Abstract
Calpain is a ubiquitous protease with potential involvement in apoptosis. We report that in human melanoma cells, cisplatin-induced calpain activation occurs early in apoptosis. Calpain activation and subsequent apoptosis were inhibited by calpeptin and PD150606, two calpain inhibitors with different modes of action. Furthermore, cisplatin induced cleavage of the BH3-only protein Bid, yielding a 14-kDa fragment similar to proapoptotic, caspase-cleaved Bid. However, Bid cleavage was inhibited by inhibitors of calpain, but not by inhibitors of caspases or of cathepsin L. Recombinant Bid was cleaved in vitro by both recombinant calpain and by lysates of cisplatin-treated cells. Cleavage was calpeptin sensitive, and the cleavage site was mapped between Gly70 and Arg71. Calpain-cleaved Bid induced cytochrome c release from isolated mitochondria. While calpeptin did not affect cisplatin-induced modulation of Bak to its proapoptotic conformation, a dominant-negative mutant of MEKK1 (dnMEKK) inhibited Bak modulation. dnMEKK did not, however, block Bid cleavage. The combination of dnMEKK and calpeptin had an additive inhibitory effect on apoptosis. In summary, calpain-mediated Bid cleavage is important in drug-induced apoptosis, and cisplatin induces at least two separate apoptotic signaling pathways resulting in Bid cleavage and Bak modulation, respectively.
During apoptosis, the mechanisms for cytochrome c release from the intermembrane space in mitochondria involve proteins of the Bcl-2 family. In the mitochondrial outer membrane, the proapoptotic functions of Bak and the related Bax protein depend at least in part on the presence of Bid, a cytosolic BH3-only protein of the Bcl-2 family. Cleavage of Bid to the mitochondrially active, truncated form, tBid, is a feature of caspase-8-mediated apoptosis induced via death receptors (10, 18). Bid can also be cleaved by caspase-3 in the intrinsic pathway to apoptosis, which is independent of death receptors (3, 27). Granzyme B is the third protease shown to cleave Bid (1). tBid translocates to mitochondria, where it is involved in oligomerization of Bak and/or Bax leading to cytochrome c release (6; reviewed in reference 16).
In addition to caspases, apoptosis may involve activation of other proteases, e.g., cathepsins and calpain. Examples of involvement of calpain in apoptosis include cleavage of p53 (17) and of Bax, the proapoptotic effect of which is thereby increased (7, 37). Calpain activation may occur downstream of caspase activation (38), but has also been reported to occur upstream of caspases in apoptosis induced by ionizing irradiation (30). However, compared to caspases, only little is known about the roles of calpain in apoptosis.
Cisplatin is a DNA-damaging agent widely used in anticancer therapy. In sensitive target cells, it induces apoptosis, which typically involves cytochrome c release from mitochondria and the subsequent activation of caspase-9 and -3. It is not known how cellular signaling from the drug-induced DNA lesions leads to these execution-phase characteristics of apoptosis, but involvement of c-Abl and stress-activated protein kinase (SAPK) pathways has been indicated (14, 22), as has induction of the expression of Bax in cell lines with retained p53 function (20). We have reported that cisplatin-induced signaling involves modulation of the mitochondrial Bcl-2 family protein Bak to a proapoptotic conformation in a panel of melanoma cell lines (19). Bak modulation was inhibitable by a dominant-negative mutant of MEKK1, dnMEKK1. Inhibition of Bak modulation did not completely block caspase activation and nuclear fragmentation, suggesting that cisplatin activates other signals in addition to the Bak pathway.
In the course of investigating cisplatin-induced modulation of Bak, we found that pretreatment of cells with calpeptin, a calpain inhibitor, inhibited cisplatin-induced apoptosis by approximately half. This prompted us to further examine cisplatin-mediated effects on calpain and, because of its role in Bak/Bax regulation, Bid. Cisplatin-mediated activation of calpain was found to occur early in the apoptotic process and to coincide with Bid cleavage. We have here characterized calpain-mediated cleavage of Bid in vitro and in vivo, and we present evidence that this mechanism is separate from the dnMEKK-sensitive Bak pathway.
MATERIALS AND METHODS
Cells.
A human metastatic melanoma cell line, 224, was used for all experiments and has been described previously, along with three other melanoma cell lines, with respect to Bak/Bax modulation and other aspects of apoptosis induction (19). Other cell lines (DFW melanoma, MDA-MB-231 breast carcinoma, U1285 lung carcinoma, and U266 myeloma cells) were used for some experiments. Cells were maintained at 37°C in 5% CO2 in RPMI medium supplemented with fetal calf serum (10%), l-glutamate, penicillin, and streptomycin.
Assessment of calpain activity in cell lysates and in intact cells.
Control and cisplatin-treated cells were resuspended in cold lysis buffer (10 mM HEPES [pH 7.4], 5 mM MgCl2, 42 mM KCl, 0.32 M sucrose) and lysed by repeated passage through a 27-gauge syringe. For each sample, 40 μg of protein was incubated with 150 μM calpain substrate (N-Suc-Leu-Tyr-AMC; Sigma Chemical Co.) in assay buffer (10 mM HEPES [pH 7.4], 1% Triton X-100, 100 μM CaCl2) at 37°C for 2 h. In assays with intact cells, control and treated cells were harvested and incubated for 20 min with cell-permeable calpain substrate Boc-Leu-Met-CMAC (10 μM; Molecular Probes, Inc.), which fluoresces upon cleavage by calpain. Intracellular fluorescence was measured by flow cytometry (FACS-Vantage).
Calcium measurement.
Cells were treated as indicated and harvested with cell dissociation solution (Sigma Aldrich), and the suspended cells were then incubated with the Ca2+ indicator FLUO-3 (Molecular Probes, Inc.) for 60 s. The intracellular Ca2+ levels, seen as fluorescent signal, were then assessed by flow cytometry with the FL1 channel. Data are presented as fold increase in fluorescence compared to that in untreated cells.
Assessment of apoptosis.
Cells were harvested with cell dissociation solution, resuspended in 50 to 100 μl of hypotonic salt solution with 30 mM glycerol, and smeared on glass slides. Air-dried smears were fixed in acetone-methanol (2:1) for 5 min and then covered with ethidium bromide (5 ng/ml in distilled water) for 5 min. After rinsing in tap water, stained cells were examined by UV microscopy. At least 200 cells per sample were counted, and the percentage of cells with fragmented nuclei was assessed in each sample.
Apoptosis was also assessed by quantitation of DEVDase activity against the Ac-DEVD-AMC substrate, which fluoresces upon cleavage by DEVDase (CaspACE assay; Promega). Harvested cells were washed in ice-cold phosphate-buffered saline (PBS) and resuspended in lysis buffer (25 mM HEPES [pH 7.5], 5 mM MgCl2, 5 mM EDTA, 5 mM dithiothreitol [DTT], 2 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg each of pepstatin and leupeptin per ml). Cells were lysed by three cycles of freeze-thawing in liquid N2. Lysates were centrifuged (16,000 × g, 20 min), and caspase activity in supernatants was assayed according to the manufacturer's instructions. Addition of DEVD-CHO to samples with caspase activity abrogated the whole signal.
Loss of mitochondrial membrane potential as an indication of apoptosis was also assessed. Cells were incubated with 25 nM TMRE (tetramethylrhodamine ethyl ester; Molecular Probes, Inc.) for 30 min, washed and resuspended in PBS containing 25 nM TMRE. Changes in mitochondrial membrane potential (ΔΨ) were detected by flow cytometry.
Calpain-mediated cleavage of Bid.
The presence of full-length and truncated Bid in cell extracts was analyzed by Western blotting with an anti-Bid antibody (Cell Signaling Technology) that recognizes both full-length and cleaved fragments of human Bid. For in vitro cleavage experiments, bacterially expressed human glutathione S-transferase (GST)-bound Bid was coupled to glutathione-Sepharose beads (Amersham Pharmacia Biotech AB). GST-Bid beads (100 μg of GST-Bid) were incubated for 10 to 60 min at 37°C with 10 μg of recombinant m-calpain (Calbiochem) in cleavage buffer (20 mM HEPES [pH 7.5], 50 mM KCl, 2 mM MgCl2, 1 mM DTT, 5 mM CaCl2) in the presence or absence of 10 μM calpeptin. A similar calpain activity in cell lysates was examined by incubating beads (10 μg of GST-Bid) with 500 μg of cell extract for 60 min at 37°C. Extracts were made from control and cisplatin-treated cells (20 μM, 5 h), as well as from cells cotreated with cisplatin and 10 μM calpeptin. After each incubation with beads, supernatants were collected, and the presence of Bid fragments was identified by Western blotting. For identification of the cleavage site, C-terminal Bid fragments of recombinant calpain-cleaved Bid were eluted from the membrane and subjected to NH2-terminal Edman degradation.
Expression of dnMEKK1.
An adenovirus vector system for the inducible expression of a 37-kDa dnMEKK1 mutant (with a K432M point mutation) was prepared and used as described earlier (19). To induce dnMEKK1 expression, doxycycline (1 μM) was added to infected cells 20 h prior to cisplatin treatment.
Isolation of mitochondria and analysis of cytochrome c release.
Cells were resuspended in buffer A (250 mM mannitol, 70 mM sucrose, 0.5 mM EGTA, 5 mM HEPES [pH 7.2], 0.1 mM PMSF) and lysed in a glass homogenizer. Homogenates were centrifuged at 1,000 × g for 10 min. The supernatant was then centrifuged at 10,000 × g for 30 min, and the mitochondrial pellet was resuspended in buffer B (250 mM sucrose, 10 mM HEPES [pH 7.5], 2 mM KH2PO4, 5 mM sodium succinate, 25 mM EGTA, 0.1 mM PMSF, 4 mM MgCl2). Mitochondria (85 μg of protein) were left untreated or incubated either with full-length Bid or calpain-cleaved Bid (7 μg) for 40 min at 30°C. Calpeptin (10 μM) was added to prevent possible effects of calpain on mitochondrial membrane proteins. After incubation, supernatants were separated from mitochondria by centrifugation at 10,000 × g for 10 min and analyzed for the presence of cytochrome c by Western blotting (antibody from PharMingen, Intl.). Lack of mitochondrial contamination in supernatants was confirmed with an anti-Cox IV antibody (Molecular Probes, Inc.).
Flow cytometric analysis of Bak-associated immunofluorescence.
Upon induction of apoptosis, the proapoptotic Bak protein undergoes a conformational change that exposes an otherwise inaccessible N-terminal epitope (9). In the present study, we have used the same antibody that was shown to specifically recognize this epitope (amino acids 1 to 52 of Bak; Oncogene Research Products; no. AM03). By using a fluorescein isothiocyanate (FITC)-conjugated secondary antibody, the increases in accessibility of the epitope were monitored by flow cytometry as described earlier (19). Data are presented as fold increase in immunofluorescence from control levels.
RESULTS
Cisplatin induces calpain activation via increased intracellular Ca2+.
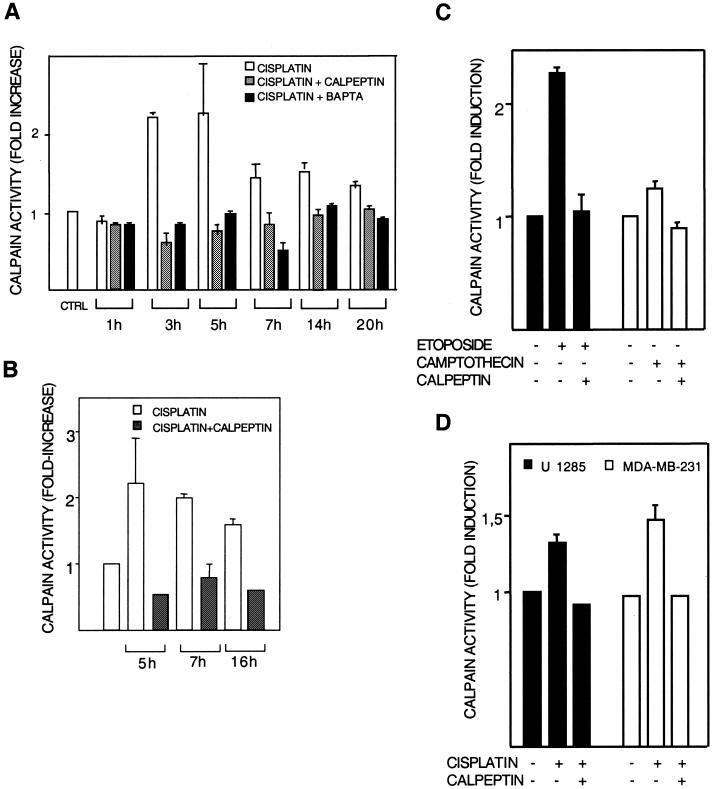
The human melanoma cell line 224 was treated with 20 μM cisplatin. At different time points posttreatment, calpain activity was assessed in cell lysates as well as in intact cells with two different fluorigenic calpain substrates (Fig. 1A and B). Cisplatin treatment for 1 h did not yield calpain activation, while a more than twofold induction was observed at 3 and 5 h, and the activity then decreased somewhat (Fig. 1A). Because calpain may be activated by association with membrane phospholipids, we also assessed calpain activity in situ. Intact cells were briefly incubated with a cell-permeable substrate, and the resulting fluorescence was analyzed by flow cytometry. A 2.2-fold induction was observed at 5 h by this method (Fig. 1B). In both assays, activation was blocked by cotreatment with calpain inhibitor calpeptin (10 μM) (Fig. 1A and B). Calpain activation was blocked also by Ca2+ chelator BAPTA-AM (Fig. 1A), in accordance with a role for Ca2+ in calpain activation (see below). BAPTA-AM was furthermore found to inhibit cisplatin-induced apoptosis to the same extent as calpeptin (Table 1 [and see below]).
FIG. 1.
Calpain activation. Calpain activation by cisplatin was studied in human melanoma 224 cells (A to C) and U1285 lung and MDA-MB-231 breast carcinoma (D) cells. After the indicated treatments in the presence or absence of the calpain inhibitor calpeptin (10 μM), calpain activity in cell lysates (A, C, and D) or in intact cells (B) was measured with substrates that become fluorescent after cleavage by calpain. Results are shown as fold activation of calpain compared to activity in untreated controls. (A) Lysates of cells treated with 20 μM cisplatin for the indicated time periods were incubated with N-Suc-Leu-Tyr-AMC, and the resulting fluorescence was assessed fluorimetrically. The effect of cotreatment with BAPTA-AM (10 μM) is also shown. (B) Cells were treated with 20 μM cisplatin for the indicated time periods and were then incubated further with cell-permeable Boc-Leu-Met-CMAC for 20 min. Fluorescence was assessed by flow cytometry. (C) Cells were treated with etoposide (15 μM) or camptothecin (1.6 μM) in the presence or absence of calpeptin. Calpain activity was assessed in lysates after 5 h. (D) Lung (U1285) and breast (MDA-MB-231) carcinoma cells were treated with 25 and 40 μM cisplatin, respectively, in the presence or absence of calpeptin. Calpain activity was assessed in lysates after 5 h.
TABLE 1.
Summary of effects of inhibitors on cisplatin-induced apoptosis and Bid cleavage
| Inhibitor | Protease inhibited | Concn (μM) | Inhibition of apoptosis (% inhibition)a | Inhibition of Bid cleavage |
|---|---|---|---|---|
| Calpeptin | Calpain | 10 | 52 | Yes |
| PD150606 | Calpain | 20 | 45 | Yes |
| BAPTA-AM (calcium chelator) | 10 | 55 | NDb | |
| NSIT | Cathepsin L | 5 | 0 | No |
| IETD-fmk | Caspase-8 | 10 | 0 | No |
| DEVD-fmk | Caspase-3 | 10 | 50 | No |
| dnMEKK1 | NAc | NA | 47 | No |
Apoptosis was assessed as the percentage of cells with fragmented nuclei at 16 h of cisplatin treatment (20μM). Figures represent the averages of two to five experiments with each agent.
ND, no data.
NA, not applicable.
We also assessed the effect of other DNA-damaging agents on calpain activation. Lysates of 224 cells treated with camptothecin (1.6 μM) or etoposide (15 μM) for 5 h showed calpain activation levels (Fig. 1C) that correlated with apoptosis induced by either dose (see below). Calpeptin inhibition of the activation confirmed the specificity of the assay (Fig. 1C).
Finally, we examined cisplatin-induced calpain activation in other cell types. U1285 lung carcinoma cells and MDA-MB-231 breast carcinoma cells were treated with 25 and 40 μM cisplatin, respectively, and calpeptin-inhibitable calpain activation was seen after 5 h of treatment (Fig. 1D).
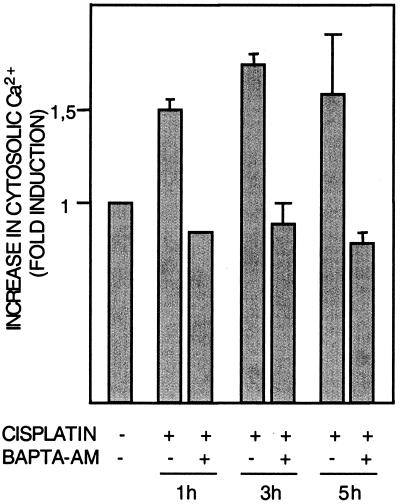
Although other factors are likely also involved in the physiological activation of calpain, activation requires increased Ca2+ levels, which in vitro are in the millimolar range for the m-calpain isoform and in the micromolar range for μ-calpain (4). Using FLUO-3-AM, a cell-permeable Ca2+ indicator (Molecular Probes), we then examined the effect of cisplatin treatment on intracellular Ca2+. After 1 h of cisplatin treatment, intracellular Ca2+ had increased by 50% (Fig. 2), and this response thus preceded activation of calpain. As expected, the increase in Ca2+ was abrogated by Ca2+ chelator BAPTA-AM (10 μM) (Fig. 2). Treatment with Ca2+ ionophore ionomycin (10 μM, 20 min) similarly induced a 1.5-fold increase in Ca2+, and in accordance with a requirement for Ca2+ in calpain activation, treatment with ionomycin resulted in a 1.7-fold increase in calpain activity (data not shown).
FIG. 2.
Cisplatin induces increased intracellular calcium levels. Human 224 melanoma cells were treated with cisplatin (20 μM) for the indicated time periods in the presence or absence of BAPTA-AM (10 μM). Intracellular Ca2+ levels indicated by FLUO-3 fluorescence were then assessed by flow cytometry.
Other candidate mediators of calpain activation and/or increased intracellular Ca2+ include reactive oxygen (13) and nitric oxide (8). We found that the reactive oxygen scavenger NAC, the nitric oxide antagonist PTIO, and the nitric oxide synthase inhibitor L-NNMA did not inhibit cisplatin-induced calpain activation (data not shown).
Inhibitors of calpain, but not of cathepsin L, inhibit cisplatin-induced apoptosis.
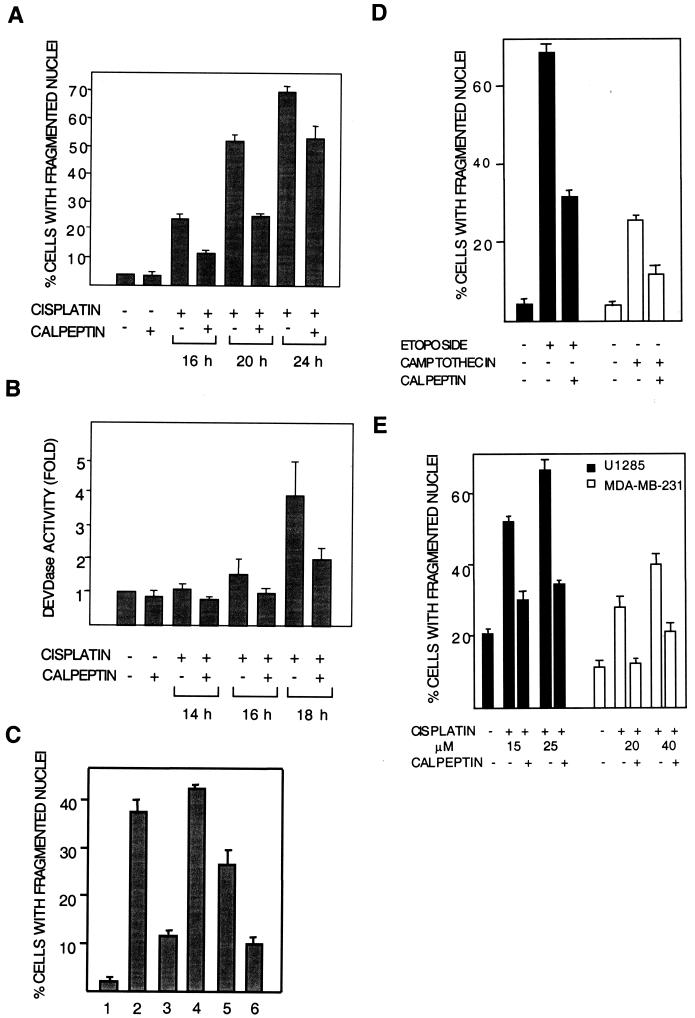
The effect of calpeptin on cisplatin-induced apoptosis was then studied in 224 cells. At 16 h posttreatment, apoptosis seen as nuclear fragmentation was inhibited by calpeptin cotreatment (Fig. 3A). In accordance with its effect on calpain activity and with a role for calpain in cisplatin-induced apoptosis, BAPTA-AM also inhibited apoptosis (Table 1). Calpeptin inhibition of apoptosis at different time points was corroborated by a DEVDase/caspase-3 enzymatic activity assay (Fig. 3B). The sensitivity of cisplatin-induced apoptosis to calpain inhibition was also tested with PD150606, another calpain-specific inhibitor with a different mode of action. Whereas calpeptin acts on the active site of calpain, PD150606 blocks its calcium-binding site (31). Cotreatment of cells with PD150606 was found to inhibit cisplatin-induced apoptosis, similar to calpeptin (Table 1). Because calpeptin may inhibit cathepsin L (34), a protease with a potential role in apoptosis, the effects of cathepsin L inhibitor NapSul-Ile-Trp-CHO (NSIT; 0.5, 1, and 5 μM) were also investigated. NSIT did not affect cisplatin-induced apoptosis (Table 1).
FIG. 3.
Calpeptin inhibits cisplatin-induced apoptosis. Cells (224 cells in panels A to D and U1285 and MDA-MB-231 cells in panel E) were treated with cisplatin (20 μM in panels A to C and as indicated in panels D and E) for different time periods with or without calpeptin (10 μM). (A) Apoptosis quantitated as percentage of cells with fragmented nuclei. Data are from four experiments. (B) Apoptosis quantitated as fold activation of DEVDase activity. Data from two experiments are shown. (C) Effects of late and early addition of calpeptin on apoptosis, seen asnuclear fragmentation. Bars: 1, control; 2, cisplatin for 20 h; 3, cisplatin for 20 h with calpeptin present throughout; 4, cisplatin for 20 h with calpeptin added at 8 h; 5, cisplatin for 8 h and, after rinsing, continued incubation in fresh medium until 20 h; 6, cisplatin and calpeptin for 8 h and, after rinsing, continued incubation in fresh medium until 20 h. This experiment was repeated with similar results. (D) 224 cells were treated with etoposide (15 μM) or camptothecin (1.6 μM) in the presence or absence of calpeptin for 20 h. Apoptosis was quantitated as the percentage of cells with fragmented nuclei. (E) U1285 lung carcinoma cells were treated with 15 and 25 μM cisplatin, and MDA-MB-231 breast carcinoma cells were treated with 20 and 40 μM cisplatin for 20 h in the presence or absence of calpeptin. Apoptosis was quantitated as the percentage of cells with fragmented nuclei. The high basal level of apoptosis in U1285 is normal for this cell line.
To confirm that early activation of calpain plays a role in cisplatin-induced apoptosis, calpeptin was added 8 h after cisplatin addition. This treatment did not affect apoptosis assessed at 20 h (Fig. 3C), showing that calpain is required early in the apoptotic process. In another experiment, cells were treated for 8 h with cisplatin in the presence or absence of calpeptin, after which both drugs were removed. Also with this protocol, calpeptin treatment inhibited apoptosis at 20 h by more than half (Fig. 3C), showing that inhibition of calpain early in the apoptotic process is sufficient to inhibit apoptosis.
In accordance with calpain involvement in apoptosis induced by camptothecin and etoposide, calpeptin cotreatment was found to inhibit apoptosis induced by these two agents (Fig. 3D).
We also examined the role of calpain in cisplatin-induced apoptosis in other tumor cell lines. Similar to the results seen with the 224 melanoma cell line and with DFW, another human melanoma cell line (data not shown), cisplatin-induced apoptosis was inhibited by calpeptin both in U1285 lung carcinoma cells and in MDA-MB-231 breast carcinoma cells (Fig. 3E).
Cisplatin induces calpain-mediated Bid cleavage.
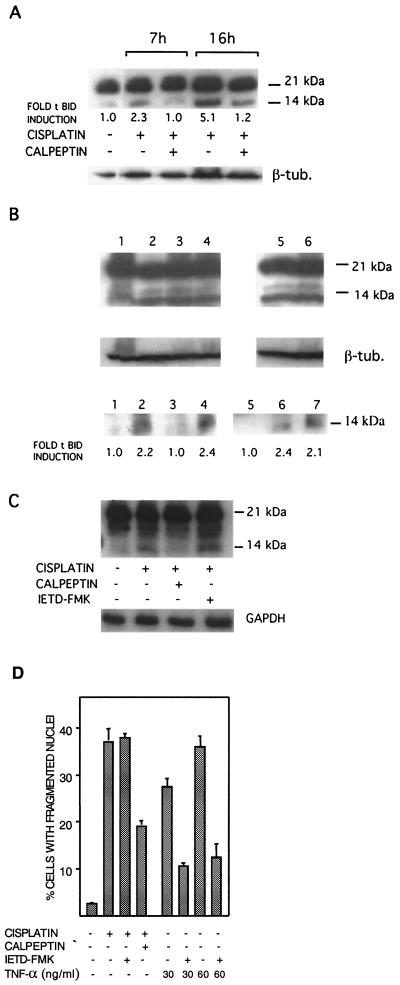
BH3-only proteins are implicated as essential regulators of apoptosis (12). The BH3-only protein Bid has a key role in apoptotic signaling, by virtue of its ability to induce the proapoptotic functionality of Bak and Bax, leading to cytochrome c release (16). By Western blot analysis, we found that in 224 cells, cisplatin induced Bid cleavage at 7 h posttreatment (Fig. 4A). Quantification by laser densitometry showed that the more than two- and fivefold increases in tBid at 7 and 16 h, respectively, were abolished by cotreatment with calpeptin (Fig. 4A). Calpain involvement was corroborated by the second calpain inhibitor used, PD150606, which also blocked Bid cleavage (Table 1). The specificity for calpain was also indicated by the findings that Bid cleavage was not inhibited by inhibitors of either cathepsin L or DEVDase/caspases-3 and -7 (Table 1 and Fig. 4B). The functionality of DEVD-fmk was confirmed by its ability to partially block apoptosis (Table 1). Furthermore, Bid cleavage was not blocked by dnMEKK1 (Fig. 4B). Finally, cisplatin-induced Bid cleavage was seen in caspase-3-deficient MCF-7 breast carcinoma cells (data not shown).
FIG. 4.
Cisplatin induces calpeptin-sensitive Bid cleavage. 224 cells were treated with 20 μM cisplatin for the indicated time periods in the presence or absence of inhibitors. Cleavage of full-length 21-kDa Bid to a 14-kDa truncated form was analyzed by Western blotting of cell lysates. Tubulin (β-tub.) or GAPDH was used as a loading control. (A) Cells were treated with cisplatin in the presence or absence of calpeptin (10 μM) as indicated. Also shown are the relative levels of tBid as assessed by laser densitometry and corrected for loading inequalities. (B) Cells were treated with cisplatin for 16 h in the presenceor absence of inhibitors. (Upper panel) Lanes: 1, control; 2, cisplatin; 3, cisplatin and DEVD-fmk (10 μM); 4, cisplatin and NSIT (0.5 μM); 5, cisplatin and NAC (5 mM); 6, cisplatin and dnMEKK1. An irrelevant slot has been removed between slots 4 and 5; thus, all samples are on the same filter. (Lower panel) Lanes: 1, control; 2, cisplatin; 3, cisplatin and calpeptin; 4, cisplatin and DEVD-fmk (10 μM). Lanes 5 to 7 show the results of the same experiment, but with a different filter: 5, cisplatin and calpeptin; 6, cisplatin and DEVD-fmk; 7, cisplatin and dnMEKK1. The relative tBid induction levels are also indicated. (C) Cells were treated with cisplatin for 16 h in the presence or absence of caspase-8 inhibitor IETD-fmk (10 μM) or calpeptin. The effects on Bid cleavage were analyzed by Western blotting. (D) The effect of IETD-fmk (10 μM) on cisplatin-induced (20 μM, 20 h) apoptosis was examined, and to ascertain the efficiency of the inhibitor, it was also used to block TNF-α-induced apoptosis. Cells were treated with TNF-α (30 and 60 ng/ml, 24 h, in combination with cycloheximide at 10 μM) in the presence or absence of IETD-fmk (10 μM).
In death receptor-mediated cell death, Bid is cleaved by caspase-8 to generate two forms of truncated Bid (p15-Bid and p13-Bid) (3). We have not observed any activation of caspase-8 by cisplatin (not shown). Furthermore, a caspase-8 inhibitor (IETD-fmk) did not affect cisplatin-induced Bid cleavage (Fig. 4C) or apoptosis (Table 1 and Fig. 4D). The functionality of IETD-fmk was demonstrated by its ability to inhibit tumor necrosis factor alpha (TNF-α)-induced apoptosis (Fig. 4D).
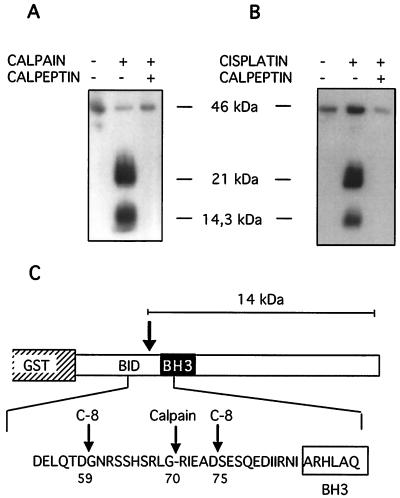
Calpain cleaves Bid between Gly70 and Arg71.
Human recombinant full-length Bid was expressed as a GST fusion protein (GST-flBid; approximately 46 kDa) and bound to glutathione-Sepharose beads. In order to investigate calpain cleavage of Bid, recombinant human m-calpain was incubated for various time intervals (10 to 60 min) with GST-flBid beads. After incubation, proteins released from the beads into the supernatant were analyzed by Western blotting with a polyclonal antibody that recognizes both full-length and cleaved Bid. Efficient Bid cleavage was seen at all time points; in the subsequent experiments, samples were incubated for 30 min.
Analysis of the supernatants showed a cleavage product that comigrated with the 14.3-kDa marker, along with a 21-kDa protein (Fig. 5A). Coincubation of samples with calpeptin (10 μM) prevented the appearance of both these bands (Fig. 5A). Some flBid was also released (Fig. 5A), likely due to the DTT-containing buffer. GST-flBid beads were similarly incubated with lysates of cells treated with cisplatin for 5 h. Lysate-mediated cleavage resulted in bands that comigrated with those induced by recombinant calpain (Fig. 5B). Calpeptin pretreatment inhibited lysate-mediated Bid cleavage (Fig. 5B), indicating that endogenous, cisplatin-activated calpain cleaves Bid.
FIG. 5.
Calpain cleavage of Bid in vitro. (A) GST-flBid (100 μg, 46 kDa), coupled to glutathione-Sepharose beads, was incubated for 30 min with human recombinant m-calpain (10 μg) as indicated. Calpeptin (10 μM) was added to the incubation mixture where indicated. The material released into the supernatants was analyzed by Western blotting for the presence of Bid fragments. This cleavage reaction was used to prepare protein for N-terminal sequencing. (B) GST-flBid (10 μg, 46 kDa), coupled to glutathione-Sepharose beads, was incubated for 60 min with lysates (500 μg) from cells treated with cisplatin. The material released into the supernatants was analyzed by Western blotting for the presence of Bid fragments. The left lane was loaded with supernatant from beads incubated with cleavage buffer only. (C) Representation of the GST-flBid protein and partial amino acid sequence showing the calpain cleavage site located N terminally of the BH3 domain. Reported caspase-8 cleavage sites are also indicated (10).
Release of a 21-kDa product indicated that calpain may cleave GST-flBid either near the N-terminal of Bid, in GST, or in the linker region; none of these products is likely to have a tBid-like effect on cytochrome c release (see Discussion). In contrast, the size of the smaller product is similar to that of caspase-cleaved Bid (14 kDa and 13 to 15 kDa, respectively).
In order to identify the calpain cleavage site, the 14-kDa fragment was eluted from the polyvinylidene difluoride membrane and subjected to five cycles of N-terminal sequencing. The cleavage site was thereby mapped between Gly70 and Arg71 in human Bid (Fig. 5C). The cleavage sites of caspase-8, which have previously been mapped to Asp59/Gly60 (in p15-Bid) and Asp75/Ser76 (in p13-Bid) (10), are also indicated.
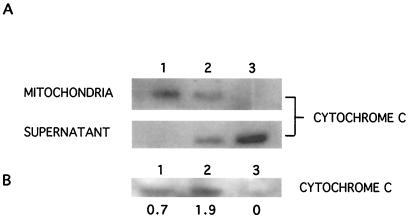
Calpain-cleaved Bid induces cytochrome c release from mitochondria.
Cleavage of Bid by caspases increases its ability to lead to release of cytochrome c from mitochondria (3). To examine whether cleavage of Bid by calpain has the same effect, mitochondria were isolated from 224 cells and incubated for 30 min with 7 μg of either flBid or of cleaved Bid obtained from GST-flBid cleavage by recombinant calpain. Nontreated mitochondria retained all cytochrome c (Fig. 6A, lane 1), whereas recombinant full-length Bid induced some cytochrome c release into the supernatant (Fig. 6A, lane 2), consistent with previous reports (26). In contrast, calpain-cleaved Bid resulted in a complete loss of cytochrome c from mitochondria (Fig. 6A, lane 3).
FIG. 6.
Calpain-cleaved Bid induces cytochrome c release from isolated mitochondria. (A) Mitochondria (85 μg of protein) isolated from 224 human melanoma cells were incubated with 7 μg of GST-flBid or cleaved Bid obtained from GST-flBid cleavage by recombinant calpain. After 40 min, the presence of cytochrome c in mitochondria and in the supernatant was assessed by Western blotting (42 μg/lane for mitochondrial samples, and 8 μl, or half the total volume, of each supernatant). Lack of mitochondrial contamination in the supernatant was confirmed with an antibody against mitochondrial COX subunit IV (not shown). Lanes: 1, control mitochondria and supernatant; 2, mitochondria treated with GST-flBid and the resulting supernatant; 3, mitochondria treated with calpain-cleaved Bid and the resulting supernatant. (B) Cytochrome c levels in cytosols prepared from control cells (lane 1) and cells treated with cisplatin (20 μM, 17 h) in the absence (lane 2) or presence (lane 3) of calpeptin. The relative amounts are indicated, as assessed by laser densitometry scanning and corrected for loading.
In intact cells, cisplatin induced cytochrome c release from mitochondria, as evidenced by analysis of cytochrome c levels in cytosol fractions of cell lysates (Fig. 6B). This release was blocked in cells cotreated with calpeptin (Fig. 6B).
Evidence for two separate signaling pathways.
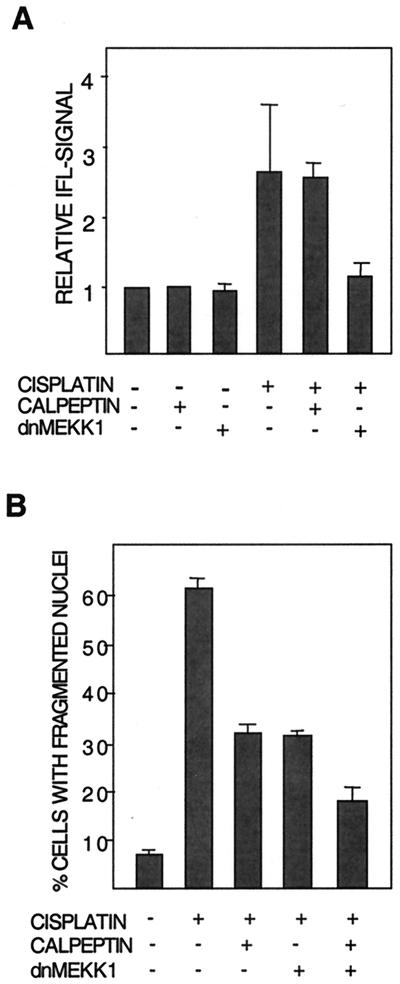
tBid has been reported to stimulate the formation of Bak oligomer pores in the mitochondrial membrane, which likely constitute a means for cytochrome c release (35). tBid might therefore be necessary for the required conformational modulation of Bak. To examine this possibility, we made use of our earlier finding that cisplatin induces modulation of Bak (19). This modulation is assessed with an antibody recognizing a specific N-terminal epitope that is exposed only when Bak is in its proapoptotic conformation (9). Modulation is quantitated with a fluorescent secondary antibody and subsequent flow cytometry analysis. The results showed that although calpeptin blocked cisplatin-induced Bid cleavage, calpeptin had no effect on cisplatin-induced Bak modulation (Fig. 7A). Similarly, calpeptin did not affect Bak modulation induced in U266 myeloma cells by genotoxic doxorubicin treatment (not shown).
FIG. 7.
Evidence of two separate signaling pathways sensitive to either calpeptin or dnMEKK1. (A) Modulation of Bak in 224 cells after 16 h of treatment with cisplatin in the presence or absence of calpeptin (10 μM) or kinase-inactive MEKK1 (dnMEKK1). Bak modulation was monitored by flow cytometry as described. Results are shown as fold increase in Bak-associated immunofluorescence (IFL). Expression of dnMEKK1 was confirmed by Western blotting (data not shown). The experiment was performed three times with similar results. (B) The effects of calpeptin and expression of dnMEKK1 per se and in combination on cisplatin-induced nuclear fragmentation were examined. Cells were treated for 20 h with 20 μM cisplatin in the presence or absence of calpeptin (10 μM) and/or dnMEKK1 expression.
We have earlier shown that dnMEKK1 inhibits the cisplatin-induced modulation of Bak and apoptosis by approximately 50% (19). Cisplatin-induced Bid cleavage was therefore examined in cells induced to express adenovirus-encoded dnMEKK1. Expression of dnMEKK1 did not inhibit Bid cleavage, showing that dnMEKK1 does not act upstream of Bid cleavage (Fig. 4B).
These data suggested that the calpeptin- and dnMEKK-sensitive pathways are independent of each other. We therefore examined whether cotreatment with both agents has an additive inhibitory effect on apoptosis. This was found to be the case (Fig. 7B).
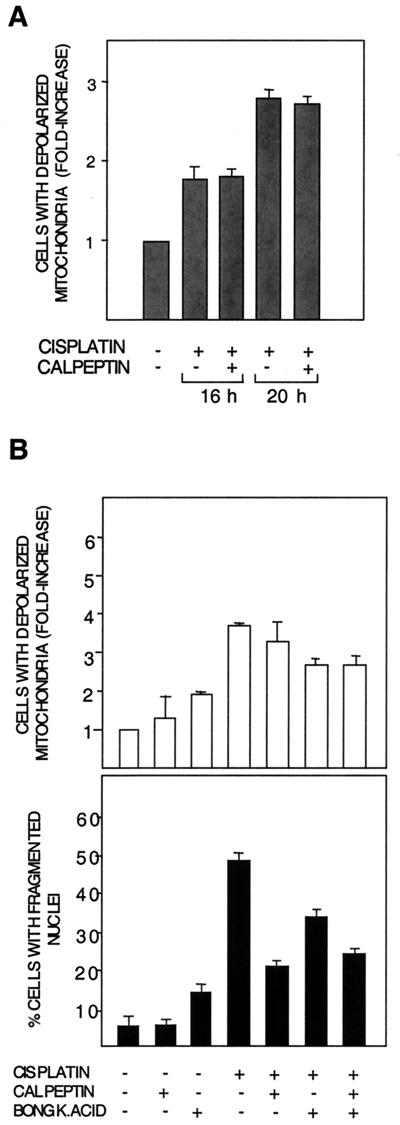
Calpeptin does not inhibit cisplatin-induced ΔΨ.
The ability of Bid to cause cytochrome c release is independent of mitochondrial permeability transition (MPT), i.e., loss of mitochondrial inner membrane potential ΔΨ (15, 26, 29). We therefore investigated the effect of calpeptin on cisplatin-induced loss of ΔΨ, seen as an increase in the population of cells with loss of TMRE staining of mitochondria. In a time course experiment, calpeptin had no effect on cisplatin-induced loss of ΔΨ, despite its inhibitory effect on apoptosis (Fig. 8A).
FIG. 8.
Calpeptin does not affect loss of ΔΨ. (A) Cisplatin-induced (20 μM) loss of mitochondrial inner membrane potential (ΔΨ) at 16 and 20 h posttreatment in the presence or absence of calpeptin (10 μM) was assessed by flow cytometry with TMRE staining of intact mitochondria. Results are shown as fold increase in the population of cells with completely depolarized mitochondria, as compared to control cells. The experiment was performed three times with similar results. (B) The effects of calpeptin and the MPT inhibitor BA(BONGK.ACID) per se and in combination were examined. BA (13 μM) was added where indicated at 12 h post-cisplatin treatment. (Upper panel) Effects of inhibitors on ΔΨ. (Lower panel) Effects on apoptosis seen as nuclear fragmentation. Note that calpeptin does not influence cisplatin-induced ΔΨ, but reduces nuclear fragmentation both in cells treated with only cisplatin and in cells treated with cisplatin and BA.
The role of loss of ΔΨ is generally studied with MPT inhibitor bongkrekic acid (BA) or cyclosporin. In distinction to calpeptin, BA inhibited cisplatin-induced loss of ΔΨ at 16 h and, similar to calpeptin, reduced nuclear fragmentation (Fig. 8B). It should be noted that BA had a toxic effect of its own (Fig. 8B). Taking into account the toxic effect of BA, the combination of BA and calpeptin had an additive inhibitory effect on nuclear fragmentation (Fig. 8B). In contrast, the combination treatment did not augment the loss of membrane potential. A similar trend was seen also with cyclosporin (data not shown).
DISCUSSION
Calpain is a ubiquitous cysteine protease with two major isoforms—m- and μ-calpain—which has recently been shown to be activated by a number of apoptosis-inducing agents, such as IR, etoposide, or staurosporine (7, 33, 34). The potential role of calpain in apoptosis is also indicated by the growing list of calpain substrates, including p53, IκB, PARP, and several cytoskeletal proteins (11, 17, 23). Likewise, calpain-mediated cleavage of Bax promotes the proapoptotic effect of Bax (37), and calpain cleavage of pro-caspase-7 and pro-caspase-3 leads to activation of these proteases (2, 25). Although the two major isoforms are known to differ in their calcium requirement for activation in vitro, the 30-kDa regulatory subunit is common to both isoforms. The isoform of importance for apoptotic signaling has not been identified. Cellular localization may also play a role, since calpain-mediated cleavage of Bax appears to depend on an as yet uncharacterized but specifically mitochondrial calpain pool (7, 37). Importantly, the mechanisms for in vivo activation of calpain are not clear. In addition to a requirement for increased calcium levels, activation may involve one or more of the factors, such as an unidentified phospholipid(s), intracellular redistribution (11), and caspase-mediated degradation of the endogenous inhibitor calpastatin (32).
Little is known about cisplatin-induced apoptotic signaling located downstream of the actual DNA damage, but upstream of cytochrome c release and caspase activation. This is the first report to show that cisplatin induces calpain activation. Using two different calpain inhibitors, we show that cisplatin-induced apoptosis is, to a significant extent, dependent on calpain activity. Together with earlier findings (19), we have now shown that calpain activation, which starts around 3 h posttreatment, is an early event in cisplatin-induced apoptosis in human melanoma cells and occurs after JNK activation, but hours before Bak modulation, cytochrome c release, and activation of caspase-9. Furthermore, calpain activation was preceded by and dependent on the increase in intracellular Ca2+, which cisplatin induced as early as 1 h posttreatment.
In contrast to this early activation, it has been reported that calpain activation by ionizing irradiation occurs after PARP cleavage and DNA fragmentation (i.e., during the late execution phase) (33). Our data show that calpeptin treatment during the first 8 h of cisplatin treatment is sufficient to inhibit apoptosis assessed at 20 h, while late addition of calpeptin had no effect. In addition, we did not see any biphasic activation of calpain up to 20 h posttreatment, when apoptosis levels are nearly 50%. Although a biphasic activation of calpain cannot be ruled out, it is unlikely to be of importance to apoptosis, since late addition of calpeptin did not protect.
Because Bid has a key role in inducing oligomerization of proapoptotic Bcl-2 family members Bak and/or Bax, which in turn leads to cytochrome c release, cleavage of Bid to tBid is of importance in apoptosis induced by many types of agent. We show here that Bid is cleaved in cisplatin-treated cells. Candidate caspases to perform this cleavage were caspase-3 and -8, while caspase-6 and -7 have been shown not to cleave Bid (3). Experiments with caspase inhibitors indicated that in this system neither caspase-3 nor caspase-8 mediates Bid cleavage. Furthermore, we did not observe DEVDase/caspase-3 activation at 7 h posttreatment (19; this study), and because there was cisplatin-induced Bid cleavage as well as in caspase-3-deficient MCF-7 cells, caspase-3 is not a likely candidate. Instead, the two functionally distinct calpain inhibitors calpeptin and PD150606 both blocked Bid cleavage, indicating that calpain is the mediator. Calpeptin may in addition to calpain also inhibit cathepsin L; however, a cathepsin L inhibitor had no effect on Bid cleavage or on apoptosis.
We show that, similar to caspases 3 and -8, human recombinant m-calpain can directly cleave human recombinant Bid (generating a 14.4-kDa fragment, as calculated from the amino acid sequence). The calpain cleavage site was mapped between Gly70 and Arg71, or N-terminal to the BH3 domain, and 11 residues C-terminal to the caspase-8 site for p15-tBid. As with caspase-cleaved tBid, calpain-cleaved Bid was able to induce cytochrome c release from isolated mitochondria, and in intact cells, calpeptin cotreatment blocked cisplatin-induced release of cytochrome c from mitochondria. Importantly, we show that lysates from cells treated with cisplatin for only 5 h can cleave Bid and that this activity is sensitive to calpeptin.
Although calpain may cleave Bax, we have not been able to detect any cisplatin-induced Bax fragments in the 224 cells, nor in AA, a p53-wt melanoma cell line, which, unlike 224 cells, shows increased Bax protein levels in response to cisplatin (data not shown). If calpain cleaves Bid, but not Bax, even though both are cytosolic before cleavage, regulation of the calpain-Bid-Bax relationship may be very intricate.
Shortly before the submission of the present work, it was reported that reperfusion of rabbit heart after myocardial ischemia led to Bid cleavage by a calpain-like activity, and in vitro experiments mapped the calpain cleavage site to Gly70 (5). Our results show that calpain activation is involved in the very different setting of genotoxin-induced apoptosis and that this activity is a physiological mechanism for Bid cleavage in human cells.
Unlike caspases and granzyme B, calpain does not recognize a well-defined cleavage motif. However, the last three residues of the N-terminal fragment are often aliphatic, and P1 on the C-terminal fragment is aliphatic or basic; consequently, there may be many potential sites within a protein, but usually only a few are actually cleaved (4). The calpain cleavage site in Bid (LG70-RIEAD) is typical in that it is preceded by two aliphatic residues, and P1 on the C-terminal side is basic. In contrast, the calpain cleavage site in Bax is within the caspase motif FIQD33 (37). In CaMK-IV, another protein that during apoptosis may be cleaved by caspases as well as calpain, the calpain sites are also less than canonical (CG201-TPG and EN23-LVP) (21). In mouse Bid, the sequence corresponding to the calpain cleavage site in human Bid is QG70-RIEP, which should also be a potential calpain site.
In the cleavage assay, the release of a 21-kDa product indicated that calpain can cleave GST-flBid at a second site. One possibility is that in addition to the Gly70 site, calpain also cleaves within the GST protein, yielding a chimeric protein consisting of the C-terminal 14 kDa of GST and an N-terminal Bid fragment up to Gly70. The Bid N-terminal fragment does not induce cytochrome c release and instead may have an inhibitory function (28); thus, the chimeric protein should not have any cytochrome c-releasing activity. Another, less likely possibility is that the secondary calpain cleavage site is either very near the N-terminal part of Bid, in the linker region, or within the GST protein close to the C-terminal part. In either case, the 21-kDa product would be nearly identical to flBid, which has little or no effect on mitochondria (10, 28) and as seen in our data. The 21-kDa protein would furthermore be cleaved as well at Gly70, thus, after all yielding the 14-kDa product of interest.
No caspase activity can be expected to be present in the in vitro assay for GST-Bid cleavage by recombinant calpain. Nevertheless, addition of zVAD (10 μM) to the reaction mixture also decreased Bid cleavage by calpain (not shown). Similarly, YVAD-CHO has been reported to reduce calpain-mediated Bax cleavage (37). In fact, zVAD at 100 μM blocked calpain-mediated cleavage in intact cells, and at 5 μM, it blocked calpain activity in vitro (33). Obviously, this raises questions about a possible overestimation of caspase involvement in many apoptosis studies. Furthermore, as in the cases of Bid, Bax, and fodrin (33), the caspase and calpain cleavage sites are so close to each other that cleavage products may be nearly identical on a Western blot.
That cisplatin induces at least two apoptotic signaling pathways was suggested by our previous work (19), showing that expression of a dnMEKK mutant inhibits cisplatin-induced Bak modulation almost completely, whereas caspase-3 activation and nuclear fragmentation are reduced by approximately half. The present work on calpain supports and extends this hypothesis. First, dnMEKK did not affect formation of tBid. Second, inhibition of calpain with calpeptin did not affect Bak modulation, but similar to dnMEKK1, it did inhibit apoptosis by approximately half. Third, the combination of dnMEKK1 and calpeptin had a clearly additive effect on inhibition of apoptosis. Thus, calpeptin and dnMEKK do not act on the same pathway.
It may be noted that although the inhibitory effect of calpeptin on apoptosis was significant at earlier time points, at 24 h, apoptosis also proceeded in the presence of calpeptin and despite its simultaneous inhibitory effect on calpain. This is compatible with the hypothesis of two pathways, which stipulates that in the presence of calpeptin, the MEKK1-Bak pathway is still active, causing cytochrome c release and DEVDase activity, which in turn can cleave and activate Bid. An amplification loop via cytochrome c to DEVDase-mediated Bid cleavage has indeed been reported for apoptosis induced by etoposide and other drugs (27).
Bid-mediated apoptosis is independent of mitochondrial MPT (or loss of ΔΨ) (15, 26, 29). In agreement with this and with calpain-mediated Bid activation, calpeptin could partially block cisplatin-induced apoptosis, but it did not affect MPT. In contrast, MPT inhibitors BA and cyclosporin partially reduced loss of membrane potential as well as apoptosis. In our system, both agents per se induced some depolarization and apoptosis. However, taking into account this toxic effect, the combination of MPT inhibitor and calpeptin had an additive effect on inhibition of apoptosis, again indicating involvement of two distinct pathways.
Bax-Bak double-knockout mouse embryo fibroblast (MEF) cells were recently shown to be resistant to a number of apoptotic stimuli, including staurosporine, etoposide, and growth factor deprivation (36). In contrast, Bid-deficient MEF cells were susceptible to these stimuli, showing that Bax or Bak can be activated by factors or pathways other than Bid (30, 36). Together, these data clearly show that Bak may be activated by at least two distinct signals, one of which is independent of tBid. Our data are in agreement with this model, since they suggest that tBid-mediated apoptosis does not necessarily require Bak modulation, and Bak modulation does not necessarily require tBid.
It is of general interest to apoptosis research that calpain activation may play an important role in apoptosis, by linking early, cytosolic events and the apoptotic machinery located in mitochondria. As an activator of Bid, calpain may be comparable to caspases. Our findings also contribute to the understanding of early apoptotic signaling induced by cisplatin, and we have identified a signaling pathway of potential importance in prediction as well as in treatment. With calpain as a potential regulator of the key role of Bid protein in chemotherapy-induced apoptosis, targeting of calpain and/or Bid is a conceivable strategy for sensitization of resistant tumors. This may in fact already have been done, because the radiosensitizing and DNA-damaging agent β-lapachone was recently shown to induce apoptosis via a calpain or a calpain-like non-caspase activity (23).
Acknowledgments
We thank Y. Tsujimoto for generously providing the GST-Bid vectors.
This work was funded by grants from the King Gustaf V Jubilee Foundation, the Cancer Society Stockholm and the Swedish Cancer Society.
REFERENCES
- 1.Barry, M., J. A. Heibein, M. J. Pinkoski, S.-F. Lee, R. W. Moyer, D. R. Green, and R. C. Bleackley. 2000. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol. Cell. Biol. 20:3781-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomgren, K., C. Zhu, X. Wang, J. O. Karlsson, A. L. Leverin, B. A. Bahr, C. Mallard, and H. Hagberg. 2001. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J. Biol. Chem. 276:10191-10198. [DOI] [PubMed] [Google Scholar]
- 3.Bossy-Wetzel, E. and D. R. Green. 1999. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem. 274:17484-17490. [DOI] [PubMed] [Google Scholar]
- 4.Carafoli, E., and M. Molinari. 1998. Calpain: a protease in search of a function? Biochem. Biophys. Res. Commun. 247:193-203. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M., H. Huaping, S. Zhan, S. Krajewski, J. C. Reed, and R. A. Gottlieb. 2001. Bid is cleaved by calpain into an active fragment in vitro, and during myocardial ischemia/reperfusion. J. Biol. Chem. 276:30724-30728 [DOI] [PubMed] [Google Scholar]
- 6.Eskes, R., S. Desagher, B. Antonsson, and J.-C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, G., and Q. P. Dou. 2001. N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes Bcl-2-independent cytochrome c release and apoptotic cell death. J. Cell. Biochem. 80:53-72. [DOI] [PubMed] [Google Scholar]
- 8.Ghafourifar, P., U. Bringold, S. D. Klein, and C. Richter. 2001. Mitochondrial nitric oxide synthase, oxidative stress and apoptosis. Biol. Signals Recept. 10:57-65. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths, G. J., L. Dubrez, C. P. Morgan, N. A. Jones, J. Whitehouse, B. M. Corfe, C. Dive, and J. A. Hickman. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 11.Han, Y., S. Weinman, I. Boldogh, R. K. Walker, and A. R. Brasier. 1999. Tumor necrosis factor-α-inducible IκBα proteolysis mediated by cytosolic m-calpain. J. Biol. Chem. 274:787-794. [DOI] [PubMed] [Google Scholar]
- 12.Huang, D. C., and A. Strasser. 2000. BH3-only proteins—essential initiators of apoptotic cell death. Cell 103:839-842. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara, I., Y. Minami, T. Nishizaki, T. Matsuoka, and H. Yamamura. 2000. Activation of calpain precedes morphological alterations during hydrogen peroxide-induced apoptosis in neuronally differentiated mouse embryonal carcinoma P19 cell line. Neurosci. Lett. 279:97-100. [DOI] [PubMed] [Google Scholar]
- 14.Kharbanda, S., R. Ren, P. Pandey, T. D. Shafman, S. M. Feller, R. R. Weichselbaum, and D. W. Kufe. 1995. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376:785-788. [DOI] [PubMed] [Google Scholar]
- 15.Kim, T. H., Y. Zhao, M. J. Barber, D. K. Kuharsky, and X. M. Yin. 2000. Bid-induced cytochrome c release is mediated by a pathway independent of mitchondrial permeability transition pore and Bax. J. Biol. Chem. 275:39474-39481. [DOI] [PubMed] [Google Scholar]
- 16.Korsmeyer, S. J., M. C. Wei, M. Saito, S. Weiler, K. J. Oh, and P. H. Schlesinger. 2000. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7:1166-1173. [DOI] [PubMed] [Google Scholar]
- 17.Kubbutat, M. H. G., and K. H. Vousden. 1997. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol. Cell. Biol. 17:460-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 19.Mandic, A., K. Viktorsson, M. Molin, G. Akusjärvi, H. Eguchi, S.-I. Hayashi, M. Toi, J. Hansson, S. Linder, and M. C. Shoshan. 2001. Cisplatin induces the proapoptotic conformation of Bak in a ΔMEKK1-dependent manner. Mol. Cell. Biol. 21:3684-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCurrach, M. E., T. M. Connor, C. M. Knudson, S. J. Korsmeyer, and S. W. Lowe. 1997. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinnis, K. M., M. M. Whitton, M. E. Gnegy, and K. K. W. Wang. 1998. Calcium/calmodulin-dependent protein kinase IV is cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. J. Biol. Chem. 273:19993-20000. [DOI] [PubMed] [Google Scholar]
- 22.Nehmé, A., R. Baskarna, S. Aebi, D. Fink, S. Nebel, B. Cenni, J. Y. J. Wang, S. B. Howell, and R. D. Christen. 1997. Differential induction of c-Jun NH2-terminal kinase and c-Abl kinase in DNA mismatch repair-proficient and -deficient cells exposed to cisplatin. Cancer Res. 57:3253-3257. [PubMed] [Google Scholar]
- 23.Pink, J. J., S. Wuerzberger-Davis, C. Tagliarino, S. M. Planchon, X. Yang, C. J. Froelich, and D. A. Boothman. 2000. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during beta-lapachone-mediated apoptosis. Exp. Cell. Res. 255:144-155. [DOI] [PubMed] [Google Scholar]
- 24.Ruffolo, S. C., D. G. Breckenridge, M. Nguyen, I. S. Goping, A. Gross, S. J. Korsmeyer, H. Li, J. Yuan, and G. C. Shore. 2000. BID-dependent and BID-independent pathways for BAX insertion into mitochondria. Cell Death Differ. 7:1101-1108. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Vela, A., G. Gonzalez de Buitrago, and A. C. Martinez. 1999. Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 18:4988-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu, S., and Y. Tsujimoto. 2000. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release but not mitochondrial membrane potential loss and do not directly modulate voltage-dependent anion channel activity. Proc. Natl. Acad. Sci. USA 90:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slee, E. A., S. A. Keogh, and S. J. Martin. 2000. Cleavage of BID curing cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7:556-565. [DOI] [PubMed] [Google Scholar]
- 28.Tan, K. O., K. M. Tan, and V. C. Yu. 1999. A novel BH3-like domain in BID is required for intramolecular interaction and autoinhibition of pro-apoptotic activity. J. Biol. Chem. 274:23687-23690. [DOI] [PubMed] [Google Scholar]
- 29.von Ahsen, O., C. Renken, G. Perkins, R. M. Kluck, E. Bossy-Wetzel, and D. D. Newmeyer. 2000. Preservation of mitochondrial structure and function after Bid- or Bax-induced cytochrome c release. J. Cell Biol. 150:1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, G. Q., B. R. Gastman, E. Wieckowski, L. Goldstein, A. Gambotto, T. H. Kim, B. Fang, A. Rabinovitz, X. M. Yin, and H. Rabinowich. 2001. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J. Biol. Chem. 276:34307-34317. [DOI] [PubMed] [Google Scholar]
- 31.Wang, K. K., R. Nath, A. Posner, K. J. Raser, M. Buroker-Kilgore, I. Hajimohammadreza, A. W. Probert, F. W. Marcoux, Q. Ye, E. Takano, M. Hatanaka, M. Maki, H. Caner, J. L. Collins, A. Fergus, K. S. Lee, E. A. Lunney, S. J. Hays, and P. Yuen. 1996. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. USA 93:6687-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, K. K., R. Posmantur, R. Nadimpalli, R. Nath, P. Mohan, R. A. Nixon, R. V. Talanian, M. Keegan, L. Herzog, and H. Allen. 1998. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch. Biochem. Biophys. 356:187-196. [DOI] [PubMed] [Google Scholar]
- 33.Waterhouse, N. J., D. M. Finucane, D. R. Green, J. S. Elce, S. Kumar, E. S. Alnemri, G. Litwack, K. Khanna, M. F. Lavin, and D. J. Watters. 1998. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 5:1051-1061. [DOI] [PubMed] [Google Scholar]
- 34.Watters, D. 1999. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol. Cell Biol. 77:263-271. [DOI] [PubMed] [Google Scholar]
- 35.Wei, M. C., T. Lindsten, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 36.Wei, M. C., W. X. Zong, E. H. Y. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood, D. E., A. Thomas, L. A. Devi, Y. Berman, R. C. Beavis, J. C. Reed, and E. W. Newcomb. 1998. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17:1069-1078. [DOI] [PubMed] [Google Scholar]
- 38.Wood, D. E., and E. W. Newcomb. 1999. Caspase-dependent activation of calpain during drug-induced apoptosis. J. Biol. Chem. 274:8309-8315. [DOI] [PubMed] [Google Scholar]