Abstract
In a yeast two-hybrid screen to identify proteins that bind to the KIX domain of the coactivator p300, we obtained cDNAs encoding nucleosome assembly protein 1 (NAP-1), a 60-kDa histone H2A-H2B shuttling protein that promotes histone deposition. p300 associates preferentially with the H2A-H2B-bound form of NAP-1 rather than with the unbound form of NAP-1. Formation of NAP-1-p300 complexes was found to increase during S phase, suggesting a potential role for p300 in chromatin assembly. In micrococcal nuclease and supercoiling assays, addition of p300 promoted efficient chromatin assembly in vitro in conjunction with NAP-1 and ATP-utilizing chromatin assembly and remodeling factor; this effect was dependent in part on the intrinsic histone acetyltransferase activity of p300. Surprisingly, NAP-1 potently inhibited acetylation of core histones by p300, suggesting that efficient assembly requires acetylation of either NAP-1 or p300 itself. As p300 acted cooperatively with NAP-1 in stimulating transcription from a chromatin template in vitro, our results suggest a dual role of NAP-1-p300 complexes in promoting chromatin assembly and transcriptional activation.
The coactivator p300 and its paralog, CBP, mediate transcriptional induction via a number of signal-dependent activators, including CREB (4, 5, 31), c-Jun (4, 6), c-Fos (7), and a variety of nuclear hormone receptors (25). Consistent with their considerable size (265 kDa), p300 and CBP contain numerous interaction surfaces that function in protein recognition. The highly conserved KIX domain, for example, has been shown to associate with phospho (Ser133) CREB (39, 40, 42, 45), c-Myb (41), SREBP-1 (38), and cubitus interruptus (2). Other domains in p300 and CBP named CH1 and CH3 function in a similar manner to promote cellular gene expression (14).
Following their association with various transcription factors, p300 and CBP appear to mediate transcriptional activation of signal-dependent genes, in part via their association with RNA polymerase II complexes (10, 27, 33, 36) and via intrinsic histone acetyltransferase (HAT) activities that destabilize the higher-order structure of nucleosomal arrays (8, 29, 37). p300−/− fibroblasts show a cell cycle defect (53); progression through S phase is severely attenuated, suggesting a potential role for this HAT in chromatin assembly during DNA replication.
Chromatin assembly during S phase is tightly coupled to DNA replication. The deposition of H3-H4 tetramers proceeds via redistribution of parental H3-H4 histone tetramers and via assembly of newly synthesized H3 and H4 (16, 23, 51). Newly synthesized H3 and H4 are transiently acetylated at sites in their N-terminal tails and then are deacetylated by histone deacetylases after deposition onto DNA (15, 43). Histone H4 is acetylated by the type B HAT holoenzyme complex Hat1 (48). The mammalian Hat1 holoenzyme contains the catalytic Hat1 enzyme and p46, a core histone binding protein that functions importantly in stabilizing complex formation with histones (48). Following their acetylation by Hat1, H3-H4 complexes are deposited onto newly replicated DNA by chromatin assembly factor 1 (CAF-1) (49). The subsequent addition of two H2A-H2B dimers to the H3-H4 tetramer completes the assembly of the nucleosome on newly replicated DNA (16, 23, 51).
The H2A-H2B histone chaperone protein NAP-1 (nucleosome assembly protein 1) acts in concert with CAF-1 or replication-coupling assembly factor (RCAF) to deliver these core histones to the chromatin assembly apparatus during S phase (17, 21, 22, 26, 35, 47). NAP-1 has been shown to enter the nucleus during early S phase, complexed with H2A-H2B (9, 22), and to exit the nucleus during G2 (22). By contrast with assembly of the H3-H4 tetramer, however, the involvement of H2A-H2B acetylation during chromatin assembly has not been fully characterized (15, 43).
In yeast two-hybrid screening experiments to identify cellular proteins that bind to the KIX domain of CBP/p300, we obtained a number of cDNAs encoding the histone chaperone protein NAP-1. Here we examine the formation of NAP-1-p300 complexes in the cell cycle, and we evaluate the importance of these complexes for chromatin assembly and for transcriptional activation on a chromatin template. Our results suggest that formation of NAP-1-p300 complexes facilitates chromatin assembly and stimulates gene expression, in part by altering nucleosome structure.
MATERIALS AND METHODS
Two-hybrid screen and plasmids.
Plasmid construction, cloning, site-directed mutagenesis, and DNA sequencing were carried out with standard protocols. A two-hybrid screen using the yeast strain L40 was performed as described previously (12, 50) to identify cellular proteins that specifically interact with the KIX domain of CBP. Briefly, a fusion between the LexA DNA binding domain and the KIX domain of CBP (amino acids [aa] 455 to 679) was constructed (LexA-KIX) and used as bait to screen a human activated B lymphocyte cDNA library cloned into the two-hybrid vector pACT2 (Clontech). Of 106 colonies screened, 62 showed specific interaction with LexA-KIX. The full-length NAP-1 cDNA (aa 1 to 391) was obtained by reverse transcription and PCR amplification of human Jurkat T cell RNA with specific primers for the coding sequence of NAP-1. A myc tag was fused in frame to the C terminus of NAP-1 by cloning the full-length NAP-1 cDNA into the mammalian expression vector pcDNA3.1/myc (Invitrogen). A plasmid encoding a glutathione S-transferase (GST)-NAP-1 fusion protein was constructed by PCR amplification of full-length NAP-1 followed by being subcloned into pGEX-4T2 (Pharmacia Biotech).
Cell culture and synchronization.
HeLa cells were maintained in Dulbecco's modified Eagle medium (Gibco) containing 10% fetal calf serum. Cells were synchronized by two sequential 12-h blocks in 2 mM thymidine (Sigma) separated by a 10-h interval in 24 μM thymidine and 24 μM deoxycytidine (Sigma) (34). Cells at various stages of the cell cycle were obtained by releasing G1/S cells into culture medium for the times indicated in Results. Cell cycle synchronization was verified by flow cytometric analysis as described previously (24).
Coimmunoprecipitations and pull-down assays.
Western blotting, coimmunoprecipitation, and GST pull-down assays were performed as previously described (36). HeLa cells were lysed by incubation at 4°C for 30 min in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM Na4P2O7, 20 mM NaF, 1 mM Na3VO4, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 U of aprotinin/ml, 20 μg of leupeptin/ml, 1.5 mM MgCl2, and 0.5% Triton X-100). The cytoplasmic fraction was collected, and nuclear extract was prepared by resuspending nuclei in lysis buffer containing 450 mM NaCl. A volume of 500 μg of lysates were then incubated with the anti-NAP-1 mouse monoclonal antibody (a kind gift from Ishimi) or the anti-CBP rabbit antibody 5728 and 10 μl of protein A/G Sepharose beads (Sigma) for 4 h at 4°C. Samples were washed four times in lysis buffer, and precipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. For GST pull-down assays, polypeptides translated in vitro with the TNT Lysate Reaction Kit (Promega) with [35S]methionine were incubated with GST resin in binding buffer (100 mM NaCl, 20 mM HEPES, pH 7.0, 2 mM glycerol, 0.2 mM EDTA, 0.05% NP-40, 1 mM mercaptoethanol) for 30 min at room temperature. Binding reactions were then washed four times with binding buffer, and bound reactions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Nucleosome assembly, micrococcal nuclease assay, and supercoiling analysis.
Chromatin assembly and micrococcal nuclease reactions were performed as described previously (18, 19). A standard reaction mixture contained supercoiled plasmid DNA (0.4 μg), purified core histones from Drosophila melanogaster embryos (0.33 μg), purified recombinant Drosophila NAP-1 (dNAP-1), purified recombinant ATP-utilizing chromatin assembly and remodeling factor (ACF), ATP (3 mM), and an ATP-regenerating system (30 mM phosphocreatine and 1 μg of creatine phosphokinase/ml). Where specifically indicated, unlabeled acetyl-coenzyme A (CoA) (10 μM) and p300 were added. For supercoiling assays, the products of the reaction were deproteinized and analyzed by agarose gel electrophoresis and subsequent ethidium bromide staining. For micrococcal nuclease assays, different amounts of enzyme were added, and the products of the reaction were analyzed by agarose gel electrophoresis and by Southern blotting.
Purification of general transcription factors and recombinant proteins.
Drosophila nuclear extract from 0- to 12-h-old embryos (200 g) was applied to a 20-ml Q Sepharose FF (Pharmacia) column preequilibrated with 0.1 M KCl HEG (25 mM HEPES-KCl, pH 7.6, 0.1 mM EDTA, and 10% glycerol). After washing the column with 0.1 M KCl in HEG, the bound protein was eluted with a linear KCl gradient (10 column volumes) from 0.1 to 1 M KCl in HEG. TFIIA and TFIIH fractions were applied to 2-ml POROS heparin columns preequilibrated with 0.1 M KCl HEG, and the fractions were eluted as described above. TFIID, TFIIE, and TFIIF fractions from Q Sepharose columns were further purified with 2-ml SP Sepharose FF columns and 1-ml POROS heparin columns. Individual protein fractions were analyzed by immunoblot assay with rabbit polyclonal anti-Drosophila TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, and RNA polymerase II antibodies. Fractions were also analyzed by in vitro transcription assay. Absence of NAP-1 in all Drosophila general transcription factor (GTF) fractions was confirmed by Western blot analysis. Bacterially expressed His-tagged TFIIB was purified by Ni-NTA affinity chromatography. Recombinant p300 and dNAP-1 was prepared essentially as described previously (20, 29). Flag-tagged ecdysone receptor/ultraspiracle heterodimer complex (EcR/USP) was purified from baculovirus-infected Sf9 cells by anti-Flag resin agarose (Sigma).
In vitro transcription.
Salt-dialyzed chromatin was prepared as described previously (19). pEcE4 DNA template containing five ecdysone response elements (EcRE) upstream of the adenovirus E4 promoter was incubated with purified Drosophila core histones in the presence of 1 M NaCl. This mixture was dialyzed against 50 mM NaCl, and the resulting chromatin was subjected to centrifugation on a linear 15 to 40% glycerol gradient. Purified chromatin was employed for the in vitro transcription assay in which partially purified Drosophila general transcription factors and recombinant TFIIB were used. Chromatin was preincubated with p300 and dNAP-1 where indicated before transcription. The chromatin template was transcribed by adding GTFs, and the RNA products were evaluated by primer extension analysis. All reactions were performed in duplicate, and each experiment was performed a minimum of three separate times to ensure reproducibility.
RESULTS
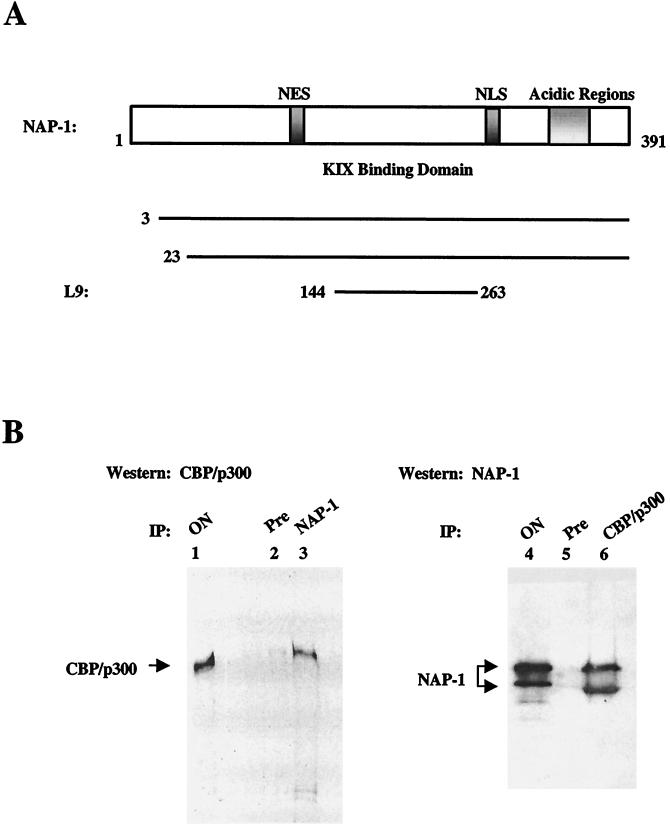
In the course of yeast two-hybrid screening experiments to identify cellular proteins that associate with the KIX domain of p300/CBP (aa 455 to 679 of CBP), we obtained four overlapping clones encoding NAP-1, a highly conserved protein that functions in histone shuttling (1, 17, 19, 30), cell cycle progression (3), and transcriptional regulation (52). NAP-1 contains a C-terminal acidic domain that associates with the positively charged N-terminal tails of histones H2A and H2B (17) (Fig. 1A). The central region of NAP-1 appears to function specifically in complex formation with KIX; one weakly positive NAP-1 cDNA identified in the two-hybrid screen (L9; aa 144 to 263) encompassed only the central region of NAP-1 (Fig. 1A).
FIG. 1.
NAP-1 interacts with p300 in vivo. (A) The top portion shows the structure of mammalian NAP-1 acidic regions that mediate histone H2A-H2B binding in hatched NAP-1. Consensus nuclear export (NES; aa 78 to 86) and nuclear import (NLS; aa 307 to 327) signals are indicated. The KIX binding domain (aa 144 to 263) is shown. Amino acid endpoints of independent human NAP-1 cDNA clones identified in two-hybrid screen are shown. (B) Coimmunoprecipitation studies of NAP-1 and p300 with whole-cell extracts prepared from HeLa cells. The left panel shows a Western blot assay of p300 recovered from immunoprecipitates prepared with preimmune (Pre; lane 2) or NAP-1 (lane 3) antiserum. The right panel shows a Western blot assay of NAP-1 recovered from immunoprecipitates prepared with preimmune (lane 5) or p300 (lane 6) antiserum. ON, onput protein from HeLa whole cell extract. IP, immunoprecipitate.
To determine whether NAP-1 associates with p300 in vivo, we performed coimmunoprecipitation studies on whole-cell extracts from HeLa cells. p300 was recovered from immunoprecipitates of NAP-1 but not from preimmune serum (Fig. 1B, left panel; compare lanes 2 and 3). Conversely, NAP-1 was readily detected in immunoprecipitates of p300; two NAP-1 immunoreactive bands, likely corresponding to differentially phosphorylated forms of the protein, were observed (Fig. 1B, right panel; compare lanes 5 and 6). NAP-1-p300 complexes were also detected in extracts of CV-1 cells, suggesting that this interaction is not restricted to cell type (data not shown).
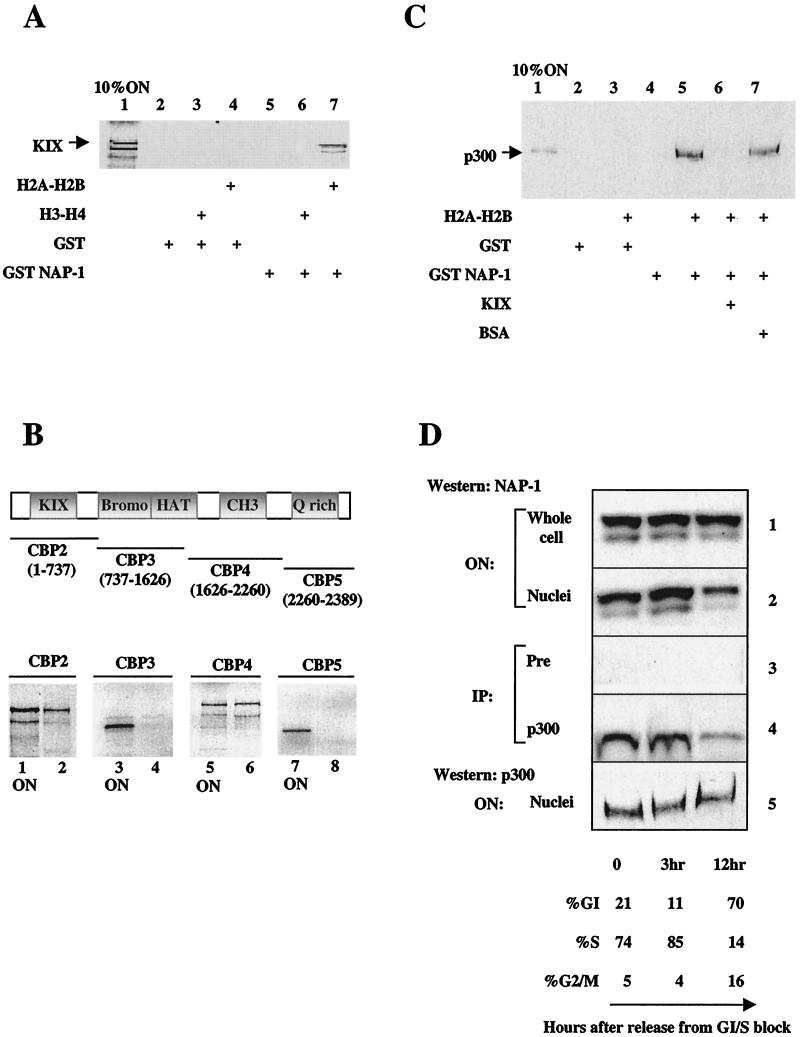
To assess whether the NAP-1-p300 interaction is direct, we performed affinity interaction assays. Reflecting a requirement for additional cofactors that stabilize complex formation between p300 and NAP-1, 35S-labeled KIX domain peptide did not bind detectably to glutathione beads containing GST-NAP-1 protein (Fig. 2A, lane 5). Addition of histone H2A-H2B to binding reactions, however, stabilized the association of NAP-1 with KIX (Fig. 2A, lane 7). The KIX domain did not appear to bind H2A-H2B directly; no retention of either H2A or H2B was observed in pull-down assays with GST-KIX beads (data not shown). Moreover, the effect of H2A-H2B on the binding of KIX to GST-NAP-1 resin appeared to be specific; H2A-H2B did not stimulate retention of 35S-labeled KIX on glutathione beads containing GST only (Fig. 2A, lane 4). Consistent with the higher affinity of NAP-1 for histone H2A and H2B (17, 22, 35), histones H3 and H4 were far less active in promoting formation of NAP-1-KIX complexes (Fig. 2A, lane 6).
FIG. 2.
H2A-H2B stabilizes binding of NAP-1 to p300. (A) Pull-down assay of 35S-labeled KIX peptide (aa 553 to 679) with glutathione Sepharose beads containing GST or human GST-NAP-1. Addition of purified histone H2A-H2B or H3-H4 to binding reactions are indicated. (B) Pull-down assay of 35S-labeled CBP fragments with indicated amino acid endpoints. Fragments were incubated with GST-NAP-1 beads plus purified histone H2A-H2B. Retained fractions and 10% onput (ON) are shown. (C) GST pull-down assay of recombinant full-length p300 with resins containing GST only or GST-NAP-1 plus histone H2A-H2B are also shown. The effect of KIX polypeptide or nonspecific (bovine serum albumin; BSA) competitor (1 μg) on NAP-1-p300 complex formation is shown. ON, 10% of input recombinant p300 protein. (D) Coimmunoprecipitation studies of NAP-1 recovered from immunoprecipitates of preimmune (Pre; panel 3) or p300 antiserum (panel 4) prepared from HeLa cells synchronized by double thymidine block. The number of hours (0, 3, or 12) following release are shown below the panel. Percent of cells in G1, S, or G2 phase is estimated by fluorescence-activated cell sorter analysis of propidium iodide-stained cells. Total cellular levels of NAP-1 (panel 1), nuclear NAP-1 (panel 2), and p300 (panel 5) are shown. IP, immunoprecipitate.
To determine whether other domains of p300/CBP in addition to KIX bind to NAP-1, we performed GST pull-down assays with fragments of CBP spanning the entire protein. In addition to the KIX domain (Fig. 2B, lanes 1 and 2), NAP-1 was also found by GST pull-down assay to interact with a second region of CBP containing the CH3 domain (Fig. 2B, CBP4 [aa 1626 to 2260], lanes 5 and 6). These results support the notion that NAP-1-CBP complex formation is stabilized by multiple surface contacts between the two proteins. To evaluate the importance of the KIX domain for association with NAP-1 in the context of the full length of the p300 protein, we performed interaction studies with baculovirus-expressed p300. In pull-down assays using GST-NAP-1 Sepharose beads, full-length p300 was efficiently recovered from binding reactions containing H2A-H2B (Fig. 2C, compare lanes 4 and 5). Addition of excess KIX polypeptide, but not unrelated competitor, to binding reactions completely blocked complex formation between NAP-1 and p300, confirming the importance of the KIX domain for this interaction (Fig. 2C, compare lanes 5, 6, and 7).
In somatic cells, nucleosome assembly is thought to occur in a two-step process, with deposition of an acetylated H3-H4 tetramer preceding that of two H2A-H2B dimers (35). NAP-1 is proposed to act in concert with CAF-1 or RCAF to deliver these core histones to the chromatin assembly apparatus during S phase (17, 21, 22, 26, 35, 47). In the early Drosophila embryo, NAP-1 is predominantly cytosolic in the first G2 phase, migrating into the nucleus during early S phase (22). Consistent with results from Drosophila studies (22), the level of NAP-1 protein increased in the nuclear fraction at the G1/S boundary (Fig. 2D, panel 2), without any change in the total level of NAP-1 being observed (Fig. 2D, panel 1). Based on the G1/S-dependent nuclear localization of NAP-1, we examined whether the levels of NAP-1-p300 complexes are cell cycle regulated. HeLa cells were synchronized by double thymidine block, and immunoprecipitates of p300 were prepared at various times after release. The highest levels of NAP-1 were detected in immunoprecipitates of p300 from cells in S phase, and the lowest levels of NAP-1 were observed in immunoprecipitates prepared from G1-phase cells (Fig. 2D, panel 4; compare lanes 2 and 3), without any noticeable change in total levels of p300 throughout the cell cycle (Fig. 2D, panel 5).
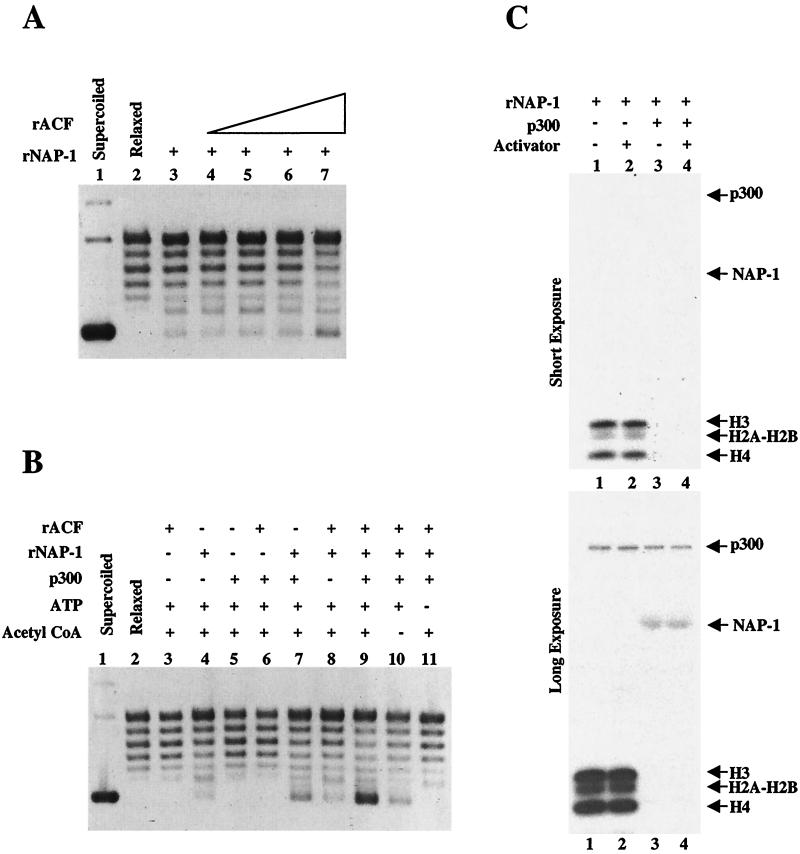
The S phase-dependent increase in NAP-1-p300 complexes prompted us to examine whether p300 may function in conjunction with NAP-1 during chromatin assembly. In a purified assembly system, NAP-1 has been found to promote chromatin assembly in conjunction with the ATP-dependent remodeling complex ACF (18, 19). Chromatin assembly and supercoiling assays were performed with NAP-1 and various concentrations of purified recombinant ACF (Fig. 3A). Using a concentration of ACF with minimal activity (Fig. 3A, lane 5), we examined the effects of NAP-1 and p300, either alone or in combination, on the assembly of nucleosomes. In the absence of additional ACF, p300 was found to promote assembly over a 3.2-kb plasmid (pEcE4) in conjunction with NAP-1 by supercoiling assay (Fig. 3B, lane 7).
FIG. 3.
p300 promotes efficient chromatin assembly in conjunction with NAP-1. (A) Chromatin assembly and supercoiling assays were performed with dNAP-1 and various concentrations of purified recombinant ACF (rACF) on a 3.2-kb plasmid template (pEcE4). (B) Using the ACF concentrations with minimal activity as described for panel A, chromatin assembly reactions were performed either in the presence or absence of indicated factors. Addition of ATP and acetyl-CoA to assembly reactions is indicated over each lane. (C) All four core histones were incubated with p300 and 3H-acetyl-CoA in the presence or absence of indicated factors. The samples were analyzed by 12% polyacrylamide gel electrophoresis, and acetylated proteins were detected by fluorography. Both short and long exposures are shown. rNAP-1, recombinant NAP-1.
The increased efficiency of assembly became most distinct when NAP-1, p300, and minimal levels of ACF were added in combination (Fig. 3B, lane 9). p300 HAT activity contributed somewhat to this process; assembly was less efficient in samples lacking acetyl-CoA (Fig. 3B, lane 10). In the absence of NAP-1, p300 could acetylate all free core histones whether or not an activator, such as GAL4-VP16, was included (Fig. 3C, lanes 1 and 2). Addition of NAP-1 strongly inhibited acetylation of core histones by p300 (Fig. 3C, lanes 3 and 4). Under longer exposure, both p300 autoacetylation and NAP-1 acetylation were detectable (Fig. 3C, lanes 3 and 4), suggesting that the importance of acetyl-CoA during the assembly reaction might reflect, in part, acetylation of NAP-1 or p300 as opposed to core histone acetylation.
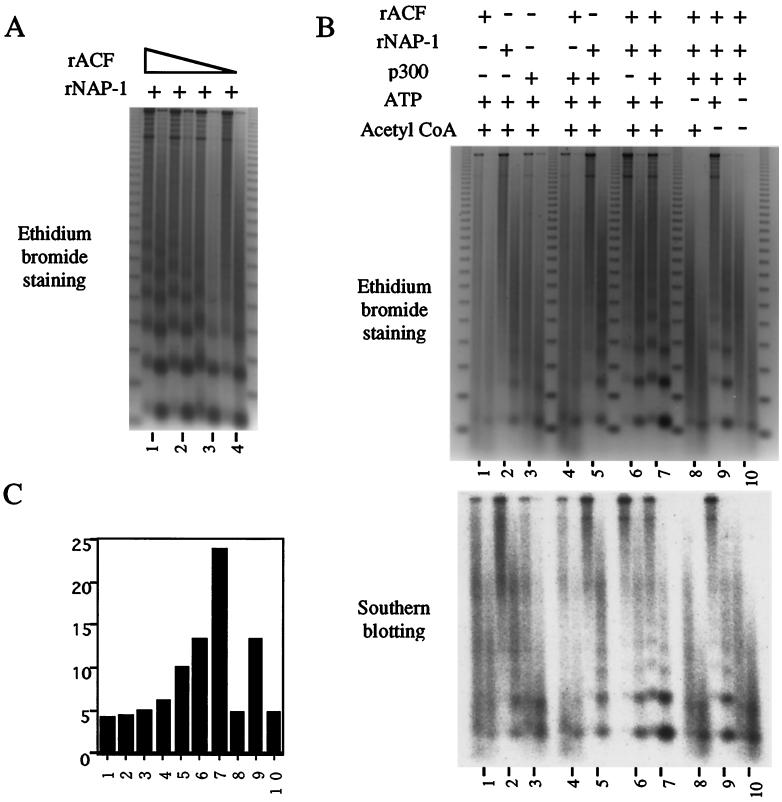
To confirm the notion that p300 stimulates chromatin assembly in conjunction with NAP-1, we performed micrococcal nuclease assays. Chromatin assembly and micrococcal nuclease digestion assays were performed with NAP-1 and various concentrations of purified recombinant ACF (Fig. 4A). In the absence of additional ACF, p300 promoted nucleosome assembly over an adenovirus E4 promoter construct in conjunction with NAP-1 both by ethidium bromide staining (Fig. 4B, lane 5) and by Southern blotting followed by phosphorimaging (Fig. 4B and C, lanes 5). In agreement with supercoiling assays, the nucleosomal ladder became most distinct when NAP-1, p300, and minimal levels of ACF (Fig. 4A, lane 4) were added in combination (Fig. 4B and C, lanes 7). Similarly, the nucleosomal ladder was less evident in samples lacking acetyl-CoA, suggesting that p300 HAT activity promotes assembly in vitro (Fig. 4B and C, compare lanes 7 and 9).
FIG. 4.
p300 stimulates assembly of periodic nucleosomal arrays with NAP-1. (A) Chromatin assembly and micrococcal nuclease digestion assays were performed with NAP-1 and various concentrations of purified recombinant ACF (rACF) on an adenovirus E4 promoter template. (B and C) Chromatin assembly reactions were performed either in the presence or absence of indicated factors. Where indicated, ACF concentrations with minimal activity, as determined for panel A, were added. Addition of ATP and acetyl-CoA to assembly reactions is indicated over each lane. Two concentrations of micrococcal nuclease were employed for each condition. Digestion products were analyzed by ethidium bromide staining (top) and by Southern blot analysis (bottom), with subsequent quantitation by a PhosphorImager (C). rNAP-1, recombinant NAP-1.
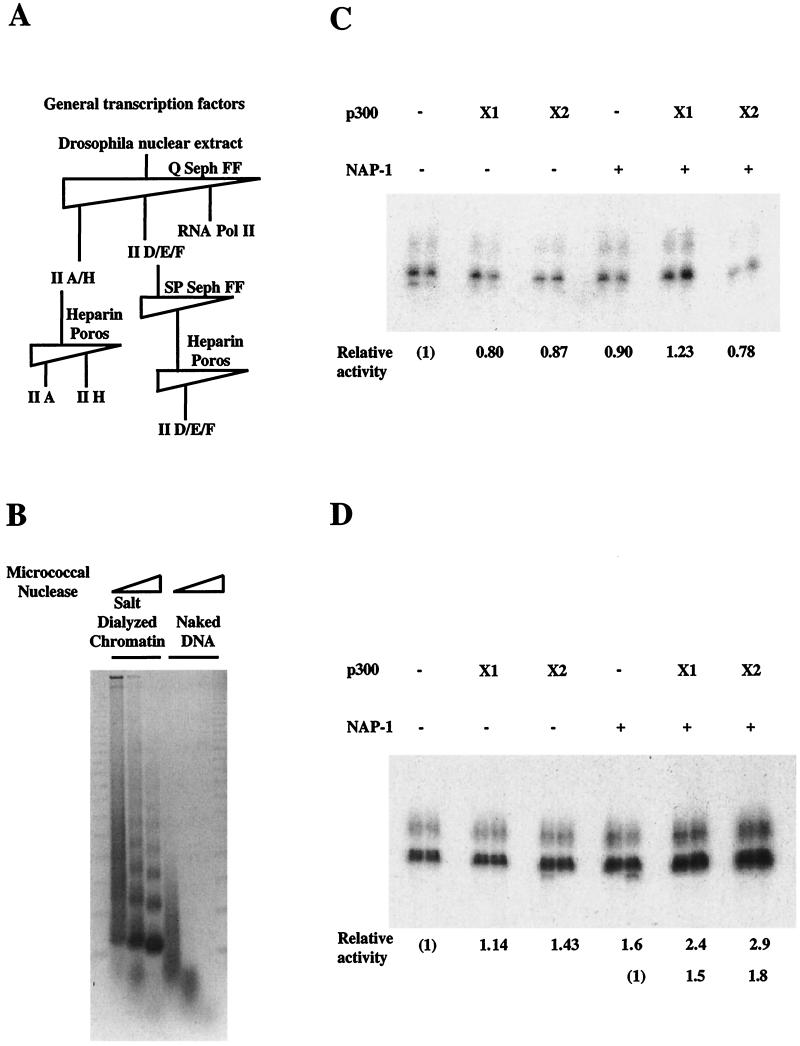
The ability of NAP-1 to promote chromatin assembly and disassembly in conjunction with p300 in vitro prompted us to examine whether NAP-1 potentiates p300 transcriptional activity on a chromatin template. To assess the role of NAP-1 in p300-dependent transcription, we purified GTFs (Fig. 5A) lacking NAP-1 and employed these fractions in in vitro transcription assays with a chromatin template containing five EcREs fused to the adenovirus E4 promoter.
FIG. 5.
NAP-1 and p300 stimulate transcription cooperatively on a chromatin template. (A) Partial purification of GTFs. Each GTF was evaluated both by Western blot and in vitro transcription assay. TFIIB was supplied as a purified recombinant protein. II A/H, TFIIA and -H; II D/E/F, TFIID, -E, and F; Seph, Sepharose. (B) Micrococcal nuclease assay of the chromatin template assembled by the salt dialysis method and naked DNA template was used for the transcription study. pEcE4 template containing five EcRE fused to the adenovirus E4 promoter was assembled into chromatin by salt dialysis. Naked DNA template (C) or salt-dialyzed chromatin (D) was incubated with various combinations of p300 and dNAP-1 as indicated in addition to the EcR/USP heterodimer, 0.7 mM acetyl-CoA, 3 mM ATP, 20-hydroxy-ecdysone ligand. After incubation chromatin template was subjected to transcription by adding partially purified GTFs and recombinant TFIIB. Fold induction over basal transcription (lane 1) was estimated by phosphorimaging. X1, one time; X2, two times.
The EcR, a member of the nuclear hormone receptor family, has been shown to stimulate cellular gene expression in a ligand-dependent manner following heterodimerization with the RXR homologue USP (54). Using EcR/USP as an activator, we established an in vitro transcription system in which transcriptional activation is EcR/USP and ligand dependent (data not shown). Following assembly into chromatin by salt dialysis with Drosophila histone octamers, micrococcal digestion of a 5× EcRE template showed nucleosomal ladders characteristic of the naked DNA template (Fig. 5B). Preincubation with NAP-1 and p300 had no effect on EcR/USP-dependent transcription from a naked DNA template, either alone or in combination, in reactions supplemented with purified GTFs (Fig. 5C). Addition of p300 or NAP-1 alone had little effect on transcription from a chromatin template, but NAP-1 and p300 together stimulated transcription via EcR/USP threefold in vitro (Fig. 5D), demonstrating the ability of NAP-1 to potentiate p300 transcriptional activity.
DISCUSSION
The modification of chromatin structure by HATs and ATP-dependent chromatin remodeling complexes constitutes a critical component in the regulation of cellular gene expression (28). The sequential recruitment of chromatin assembly complexes and HATs to the promoter appears to be important for preinitiation complex formation. In yeast, for example, activation of the HO promoter requires activator-mediated recruitment of SWI/SNF complexes followed by histone acetylation (11). Trans-activation by RAR/RXR heterodimers also requires prior SWI/SNF-mediated remodeling followed by p300/TIF2-dependent acetylation of promoter-bound nucleosomes (13). Using a purified recombinant chromatin assembly system, we have observed that prior chromatin remodeling is required for subsequent activator-dependent nucleosome acetylation via p300 (20). Nucleosome acetylation, in turn, promotes transfer of H2A-H2B dimers from the acetylated nucleosome onto NAP-1. Results outlined in this report indicate that NAP-1-p300 complexes function not only in chromatin assembly during S phase but also in chromatin remodeling events over the promoter prior to recruitment of the transcriptional apparatus. Although NAP-1 is most abundant in the nucleus during early S phase, a small fraction of NAP-1 remains nuclear throughout the cell cycle, potentially reflecting an ongoing role for this protein in transcriptional regulation.
In a recent study, NAP-2, which is closely related to NAP-1, was found to potentiate transcriptional induction via CBP/p300-dependent activators, including p53 and E2F, presumably via formation of NAP-2-p300 complexes (46). p300 interaction with NAP-2 in this study was found to require the C-terminally located CH3 region in p300 (46) rather than the N-terminally located KIX domain. Indeed, in our studies both domains of CBP/P300 appeared capable of binding to NAP-1, suggesting that this complex is stabilized by multiple surface contacts.
In addition to NAP-1, p300 has been found to interact with the CAF-1-associated RbAp46 and its homologue, RbAp48 (55), in a histone-dependent manner. In that study RbAp46 and RbAp48 were found to potentiate transcription via p300 by enhancing histone acetylation on a chromatin template (55). Moreover, binding of RbAp48 to KIX strongly potentiated complex formation with the cyclic AMP-responsive activator CREB (55), suggesting that RbAp48 and perhaps NAP-1 may bind to a distinct surface in KIX from CREB. Nevertheless, both studies point to a potential role for histone chaperones in transcriptional regulation via CBP/p300 coactivators.
The NAP-1 family member SET has been found to inhibit core histone acetylation via p300, evidently by direct binding to histone tails (44). Taken together with our observation that NAP-1 blocks acetylation of core histones via p300 (20) (also see Fig. 3C), it appears unlikely that p300 acetylates core histones when complexed with NAP-1. Given its importance as a core histone chaperone, NAP-1 may prevent modification of core histones during their transfer from cytoplasm to replicated DNA or even from chromatin to NAP-1 after remodeling coupled with transcriptional activation (20). The requirement for p300 HAT activity which we observed in chromatin assembly studies may reflect acetylation of other factors, such as NAP-1 or p300 itself, as shown in Fig. 3C.
p300 has recently been found to form a stable template-committed complex with chromatin in the absence of NAP-1 (32). In preliminary experiments, however, p300 appears unable to associate with chromatin in the presence of NAP-1 (data not shown). Thus, NAP-1-p300 complexes may function both in chromatin assembly and in transcriptional activation partially by tuning the HAT activity of p300 by protecting the core histones from inappropriate modifications and by regulating p300 localization. Investigation into the potential role of NAP-1 and RbAp46 and RbAp48 acetylation by p300 or autoacetylation of p300 itself should provide further insight into how this process is regulated.
Acknowledgments
We thank A. Vojtek for providing plasmids and the yeast strain L40 and M. Deckert for valuable discussions and help with confocal laser microscopy. We thank Carl Wu for ISWI cDNA and Y. Nakatani (Dana Farber) for recombinant p300 protein. We also thank Y. Ishimi and J. Kadonaga for the gift of NAP-1 antiserum. We are indebted to David Chambers for help with fluorescence-activated cell sorter analysis.
This work was supported by National Institutes of Health grants and by grants from the Japanese Society for the Promotion of Science (H.A.) and the Research for the Future Program (T.I.).
H.A. is on sabbatical leave from Okayama University Medical School. S.T.-D. is an investigator of Institut National de la Santé et de la Recherche Médicale (INSERM). T.H. is a Frank and Else Schilling American Cancer Society Research Professor.
Hiroshi Asahara, Sophie Tartare-Deckert, Takeya Nakagawa, and Tsuyoshi Ikehara contributed equally to this work.
REFERENCES
- 1.Adams, C. R., and R. T. Kamakaka. 1999. Chromatin assembly: biochemical identities and genetic redundancy. Curr. Opin. Genet. Dev. 9:185-190. [DOI] [PubMed] [Google Scholar]
- 2.Akimaru, H., Y. Chen, P. Dai, D. X. Hou, M. Nonaka, S. M. Smolik, S. Armstrong, R. H. Goodman, and S. Ishii. 1997. Drosophila CBP is a coactivator of cubitus interruptus in hedgehog signalling. Nature 386:735-738. [DOI] [PubMed] [Google Scholar]
- 3.Altman, R., and D. Kellogg. 1999. Control of mitotic events by Nap1 and the Gin4 kinase. J. Cell Biol. 138:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias, J., A. S. Alberts, P. Brindle, F. X. Claret, T. Smeal, M. Karin, J. Feramisco, and M. Montminy. 1994. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 370:226-228. [DOI] [PubMed] [Google Scholar]
- 5.Asahara, H., B. Santoso, E. Guzman, K. Du, P. A. Cole, I. Davidson, and M. Montminy. 2001. Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Mol. Cell. Biol. 21:7892-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannister, A. J., T. D. W. Oehler, P. Angel, and T. Kouzarides. 1995. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. EMBO J. 11:2509-2514. [PubMed] [Google Scholar]
- 7.Bannister, A. J., and T. Kouzarides. 1995. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 14:4758-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister, A. J., and T. Kouzarides. 1996. The CBP coactivator is a histone acetyltransferase. Nature 384:641-643. [DOI] [PubMed] [Google Scholar]
- 9.Chang, L., et al. 1997. Histones in transit: cytosolic histone complexes and diacetylation of H4 during nucleosome assembly in human cells. Biochemistry 36:469-480. [DOI] [PubMed] [Google Scholar]
- 10.Cho, H., G. Orphanides, X. Sun, X. J. Yang, V. Orgyzko, E. Lees, Y. Nakatani, and D. Reinberg. 1998. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol. Cell. Biol. 18:5355-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosma, M., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 12.Deckert, M., S. Tartare-Deckert, J. Hernandez, R. Rottapel, and A. Altman. 1998. Adaptor function for the Syk kinases-interacting protein 3BP2 in IL-2 gene activation. Immunity 9:595-605. [DOI] [PubMed] [Google Scholar]
- 13.Dilworth, F., C. Fromental-Ramain, K. Yamamoto, and P. Chambon. 2000. ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR in vitro. Mol. Cell 6:1049-1058. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, R., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 15.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 16.Holde, K. E. 1989. Chromatin. Springer-Verlag, New York, N.Y.
- 17.Ishimi, Y., M. Kojima, M. Yamada, and F. Hanaoka. 1987. Binding mode of nucleosome-assembly protein (AP-I) and histones. Eur. J. Biochem. 162:19-24. [DOI] [PubMed] [Google Scholar]
- 18.Ito, T., et al. 1999. ACF consists of two subunits, Acf1 and ISWI, that function cooperatively in the ATP-dependent catalysis of chromatin assembly. Genes Dev. 13:1529-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, T., M. Bulger, M. Pazin, R. Kobayashi, and J. T. Kadonaga. 1997. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell 90:145-155. [DOI] [PubMed] [Google Scholar]
- 20.Ito, T., T. Ikehara, T. Nakagawa, W. Kraus, and M. Muramatsu. 2000. p300-mediated acetylation facilitates the transfer of histone H2A-H2B dimers from nucleosomes to a histone chaperone. Genes Dev. 14:1899-1907. [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, T., J. K. Tyler, M. Bulger, R. Kobayashi, and J. T. Kadonaga. 1996. ATP-facilitated chromatin assembly with a nucleoplasmin-like protein from Drosophila melanogaster. J. Biol. Chem. 271:25041-25048. [DOI] [PubMed] [Google Scholar]
- 22.Ito, T., M. Bulger, R. Kodbayashi, and J. T. Kadonaga. 1996. Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Mol. Cell. Biol. 16:3112-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito, T., J. K. Tyler, and J. T. Kadonaga. 1997. Chromatin assembly factors: a dual function in nucleosome formation and mobilization? Genes Cells 2:593-600. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, W., and T. Hunter. 1997. Identification and characterization of a human protein kinase related to budding yeast Cdc7p. Proc. Natl. Acad. Sci. USA 94:14320-14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamei, Y. X. L., T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfield. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman, P. D., R. Kobayashi, N. Kessler, and B. Stillman. 1995. The p150 and p60 subunits of chromatin assembly factor 1: a molecular link between newly synthesized histones and DNA replication. Cell 81:1105-1114. [DOI] [PubMed] [Google Scholar]
- 27.Kee, B., J. Arias, and M. Montminy. 1996. Adaptor mediated recruitment of RNA polymerase II to a signal dependent activator. J. Biol. Chem. 271:2373-2375. [DOI] [PubMed] [Google Scholar]
- 28.Kingston, R. E., and G. J. Narlikar. 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 15:2339-2352. [DOI] [PubMed] [Google Scholar]
- 29.Kraus, W., and J. Kadonaga. 1998. p300 and estrogen receptor cooperatively actiate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 12:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krude, T. 1999. Chromatin assembly during DNA replication in somatic cells. Eur. J. Biochem. 263:1-5. [DOI] [PubMed] [Google Scholar]
- 31.Kwok, R., J. R. Lunblad, J. C. Chrivia, J. P. Richards, H. P. Bachinger, R. G. Brennan, S. G. Roberts, M. R. Green, and R. H Goodman. 1994. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370:223-226. [DOI] [PubMed] [Google Scholar]
- 32.Manning, E., T. Ikehara, T. Ito, J. Kadonaga, and W. Kraus. 2001. p300 forms a stable, template-committed complex with chromatin; a role for the bromodomain. Mol. Cell. Biol. 21:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna, N., Z. Nawaz, S. Tsai, M. Tsai, and B. O'Malley. 1998. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc. Natl. Acad. Sci. USA 95:11697-11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motokura, T., et al. 1991. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 350:512-515. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa, T., M. Bulger, M. Muramatsu, and T. Ito. 2001. Multistep chromatin assembly on supercoiled plasmid DNA by nucleosome assembly protein-1 and ACF. J. Biol. Chem. 276:27384-27391. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima, T., C. Uchida, S. Anderson, J. Parvin, and M. Montminy. 1997. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 11:738-747. [DOI] [PubMed] [Google Scholar]
- 37.Ogryzko, V. V. S. R., V. Russanova, B. H. Howard, and M. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetytransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 38.Oliner, J., J. Andresen, S. Hansen, S. Zhou, and R. Tjian. 1996. SREBP transcriptional activity is mediated through an interaction with the CREB-binding protein. Genes Dev. 10:2903-2911. [DOI] [PubMed] [Google Scholar]
- 39.Parker, D., K. Ferreri, T. Nakajima, V. J. LaMorte, R. Evans, S. C. Koerber, C. Hoeger, and M. R. Montminy. 1996. Phosphorylation of CREB at Ser133 induces complex formation with CBP via a direct mechanism. Mol. Cell. Biol. 16:694-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker, D., U. S. Jhala, I. Radhakrishnan, M. B. Yaffe, C. Reyes, A. I. Shulman, L. C. Cnatley, P. E. Wright, and M. Montminy. 1998. Analysis of an activator:coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol. Cell 2:353-359. [DOI] [PubMed] [Google Scholar]
- 41.Parker, D., M. Rivera, T. Zor, A. Henrion-Caude, I. Radhakrishnan, A. Kumar, L. H. Shapiro, P. E. Wright, M. Montminy, and P. K. Brindle. 1999. Role of secondary structure in discrimination between constitutive and inducible activators. Mol. Cell. Biol. 19:5601-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radhakrishnan, I., G. C. Perez-Alvarado, D. Parker, H. J. Dyson, M. R. Montminy, and P. E. Wright. 1997. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91:741-752. [DOI] [PubMed] [Google Scholar]
- 43.Roth, S., and C. D. Allis. 1996. Histone acetylation and chromatin assembly: a single escort, multiple dances? Cell 87:5-8. [DOI] [PubMed] [Google Scholar]
- 44.Seo, S., P. McNamara, S. Heo, A. Turner, W. S. Lane, and D. Chakravati. 2001. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 104:119-130. [DOI] [PubMed] [Google Scholar]
- 45.Shih, H. M., P. S. Goldman, A. J. DeMaggio, S. M. Hollenberg, R. H. Goodman, and M. F. Hoekstra. 1996. A positive genetic selection for disrupting protein-protein interactions: identification of CREB mutations that prevent association with the coactivator CBP. Proc. Natl. Acad. Sci. USA 93:13896-13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shikama, N., et al. 2000. Functional interaction between nucleosome assembly, proteins and p300/CREB-binding protein family coactivators. Mol. Cell. Biol. 20:8933-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyler, J. K., C. R. Adams, S. R. Chen, R. Kobayashi, R. T. Kamakaka, and J. T. Kadonaga. 1999. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402:555-560. [DOI] [PubMed] [Google Scholar]
- 48.Verreault, A., P. D. Kaufman, R. Kobayashi, and B. Stillman. 1998. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Curr. Biol. 8:96-108. [DOI] [PubMed] [Google Scholar]
- 49.Verreault, A., P. D. Kaufman, R. Kobayashi, and B. Stillman. 1996. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 87:95-104. [DOI] [PubMed] [Google Scholar]
- 50.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 51.Wolffe, A. P. 1998. Chromatin: structure and function. Academic Press, San Diego, Calif.
- 52.Walter, P., T. Owen-Hughes, J. Cote, and J. Workman. 1995. Stimulation of transcription factor binding and histone displacement by nucleosome assembly protein 1 and nucleoplasmin requires deposition of the histone octamer. Mol. Cell. Biol. 15:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsom, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 54.Yao, T., B. M. Forman, Z. Jiang, L. Cherbas, J. D. Chen, M. McKeown, P. Cherbas, and P. Evans. 1993. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 366:476-479. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, Q., N. Vo, and R. Goodman. 2000. Histone Binding Protein RbAp48. Interacts with a complex of CREB binding protein and phosphorylated CREB. Mol. Cell. Biol. 20:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]