Abstract
To understand how cellular differentiation is coupled to withdrawal from the cell cycle, we have focused on two negative regulators of the cell cycle, the MYC antagonist MAD1 and the cyclin-dependent kinase inhibitor p27KIP1. Generation of Mad1/p27KIP1 double-null mice revealed a number of synthetic effects between the null alleles of Mad1 and p27KIP1, including embryonic lethality, increased proliferation, and impaired differentiation of granulocyte precursors. Furthermore, with granulocyte cell lines derived from the Mad1/p27KIP1 double-null mice, we observed constitutive Myc expression and cyclin E-CDK2 kinase activity as well as impaired differentiation following treatment with an inducer of differentiation. By contrast, similar treatment of granulocytes from Mad1 or p27KIP1 single-null mice resulted in differentiation accompanied by downregulation of both Myc expression and cyclin E-CDK2 kinase activity. In the double-null granulocytic cells, addition of a CDK2 inhibitor in the presence of differentiation inducer was sufficient to restore differentiation and reduce Myc levels. We conclude that Mad1 and p27KIP1 operate, at least in part, by distinct mechanisms to downregulate CDK2 activity and Myc expression in order to promote cell cycle exit during differentiation.
A common feature of differentiating cells is the association of withdrawal from the cell cycle with acquisition of the differentiated phenotype. However, the precise molecular mechanisms by which differentiation and cell cycle exit are coupled remain poorly understood. Recently, a number of families of molecules that may play a role in these processes have been identified. These include the cyclin-dependent kinase inhibitors (CKIs) (37), the Rb family of transcriptional repressors (26), and the Mad family of Max-interacting transcriptional repressors (22). A critical question remains as to how these various families of molecules functionally interact to mediate withdrawal from the cell cycle during differentiation.
Recent studies utilizing gene targeting strategies in the mouse have clearly implicated the CKIs p21CIP1, p27KIP1, and p57KIP2 in cell cycle arrest associated with differentiation (4, 24, 44-46). The role of the INK4 family of CKIs in differentiation is less clear. However, consistent with a function for these molecules in negative regulation of the cell cycle during differentiation, p16INK4b and p18INK4c null mice demonstrate an increase in the numbers of some differentiated cells (12, 36) and p19INK4d mice display dysregulation of the proliferation and differentiation of neural cells when crossed with p27KIP1-null mice (47). Moreover, in some experimental systems, overexpression of members of the INK4 family can promote differentiation (1).
The MAX network of transcription factors has also been implicated in mediating cell cycle arrest during differentiation. During the transition from an undifferentiated to a differentiated state, there is a switch from MYC-MAX to MAD-MAX complexes that function as transcriptional repressors. Deregulated expression of c-MYC inhibits terminal differentiation and prevents cell cycle arrest, consistent with its integral role in regulating the cell cycle during differentiation (reviewed in references 9 and 14). By contrast, overexpression of the Mad family transcriptional repressors MAD1 and MXI1 arrests cells in the G1 phase of the cell cycle (5, 32) and promotes differentiation of some cell types (9). Consistent with a role of MAD proteins in cell cycle control during differentiation, gene targeting to generate Mad1 null mice delays cell cycle exit during a late stage of granulocyte differentiation (11). Moreover, mice with homozygous disruption of Mxi1 develop hyperplasia of a variety of cell types, including lymphoid and prostatic cells (34).
A major question is the relationship between members of the Max network and the CKIs. The expression of both MAD1 (reviewed in reference 22) and p27KIP1 (4, 13, 16, 21) are upregulated during differentiation. Moreover c-MYC is downregulated during cellular differentiation as levels of p27KIP1 increase (see below). These correlative observations raise the possibility that p27KIP1 and MAD1 cooperate in regulating the cell cycle during differentiation. In further support of this hypothesis, studies in rodent fibroblasts have indicated that c-MYC is able to activate CDK2 (39), a major molecular target for inhibition by p27KIP1 (28). Taken together with the notion that Mad antagonizes Myc function, these data support the possibility of cooperation between Mad1 and p27KIP1 in negatively regulating CDK2 during differentiation. Here we employed a genetic approach to define the relationship between Mad1 and p27KIP1 during differentiation. Mad1 null mice were crossed with p27KIP1 null mice to generate Mad1/p27KIP1 double-null mice. Our analyses reveal that p27KIP1 and MAD1 have partly redundant functions converging on regulation of cyclin E-CDK2 as well as c-Myc.
MATERIALS AND METHODS
Mice.
The generation of Mad1−/− (11) and p27−/− (10) mice have been described previously. All mice used in hematopoietic studies and for determinations of organ weights were males aged 9 to 11 weeks. Mice had been backcrossed a minimum of three generations to the C57BL/6 background. Mice homozygous for each null allele were generated by breeding p27KIP1+/− males and females to produce mice of Mad1+/+ p27KIP1+/+ (wild-type) and Mad1+/+;p27KIP1−/− (p27 null) genotypes. Mad1−/− p27KIP1+/+ (Mad1 null) and Mad1−/−;p27KIP1−/− (Mad1/p27 double-null) mice were generated by breeding male and female Mad1−/− p27KIP1+/− mice. Use of mice in these experiments complied with federal and institutional policies.
Studies on hematopoietic cells.
Flow cytometry and cell cultures in semisolid media were performed as previously described (11). Myeloid cell cultures contained 25,000 cells/1 ml of culture. Erythroid cultures in methylcellulose medium contained 50,000 cells/1 ml of culture. The following recombinant cytokines were kindly provided by Immunex Corporation (Seattle, Wash.): granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), interleukin-6 (IL-6), and partially purified IL-3. Epo and G-CSF were obtained from Amgen (Thousand Oaks, Calif.). The M-CSF used was a baculovirus supernatant (L. Rohrschneider). All statistical analyses were performed using a two-sided unpaired Student t test.
Generation and differentiation of granulocyte cell lines.
Granulocyte cell lines were generated using the technique described by Tsai and Collins (41). Briefly, bone marrow cells were obtained from age- and sex-matched mice of different genotypes 5 days after intravenous injection with 100 mg of 5-fluorouracil/kg of body weight. Cells were stimulated with 1,000 U of GM-CSF (Immunex)/ml while being cocultured with PA-317 packaging cell lines encoding a control LXSN or dominant-negative LRARα403SN-retrovirus. After 3 days, nonadherent cells were transferred to separate plates and serially passaged every 2 to 3 days into fresh media. Granulocyte culture media consisted of Dulbecco's modified Eagle's media containing 20% bovine calf serum (BCS; HyClone, Logan, Utah) and BHK-HM5-conditioned media containing 1,000 U of GM-CSF/ml and 10 μg of ciprofloxacin/ml. Pools of cells were analyzed as described below. Clones were generated by limit dilution or by micromanipulation in methylcellulose media. pBabe-puro and pBabe-puro-MYCER cells were generated by coculturing granulocyte cell lines with 293T cells transfected with ecotropic helper plasmid and pBabe-puro retroviral plasmids (kindly provided by T. Littlewood) as previously described (32). Following coculture, cells were selected in granulocyte culture media containing 1 μg of puromycin/ml.
Granulocyte cell lines were induced to differentiate by transfer to Dulbecco's modified Eagle's media containing 10% BCS and a 10−8 M concentration of the RXR agonist AGN194204 (kindly provided by Allergan, Irvine, Calif.). Roscovitine was obtained from Calbiochem (San Diego, Calif.). Differentiation was analyzed by staining with biotin-anti-Gr-1 antibody (Ly-6G) and phosphatidylethanolamine (PE)-streptavidin (Caltag) followed by analysis on a FACScan (Becton Dickinson, San Jose, Calif.). Morphological examination was performed on cytocentrifuge preparations that were stained with May-Grünwald Giemsa. S-phase fractions were determined by fixing cells in 85% ethanol in phosphate-buffered saline followed by staining with 1 μg of propidium iodide/ml and 0.25 mg of RNase A/ml in phosphate-buffered saline containing 2% BCS. Cells were analyzed on a FACScan, and S-phase fractions were calculated using MultiCycle software (Phoenix Flow Systems, San Diego, Calif.).
Kinase assays, Northern blottings, and immunoblottings.
Assays of kinase activity associated with cyclin E or cyclinD3 were performed as previously described (7). Northern analysis was performed using a full-length cDNA probe of human c-Myc following extraction of total RNA by using Trizol reagent (Gibco, Grand Island, N.Y.). Immunoblotting was performed on protein extracts lysed in cyclin E-lysis buffer (see above) unless otherwise described. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, cellular proteins were transferred and analyzed using the antisera listed below and enhanced chemiluminescence (ECL) detection. The following antibodies were utilized: rabbit anti-p27 (28), rabbit anti-cyclin D3 (kindly provided by C. Sherr); rabbit anti-Cyclin E (sc-198 or 481), p107 (sc-318), p130 (sc-317), CDK2 (sc-163), cdc25a (sc-97), p21 (sc-756 or sc-6246), and pRb (sc-50) (Santa Cruz, Santa Cruz, Calif.); mouse anti-pRb 14001A (Pharmigen, San Diego, Calif.); mouse anti-pRb 28-0007 (Zymed, San Diego, Calif.); and rabbit anti c-MYC 06-213 (UBI, Lake Placid, N.Y.).
RESULTS
Null alleles of Mad1 and p27KIP1 display synthetic effects.
To examine the functional interaction between Mad1 and p27KIP1 in regulation of the cell cycle, we generated animals lacking both genes. Although we have previously observed that Mad1 null and p27KIP1 null single-knockout mice are completely viable on the C57BL/6J background employed here (11; M. L. Fero and K. P. Foley, unpublished data), only 12.5% of progeny from mating male and female Mad1−/− p27KIP1+/− mice represented Mad1/p27KIP1 double-knockout animals, rather than the expected frequency of 25%. This underrepresentation was highly statistically significant (P < 0.001) as analyzed by the Chi-square test, consistent with a phenotype of partially penetrant embryonic lethality. However, surviving Mad1/p27KIP1 double-knockout mice appeared outwardly normal, with the exception that they shared the approximately 20% increase in body weight displayed by p27KIP1 null animals on this background (data not shown).
Mice homozygous for single-null alleles of Mad1 and p27KIP1 display abnormalities in their hematopoietic systems. We therefore examined the hematopoietic organs from mice aged 10 to 11 weeks. The most striking abnormality in p27KIP1 null mice is hyperplasia of the thymus and spleen (10, 19, 27). Mad1 null mice displayed a modest defect in cell cycle exit during granulocyte differentiation but had normal cellularity of the hematopoietic organs thymus, spleen, and bone marrow (11). Mad1/p27KIP1 double-null mice exhibited similar degrees of thymic and splenic hyperplasia to that seen in p27KIP1-null mice (data not shown). However, Mad1/p27KIP1 double-null mice had mild bone marrow hyperplasia predominantly involving myeloid cells (11.9 × 106 ± 1.2 × 106 CD11b-positive cells/femur compared to 7.8 × 106 ± 0.8 × 106 in wild-type mice). Mice homozygous for single-null alleles of Mad1 and p27KIP1 had relatively normal bone marrow cellularity (8.6 × 106 ± 0.8 × 106 and 8.7 × 106 ± 1.0 × 106 CD11b-positive cells/femur, respectively), indicating a synthetic effect of these null alleles on the numbers of myeloid cells in the bone marrow.
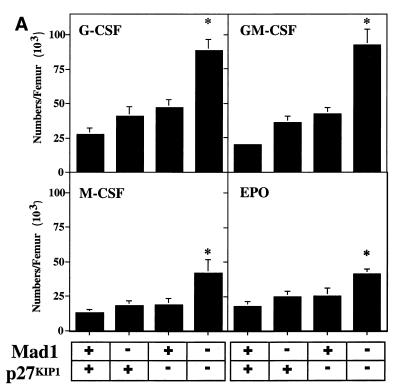
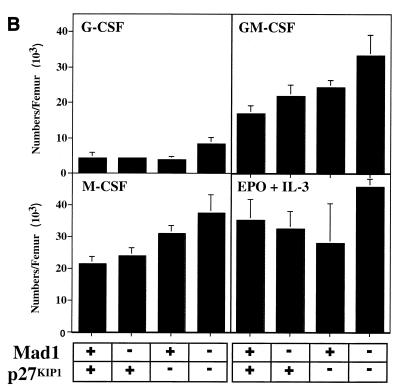
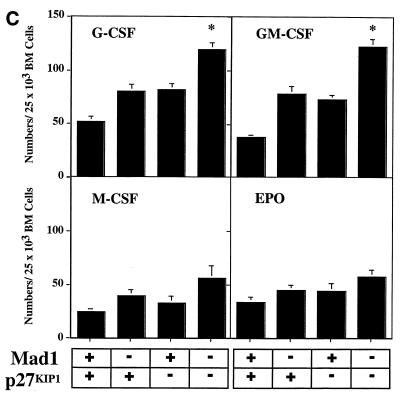
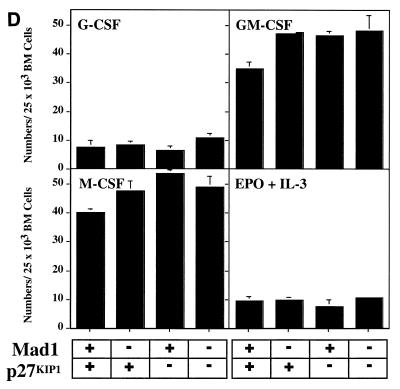
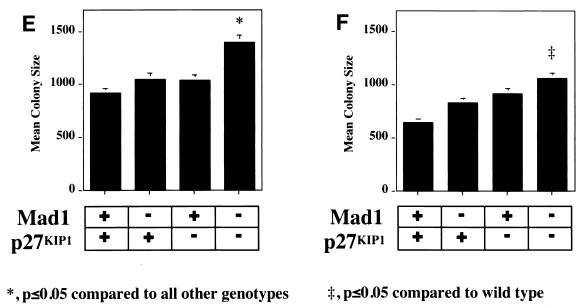
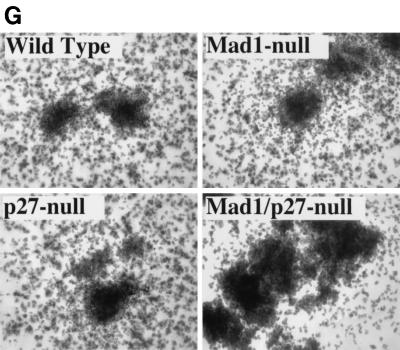
The defect in cell cycle exit during granulocyte differentiation of Mad1 null mice is characterized by an increased frequency of limited proliferative potential cluster-forming cells, which represent a population of cells approaching terminal differentiation but that still have some proliferative ability (11). When bone marrow cells from Mad1/p27KIP1 double-null mice were stimulated with various cytokines, there was a highly significant increase in the numbers and frequency of limited proliferative potential cells (Fig. 1a and c), as enumerated in semisolid cultures. This was most dramatic for the cytokines active on granulocyte precursors, G-CSF and GM-CSF (Fig. 1a and c). In contrast, the number and frequency of the more immature colony-forming cells, which have greater proliferative potential, were more modestly increased in Mad1/p27KIP1 double-null mice (Fig. 1b and d). An increased proliferative capacity of myeloid precursor cells from Mad1/p27KIP1 double-null mice would be expected to increase the size of myeloid colonies in culture. Therefore, we examined the sizes of colonies stimulated with various cytokines. Colonies composed of both granulocytes and macrophages from Mad1/p27KIP1 double-null mice that were stimulated with GM-CSF or the combination of IL-3/IL-6 and SCF were significantly increased in size compared to colonies from wild-type mice (Fig. 1e and f). Moreover, granulocyte-macrophage colonies from Mad1/p27KIP1 double-null mice that were stimulated with IL-3/IL-6 and SCF were composed of a large fraction of cells with a more immature morphology (i.e., possessing large nuclei) than colonies derived from mice of other genotypes (which possess doughnut-shaped nuclei characteristic of differentiatied granulocytes) (Fig. 1g and h). Taken together, these data support a synthetic effect between the null alleles of Mad1 and p27KIP1 acting to increase the population size of late-stage granulocyte progenitor cells, most likely by delaying cell cycle exit and terminal differentiation (see below). As p27KIP1 null mice also display hyperplasia of a number of nonhematopoietic organs (10, 19, 27), we performed measurements of organ mass of 10- to 11-week-old male mice of various genotypes. Interestingly, we also observed a synthetic effect between the null alleles of Mad1 and p27KIP1 leading to an increase in kidney mass (0.64 ± 0.3 g for two kidneys in Mad1/p27KIP1 double-null mice compared to 0.36 ± 0.1 g in wild-type mice and 0.42 ± 0.06 g and 0.44 ± 0.05 g in Mad1-null and p27-null mice, respectively). Histological examination of these kidneys indicated normal proportions of cells of different lineages, suggesting that the synthetic effect resulted in hyperplasia of all types of cells normally present in the kidneys.
FIG. 1.
Cellular abnormalities in Mad1/p27KIP1 double-null mice. (A) Total numbers of limited proliferative potential cluster-forming cells stimulated with different cytokines from femurs of mice of each genotype. The P value was <0.01 for numbers of precursors stimulated with G-CSF or GM-CSF from Mad1/p27KIP1 double-null mice compared to those of other genotypes. The P value was ≤0.05 for Epo or M-CSF. (B) Total numbers of colony-forming cells stimulated with different cytokines from femurs of mice of each genotype. The P value was ≤0.05 for numbers of Mad1/p27KIP1 double-null colony-forming cells compared to those of other genotypes. (C) Numbers of limited proliferative potential cluster-forming cells per 25,000 total bone marrow cells. The P value was <0.01 for numbers of precursors stimulated with G- or GM-CSF from Mad1/p27KIP1 double-null mice compared to those of other genotypes. (D) Numbers of colony-forming cells per 25,000 total bone marrow cells. (E) Size of granulocyte-macrophage colonies stimulated with IL-3/IL-6 and SCF, expressed as mean number of cells/colony. The P value was <0.05 for Mad1/p27KIP1 double-null colonies compared to those of all other genotypes. (F) Size of granulocyte-macrophage colonies stimulated with GM-CSF. The P value was <0.05 for Mad1/p27KIP1 double-null colonies compared to wild-type colonies. (G) Morphology of granulocyte-macrophage colonies stimulated with IL-3/IL-6 and SCF. (H) Morphology of cells from granulocyte-macrophage colonies stimulated with IL-3/IL-6 and SCF.
Impaired differentiation and cell cycle arrest in granulocyte cell lines from Mad1/p27 double-null mice.
To understand the mechanisms underlying the synthetic effects observed between the null alleles of Mad1 and p27KIP1, we focused our attention on cells of the granulocyte lineage. Granulocytes from mouse bone marrow can be immortalized at the promyelocyte stage of differentiation by retroviral transfer of a gene encoding a truncated retinoic acid receptor (RARα403) (41). This block to differentiation can be overcome by stimulation with retinoid agonists, such as the RXR agonist AGN 194024, allowing terminal differentiation to proceed (17). We had previously demonstrated that expression of MAD1 in these immortalized granulocytes was tightly correlated with exit from the cell cycle (11). To further examine the role of Mad1 and p27KIP1 in granulocyte differentiation and cell cycle control, we generated additional granulocyte cell lines derived from wild-type, Mad1 null, p27KIP1 null, and Mad1/p27KIP1 double-null mice.
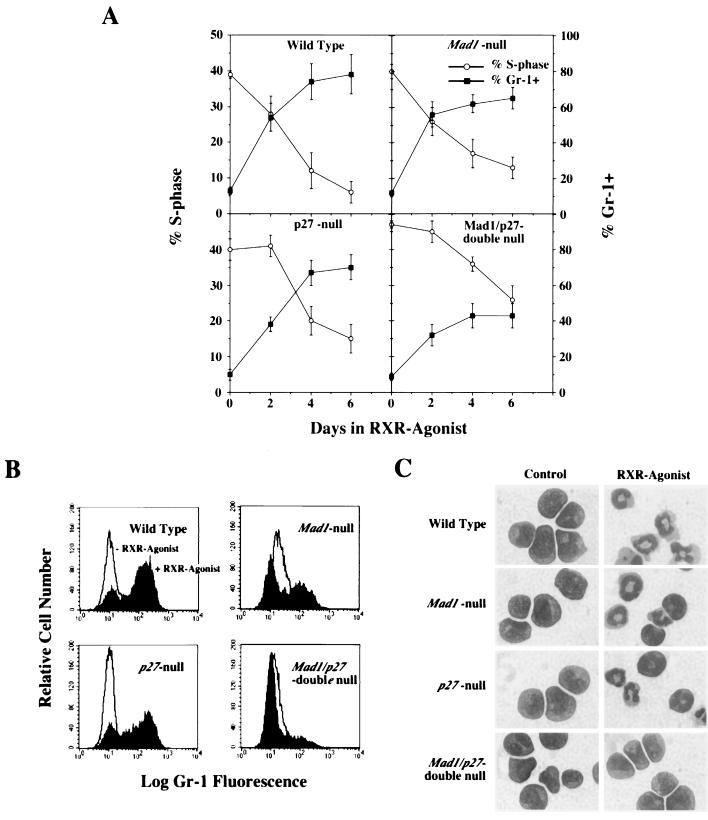
Granulocytic cells infected with the LRARα403SN-retrovirus were analyzed for their ability to differentiate in response to retinoids. Cells were stimulated with the RXR agonist AGN194024, and 2, 4, and 6 days later, cell populations were analyzed by flow cytometry and examined for morphological differentiation. When granulocytic cells derived from wild-type mice were stimulated with the RXR agonist, they underwent cell cycle arrest (Fig. 2a), upregulated Gr-1 (Fig. 2a and b), and terminally differentiated into mature granulocytes, as shown by the acquisition of the characteristic doughnut-shaped nucleus (Fig. 2c). Cells derived from single-null Mad1 null and p27KIP1 null mice also reduced their S-phase fraction and terminally differentiated, albeit with slightly reduced efficiency in the case of Mad1 null cells (Fig. 2). In contrast, cells derived from Mad1/p27KIP1 double-null mice failed to arrest and displayed little ability to differentiate (Fig. 2a to c). Similar findings were obtained for seven or more clones of each genotype and in independently derived pools of cells that were examined prior to cloning within the first 5 to 6 weeks following retroviral infection. Taken together, these results demonstrated a marked impairment of Mad1/p27KIP1 double-null granulocyte cell lines to differentiate and exit the cell cycle in response to attempted differentiation induction with an RXR agonist.
FIG. 2.
Impaired differentiation and cell cycle exit in cloned granulocyte cell lines from Mad1/p27 double-null mice. Granulocytic cell lines were stimulated with a 10−8 M concentration of the RXR agonist AGN-194204 (or carrier as a control), and cells were analyzed after 2, 4, and 6 days in culture. (A) S-phase fractions and Gr-1 expression as determined by flow cytometry. Gr-1+ cells were defined as in Fig. 2b. The P value was <0.05 for S-phase and Gr-1 expression in Mad1/p27KIP1 double-null cells compared to other genotypes at 4 and 6 days. Error bars represent standard errors from a minimum of seven clones analyzed of each genotype. (B) Representative FACS profiles of Gr-1 expression. (C) Representative morphology of clones 6 days after stimulation with the RXR agonist.
Granulocyte cell lines from Mad1/p27 double-null mice display constitutive expression of c-Myc.
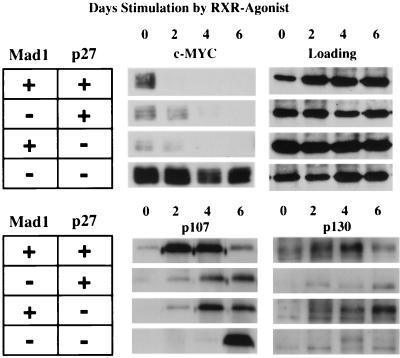
As both p27KIP1 and MYC have been implicated in the regulation of CDK2 (25, 28, 39, 42), we next examined the expression of molecules known to regulate CDK2. We had previously demonstrated that MAD1 protein is upregulated in the wild-type granulocyte cell lines during differentiation (11). We have also found that p27KIP1, but not p21CIP1, is upregulated during granulocyte differentiation. In addition, we did not detect any significant changes in the total levels of CDK2, cyclin E, or cdc25a proteins during differentiation of wild-type cells or cells derived from single- or double-null mice (data not shown). In contrast, we observed a marked upregulation of c-MYC protein in Mad1/p27KIP1 double-null cell lines that was maintained following stimulation with the RXR agonist consistent with the inability of these cells to undergo terminal differentiation (Fig. 3). Comparison of Western blots loaded with equal amounts of protein showed that c-MYC was at lower levels in undifferentiated cells of other genotypes and was abruptly downregulated following stimulation with the RXR agonist, albeit slightly more slowly in the single-null cells than in the wild-type cells (Fig. 3). Finally, we examined expression of members of the Rb family of transcription repressors, pRb, p107, and p130, which have been found in complexes with cyclin E and CDK2 (8, 43). Rb protein could not be detected in these cells by using three different antisera; however, we readily detected p107 and p130, both of which were upregulated following induction of differentiation of wild-type cell lines (Fig. 3). In contrast, induction of p107 and p130 was perturbed in single-null cells and markedly attenuated in Mad1/p27KIP1 double-null cells (Fig. 3). Taken together, these data support a role for MAD1 and p27KIP1 in the regulation of c-MYC, p107, and p130 that may in turn influence the activity of CDK2.
FIG. 3.
Expression of c-MYC and Rb family proteins during granulocyte differentiation. Granulocyte cell lines were stimulated with 10−8 M AGN-194204 to induce differentiation. Cell lysates were prepared, and following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blots were probed with antisera as indicated. Approximately 10 μg of total protein was loaded in each lane. An antibody to HDAC1 was used to confirm equal loading.
Downregulation of kinase activity associated with cyclin E-CDK2 complex is required for granulocyte differentiation in response to retinoids.
We next examined kinase activity associated with the cyclin E-CDK2 complex. Wild-type, Mad1-null, and p27KIP1 null granulocyte cell lines strongly downregulated kinase activity associated with the cyclin E-CDK2 complex during differentiation (Fig. 4). In multiple experiments, we observed that wild-type and mad1 null cells retained less than 20% of initial kinase activity by day 6 after induction of differentiation, while p27 null cells retained, on average, less than 35% of their kinase activity (Fig. 4B). In contrast, kinase activity in Mad1/p27KIP1 double-null cells was maintained at substantial levels out to day 6 following stimulation with the RXR agonist. (Fig. 4A and B). Kinase activity associated with cyclin D3, the major D-cyclin species expressed in granulocytes, was only modestly downregulated during granulocyte differentiation. Furthermore, there were no significant differences between cyclin D3-associated kinase activity in Mad1/p27 double-null cells compared with cells of other genotypes (data not shown).
FIG. 4.
Downregulation of cyclin E-CDK2-associated kinase activity is abrogated in Mad1/p27KIP1-double null cell lines. Representative clones of granulocytic cell lines were stimulated with a 10−8 M concentration of the RXR agonist AGN-194204 and analyzed after 2, 4, and 6 days in culture. Control (zero time point) cultures were stimulated with ethanol carrier alone for 4 days. (A) Cyclin E-associated kinase activity as determined by using histone H1 as a substrate from a representative experiment. (B) Quantification of cyclin E-associated kinase activity by using a phosphorimager. Results shown are means ± standard error of the mean from a total of five separate experiments performed on two clones of each genotype. Data shown are relative to kinase activity at day 0.
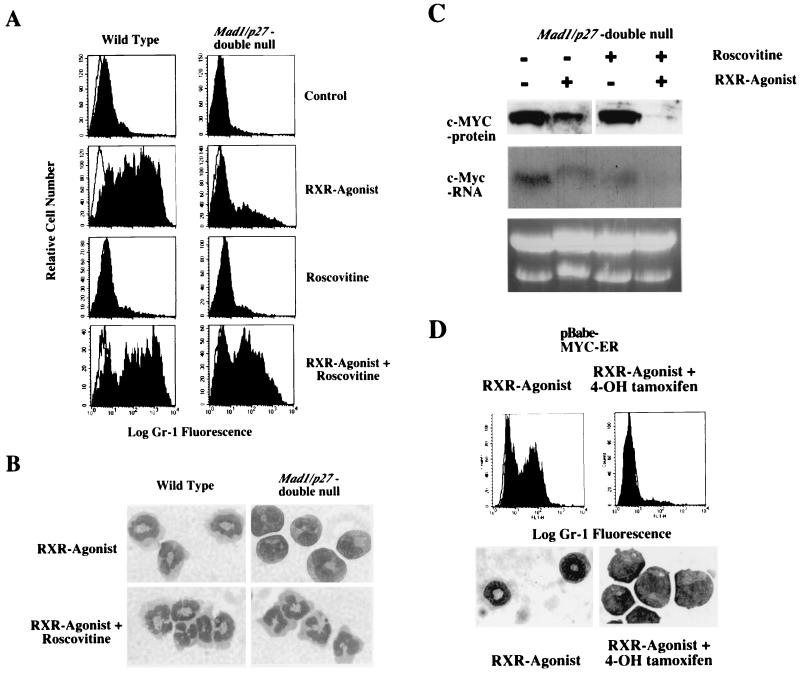
The constitutive CDK2 activity found in granulocyte cell lines from Mad1/p27KIP1 double-null mice raised the possibility that downregulation of CDK2 activity was required for retinoid-induced granulocyte differentiation. To investigate this possibility, we treated the granulocyte cell lines with the CDK2-inhibitory drug Roscovitine (23). Roscovitine is an isopropylpurine compound that inhibits CDK2 and cdc2 at micromolar concentrations. At similar concentrations, Roscovitine does not inhibit CDK4 or CDK6 or other non-CDK kinases (23). Strikingly, Roscovitine restored the ability of Mad1/p27KIP1 double-null granulocyte cell lines to differentiate in response to the RXR agonist. Treatment of the Mad1/p27KIP1 double-null cells with the CDK2-inhibitor and the RXR agonist upregulated the expression of Gr-1 (Fig. 5a) and induced differentiation as assessed by cell morphology (Fig. 5b). However, CDK2 inhibition alone was not sufficient to induce differentiation as treatment with Roscovitine without concomitant addition of the RXR agonist failed to differentiate the Mad1/p27KIP1 double-null cells (Fig. 5a). We then examined expression of c-MYC protein and mRNA in Mad1/p27KIP1 double-null granulocyte cell lines treated with the RXR agonist and Roscovitine. c-MYC expression was high in the untreated Mad1/p27KIP1 double-null cells and showed some reduction following treatment with the RXR agonist alone (Fig. 5c). Significantly, the combination of CDK inhibition and retinoid treatment completely abrogated the expression of c-MYC (Fig. 5c), which was associated with restoration of the ability of the Mad1/p27KIP1 double-null cells to differentiate. In all cases, c-MYC mRNA levels as detected by Northern analysis changed in parallel to the levels of c-MYC protein (Fig. 5C and data not shown). Finally, we examined whether constitutive expression of c-MYC was sufficient to inhibit differentiation of wild-type cells. Cells were infected with pBabe-puro and pBabepuro-MYCER retroviruses. Following selection in puromycin, cells were induced to differentiate by stimulation with the RXR agonist with or without treatment with 4-hydroxy-tamoxifen (4OH-tamoxifen). The RXR-agonist-induced differentiation of cells infected with pBabe-puro-MYCER in the absence of 4OH-tamoxifen. The RXR agonist also induced differentiation of cells infected with pBabe-puro with or without treatment with 4OH-tamoxifen (not shown). However, induction of MYCER activity by treatment with 4OH-tamoxifen completely inhibited differentiation induced by the RXR agonist (Fig. 5d), demonstrating that constitutive expression of c-MYC was sufficient to inhibit differentiation.
FIG. 5.
Downregulation of cyclin E-CDK2-associated kinase activity and c-MYC expression is required for granulocyte differentiation in response to retinoids. Representative clones of granulocytic cell lines were stimulated with a 10−8 M concentration of the RXR agonist AGN-194204, and cells were analyzed after 4 days in culture. Control or zero time point cultures were stimulated with ethanol carrier alone for 4 days. (A) Expression of Gr-1 in wild-type and Mad1/p27KIP1 double-null cells after treatment with a 15 μM concentration of the CDK2 inhibitor roscovitine. CDK2 inhibition restored the ability of Mad1/p27KIP1 double-null cells to differentiate in response to the RXR agonist. (B) Morphology of wild-type and Mad1/p27KIP1 double-null cells after treatment with 15 μM roscovitine and the RXR agonist. (C) Expression of c-MYC protein as detected by Western blotting and mRNA as detected by Northern blotting in Mad1/p27KIP1 double-null cells 4 days after stimulation with the RXR agonist AGN-194204 and/or 15 μM roscovitine. Samples were normalized for equal amounts of protein or total RNA. (D) c-MYCER protein was activated in wild-type cells infected with pBabe-puro-MYCER retrovirus by the addition of 200 nM 4OH-tamoxifen. Expression of Gr-1 and cell morphology were examined 4 days after stimulation with the RXR agonist AGN-194204.
DISCUSSION
Synthetic effects between null alleles of Mad1 and p27KIP1.
The generation of Mad1/p27KIP1 double-null mice revealed synthetic effects between the null alleles of Mad1 and p27KIP1. First, Mad1/p27KIP1 double-null mice were present at significantly lower frequencies, suggesting embryonic, fetal, or perinatal mortality of a subset of Mad1/p27KIP1 double-null mice. Second, kidney size was greater in Mad1/p27KIP1 double-null mice than in mice of other genotypes. Third, there was bone marrow hyperplasia in Mad1/p27KIP1 double-null mice, which was composed mostly of an increase in myeloid cells. Further examination of hematopoiesis in these animals by using colony assays on bone marrow cells suggested an increased number of cell divisions occurring in vivo between the immature colony-forming cells and the fully differentiated mature cells. This finding was extended by deriving granulocyte cell lines from wild-type, Mad1-null, p27KIP1-null, and Mad1/p27KIP1 double-null mice. When granulocyte cell lines were derived from Mad1/p27KIP1 double-null mice, the cell lines were markedly impaired in their capacity to differentiate into mature granulocytes. The phenotype of the Mad1/p27KIP1 double-null granulocyte cell lines that were obtained by enforced expression of a truncated retinoic acid receptor was more severe than the phenotype observed for primary cells. This suggests genetic interaction between Mad1 and p27KIP1and pathways downstream of the retinoic acid receptor.
Other cell types were not subject to synthetic effects between these null alleles. Hyperplasia in thymus, spleen, liver, and testis seen in p27KIP1 null mice was no more severe in Mad1/p27KIP1 double-null mice. This may represent a fundamental difference in the molecular regulation of cell cycle withdrawal in these cells. Alternatively, other members of the Mad family may play a redundant role in these cell types. Consistent with this possibility, abnormalities in lymphoid cell proliferation have been noted for Mxi1 null mice (34). Moreover, analysis of Mad family gene expression in spleens from Mad1 null mice detected upregulation of Mxi1 and Mad3 (11). Hence, there is likely to be cross-regulation between members of the Mad gene family. Further examination of this possibility will have to await the generation of mice carrying null alleles of multiple Mad family members. It will also be interesting to examine genetic interaction of other CKIs with members of the Mad family.
Mad1 and p27KIP1 cooperate to inhibit cyclin E-CDK2 activity.
Strikingly, granulocyte cell lines from Mad1/p27KIP1 double-null mice had kinase activity associated with cyclin E-CDK2 that was inefficiently downregulated following the attempted induction of differentiation. The mechanism of inhibition of CDK activity by p27KIP1 is reasonably well established; p27KIP1 binds to CDK2 close to the ATP binding site and in doing so prevents ATP binding and catalytic activity of the kinase (33). The mechanisms by which Mad1 inhibits CDK2 activity or by which Myc stimulates CDK2 activity are not completely resolved. It has been suggested that the ability of c-MYC to induce CDK2 activity is mediated through degradation of p27KIP1 (25, 29), possibly through a Cul1-dependent mechanism (29) or through sequestration of p27KIP1 into alternate protein complexes, such as those containing cyclin-D2 and CDK4, both of which have been shown to be transcriptionally regulated by MYC (3, 15, 31). If we assume that Mad1 and c-MYC have similar sets of target genes, this model would predict that loss of Mad1 should not have any synthetic effects with the loss of p27 in inducing kinase activity associated with cyclin E-CDK2. The fact that we did observe synthetic effects suggests that, at least in part, Mad1 and p27KIP1 can inactivate CDK2 by independent mechanisms. The hypothesis that Mad1 has effects on cell cycle progression independent of p27KIP1 is strengthened by the observation that overexpression of Mad1 can induce a G1 arrest in p27KIP1 null fibroblasts (G. A. McArthur, unpublished data). These data are also consistent with recent evidence that Mad and Myc transcriptional factors may not have completely overlapping targets (30) (L. James and R. N. Eisenman, unpublished data).
Data obtained from biochemical analyses of cellular lysates from Mad1 null and Mad1/p27KIP1 double-null granulocyte cell lines suggest that Mad1 may regulate the expression of two members of the Rb family, p107 and p130. As these molecules may have some role in direct inhibition of cyclin E-CDK2 complexes (2, 8, 43), the loss of p107 and p130 expression may further contribute to the activation of CDK2 in Mad1/p27KIP1 double-null cells. Constitutive activation of cyclin E-CDK2 complexes may also affect the function of p107 and p130 (6, 40). The mechanism by which the levels of p107 and p130 were reduced in Mad1 null and Mad1/p27KIP1 double-null cells has not been addressed but could involve indirect effects on transcription of p107 or p130 mRNAs or alternately may involve posttranscriptional effects on the stability or synthesis of p107 or p130 proteins. At least two of these mechanisms have been suggested to play a role in the regulation of these Rb family proteins (38).
There was a marked increase in c-MYC levels in the Mad1/27KIP1 double-null cells. As enforced expression of c-MYC was sufficient to inhibit differentiation of wild-type cells, c-MYC may have played a major role in inhibiting differentiation of the Mad1/27KIP1 double-null cells. The increased expression of c-MYC was not due to simple derepression of c-MYC transcription secondary to the loss of Mad1, as the loss of p27KIP1 was also required to induce upregulation of c-MYC. One possibility is that c-MYC transcription is being directly regulated by E2F proteins released through cyclin E-CDK2 phosphorylation of Rb family members. Indeed, positive regulation of the c-myc promoter by E2F has been reported for other cellular systems (48). However, CDK2 activity is clearly not the only mechanism contributing to enhanced c-MYC expression as the CDK2 inhibitory compound Roscovitine, in the absence of the differentiation inducer, was not sufficient to downregulate c-MYC protein levels.
Coupling of differentiation to withdrawal from the cell cycle.
During granulocyte differentiation, there is a tight coupling of inhibition of cellular proliferation and the development of the terminally differentiated state. Our data indicate that withdrawal from the cell cycle by downregulation of CDK2 activity is required for granulocyte differentiation. This suggests that downregulation of CDK2 activity by the cooperative effects of MAD1 and p27KIP1 may be a critical step in allowing differentiation to proceed and a key nodal point in linking regulation of the cell cycle to the regulation of differentiation. In this regard, the apparent deregulation of Myc in the double knockout cells is intriguing. Myc is generally considered to be upstream of E2F1, E2F2, and E2F3 and to drive cell cycle progression and cell growth (18, 20, 35). Our results with the double-null cells may reveal a positive feedback system that participates in maintaining Myc expression as cells progress from G1 into S phase. It will be important to identify the transcriptional targets of MAD1 and the substrates of CDK2 in order to further elucidate the molecular circuitry underlying the connection between MAD1, CDK2, MYC, and differentiation.
Acknowledgments
We thank L. Ramos, L. Purton, S. Collins, L. Rohrschneider, G. Sale, P. Waring, and S. Tsai for technical assistance, advice, or reagents and Amir Oryan for a critical reading of the manuscript. Cytokines were generously provided by Immunex Corp., and the RXR agonist AGN 194204 was kindly provided by Allergan Corp. We are grateful to Santa Cruz Biotechnology for help with antibodies.
This work was supported by fellowships from the American Cancer Society (K.P.F.) and the Damon Runyon-Walter-Winchell Foundation DRG-076, by a Special Fellowship of the Leukemia Society of America (G.A.M.), and by grants from the National Institutes of Health (RO1CA57138 and HL54881 to R.N.E.) and from the National Health and Medical Research Council of Australia (G.A.M). R.N.E. is an American Cancer Research Professor.
REFERENCES
- 1.Adachi, M., M. F. Roussel, K. Havenith, and C. J. Sherr. 1997. Features of macrophage differentiation induced by p19INK4d, a specific inhibitor of cyclin D-dependent kinases. Blood 90:126-137. [PubMed] [Google Scholar]
- 2.Beier, R., A. Burgin, A. Kiermaier, M. Fero, H. Karsunky, R. Saffrich, T. Moroy, W. Ansorge, J. Roberts, and M. Eilers. 2000. Induction of cyclin E-cdk2 kinase activity, E2F-dependent transcription and cell growth by Myc are genetically separable events. EMBO J. 19:5813-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchard, C., K. Thieke, A. Maier, R. Saffrich, J. Hanley-Hyde, W. Ansorge, S. Reed, P. Sicinski, J. Bartek, and M. Eilers. 1999. Direct induction of cyclin D2 by myc contributes to cell cycle progression and sequestration of p27. EMBO J. 18:5321-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casaccia, B. P., R. Tikoo, H. Kiyokawa, V. J. Friedrich, M. V. Chao, and A. Koff. 1997. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 11:2335-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., T. Willingham, L. R. Margraf, A. N. Schreiber, R. A. DePinho, and P. D. Nisen. 1995. Effects of the MYC oncogene antagonist, MAD, on proliferation, cell cycling and the malignant phenotype of human brain tumour cells. Nat. Med. 1:638-643. [DOI] [PubMed] [Google Scholar]
- 6.Claudio, P. P., A. De Luca, C. M. Howard, A. Baldi, E. J. Firpo, A. Koff, M. G. Paggi, and A. Giordano. 1996. Functional analysis of pRb2/p130 interaction with cyclins. Cancer Res. 56:2003-2008. [PubMed] [Google Scholar]
- 7.Coats, S., W. M. Flanagan, J. Nourse, and J. M. Roberts. 1996. Requirement of p27(kip1) for restriction point control of the fibroblast cell cycle. Science 272:877-880. [DOI] [PubMed] [Google Scholar]
- 8.Coats, S., P. Whyte, M. L. Fero, S. Lacy, G. Chung, E. Randel, E. Firpo, and J. M. Roberts. 1999. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 9:163-173. [DOI] [PubMed] [Google Scholar]
- 9.Cultraro, C. M., T. Bino, and S. Segal. 1997. Function of the c-myc antagonist mad1 during a molecular switch from proliferation to differentiation. Mol. Cell. Biol. 17:2353-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 11.Foley, K. P., G. A. McArthur, C. Queva, P. J. Hurlin, P. Soriano, and R. N. Eisenman. 1998. Targeted disruption of the myc antagonist mad1 inhibits cell cycle exit during granulocyte differentiation. EMBO J. 17:774-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin, D. S., V. L. Godfrey, H. Lee, G. I. Kovalev, R. Schoonhoven, S. Chen-Kiang, L. Su, and Y. Xiong. 1998. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 12:2899-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser, P. J., D. Agrawal, M. Flanagan, and W. J. Pledger. 1997. The role of p27(kip 1) in the in vitro differentiation of murine keratinocytes. Cell Growth Differ. 8:203-211. [PubMed] [Google Scholar]
- 14.Henriksson, M., and B. Luscher. 1996. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv. Cancer Res. 68:109-182. [DOI] [PubMed] [Google Scholar]
- 15.Hermeking, H., C. Rago, M. Schuhmacher, Q. Li, J. F. Barrett, A. J. Obaya, B. C. O'Connell, M. K. Mateyak, W. Tam, F. Kohlhuber, C. V. Dang, J. M. Sedivy, D. Eick, B. Vogelstein, and K. W. Kinzler. 2000. Identification of CDK4 as a target of c-MYC. Proc. Natl. Acad. Sci. USA 97:2229-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh, F. F., L. A. Barnett, W. F. Green, K. Freedman, I. Matushansky, A. I. Skoultchi, and L. L. Kelley. 2000. Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase. Blood 96:2746-2754. [PubMed] [Google Scholar]
- 17.Johnson, B. S., R. A. Chandraratna, R. A. Heyman, E. A. Allegretto, L. Mueller, and S. J. Collins. 1999. Retinoid X receptor (RXR) agonist-induced activation of dominant-negative RXR-retinoic acid receptor alpha403 heterodimers is developmentally regulated during myeloid differentiation. Mol. Cell. Biol. 19:3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston, L. A., D. A. Prober, B. A. Edgar, R. N. Eisenman, and P. Gallant. 1999. Drosophila myc regulates cellular growth during development. Cell 98:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 20.Leone, G., R. Sears, E. Huang, R. Rempel, F. Nuckolls, C. H. Park, P. Giangrande, L. Wu, H. I. Saavedra, S. J. Field, M. A. Thompson, H. Yang, Y. Fujiwara, M. E. Greenberg, S. Orkin, C. Smith, and J. R. Nevins. 2001. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol. Cell 8:105-113. [DOI] [PubMed] [Google Scholar]
- 21.Levine, E. M., J. Close, M. Fero, A. Ostrovsky, and T. A. Reh. 2000. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev. Biol. 219:299-314. [DOI] [PubMed] [Google Scholar]
- 22.McArthur, G. A., C. Laherty, C. Quéva, P. J. Hurlin, L. Loo, L. James, C. Grandori, P. Gallant, Y. Shiio, A. Bush, P. F. Cheng, Q. Lawrence, B. Pulverer, P. Koskinen, K. P. Foley, D. E. Ayer, and R. N. Eisenman. 1998. The MAD protein family links transcriptional repression to cell differentiation. Cold Spring Harbor Symp. Quant. Biol. LXIII:423-433. [DOI] [PubMed] [Google Scholar]
- 23.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 24.Missero, C., F. Di Cunto, H. Kiyokawa, A. Koff, and G. P. Dotto. 1996. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 10:3065-3075. [DOI] [PubMed] [Google Scholar]
- 25.Muller, D., C. Bouchard, B. Rudolph, P. Steiner, I. Stuckmann, R. Saffrich, W. Ansorge, W. Huttner, and M. Eilers. 1997. Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene 15:2561-2576. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan, G., and T. Jacks. 1998. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 14:223-229. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 28.Nourse, J., E. Firpo, W. M. Flanagan, S. Coats, K. Polyak, M. H. Lee, J. Massague, G. R. Crabtree, and J. M. Roberts. 1994. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570-573. [DOI] [PubMed] [Google Scholar]
- 29.O'Hagan, R. C., M. Ohh, G. David, I. M. de Alboran, F. W. Alt, W. G. Kaelin, Jr., and R. A. DePinho. 2000. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 14:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hagan, R. C., N. Schreiber-Agus, K. Chen, G. David, J. A. Engelman, R. Schwab, L. Alland, C. Thomson, D. R. Ronning, J. C. Sacchettini, P. Meltzer, and R. A. DePinho. 2000. Gene-target recognition among members of the myc superfamily and implications for oncogenesis. Nat. Genet. 24:113-119. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Roger, I., S. H. Kim, B. Griffiths, A. Sewing, and H. Land. 1999. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1). EMBO J. 18:5310-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roussel, M. F., R. A. Ashmun, C. J. Sherr, R. N. Eisenman, and D. E. Ayer. 1996. Inhibition of cell proliferation by the Mad1 transcriptional repressor. Mol. Cell. Biol. 16:2796-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo, A. A., P. D. Jeffrey, A. K. Patten, J. Massague, and N. P. Pavletich. 1996. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382:325-331. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber-Agus, N., Y. Meng, T. Hoang, H. Hou, Jr., K. Chen, R. Greenberg, C. Cordon-Cardo, H. W. Lee, and R. A. DePinho. 1998. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature 393:483-487. [DOI] [PubMed] [Google Scholar]
- 35.Sears, R., K. Ohtani, and J. R. Nevins. 1997. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol. Cell. Biol. 17:5227-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrano, M., H. Lee, L. Chin, C. C. Cordon, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 37.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 38.Smith, E. J., G. Leone, and J. R. Nevins. 1998. Distinct mechanisms control the accumulation of the Rb-related p107 and p130 proteins during cell growth. Cell Growth Differ. 9:297-303. [PubMed] [Google Scholar]
- 39.Steiner, P., A. Philipp, J. Lukas, K. D. Godden, M. Pagano, S. Mittnacht, J. Bartek, and M. Eilers. 1995. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 14:4814-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki-Takahashi, I., M. Kitagawa, M. Saijo, H. Higashi, H. Ogino, H. Matsumoto, Y. Taya, S. Nishimura, and A. Okuyama. 1995. The interactions of E2F with pRB and with p107 are regulated via the phosphorylation of pRB and p107 by a cyclin-dependent kinase. Oncogene 10:1691-1698. [PubMed] [Google Scholar]
- 41.Tsai, S., and S. J. Collins. 1993. A dominant negative retinoic acid receptor blocks neutrophil differentiation at the promyelocyte stage. Proc. Natl. Acad. Sci. USA 90:7153-7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlach, J., S. Hennecke, K. Alevizopoulos, D. Conti, and B. Amati. 1996. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 15:6595-6604. [PMC free article] [PubMed] [Google Scholar]
- 43.Woo, M., I. Sanchez, and B. D. Dynlacht. 1997. P130 and p107 use a conserved domain to inhibit cellular cyclin-dependent kinase activity. Mol. Cell. Biol. 17:3566-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan, Y., J. Frisen, M. H. Lee, J. Massague, and M. Barbacid. 1997. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11:973-983. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, P., C. Wong, R. A. DePinho, J. W. Harper, and S. J. Elledge. 1998. Cooperation between the cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 12:3162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, P. M., N. J. Liegeois, C. Wong, M. Finegold, H. Hou, J. C. Thompson, A. Silverman, J. W. Harper, R. A. Depinho, and S. J. Elledge. 1997. Altered cell differentiation and proliferation in mice lacking p57(kip2) indicates a role in beckwith-wiedemann-syndrome. Nature 387:151-158. [DOI] [PubMed] [Google Scholar]
- 47.Zindy, F., J. J. Cunningham, C. J. Sherr, S. Jogal, R. J. Smeyne, and M. F. Roussel. 1999. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 96:13462-13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou, X. M., S. Rudchenko, K. K. Wong, and K. Calame. 1997. Induction of c-myc transcription by the v-abl tyrosine kinase requires ras, raf1, and cyclin-dependent kinases. Genes Dev. 11:654-662. [DOI] [PubMed] [Google Scholar]