 15:6595-6604. [PMC free article] [PubMed] [Google Scholar]
- 54.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warner, B. J., S. W. Blain, J. Seoane, and J. Massagué. 1999. Myc downregulation by transforming growth factor β required for activation of the p15Ink4b G1 arrest pathway. Mol. Cell. Biol. 19:5913-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]